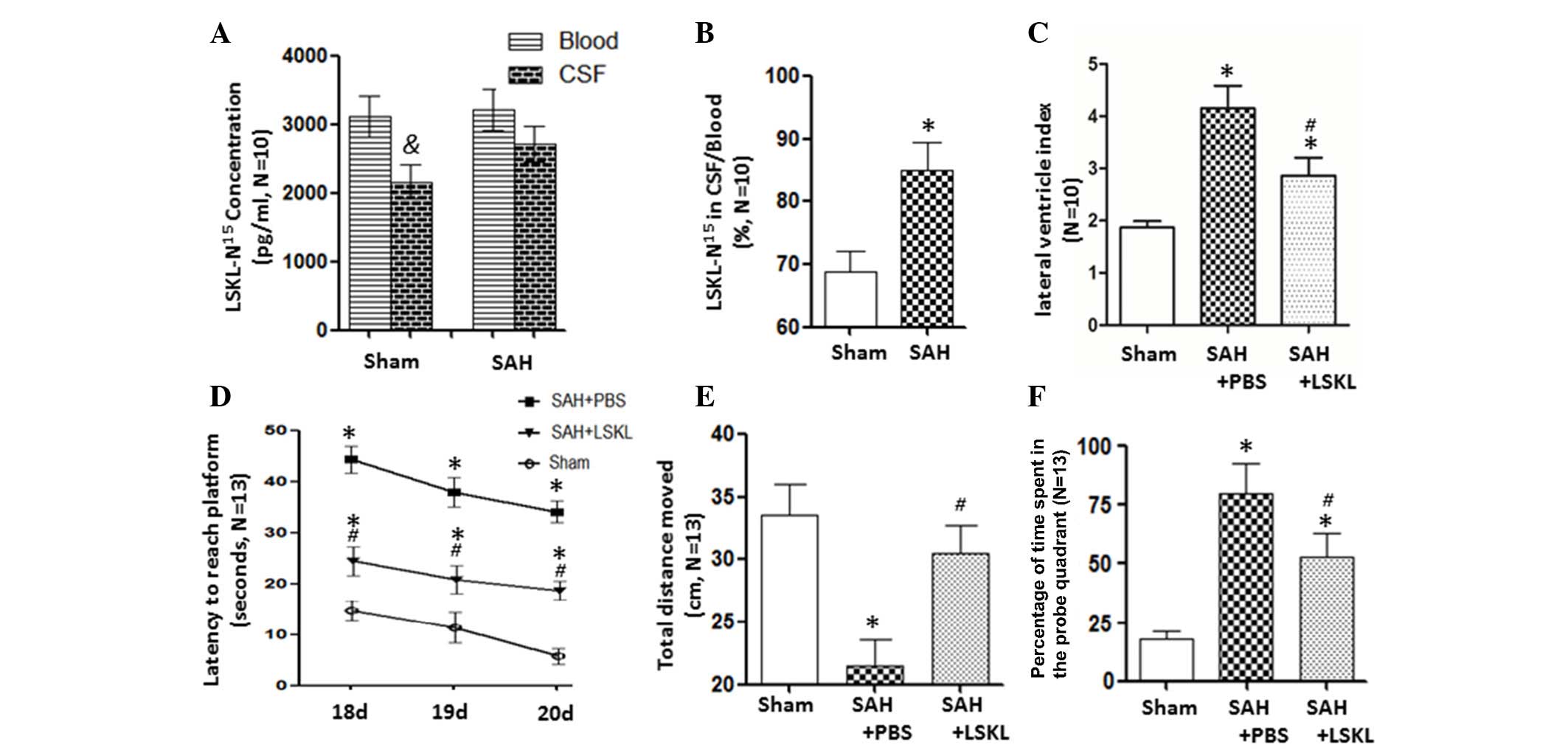
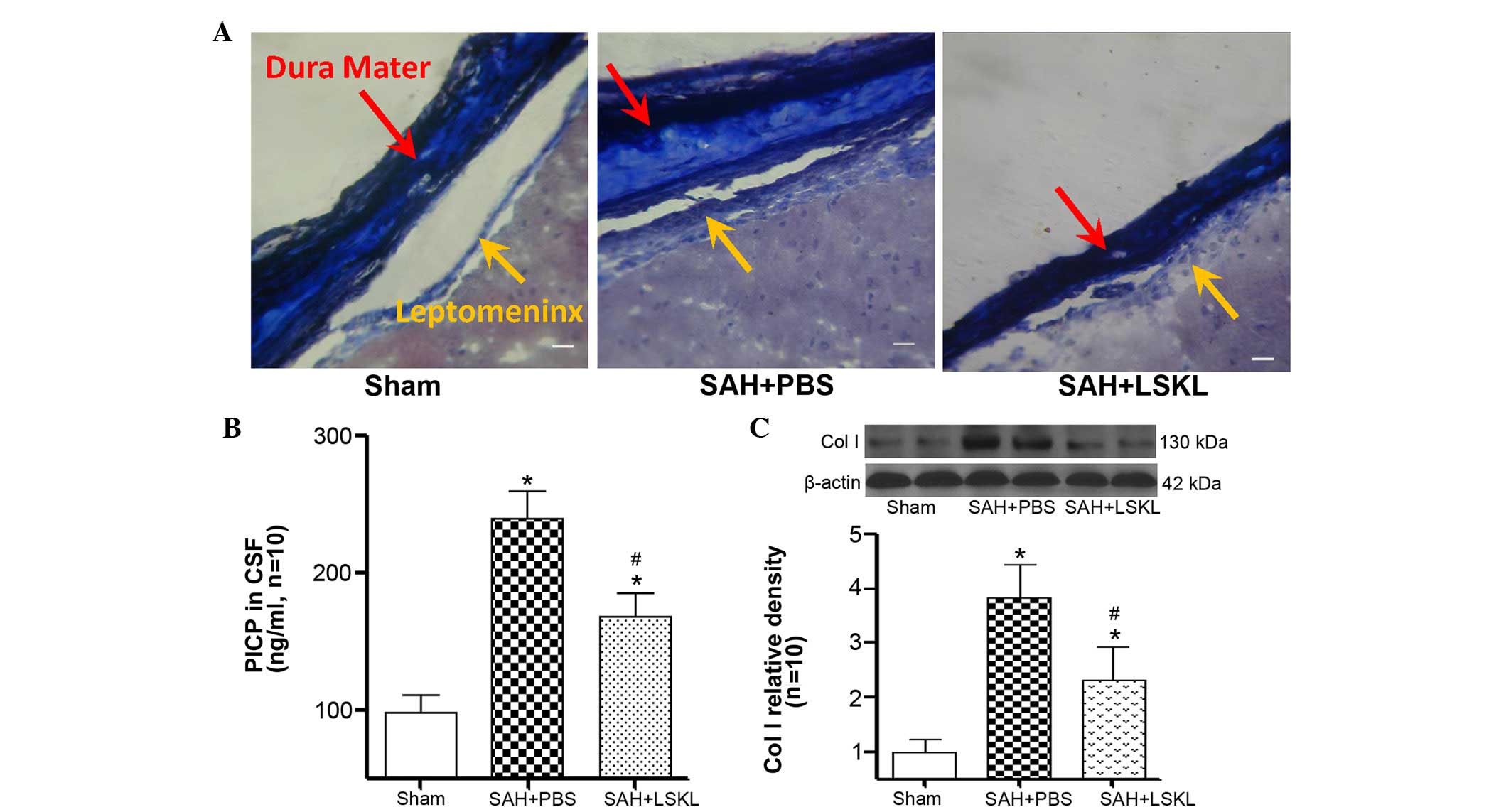
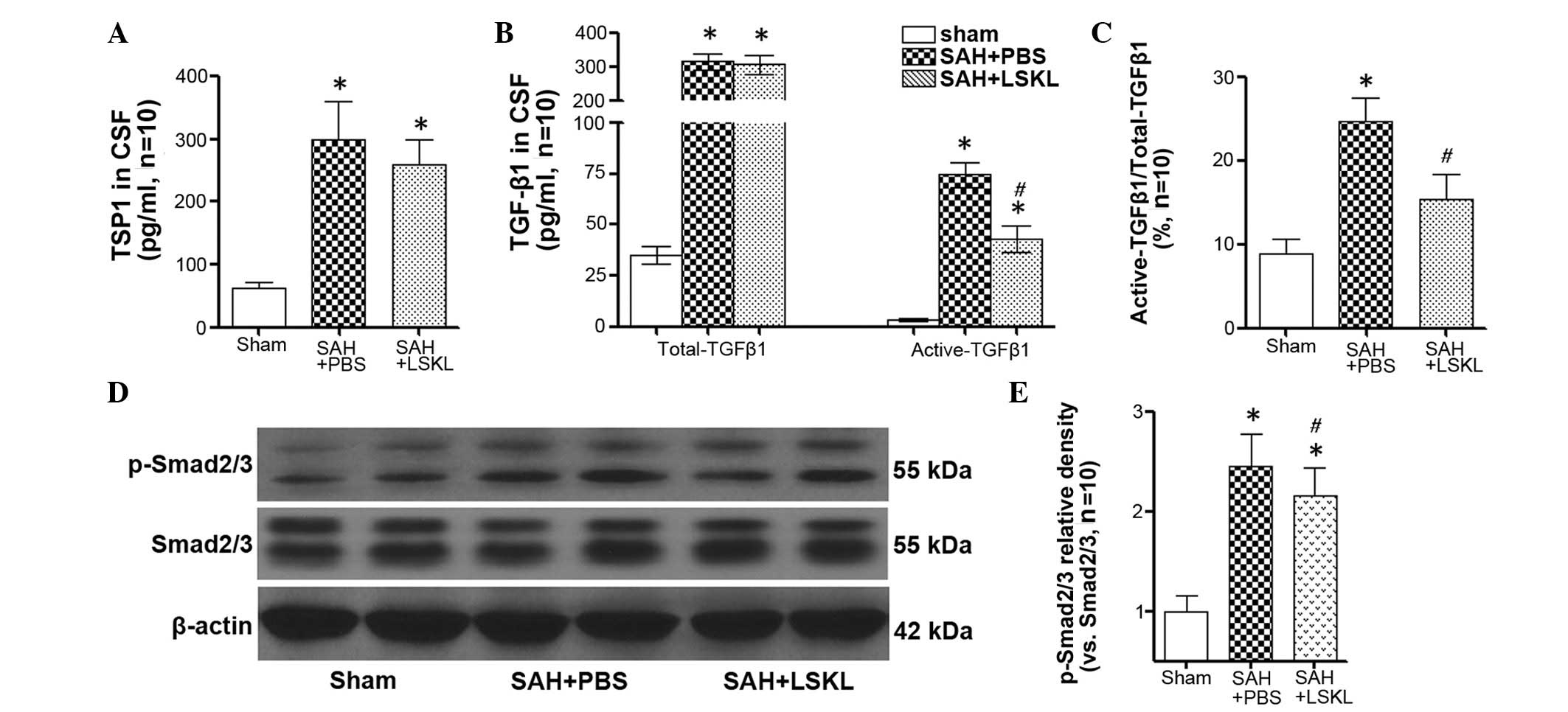
|
1
|
Fujii M, Yan J, Rolland WB, Soejima Y,
Caner B and Zhang JH: Early brain injury, an evolving frontier in
subarachnoid hemorrhage research. Transl Stroke Res. 4:432–446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giraldo EA, Mandrekar JN, Rubin MN, Dupont
SA, Zhang Y, Lanzino G, Wijdicks EF and Rabinstein AA: Timing of
clinical grade assessment and poor outcome in patients with
aneurysmal subarachnoid hemorrhage. J Neurosurg. 117:15–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah AH and Komotar RJ: Pathophysiology of
acute hydrocephalus after subarachnoid hemorrhage. World Neurosurg.
80:304–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Botfield H, Gonzalez AM, Abdullah O,
Skjolding AD, Berry M, McAllister JP II and Logan A: Decorin
prevents the development of juvenile communicating hydrocephalus.
Brain. 136:2842–2858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishii M, Suzuki S, Iwabuchi T and Julow J:
Effect of antifibrinolytic therapy on subarachnoid fibrosis in dogs
after experimental subarachnoid haemorrhage. Acta Neurochir (Wien).
54:17–24. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graff-Radford NR, Torner J, Adams HP Jr
and Kassell NF: Factors associated with hydrocephalus after
subarachnoid hemorrhage. A report of the Cooperative Aneurysm
Study. Arch Neurol. 46:744–752. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Yuan W, Liao D, Zhang T and Wang Z:
Association of chronic hydrocephalus after aneurysmal subarachnoid
hemorrhage with transforming growth factor-β1 levels and other risk
factors. Nan Fang Yi Ke Da Xue Xue Bao. 33:382–385. 2013.(In
Chinese). PubMed/NCBI
|
|
8
|
Li T, Zhang P, Yuan B, Zhao D, Chen Y and
Zhang X: Thrombin-induced TGF-β1 pathway: A cause of communicating
hydrocephalus post subarachnoid hemorrhage. Int J Mol Med.
31:660–666. 2013.PubMed/NCBI
|
|
9
|
Douglas MR, Daniel M, Lagord C, Akinwunmi
J, Jackowski A, Cooper C, Berry M and Logan A: High CSF
transforming growth factor beta levels after subarachnoid
haemorrhage: Association with chronic communicating hydrocephalus.
J Neurol Neurosurg Psychiatry. 80:545–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribeiro SM, Poczatek M, Schultz-Cherry S,
Villain M and Murphy-Ullrich JE: The activation sequence of
thrombospondin-1 interacts with the latency-associated peptide to
regulate activation of latent transforming growth factor-beta. J
Biol Chem. 274:13586–13593. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuroki H, Hayashi H, Nakagawa S, Sakamoto
K, Higashi T, Nitta H, Hashimoto D, Chikamoto A, Beppu T and Baba
H: Effect of LSKL peptide on thrombospondin 1-mediated transforming
growth factor β signal activation and liver regeneration after
hepatectomy in an experimental model. Br J Surg. 102:813–825. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie XS, Li FY, Liu HC, Deng Y, Li Z and
Fan JM: LSKL, a peptide antagonist of thrombospondin-1, attenuates
renal interstitial fibrosis in rats with unilateral ureteral
obstruction. Arch Pharm Res. 33:275–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondou H, Mushiake S, Etani Y, Miyoshi Y,
Michigami T and Ozono K: A blocking peptide for transforming growth
factor-beta1 activation prevents hepatic fibrosis in vivo. J
Hepatol. 39:742–748. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Xu X, Zhou D, Li H, You W, Wang Z
and Chen G: Possible role of raf-1 kinase in the development of
cerebral vasospasm and early brain injury after experimental
subarachnoid hemorrhage in rats. Mol Neurobiol. 52:1527–1539. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugawara T, Ayer R, Jadhav V and Zhang JH:
A new grading system evaluating bleeding scale in filament
perforation subarachnoid hemorrhage rat model. J Neurosci Methods.
167:327–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan H, Chen Y, Li L, Jiang J, Wu G, Zuo Y,
Zhang JH, Feng H, Yan X and Liu F: Decorin alleviated chronic
hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after
subarachnoid hemorrhage in rats. Brain Res. 1630:241–253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F, Chen Y, Hu Q, Li B, Tang J, He Y,
Guo Z, Feng H, Tang J and Zhang JH: MFGE8/Integrin β3 pathway
alleviates apoptosis and inflammation in early brain injury after
subarachnoid hemorrhage in rats. Exp Neurol. 272:120–127. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu F, Hu Q, Li B, Manaenko A, Chen Y,
Tang J, Guo Z, Tang J and Zhang JH: Recombinant milk fat
globule-EGF factor-8 reduces oxidative stress via integrin
β3/nuclear factor erythroid 2-related factor 2/heme oxygenase
pathway in subarachnoid hemorrhage rats. Stroke. 45:3691–3697.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Q, Liang X, Chen D, Chen Y, Doycheva D,
Tang J, Tang J and Zhang JH: Delayed hyperbaric oxygen therapy
promotes neurogenesis through reactive oxygen
species/hypoxia-inducible factor-1α/β-catenin pathway in middle
cerebral artery occlusion rats. Stroke. 45:1807–1814. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okubo S, Strahle J, Keep RF, Hua Y and Xi
G: Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke.
44:547–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Luo C, Zhao M, Li Q, Hu R, Zhang
JH, Liu Z and Feng H: Administration of a PTEN inhibitor BPV(pic)
attenuates early brain injury via modulating AMPA receptor subunits
after subarachnoid hemorrhage in rats. Neurosci Lett. 588:131–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo
C, Tang J, Feng H and Zhang JH: Norrin protected blood-brain
barrier via frizzled-4/β-catenin pathway after subarachnoid
hemorrhage in rats. Stroke. 46:529–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sehba FA, Hou J, Pluta RM and Zhang JH:
The importance of early brain injury after subarachnoid hemorrhage.
Prog Neurobiol. 97:14–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Macdonald RL: Delayed neurological
deterioration after subarachnoid haemorrhage. Nat Rev Neurol.
10:44–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Feng H, Sherchan P, Klebe D, Zhao
G, Sun X, Zhang J, Tang J and Zhang JH: Controversies and evolving
new mechanisms in subarachnoid hemorrhage. Prog Neurobiol.
115:64–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lackner P, Vahmjanin A, Hu Q, Krafft PR,
Rolland W and Zhang JH: Chronic hydrocephalus after experimental
subarachnoid hemorrhage. PLoS One. 8:e695712013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chu SH, Feng DF, Ma YB, Zhang H, Zhu ZA,
Li ZQ and Zhang ZH: Expression of HGF and VEGF in the cerebral
tissue of adult rats with chronic hydrocephalus after subarachnoid
hemorrhage. Mol Med Rep. 4:785–791. 2011.PubMed/NCBI
|
|
28
|
Strahle J, Garton HJ, Maher CO, Muraszko
KM, Keep RF and Xi G: Mechanisms of hydrocephalus after neonatal
and adult intraventricular hemorrhage. Transl Stroke Res. 3 Suppl
1:S25–S38. 2012. View Article : Google Scholar
|
|
29
|
Orešković D and Klarica M: Development of
hydrocephalus and classical hypothesis of cerebrospinal fluid
hydrodynamics: Facts and illusions. Prog Neurobiol. 94:238–258.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HS: Paracrine role for TGF-β-induced
CTGF and VEGF in mesangial matrix expansion in progressive
glomerular disease. Histol Histopathol. 27:1131–1141.
2012.PubMed/NCBI
|
|
31
|
Flood C, Akinwunmi J, Lagord C, Daniel M,
Berry M, Jackowski A and Logan A: Transforming growth factor-beta1
in the cerebrospinal fluid of patients with subarachnoid
hemorrhage: Titers derived from exogenous and endogenous sources. J
Cereb Blood Flow Metab. 21:157–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Young GD and Murphy-Ullrich JE: Molecular
interactions that confer latency to transforming growth
factor-beta. J Biol Chem. 279:38032–38039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krishna SM, Seto SW, Jose RJ, Biros E,
Moran CS, Wang Y, Clancy P and Golledge J: A peptide antagonist of
thrombospondin-1 promotes abdominal aortic aneurysm progression in
the angiotensin II-infused apolipoprotein-E-deficient mouse.
Arterioscler Thromb Vasc Biol. 35:389–398. 2015. View Article : Google Scholar : PubMed/NCBI
|