Introduction
Subarachnoid hemorrhage (SAH) is a fatal subtype of
hemorrhagic stroke associated with significant morbidity and a high
mortality rate of up to 50% (1).
Hydrocephalus is an independent risk factor for poor outcomes in
patients with SAH (2). Blockage of
cerebrospinal fluid (CSF) flow and drainage is widely considered to
play a vital role in communicating hydrocephalus (3), possibly by inducing subarachnoid
fibrosis (4,5). Therefore, to improve long-term
neurological outcomes of patients with SAH, it is important to
develop new therapies for subarachnoid fibrosis and chronic
hydrocephalus.
Antifibrinolytic therapy, commonly used for reducing
the rate of further hemorrhage in patients with SAH, has also been
reported to be associated with hydrocephalus and delayed brain
injury after SAH (6). A previous
study by the authors of the present study indicated that
transforming growth factor-β1 (TGF-β1), a key fibrogenic factor, is
significantly elevated in the CSF after SAH (7), which implies a pivotal role in the
development of chronic hydrocephalus (8,9). TGF-β
is usually stored in the extracellular matrix by binding to
latency-associated peptide (LAP). Thrombospondin-1 (TSP1) converts
the latent TGF-β/LAP complex to its active form by binding to LAP,
releasing TGF-β, and making it available to bind to and activate
the TGF-β receptor (10).
Several previous studies have reported that a small
peptide (molecular weight, 458.6 Da), the
leucine-serine-lysine-leucine (LSKL) peptide, can inhibit the
binding of TSP1 to LAP (11–13) and alleviate renal interstitial
fibrosis (12) as well as hepatic
fibrosis (13). It remains unclear,
however, whether LSKL is protective against subarachnoid fibrosis
and chronic hydrocephalus following SAH. Thus, the present study
sought to investigate the potential protective role of LSKL in
SAH-induced subarachnoid fibrosis, specifically whether it acts via
inhibition of TGF-β1 signaling, prevention of chronic
hydrocephalus, and improvement in long-term cognitive deficits in a
rat model of SAH.
Materials and methods
Animals
All experimental protocols were approved by the
Ethics Committee of Central South University (Changsha, China), and
all animal procedures were conducted in accordance with the eighth
edition of the National Institutes of Health Guide for the Care and
Use of Laboratory Animals (2011).
In total, 103 male Sprague-Dawley rats (age, 6
weeks; weight, 160–180 g; Experimental Center of Central South
University) were used. These rats were divided into four groups:
Sham group (n=34), SAH+phosphate buffer solution (PBS) group
(n=28), SAH+N15-labeled LSKL peptide
(LSKL-N15) group (n=12) and SAH+LSKL group (n=29). All
rats were kept in a quiet room at 23–25°C, 70% humidity and a 12 h
light/dark cycle and were allowed free access to normal rat chow
and filtered water.
SAH model
Rats were intubated transorally and then
anesthetized and maintained under anesthesia with 3% isoflurane in
70:30 medical air:oxygen. The rats were then placed in a supine
position on a heating pad to maintain the body temperature at
36.5±0.5°C. The SAH model was executed according to the
two-hemorrhage cisterna magna method as previously described
(14). Briefly, a small (1.0–1.5 cm)
longitudinal, midline suboccipital incision was made over the
center of the foramen magnum, and the neck muscles were dissected
until the dura was visible. Autologous unheparinized blood (0.5 ml)
drawn from the arteria cruralis was injected into the cisterna
magna (defined as day 0) with a 25-gauge butterfly needle. The rat
was then placed on an inclined board at a 45° angle with the head
down in a neutral position for 30 min. A second injection of blood
was conducted 24 h later by the same procedure. Sham-operated
animals were treated in a similar manner with the exception that
0.5 ml PBS was injected instead of 0.5 ml unheparinized blood.
The severity of SAH was assessed using an 18-point
SAH grading scale as previously reported (15). The score was based on the amount of
subarachnoid blood clot. Each of six segments of the basal cistern
were scored on a scale from 0 to 3 (total, 0–18 points). If the
score is <8, then the animal was excluded from the study.
Previous and present studies conducted in the current team's
laboratory demonstrate minimal variability in the scoring of SAH
(16–18).
LSKL peptide administration and
detection
To detect the ability of LSKL to pass the blood
brain barrier, isotope-labeled LSKL peptide (LSKL-N15;
GL Biochem Ltd., Shanghai, China), 1 mg/kg (11), was injected intraperitoneally on day
3 after SAH (n=10). At 5 min after administration, 100 µl clear and
blood-free CSF was collected via cisterna magna puncture under
microsurgery with a 27-gauge needle and immediately frozen on dry
ice and maintained at −80°C until analysis. At the same time, 1 ml
fresh venous blood was collected from the left femoral vein and
centrifuged at 4°C and 12,000 × g for 10 min. The supernatant was
collected and maintained at −80°C until analysis. The concentration
of LSKL-N15 was detected by stable isotope ratio mass
spectrometry (Finnigan MAT 253; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
For functional assessment of the LSKL peptide, LSKL
(1 mg/kg) was intraperitoneally administered immediately following
the first blood injection of the SAH modeling procedure and
repeated once every 12 h until sacrifice.
Morris water maze
Between days 17 and 20 following SAH, the Morris
water maze test (n=13 per group) was performed in a blinded setup
to evaluate SAH-induced neurocognitive deficits, as previously
described (19). Briefly, the test
consisted of three trials, including a cued learning paradigm,
spatial paradigm and probe paradigm. All trials lasted a maximum of
60 sec.
The cued learning trials were conducted on day 17
after SAH for non-associative factors that could affect scoring on
the Morris water maze, such as motivation, swimming ability and
vision. During the cued learning trials, there was a visible
platform above the water surface. Rats were placed in the tank and
required simply to swim to the platform to end the trial, and were
allowed to remain on the platform for 10 sec after finding or being
guided to it.
On the following 3 consecutive days, the spatial and
probe trials were conducted to measure the ability of the rat to
learn and remember the location of a hidden platform in the tank.
Unlike the cued learning trial, the platform in these trials was
submerged under the water. Once a rat was released in the tank, it
was allowed to swim and search for the platform. The total distance
to find the platform was measured to reflect spatial learning
ability. At 1 h after the spatial trial, the platform was removed
completely and the rats were allowed to swim again in search of the
now-absent platform. The percentage of time spent in the probe
quadrant (the previous location of the platform) was measured to
reflect spatial memory ability.
Lateral ventricle index
calculation
On day 21 after SAH, rats (n=10, shared with Morris
water maze test) were transcardially perfused with ice-cold PBS (pH
7.4), followed by perfusion with 4% paraformaldehyde. The brains
were then removed and fixed in 4% paraformaldehyde at 4°C for 3
days. Following dehydration with 30% sucrose in PBS (pH 7.4), the
brains were cut into 10 µm sections on a vibratome.
The size of the lateral ventricle was determined
using the lateral ventricle index, which was calculated on the
basis of measurements of the brain slices using ImageJ software
(version 1.48; National Institutes of Health, Bethesda, MD, USA).
Specifically, the lateral ventricle index was calculated as the
lateral ventricle volume divided by the total area of the brain
slice at the level of the preoptic chiasm. Hydrocephalus was
defined as a lateral ventricle index >3 standard deviations
above the mean in sham animals (20).
Masson staining
The brain samples (n=3, shared with Morris water
maze test) were prepared in a manner similar to that of the animals
used for the lateral ventricle index calculation, with the
exception that brain tissue from the dura mater was not removed in
order to prevent possible traction injury of the leptomeninges.
Masson staining was performed according to the manufacturer's
protocol (Leagene Biotech Co., Ltd., Beijing, China). Briefly, the
brain slices were stained with Masson composite staining solution
for 5 min, washed briefly with 0.2% acetic acid solution, for 5 min
with 5% phosphotungstic acid, and twice briefly with 0.2% acetic
acid. Then, the slices were stained with 1% aniline blue for 5 min,
followed by washing twice briefly with 0.2% acetic acid. The slices
were then dehydrated in absolute alcohol, rendered transparent by
placing in xylene, and finally mounted with neutral gum. Collagen
fibers appeared bluish green in color when observed under a light
microscope.
Enzyme-linked immunosorbent assay
(ELISA)
On days 3, 4 and 5 after SAH, 100 µl CSF was
collected (n=10) 5 min after LSKL administration. These CSF samples
were mixed and divided into three equal shares for the detection of
TSP1, activated TGF-β1 and total TGF-β1 using the respective ELISA
kit (Wuhan Boster Biological Technology, Ltd., Wuhan, China). For
total TGF-β1 detection, 1 mol/l HCl was added to the CSF at the
ratio of 3:1 for 10 min. The sample was then analyzed using the
ELISA kit used to detect activated TGF-β1.
On day 21 after SAH, 100 µl CSF was collected from
each of the rats used for the Morris water maze test (n=10). The
content of pro-collagen I c-terminal propeptide (PICP) was then
detected using a PICP ELISA kit (Wuhan Boster Biological
Technology, Ltd.).
Western blotting
Western blot analysis for superficial brain tissues
was performed as previously described (21,22).
Brain tissues were isolated on day 5 after SAH (n=10) and
homogenized in a lysis buffer containing 150 mmol/l NaCl, 50 mmol/l
Tris-HCl, 10 mmol/l ethylenediamine tetra-acetic acid, 0.1%
Tween-20, 1% Triton X-100, 0.1% β-mercaptoethanol, 0.1 mmol/l
phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin and 5 µg/ml
aprotinin, pH 7.4. Homogenates were centrifuged at 4°C for 10 min
at 10,000 × g, and supernatants were collected. Protein
concentrations were determined using a protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA). Following this, the sample (50
µg) was loaded onto 10% polyacrylamide gel with 0.1% sodium dodecyl
sulfate and separated by electrophoresis at 100 V for 120 min.
Proteins were then transferred onto nitrocellulose membranes and
probed with primary antibodies against collagen I (1:2,000;
sc-59772; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Smad2/3
and phospho (p)-Smad2/3 (1:1,000; sc-376928 and sc-101801,
respectively; Santa Cruz Biotechnology, Inc.). After washing,
membranes were incubated with secondary horseradish peroxidase
(HRP)-conjugated goat anti-rabbit antibody and HRP-conjugated goat
anti-mouse antibody (1:4,000; A0208 and A0216; Beyotime Institute
of Biotechnology, Shanghai, China). Proteins were visualized with
enhanced chemiluminescence reagents (ECL Plus; GE Healthcare Life
Sciences, Chalfont, UK), and blots were exposed to Hyperfilm. The
results were analyzed with Kodak ID image analysis software (Kodak,
Rochester, NY, USA). The relative intensity of the bands of
interest was calculated by correcting for β-actin (1:4,000;
sc-47778; Santa Cruz Biotechnology, Inc.) from the same sample.
Fold changes were then calculated against control intensities from
sham-treated animals.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS
version 16.0 software (SPSS, Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard error of the mean and analyzed by
one-way analysis of variance followed by Student-Newman-Keuls test.
Mortality data were analyzed by Fisher's exact test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Mortality
Of the 103 rats in the present study, 14 rats died
and were excluded from further experiments. One rat in the sham
group died during surgery. Five rats in the SAH+PBS group, 2 rats
in the SAH+LSKL-N15 group and 6 rats in the SAH+LSKL
group died within 72 h after SAH model initiation, perhaps due to
severe brain injury. The total mortality rates were 17.8, 16.7 and
20.7% in the SAH+PBS, SAH+LSKL-N15 and SAH+LSKL groups,
respectively, with no significant differences between the groups
(P>0.05).
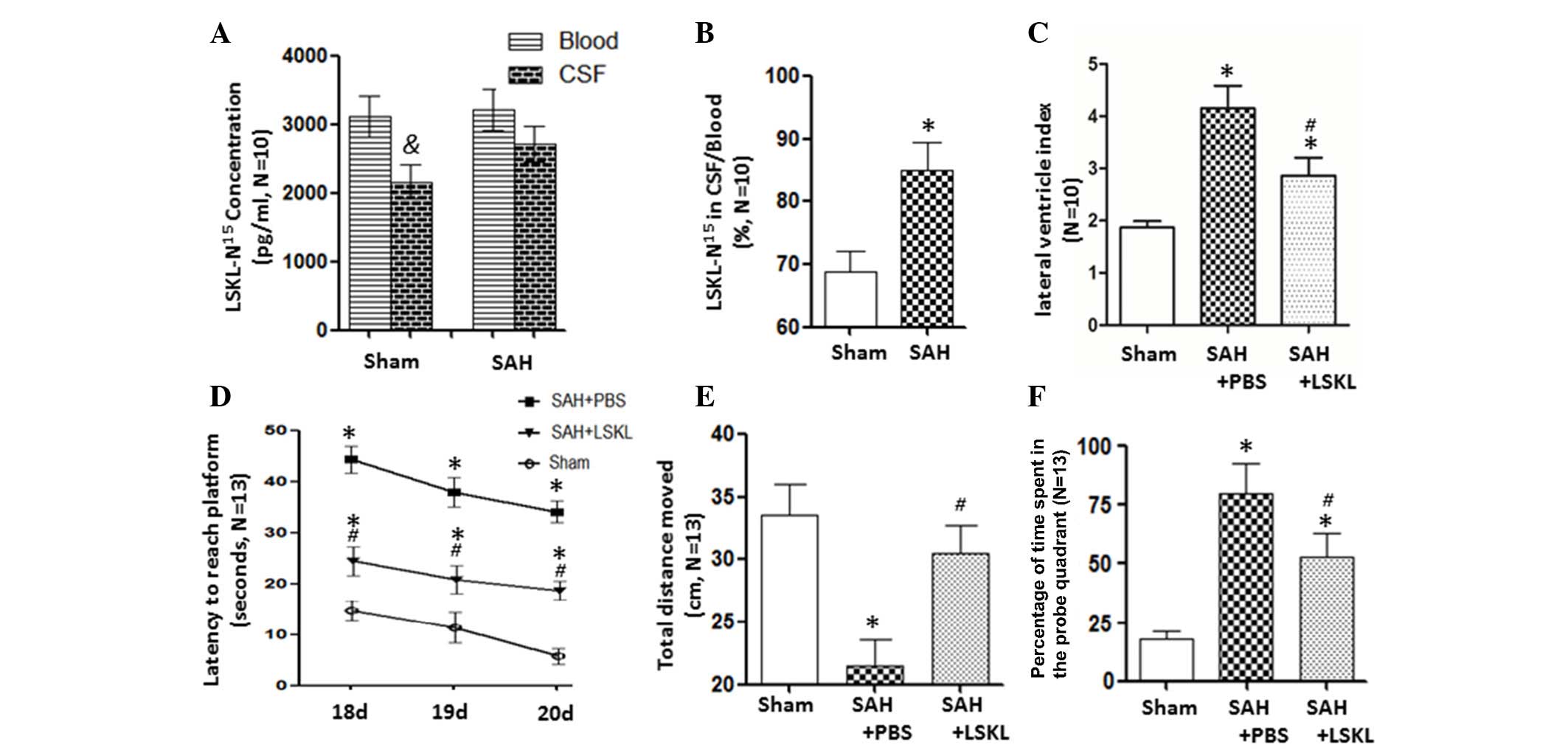
LSKL peptide can easily traverse the
blood brain barrier
At 5 min after the intraperitoneal injection of
N15-labelled LSKL into the rats, the concentration of
LSKL-N15 was lower in the CSF than in the blood in sham
rats (P<0.05); however, there was no significant difference in
LSKL-N15 concentration between the CSF and blood in the
SAH group (Fig. 1A). The ratio of
LSKL-N15 between the CSF and blood significantly
increased after SAH in comparison with that in the sham group
(P<0.05) by a mean value of 84.99% (Fig. 1B).
LSKL peptide alleviates hydrocephalus
and long-term cognitive deficits after SAH
PBS-treated rats following SAH exhibited a
significantly larger lateral ventricular index compared with the
sham rats (P<0.05). Further analysis revealed that LSKL
treatment reduced the lateral ventricular index, an indicator of
ventriculomegaly, compared with that in the SAP+PBS group
(P<0.05; Fig. 1C).
The Morris water maze results indicate that the
two-hemorrhage injection rat model of SAH induced significant
increases in latency to reach the platform on days 18, 19 and 20
after SAH (P<0.05; Fig. 1D),
decreased the total swim distance (P<0.05; Fig. 1E), and increased the number of times
that the rat entered the platform quadrant (P<0.05; Fig. 1F). However, treatment with the LSKL
peptide attenuated these unfavorable effects when compared with the
SAH+PBS group (P<0.05; Fig.
1D-F).
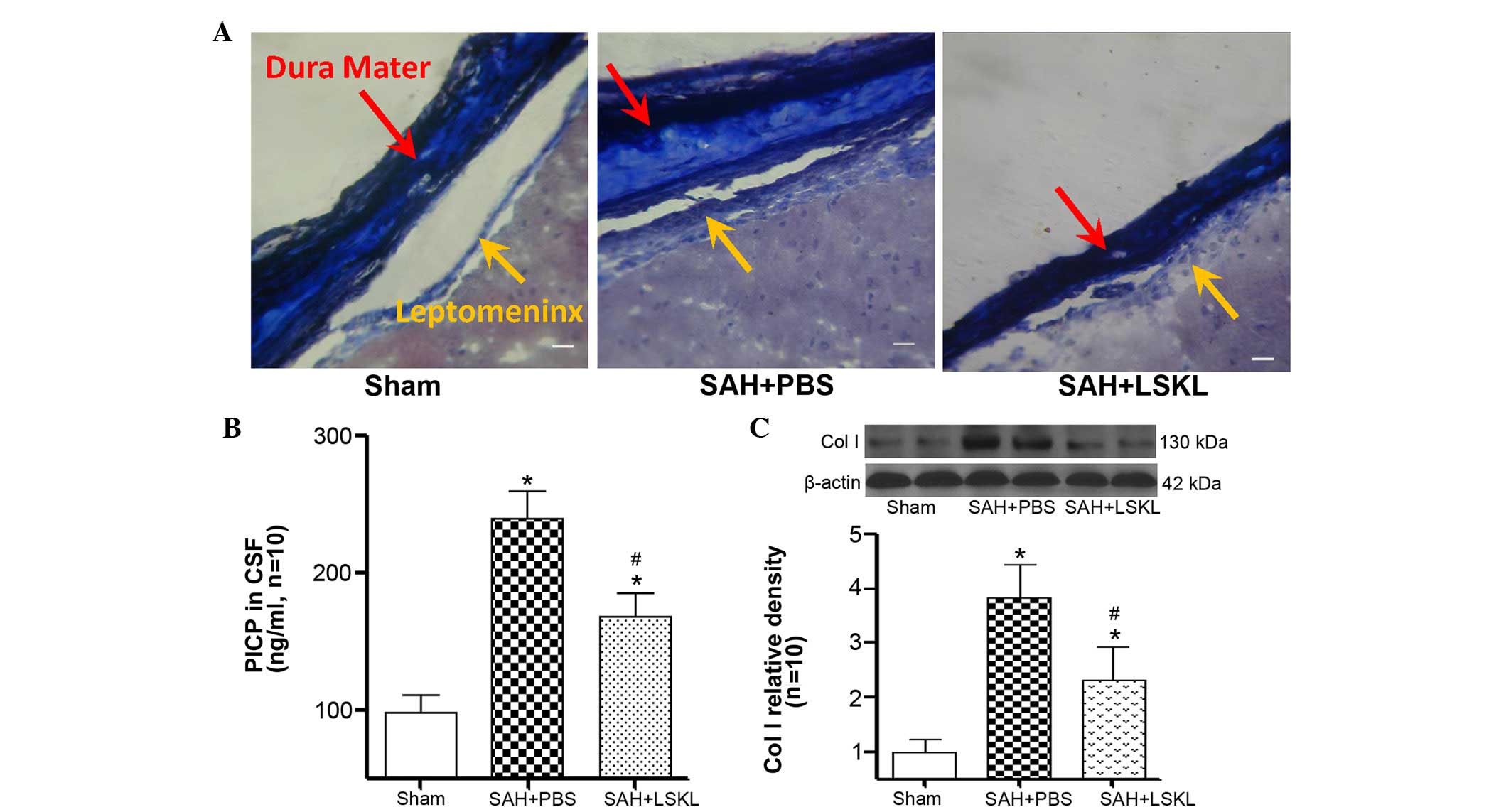
LSKL peptide inhibits subarachnoid
fibrosis after SAH
On day 21 after SAH, Masson staining revealed
greater quantities of collagen fibers in the brains of rats in the
SAH+PBS group as compared with the SAH+LSKL group (Fig. 2A). In addition, as compared with the
sham group, PICP levels in the CSF were significantly elevated on
day 21 in the SAH+PBS and SAH+LSKL treatment groups (P<0.05;
Fig. 2B). Notably, LSKL treatment
effectively reduced the concentration of PICP compared with that in
the SAH+PBS group (P<0.05; Fig.
2B). Furthermore, on day 5 after SAH, the expression level of
collagen I in the SAH+PBS group was significantly increased in
comparison with that in the sham group (P<0.05) but was reduced
by LSKL treatment (P<0.05; Fig.
2C).
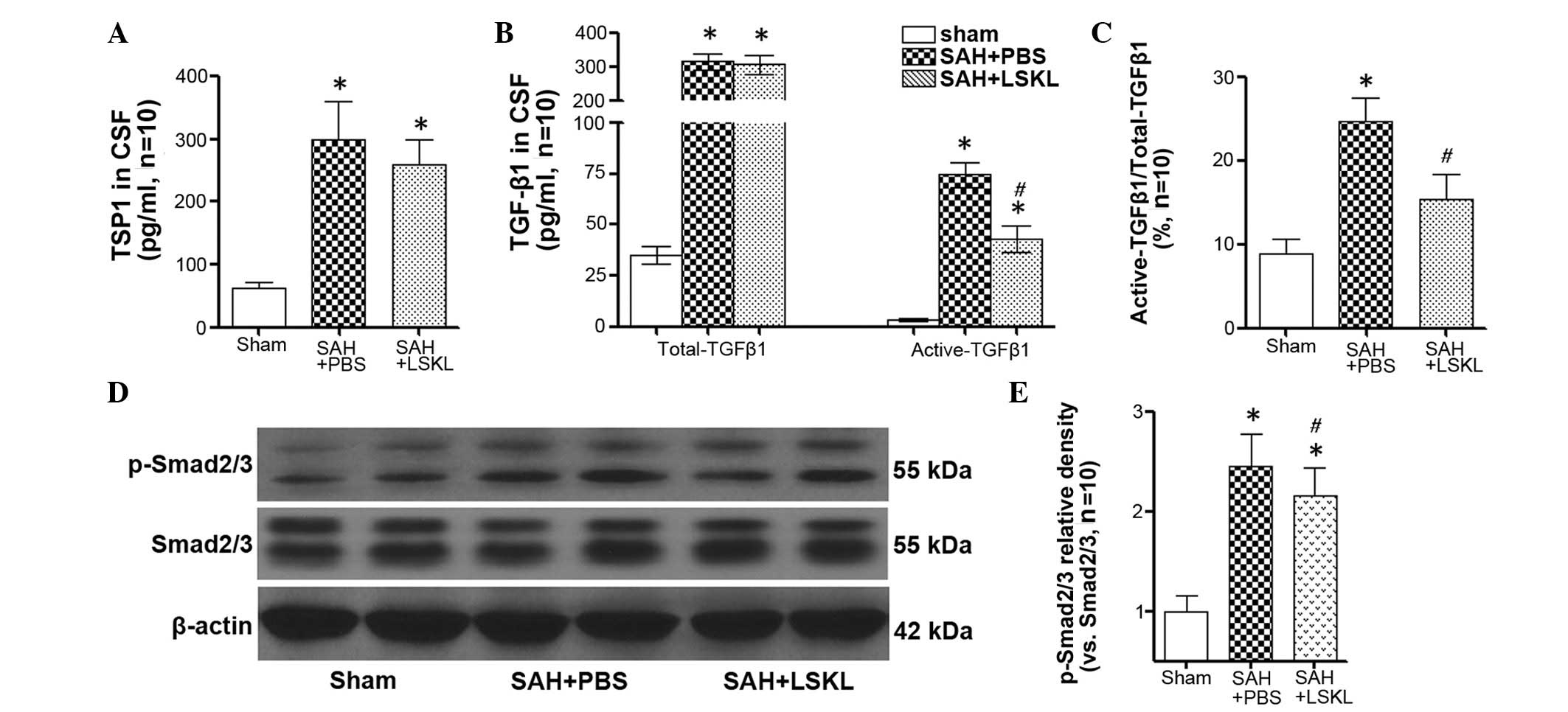
LSKL peptide inhibits TGF-β1 signaling
activity after SAH
The concentration of TSP1 in the CSF of rats in the
SAH+saline group was significantly increased compared with that in
the sham group (P<0.05), and LSKL treatment only slightly
reduced the concentration of TSP1 following SAH (P>0.05;
Fig. 3A). However, LSKL treatment
significantly decreased the activation of TGF-β1 in the CSF in
comparison with that in the SAH+PBS group (P<0.05; Fig. 3B and C). Moreover, the expression of
p-Smad2/3 in the rat brains was significantly increased at day 5
after SAH (P<0.05), and treatment with LSKL significantly
reduced the elevated level of p-Smad2/3 following SAH in comparison
with that in the SAH+PBS group (P<0.05; Fig. 3D and E).
Discussion
In the present study, it was demonstrated that LKSL
peptide easily crosses the blood brain barrier and protects against
subarachnoid fibrosis, attenuates ventriculomegaly, and effectively
suppresses the development of chronic hydrocephalus in a rat model
of SAH. In addition, it was revealed that the protective effects of
LSKL peptide are achieved primarily via inhibition of the
activating process of TGF-β1 and downstream Smad2/3 signaling
without affecting the expression of TSP1, which is critically
implicated in the pathogenesis of subarachnoid fibrosis and chronic
hydrocephalus after SAH (11–13).
Notably, it was also demonstrated that the administration of LSKL
peptide ameliorates long-term neurocognitive deficits following
SAH.
Early brain injury is currently considered the most
promising target in the treatment of SAH (23); however, delayed neurological
deterioration remains one of the major causes of severe morbidity
(24). Although it presents less
frequently than other complications of SAH, chronic communicating
hydrocephalus reportedly contributes to poor long-term neurological
outcomes, particularly severe cognitive deficits (25). Two-hemorrhage injection models of SAH
were employed in the present study, which produce a similar
incidence of hydrocephalus to other SAH models (26,27).
Evidence from previous studies indicates that fibrosis of the
leptomeninges and arachnoid granulations due to blood product
deposition may contribute to the development of post-hemorrhagic
communicating hydrocephalus via blockage of the flow of CSF,
suppression of CSF absorption, and reduction of CSF drainage
(28,29).
TGF-β is a potent fibrogenic factor in the
pathogenesis of fibrosis, with an important role in various
cellular processes such as cell proliferation, differentiation,
apoptosis, migration, and stimulation of extracellular matrix
synthesis. Members of the TGF-β family can bind to TGF-β type-1 and
type-2 receptors on the cell surface, inducing phosphorylation of
Smad2/3, initiating multiple intracellular signaling and exerting
pro-fibrotic effects (30). Marked
upregulation of TGF-β1 has been observed in the CSF of patients
following SAH, particularly in those with hydrocephalus (9,31).
However, only trace amounts of TGF-β1 are found in the CSF of
healthy individuals, although platelets are known to store excess
TGF-β1, which can be released by platelet degranulation after
aneurysm rupture to initiate the local synthesis of extracellular
matrix (31). In the present study,
an increase of TGF-β1 in the CSF of SAH model rats was observed.
This is consistent with the previous study in which the active form
of TGF-β1 was shown to be vital in activating TGF-β1/Smad2/3 and
the downstream biological effects.
One of the key regulatory factors of TGF-β activity
is its conversion from the latent precursor to the biologically
active form. The activation of TGF-β is dependent on the
interaction of a specific amino acid sequence
(K412RFK415) in TSP1, with the latent TGF-LAP
complex (10). Formation of the
TSP1-LAP complex requires the activation sequence Lys-Arg-Phe-Lys
(KRFK) and a sequence (LSKL) near the amino terminus of LAP, which
is also conserved in TGF-β. Endogenous LSKL peptides are able to
competitively inhibit the activation by TSP or other KRFK
sequence-containing peptides (32).
In addition, the interaction of LAP with TSP1 may prevent the
reformation of an inactive TGF-LAP complex because TSP-bound LAP is
not able to activate latent TGF-β (32). In the present study, it was
demonstrated that exogenous LSKL is capable of significantly
reducing the activation of latent TGF-β following SAH without
affecting the expression of TSP1 in the CSF. The experimental data
further demonstrate the potential of LSKL peptide to inhibit
fibrosis, which is consistent with previous studies of fibrosis in
other tissues (11–13,33).
Exogenous LSKL peptide is a small peptide that
consists of only four amino acids. Its simple structure and small
molecular weight allow the unsaturated, exogenous LSKL peptide to
easily cross the cell membrane and directly enter the cell without
expending energy. Therefore, the absorption, transformation and
utilization of LSKL peptide are efficient, complete and thorough,
characteristics that may be favorable for clinical application to
reduce subarachnoid fibrosis after SAH. In the present study, it
was demonstrated that LSKL can efficiently cross the blood brain
barrier, alleviate the symptoms of hydrocephalus, and ameliorate
long-term neurocognitive deficits after SAH.
In summary, the present study indicates that LSKL
peptide suppresses subarachnoid fibrosis via inhibition of
TSP1-mediated TGF-β1 activity, prevents the development of chronic
hydrocephalus, and attenuates long-term neurocognitive deficits
following SAH. Although direct translational trials of the present
study may prove impractical, given the relatively high incidence
and sustained debilitating properties of chronic hydrocephalus,
LSKL peptide represents a potentially feasible and promising
therapeutic tool. Further investigation of the protective effects
of LSKL peptide in post-hemorrhagic chronic hydrocephalus is
warranted.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81571150 to Fei Liu, grant
no. 81501002 to Yujie Chen and grant no. 81220108009 to Hua Feng)
and the Science and Technology Planning Project of Hunan (grant no.
2012FJ3125 to Fei Liu).
References
|
1
|
Fujii M, Yan J, Rolland WB, Soejima Y,
Caner B and Zhang JH: Early brain injury, an evolving frontier in
subarachnoid hemorrhage research. Transl Stroke Res. 4:432–446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giraldo EA, Mandrekar JN, Rubin MN, Dupont
SA, Zhang Y, Lanzino G, Wijdicks EF and Rabinstein AA: Timing of
clinical grade assessment and poor outcome in patients with
aneurysmal subarachnoid hemorrhage. J Neurosurg. 117:15–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah AH and Komotar RJ: Pathophysiology of
acute hydrocephalus after subarachnoid hemorrhage. World Neurosurg.
80:304–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Botfield H, Gonzalez AM, Abdullah O,
Skjolding AD, Berry M, McAllister JP II and Logan A: Decorin
prevents the development of juvenile communicating hydrocephalus.
Brain. 136:2842–2858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishii M, Suzuki S, Iwabuchi T and Julow J:
Effect of antifibrinolytic therapy on subarachnoid fibrosis in dogs
after experimental subarachnoid haemorrhage. Acta Neurochir (Wien).
54:17–24. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graff-Radford NR, Torner J, Adams HP Jr
and Kassell NF: Factors associated with hydrocephalus after
subarachnoid hemorrhage. A report of the Cooperative Aneurysm
Study. Arch Neurol. 46:744–752. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Yuan W, Liao D, Zhang T and Wang Z:
Association of chronic hydrocephalus after aneurysmal subarachnoid
hemorrhage with transforming growth factor-β1 levels and other risk
factors. Nan Fang Yi Ke Da Xue Xue Bao. 33:382–385. 2013.(In
Chinese). PubMed/NCBI
|
|
8
|
Li T, Zhang P, Yuan B, Zhao D, Chen Y and
Zhang X: Thrombin-induced TGF-β1 pathway: A cause of communicating
hydrocephalus post subarachnoid hemorrhage. Int J Mol Med.
31:660–666. 2013.PubMed/NCBI
|
|
9
|
Douglas MR, Daniel M, Lagord C, Akinwunmi
J, Jackowski A, Cooper C, Berry M and Logan A: High CSF
transforming growth factor beta levels after subarachnoid
haemorrhage: Association with chronic communicating hydrocephalus.
J Neurol Neurosurg Psychiatry. 80:545–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribeiro SM, Poczatek M, Schultz-Cherry S,
Villain M and Murphy-Ullrich JE: The activation sequence of
thrombospondin-1 interacts with the latency-associated peptide to
regulate activation of latent transforming growth factor-beta. J
Biol Chem. 274:13586–13593. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuroki H, Hayashi H, Nakagawa S, Sakamoto
K, Higashi T, Nitta H, Hashimoto D, Chikamoto A, Beppu T and Baba
H: Effect of LSKL peptide on thrombospondin 1-mediated transforming
growth factor β signal activation and liver regeneration after
hepatectomy in an experimental model. Br J Surg. 102:813–825. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie XS, Li FY, Liu HC, Deng Y, Li Z and
Fan JM: LSKL, a peptide antagonist of thrombospondin-1, attenuates
renal interstitial fibrosis in rats with unilateral ureteral
obstruction. Arch Pharm Res. 33:275–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondou H, Mushiake S, Etani Y, Miyoshi Y,
Michigami T and Ozono K: A blocking peptide for transforming growth
factor-beta1 activation prevents hepatic fibrosis in vivo. J
Hepatol. 39:742–748. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Xu X, Zhou D, Li H, You W, Wang Z
and Chen G: Possible role of raf-1 kinase in the development of
cerebral vasospasm and early brain injury after experimental
subarachnoid hemorrhage in rats. Mol Neurobiol. 52:1527–1539. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugawara T, Ayer R, Jadhav V and Zhang JH:
A new grading system evaluating bleeding scale in filament
perforation subarachnoid hemorrhage rat model. J Neurosci Methods.
167:327–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan H, Chen Y, Li L, Jiang J, Wu G, Zuo Y,
Zhang JH, Feng H, Yan X and Liu F: Decorin alleviated chronic
hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after
subarachnoid hemorrhage in rats. Brain Res. 1630:241–253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F, Chen Y, Hu Q, Li B, Tang J, He Y,
Guo Z, Feng H, Tang J and Zhang JH: MFGE8/Integrin β3 pathway
alleviates apoptosis and inflammation in early brain injury after
subarachnoid hemorrhage in rats. Exp Neurol. 272:120–127. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu F, Hu Q, Li B, Manaenko A, Chen Y,
Tang J, Guo Z, Tang J and Zhang JH: Recombinant milk fat
globule-EGF factor-8 reduces oxidative stress via integrin
β3/nuclear factor erythroid 2-related factor 2/heme oxygenase
pathway in subarachnoid hemorrhage rats. Stroke. 45:3691–3697.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Q, Liang X, Chen D, Chen Y, Doycheva D,
Tang J, Tang J and Zhang JH: Delayed hyperbaric oxygen therapy
promotes neurogenesis through reactive oxygen
species/hypoxia-inducible factor-1α/β-catenin pathway in middle
cerebral artery occlusion rats. Stroke. 45:1807–1814. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okubo S, Strahle J, Keep RF, Hua Y and Xi
G: Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke.
44:547–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Luo C, Zhao M, Li Q, Hu R, Zhang
JH, Liu Z and Feng H: Administration of a PTEN inhibitor BPV(pic)
attenuates early brain injury via modulating AMPA receptor subunits
after subarachnoid hemorrhage in rats. Neurosci Lett. 588:131–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo
C, Tang J, Feng H and Zhang JH: Norrin protected blood-brain
barrier via frizzled-4/β-catenin pathway after subarachnoid
hemorrhage in rats. Stroke. 46:529–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sehba FA, Hou J, Pluta RM and Zhang JH:
The importance of early brain injury after subarachnoid hemorrhage.
Prog Neurobiol. 97:14–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Macdonald RL: Delayed neurological
deterioration after subarachnoid haemorrhage. Nat Rev Neurol.
10:44–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Feng H, Sherchan P, Klebe D, Zhao
G, Sun X, Zhang J, Tang J and Zhang JH: Controversies and evolving
new mechanisms in subarachnoid hemorrhage. Prog Neurobiol.
115:64–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lackner P, Vahmjanin A, Hu Q, Krafft PR,
Rolland W and Zhang JH: Chronic hydrocephalus after experimental
subarachnoid hemorrhage. PLoS One. 8:e695712013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chu SH, Feng DF, Ma YB, Zhang H, Zhu ZA,
Li ZQ and Zhang ZH: Expression of HGF and VEGF in the cerebral
tissue of adult rats with chronic hydrocephalus after subarachnoid
hemorrhage. Mol Med Rep. 4:785–791. 2011.PubMed/NCBI
|
|
28
|
Strahle J, Garton HJ, Maher CO, Muraszko
KM, Keep RF and Xi G: Mechanisms of hydrocephalus after neonatal
and adult intraventricular hemorrhage. Transl Stroke Res. 3 Suppl
1:S25–S38. 2012. View Article : Google Scholar
|
|
29
|
Orešković D and Klarica M: Development of
hydrocephalus and classical hypothesis of cerebrospinal fluid
hydrodynamics: Facts and illusions. Prog Neurobiol. 94:238–258.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HS: Paracrine role for TGF-β-induced
CTGF and VEGF in mesangial matrix expansion in progressive
glomerular disease. Histol Histopathol. 27:1131–1141.
2012.PubMed/NCBI
|
|
31
|
Flood C, Akinwunmi J, Lagord C, Daniel M,
Berry M, Jackowski A and Logan A: Transforming growth factor-beta1
in the cerebrospinal fluid of patients with subarachnoid
hemorrhage: Titers derived from exogenous and endogenous sources. J
Cereb Blood Flow Metab. 21:157–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Young GD and Murphy-Ullrich JE: Molecular
interactions that confer latency to transforming growth
factor-beta. J Biol Chem. 279:38032–38039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krishna SM, Seto SW, Jose RJ, Biros E,
Moran CS, Wang Y, Clancy P and Golledge J: A peptide antagonist of
thrombospondin-1 promotes abdominal aortic aneurysm progression in
the angiotensin II-infused apolipoprotein-E-deficient mouse.
Arterioscler Thromb Vasc Biol. 35:389–398. 2015. View Article : Google Scholar : PubMed/NCBI
|