Introduction
In the development and progression of vascular
diseases such as abdominal aortic aneurysm (AAA), smooth muscle
cells (SMCs) play very important roles (1–3). Due to
lack of terminal differentiation, SMCs have the ability to
proliferate, migrate and synthesize extracellular matrix (ECM)
(4). In certain pathological
conditions such as atherosclerosis, the proliferation and cell
cycling of SMCs is considered to be dangerous (5). However, in other conditions, such as
aneurysmal dilation, the regrowth of SMSs is considered to be
beneficial (6). The microenvironment
affects the proliferation of SMCs, and SMC growth is stimulated by
a variety of extracellular and intracellular factors (7–9).
It has been identified that microRNAs (miRNAs) play
a significant role in determining the fate and behavior of SMCs
(10). miRNAs are small non-coding
RNAs comprising ~22 nucleotides that can lead to the inhibition of
target gene expression (11,12). Previous studies have demonstrated
that miRNAs are involved in various cellular functions, including
differentiation, growth and development in vascular-associated
diseases (12,13). miRNAs regulate target gene expression
in angiogenesis, endothelial cell function and vascular
inflammation (14). However, little
is known about how miRNAs function in SMCs.
In the present study, the aim was to investigate the
roles of miRNAs in SMC pathobiology. An miRNA array was used to
identify the significant miRNAs in a mouse model of AAA. The effect
of one of the most differentially expressed miRNAs identified by
the array on SMC proliferation and apoptosis was further
investigated. Its target gene was also predicted and verified.
Materials and methods
Murine models of AAA
A total of 20 male ApoE−/− mice (10 weeks
old; n=4 per group) were purchased from Better Biotechnology Co.,
Ltd. (Nanjing, China). The mice were maintained in cages with food
and water ad libitum, and with a 12 h light/dark cycle (room
temperature, 23–25°C). AAA models were established according to a
previously reported procedure (15).
Briefly, 10% hydrated chlorine aldehyde was used to anesthetize the
mice (0.3 ml/100 g body weight). The infra-renal abdominal aortas
of the mice were isolated and temporary ligatures were applied.
Saline solution containing 4.5 U/ml type 1 porcine pancreatic
elastase (E1250; Sigma-Aldrich, St. Louis, MO, USA) was instilled
for 5 min at 100 mmHg. The aortotomy was closed with a 10-0 suture,
and aortic flow was restored. Mice were sacrificed 7 days after
modeling by peritoneal injection of pentobarbital (50 mg/kg).
Aortas were harvested from the mice and immediately flash-frozen in
liquid nitrogen. The SMCs were isolated from the aortas according
to a previously described method (15). The study was approved by the Animal
Experiments Ethics Committee of the Affiliated Hospital of Nanjing
University Medical School (Nanjing, China).
Cell culture
Human SMCs (T/G HA-VSMC) obtained from X-Y
Biotechnology (Shanghai, China) were cultured in SmGM-2 Smooth
Muscle Growth Medium-2 (Lonza, Walkersville, MD, USA) with 5% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. Cells were harvested
for RNA or protein analysis at ~90% confluence.
miRNA array hybridization and
analysis
The RNA from the SMCs was isolated following
homogenization using a mirVana miRNA Isolation kit (Ambion; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. RNA quality was checked using an Agilent 2100
Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA),
which was then used to conduct the RT-qPCR. RNA samples from normal
(control) and AAA model mice were prepared for analysis using an
Agilent Mouse miRNA 8×15K Array kit (Agilent Technologies, Inc.,
Santa Clara, CA, USA). This array consisted of probes for 135 mice
miRNAs from the Sanger database, version 10.1. The analysis was
conducted according to the manufacturer's protocol. Significant
miRNAs were those that showed a >2.0-fold difference in
expression from the control. The most differentially expressed
miRNAs were confirmed by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR).
RT-qPCR
Human SMCs were transfected with miR129-5p or miR
control. Total RNA was extracted from the mouse or transfected SMCs
using an miRNeasy Mini kit (Qiagen, Valencia, CA, USA). Total RNA
was reverse transcribed to cDNA using M-MuLV reverse transcriptase
(Promega Corporation, Madison, WI, USA). A
Taqman® MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) used the
stem-loop method to detect the expression levels of mature miRs.
Total RNA (10 ng) was reverse transcribed and mixed with specific
stem-loop primers (Applied Biosystems; Thermo Fisher Scientific,
Inc.), and then qPCR was performed using an ABI PRISM 7900HT
Sequence Detection System (Thermo Fisher Scientific, Inc.). miRNA
amplification was carried out using the GenoExplorer miRNA qRT-PCR
kit (Genosensor Corporation, Tempe, AZ, USA). The primer sequences
of miR-129-5p and miRNA control were as follows: miR-129-5p,
forward: 5′-ACACTCCTTTTTGCGTCTGGGCTTGC-3′ and reverse:
5′-TGGTGTCGTGGAGTCG-3′; miRNA control, forward:
5′-CTCGCTTCGGCAGCACA-3′ and reverse: 5′-AACGCTTCACGAATTTGCGT-3′;
Wnt5a forward: 5′-CTTGGTGGTCGCTAGGTATGAAT-3′ and reverse:
5′-GGTGTTATCCACAGAGTGCTGC-3′; β-actin forward:
5′-CCTGGAGAAGAGCTATGAGCTGCCTG-3′ and reverse:
5′-CGATCCACACAGAGTACTTGCGC-3′. Relative mRNA quantification was
determined with respect to the mean of β-actin. Relative miR
quantification was determined with respect to the mean of RNU48.
The relative expression levels of the miRNAs were evaluated with
the 2−ΔΔCq method. Experiments were conducted in
triplicate.
Luciferase assay
Wnt5a was predicted as the target gene of miR-129-5p
by TargetScan (http://www.targetscan.org and http://www.mirbase.org). The 3′ untranslated region
(3′UTR) of Wnt5a containing a potential binding site for miR-129-5p
was amplified and cloned into a pGL4 luciferase reporter vector
(Promega Corporation, Madison, WI, USA). Site-directed mutagenesis
of the miR-129-5p binding site in the Wnt5a 3′UTR was performed
using the GeneTailor Site-Directed Mutagenesis System (Invitrogen;
Thermo Fisher Scientific). The pGL4 vector contains a firefly
luciferase reporter gene. SMCs were co-transfected with miR-129-5p,
or miRNA control combined with Wnt5a 3′-UTR for 24 h and then
luciferase activity was measured. Luciferase activity was measured
36 h after cell transfection using the Dual-Luciferase Reporter
Assay System (Promega Corporation). Values were normalized with
firefly luciferase activity.
Cell transfection
Lentivirus of miR-129-5p or its control were
produced in 293T cells transfected with the miRNA vector. Human
SMCs were seeded in triplicate into 6-well plates at a density of
1×105 cells/well, and were grown to 30–50% confluence at
37°C in an atmosphere containing 5% CO2. Turbofect
transfection reagent (Thermo Fisher Scientific, Inc.) was used for
transfection, according to the manufacturer's instructions. Human
SMCs were transfected with the indicated plasmid or nucleotides
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific).
After 24 h of transfection, the cells were seeded into 96-well
culture plates at a density of 2×103 cells/well. The
cell proliferation of the SMCs was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) assay according to the manufacturer's protocol.
Detection of cell apoptosis
Human SMCs were transfected with miR-129-5p or miR
control. After 48 h of transfection, the cells were washed with
phosphate-buffered saline and then stained with 5 µl Annexin V and
5 µl propidium iodide for 15 min at room temperature in the dark in
accordance with the manufacturer's protocol (BD Biosciences, San
Jose, CA, USA). The apoptosis was analyzed using flow
cytometry.
Western blot analysis
Transfected SMCs were lysed in
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing 1X protease inhibitor cocktail, and protein
concentrations were determined using the Bradford assay (Bio-Rad
Laboratories, Inc., Philadelphia, PA, USA). Protein lysates (50 µg)
were loaded onto 10% SDS-PAGE gels, fractionated, and
electroblotted onto nitrocellulose membranes (Millipore, Bedford,
MA, USA) at 80 V for 2 h. After blocking in 5% non-fat dry milk,
the membranes were incubated with primary antibodies (1:1,000)
overnight at 4°C and then incubated with goat anti-rabbit (1:2,000;
cat. no. sc-2301; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and goat anti-mouse (1:3,000; cat. no. sc-2031; Santa Cruz
Biotechnology, Inc.) secondary antibodies conjugated with
horseradish peroxidase for 1 h at room temperature. The following
primary antibodies were used: Mouse monoclonal Wnt-5a antibody
(A-5; sc-365370), rabbit polyclonal Rac 1 antibody (C-11; sc-95),
rabbit polyclonal Rho A antibody (119; sc-179) and mouse monoclonal
glucreraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (G-9;
sc-365062) were purchased from Santa Cruz Biotechnology, Inc.
Protein bands were visualized by enhanced chemiluminescence using
LumiGLO Chemiluminescent Substrate (KPL Inc., Gaithersburg, MD,
USA).
Statistical analysis
The study data were analyzed using SPSS software,
version 13.0 (SPSS, Inc., Chicago, IL, USA). Results are presented
as the mean ± standard error of the mean of at least three
independent experiments. Comparisons of two independent groups were
analyzed using the two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
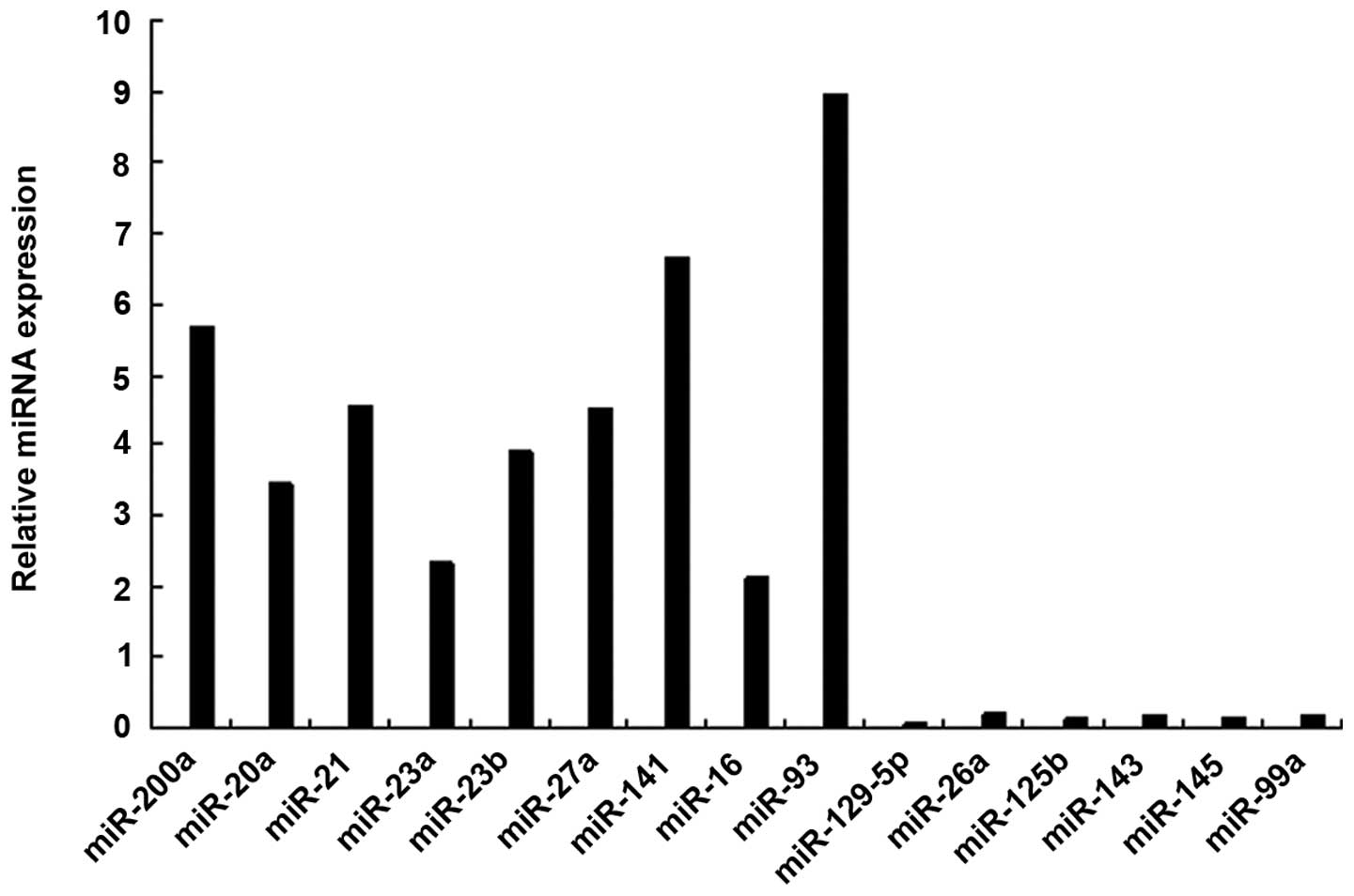
miRNA profile of SMCs from AAA
models
To identify the miRNAs that differed in expression
between AAA model and normal mice, a customized miRNA microarray
that contained 135 mouse miRNAs was used to analyze miRNA
expression levels. miRNAs were considered differentially expressed
if their M values (log2-fold change values) were >1.0 or
<-1.0. There were 15 miRNAs that showed significant changes in
≥12 of the 20 AAA models (Table I).
The upregulated miRNAs in the AAA model were miR-21, miR-20a,
miR-93, miR-200a, miR-23a, miR-23b, miR-27a, miR-141 and miR-16,
and the downregulated miRNAs were miR-129-5p, miR-26a, miR-125b,
miR-143, miR-145 and miR-99a. Of these miRNAs, the most
downregulated miRNA in the SMCs was miR-1259-5p. The results were
confirmed by RT-qPCR analysis (Fig.
1).
 | Table I.miRNA profile of AAA model mice. |
Table I.
miRNA profile of AAA model mice.
| miRNA symbol |
Upregulated/downregulated | Fold
expressiona |
|---|
| has-miR-200a | Upregulated | 3.42 |
| has-miR-20a | Upregulated | 4.72 |
| has-miR-21 | Upregulated | 9.51 |
| has-miR-23a | Upregulated | 2.52 |
| has-miR-23b | Upregulated | 4.65 |
| has-miR-27a | Upregulated | 3.45 |
| has-miR-141 | Upregulated | 7.88 |
| has-miR-16 | Upregulated | 2.23 |
| has-miR-93 | Upregulated | 10.43 |
| has-miR-129-5p | Downregulated | 0.12 |
| has-miR-26a | Downregulated | 0.34 |
| has-miR-125b | Downregulated | 0.29 |
| has-miR-143 | Downregulated | 0.32 |
| has-miR-145 | Downregulated | 0.24 |
| has-miR-99a | Downregulated | 0.15 |
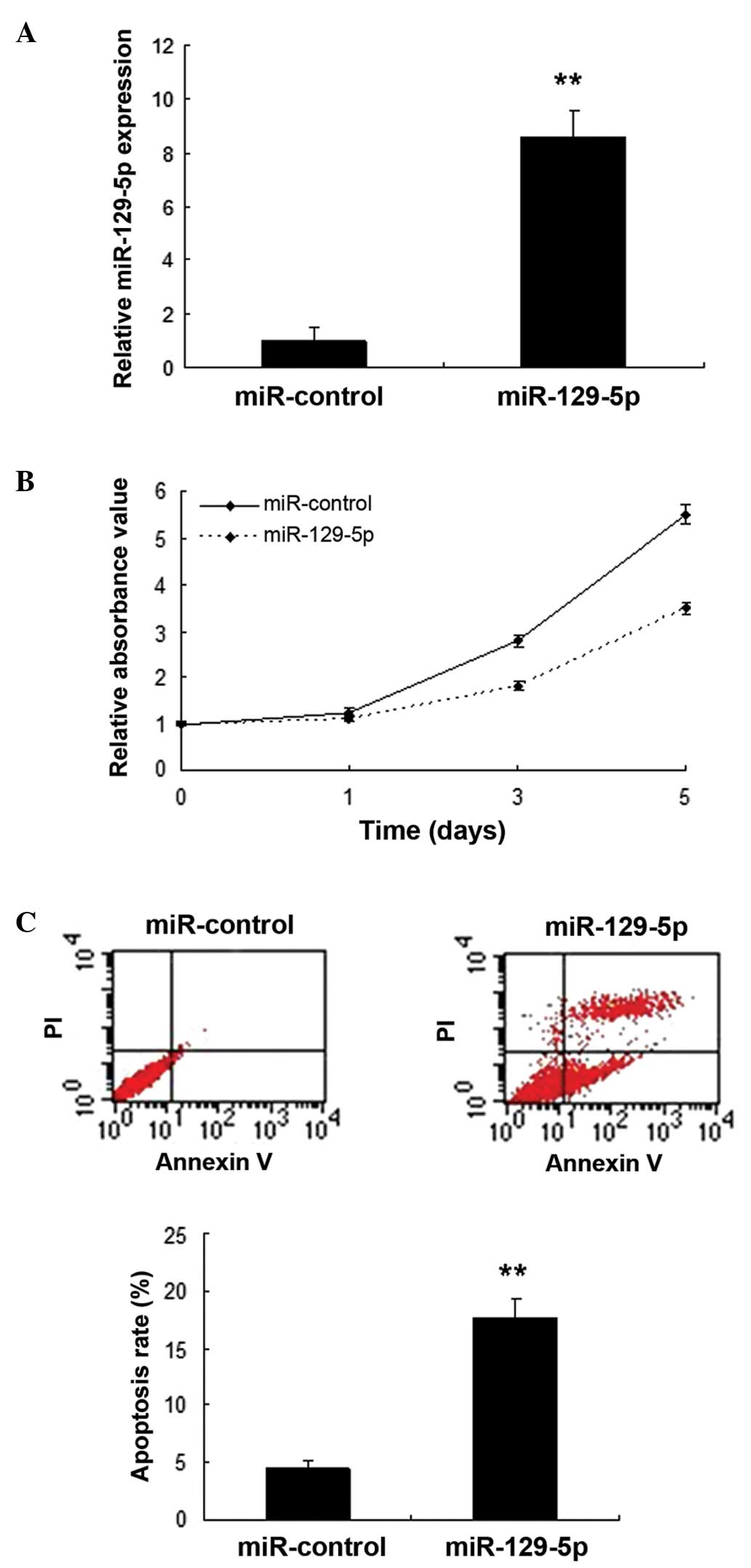
miR-129-5p inhibits SMC proliferation
and induces apoptosis
Following the identification of miR-129-5p as a
frequently downregulated miRNA in SMCs, its role in AAA was
investigated. SMCs were infected with miR-129-5p using a lentiviral
vector, and the expression of miR-129-5p was confirmed to be
increased (Fig. 2A). Cell
proliferation was evaluated by MTT assay. The results showed that
miR-129-5p inhibited the proliferation of SMCs (Fig. 2B). Furthermore, flow cytometry data
indicated that apoptosis was increased in the SMCs with miR-129-5p
overexpression compared with that in the control (Fig. 2C).
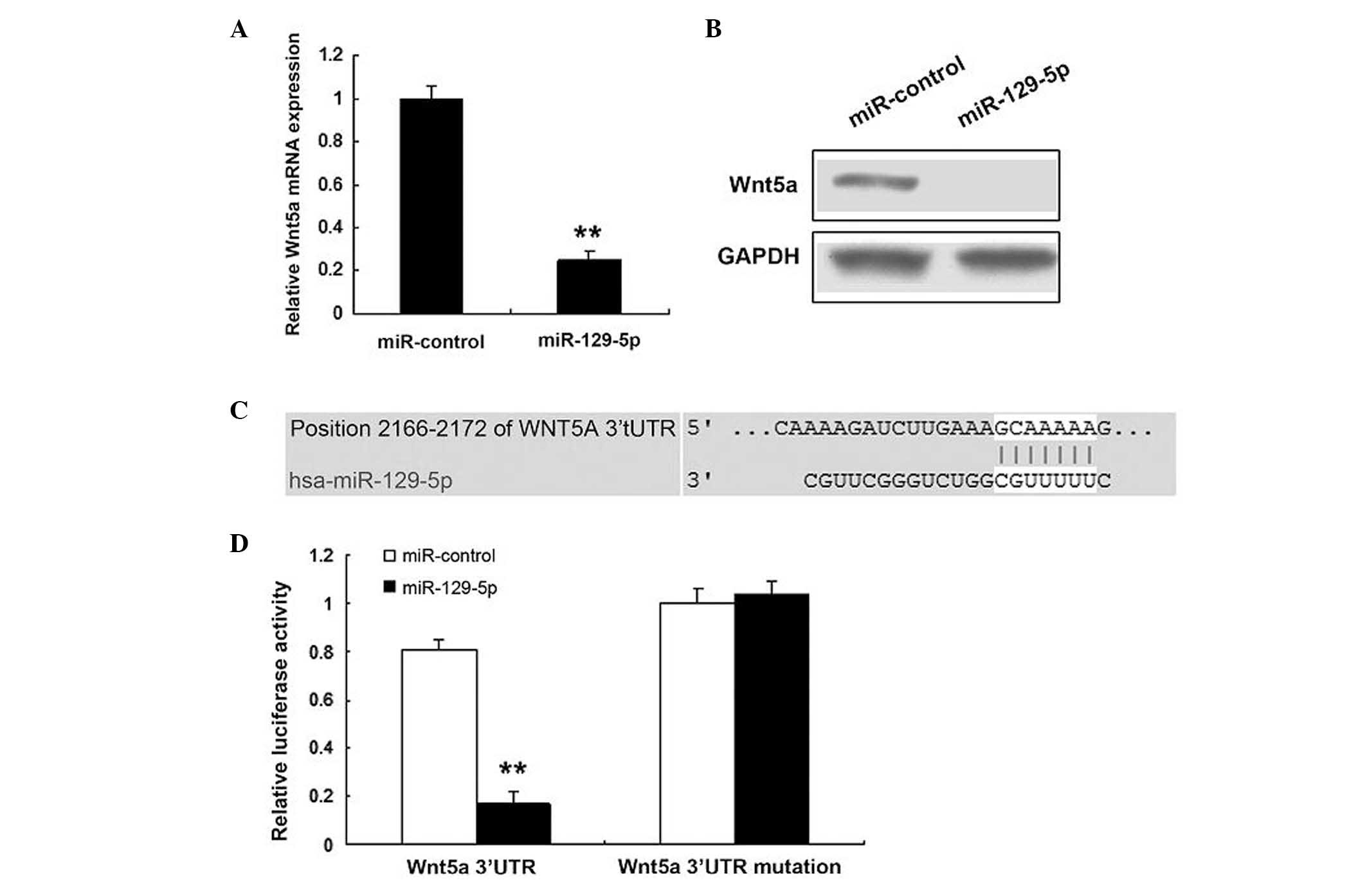
Wnt5a is a target gene of miR-129-5p
in SMCs
The prediction that miR-129-5p regulates endogenous
Wnt5a expression in SMCs was investigated. Endogenous Wn5a mRNA
levels (Fig. 3A) were downregulated
when human SMCs were transfected with miR-129-5p, compared with
those in the control. Western blotting demonstrated that the Wnt5a
protein level was low in the cells transfected with miR-129-5p
(Fig. 3B). Bioinformatic analysis
indicated that Wnt5a would be directly suppressed by binding to
miR-129-5p (Fig. 3C). As shown in
Fig. 3D, the luciferase activity of
Wnt5a in SMCs transfected with miR-129-5p was much lower than that
in control cells. The luciferase activity of mutant Wnt5a was not
affected by miR-129-5p.
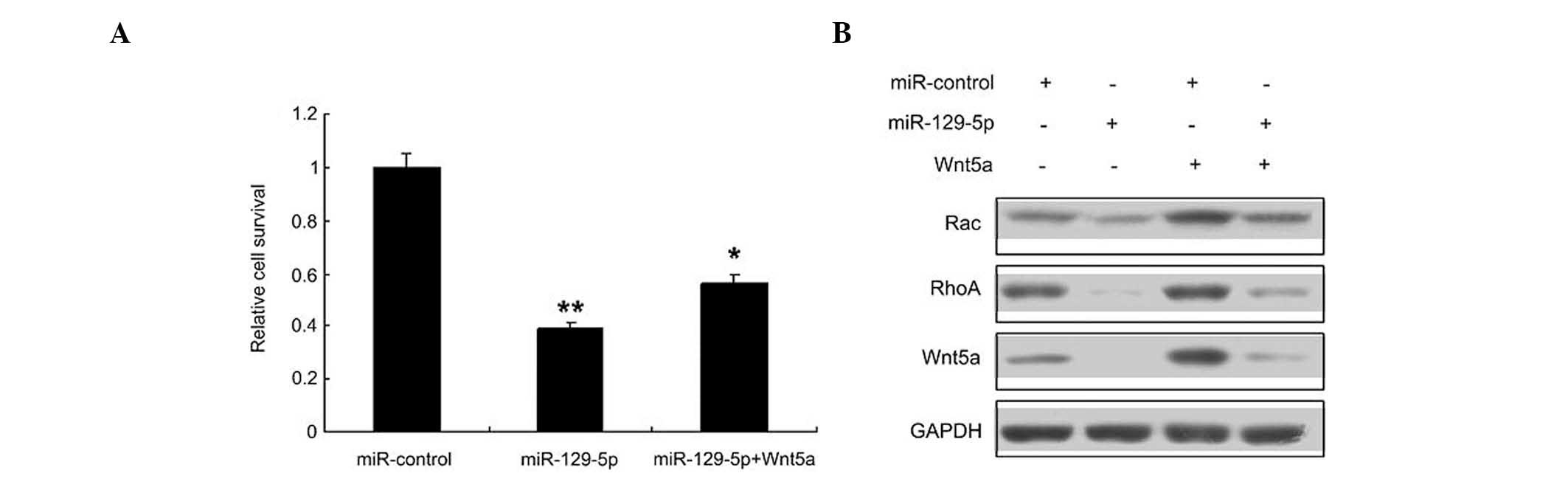
miR-129-5p inhibits SMC proliferation
by targeting the Wnt5a signaling pathway
Whether miR-129-5p inhibits proliferation by
targeting Wnt5a in SMCs was then investigated; it was found that
miR-129-5p inhibited SMC proliferation, and its inhibitory effect
was reduced when Wnt5a was overexpressed (Fig. 4A). Since the Wnt signaling pathway is
an important pathway involved in SMC regulation, the levels of
Wnt5a and downstream proteins in the Wnt signaling pathway, namely
RhoA and Rac 1, were evaluated by western blotting (Fig. 4B). The results indicated that the
Wnt5a signal pathway was inhibited in the SMCs transfected with
miR-129-5p.
Discussion
Previous studies have indicated that several miRNAs
play a role in vascular physiology, including miRNA-145, miRNA-21
and miRNA-221 (15,16). However, the mechanism of regulation
of SMC function by miRNAs in AAA remains unknown. In the present
study, the aim was to clarify this by performing miRNA microarray
analysis of vascular SMCs from AAA models. Mouse AAA models were
established and SMCs were isolated from the animals. Through the
miRNA array assay, 15 miRNAs that were significantly upregulated or
downregulated were identified. Further experiments were conducted
to investigate the most notable miRNA, namely miR-129-5p, including
its target genes and function.
miR-129-5p was selected as it was downregulated to a
greater extent than other miRNAs such as miR-26a, miR-125b, miR-99a
and miR-145. It was hypothesized that miR-129-5p could be a key
regulator of SMC physiology. Despite its downregulation during SMC
differentiation, it was found that the overexpression of miR-129-5p
inhibits SMC proliferation. It was then postulated that miR-129-5p
may be an inducer of cell apoptosis in SMCs. The results of flow
cytometric analysis indicated that miR-129-5p serves as a
stimulator of SMC apoptosis. The results of the present study
identified that Wnt5a was a direct target gene of miR-129-5p.
Previous reports demonstrated that Wnt5a-induced Wnt1-inducible
secreted protein-1 suppresses vascular smooth muscle cell apoptosis
induced by oxidative stress (16).
Wnt5a is induced by TGF-β/Smad3 in rat aortic SMC and promotes its
proliferation (17). Wnt5a is
associated with the differentiation of bone marrow mesenchymal stem
cells in vascular calcification by connecting with various
receptors (18). Curculigoside A
induces cell proliferation and angiogenesis via Wnt5a/β-catenin and
VEGF/CREB/Egr-3/VCAM-1 signaling, and promotes maturation and
stability of new blood vessels by increasing Ang1 and Tie-2
expression (19). Wnts are expressed
during the differentiation of MSCs during calcification. MSCs are
able to differentiate into various cell phenotypes when there is
direct cell-cell contact with VSMCs or calcified VSMCs, and the
Wnt5a/Ror2 pathway may be associated with the determination of
differentiation of MSCs during this process (20).
In a conclusion, numerous significantly regulated
miRNAs in a mouse model of AAA were identified using a microarray.
Notably, miR-129-5p was suggested to be a potentially critical
regulator of AAA development, an inhibitor of cell proliferation
and inducer of apoptosis in SMCs. Wnt5a was identified as a target
gene of miR-129-5p, and transfection with miR-129-5p downregulated
Wnt5a mRNA and protein expression in SMCs. Further studies are
required to investigate other biological effects of miR-129-5p in
AAA.
Acknowledgements
The authors thank the Natural Science Foundation of
Jiangsu Province, China for support (No: BK2009035).
References
|
1
|
Kim CW, Kumar S, Son DJ, Jang IH,
Griendling KK and Jo H: Prevention of abdominal aortic aneurysm by
anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused
mice. Arterioscler Thromb Vasc Biol. 34:1412–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biros E, Moran CS, Wang Y, Walker PJ,
Cardinal J and Golledge J: MicroRNA profiling in patients with
abdominal aortic aneurysms: The significance of miR-155. Clin Sci
(Lond). 126:795–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maegdefessel L, Dalman RL and Tsao PS:
Pathogenesis of abdominal aortic aneurysms: MicroRNAs, proteases,
genetic associations. Annu Rev Med. 65:49–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maegdefessel L, Azuma J and Tsao PS:
MicroRNA-29b regulation of abdominal aortic aneurysm development.
Trends Cardiovasc Med. 24:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maegdefessel L, Spin JM, Adam M, Raaz U,
Toh R, Nakagami F and Tsao PS: Micromanaging abdominal aortic
aneurysms. Int J Mol Sci. 14:14374–14394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheuk BLY and Cheng SWK: Identification
and characterization of microRNAs in vascular smooth muscle cells
from patients with abdominal aortic aneurysms. J Vasc Surg.
59:20–209. 2014. View Article : Google Scholar
|
|
7
|
Adam M, Raaz U, Spin JM and Tsao PS:
MicroRNAs in abdominal aortic aneurysm. Curr Vasc Pharmacol.
13:280–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HJ, Yi JS, Lee HJ, Lee IW, Park KC and
Yang JH: Dysregulated expression profiles of MicroRNAs of
experimentally induced cerebral aneurysms in rats. J sKorean
Neurosurg Soc. 53:72–76. 2013. View Article : Google Scholar
|
|
9
|
Golledge J and Kuivaniemi H: Genetics of
abdominal aortic aneurysm. Curr Opin Cardiol. 28:290–296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kin K, Miyagawa S, Fukushima S, Shirakawa
Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T and
Sawa Y: Tissue- and plasma-specific MicroRNA signatures for
atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc.
1:e0007452012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pahl MC, Derr K, Gäbel G, Hinterseher I,
Elmore JR, Schworer CM, Peeler TC, Franklin DP, Gray JL, Carey DJ,
et al: MicroRNA expression signature in human abdominal aortic
aneurysms. BMC Med Genomics. 5:252012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maegdefessel L, Azuma J, Toh R, Deng A,
Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell
MV, et al: MicroRNA-21 blocks abdominal aortic aneurysm development
and nicotine-augmented expansion. Sci Transl Med. 4:122ra222012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maegdefessel L, Azuma J, Toh R, Merk DR,
Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ,
et al: Inhibition of microRNA-29b reduces murine abdominal aortic
aneurysm development. J Clin Invest. 122:497–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milewicz DM: MicroRNAs, fibrotic
remodeling and aortic aneurysms. J Clin Invest. 122:490–493. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu G, Huang Y, Lu X, Lu M, Huang X, Li W
and Jiang M: Identification and characteristics of microRNAs with
altered expression patterns in a rat model of abdominal aortic
aneurysms. Tohoku J Exp Med. 222:187–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mill C, Monk BA, Williams H, Simmonds SJ,
Jeremy JY, Johnson JL and George SJ: Wnt5a-induced Wnt1-inducible
secreted protein-1 suppresses vascular smooth muscle cell apoptosis
induced by oxidative stress. Arterioscler Thromb Vasc Biol.
34:2449–2456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin Y, Wang W, Chai S, Liu J, Yang T and
Wang J: Wnt5a attenuates hypoxia-induced pulmonary arteriolar
remodeling and right ventricular hypertrophy in mice. Exp Biol Med
(Maywood). 240:1742–1751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan S, Wang Z, Xin F and Xin H: Wnt5a is
associated with the differentiation of bone marrow mesenchymal stem
cells in vascular calcification by connecting with different
receptors. Mol Med Rep. 10:1985–1991. 2014.PubMed/NCBI
|
|
19
|
Zhu H, He J, Ye L, Lin F, Hou J, Zhong Y
and Jiang W: Mechanisms of angiogenesis in a Curculigoside
A-treated rat model of cerebral ischemia and reperfusion injury.
Toxicol Appl Pharmacol. 288:313–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan S, Wang Z, Xin F and Xin H: Wnt5a is
associated with the differentiation of bone marrow mesenchymal stem
cells in vascular calcification by connecting with different
receptors. Mol Med Rep. 10:1985–1991. 2014.PubMed/NCBI
|