Introduction
Acute renal failure (ARF) is induced by various
causes, presenting as renal function rapid declining in a short
time, significant impairment of glomerular filtration function,
rapid increasing of blood urea nitrogen and creatinine, water,
electrolyte and acid-base balance disorders, and ultimately ARF
(1). Kidney injury caused by
ischemia/reperfusion induced renal injury (I/RIRI) is the primary
cause of ischemic ARF (2). The
pathogenetic mechanism underlying I/RIRI is complicated, involving
free radicals, calcium overload and energy metabolism dysfunction
(3). Previous results suggest that
inflammatory medium, adhesion molecules and various cytokines
participate in I/RIRI (4).
I/RIRI refers to damage to tissues and organs after
blood perfusion and oxygen delivery, and is the primary cause of
ischemic ARF (5,6). Kidneys are among the organs that I/RIRI
mostly frequently occurs in (7).
I/RIRI is commonly detected on ischemic diseases induced by renal
blood flow transient hypoperfusion such as renal artery sclerosis,
renal artery or vein embolism, severe trauma, cardiac arrest and
hypovolemic shock (8). Furthermore,
I/RIRI is highly frequent following organ transplantation (6). The occurrence of I/RIRI may trigger
acute rejection and induce early allograft function failure, or it
may lead to delayed recovery of allograft function, accelerate loss
of allograft function and shorten lifespan (9). The pathogenetic mechanism underlying
I/RIRI is complicated, involved in free radical, calcium
overloading, energy metabolism dysfunction, inflammatory medium and
adhesion molecule, upregulation of cytokines, endothelial
dysfunction, abnormal blood rheology and increased apoptosis
(10,11).
A variety of compounds have been extracted from the
roots of the herb Salvia miltiorrhiza for assessment of
their clinical utility (12). In
China, S. miltiorrhiza has been widely applied in the
treatment of cardiovascular and cerebrovascular diseases (13). Tanshinone IIA is a key active monomer
extracted from S. miltiorrhiza (14). Previous studies suggest that
pre-treatment tanshinone IIA exerts a nerve protective effect
against cerebral ischemia reperfusion injury (14,15). The
protection mechanism may involve B-cell lymphoma-2 (Bcl-2),
Bcl-2-associated X protein (Bax) and TRPM7 regulation (16,17).
Through increasing active removal of oxygen free radicals of
glutathione peroxidase, cell apoptosis may be inhibited (18). Thus, in the present study, tanshinone
IIA pretreatment was conducted in I/RIRI model rats, to investigate
the possible underlying mechanisms, in order to provide a
scientific basis for the development of a novel drug for the
treatment of I/RIRI.
Materials and methods
Chemicals
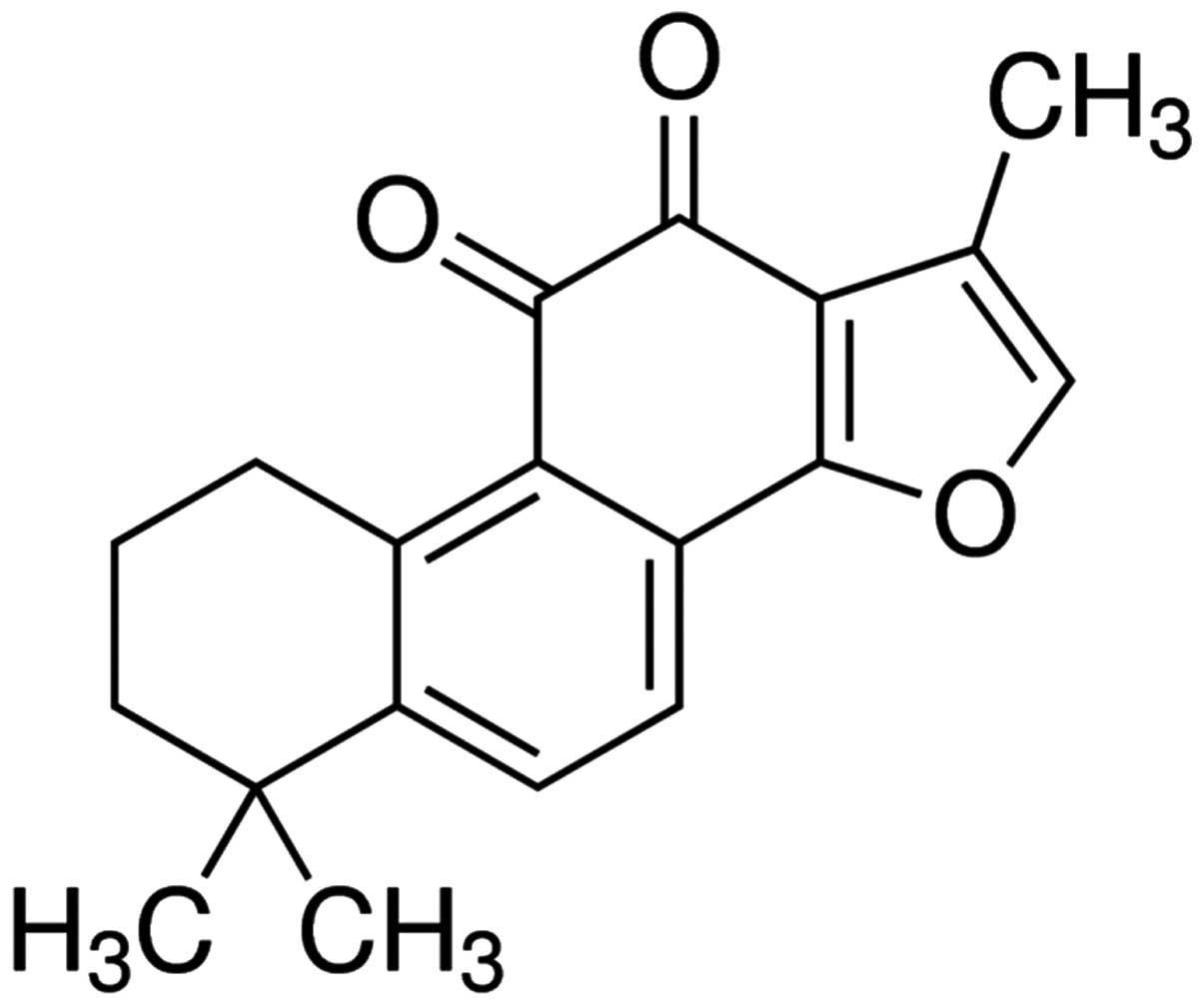
The chemical structure of tanshinone IIA is
indicated in Fig. 1 and purchased
from Sigma-Aldrich (St. Louis, MO, USA). Urea Nitrogen
Diacetylmonoxime Test Kit and Creatinine LiquiColor Test (Kinetic)
were purchase from Tiangen Biotech. Co., Ltd. (Beijing, China).
ELISA kits for the determination of myeloperoxidase (MPO), tumor
necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and macrophage
migration inhibition factor (MIF), and a bicinchoninic acid (BCA)
protein assay kit were purchased from the Nanjing Jiancheng
Bioengineering Institute (Nanjing, China).
Animals
A total of 26 adult male Sprague-Dawley rats
(weight, 250±20 g), were housed under a 12-h light/dark cycle at a
humidity of 60–65% and temperature of 22±3°C with free access to
food and water. The present experiment was approved by the animal
experimental ethics committee of Wuhan University (Wuhan,
China).
Rat model of I/RIRI
We established an I/RIRI rat model as previously
described (1). Briefly, experiment
rats were treated with isoflurane (2.5%) for anesthesia. Animal
body temperature was maintained during surgery. I/RIRI model was
induced following a right uninephrectomy and the left kidney was
ligatured using a non-traumatic aneurysm clip (FE690K; Aesculap AG,
Tuttlingen, Germany) for 25 min. Reperfusion was confirmed visually
when the color changed. Following surgery, experimental rats were
allowed to recover and had free access to water and chow.
Groups and drug administration
Experimental rats were randomly distributed into
three groups: Control group (n=6), rats were intraperitoneally
injected with saline; Model group (n=10), I/RIRI rats were
intraperitoneally injected with saline; and Tan group (n=10),
I/RIRI rats were intraperitoneally injected with tanshinone IIA (25
mg/kg body weight) for 10 days. Following treatment, rats were
sacrificed by decapitation.
Assessment of heart function
Renal tissue samples from all three groups were
measured. The blood urea nitrogen (BUN) and creatinine levels were
detected using commercial kits (Tiangen Biotech Co., Ltd.),
according to the manufacturer's protocols.
Assessment of MPO activity
Renal tissue samples from all three groups were
assessed by measuring MPO activity according to the manufacturer's
instructions (Jiancheng Bioengineering Institute). MPO activity
from all three groups was measured using a spectrophotometer at 460
nm.
ELISA for assessment of TNF-α, IL-6
and MIF activities
Renal tissue samples from all three groups were
assessed by measuring TNF-α, IL-6 and MIF activities using a
commercially obtained ELISA kits according to the manufacturer's
instructions (Jiancheng Bioengineering Institute).
Western blot analysis of cleaved
caspase-3, Bcl-2 and phosphorylated p38 mitogen-activated protein
kinase (p-p38 MAPK)
Renal injury samples from all three groups were
collected and extracted using RIPA Lysis Buffer (Beyotime Institute
of Biotechnology, Haimen, China) on ice for 30 min, and analyzed
using BCA protein assay kit (Jiancheng Bioengineering Institute).
Equal quantities of total protein (80 µg) from all three groups
were separated using 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked in 5% non-fat milk with phosphate-buffered
saline (PBS)-Tween 20 solution. Primary antibodies against cleaved
caspase-3 (1:500; cat. no. sc-9664), Bcl-2 (1:1,000; cat. no.
sc-7382), p-p38 MAPK (1:500; cat. no. sc-166182) and β-actin
(1:1,000; cat. no. sc-130300) (all purchased from Santa Cruz
Biotechnology, Inc., Carlsbad, CA, USA) were used at 4°C overnight.
The membranes were incubated with 5% nonfat milk/PBS-Tween 20
containing horseradish peroxidase-conjugated secondary antibody
(1:5,000; goat anti-mouse IgG; cat. no. 6401-05; Amyjet Scientific
Inc., Wuhan, China) for 2 h at 37°C. The membranes were visualized
using enhanced chemiluminescence (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and assessed using a Gel Doc 2000 imaging
scanner (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistics analysis
All data are expressed as the mean ± standard error
of the mean. Statistical analysis of data was performed using
one-way analysis of variance using SPSS software, version 19.0
(SPSS, Inc., Chicago, IL, USA). A P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of tanshinone IIA on renal
function
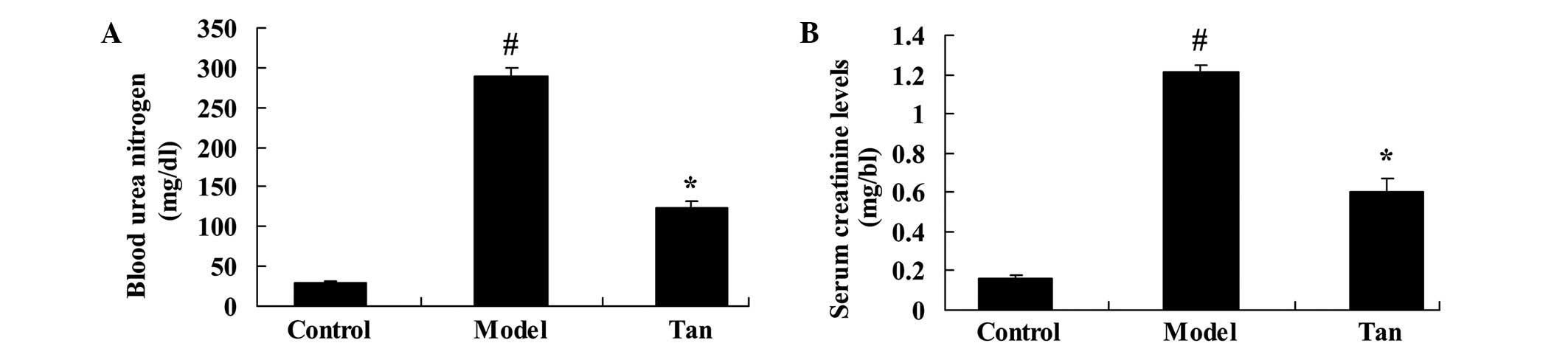
To determine the effect of tanshinone IIA on renal
function of I/RIRI rats, we measured the BUN and creatinine levels
with and without administration of tanshinone IIA. Fig. 2 showed I/RIRI significantly increased
the BUN and creatinine levels in I/RIRI rat, compared to those of
control rats. Then, relative to I/RIRI group, the BUN and
creatinine levels were significantly reduced with administration of
tanshinone IIA (Fig. 2).
Effect of tanshinone IIA on MPO
activity
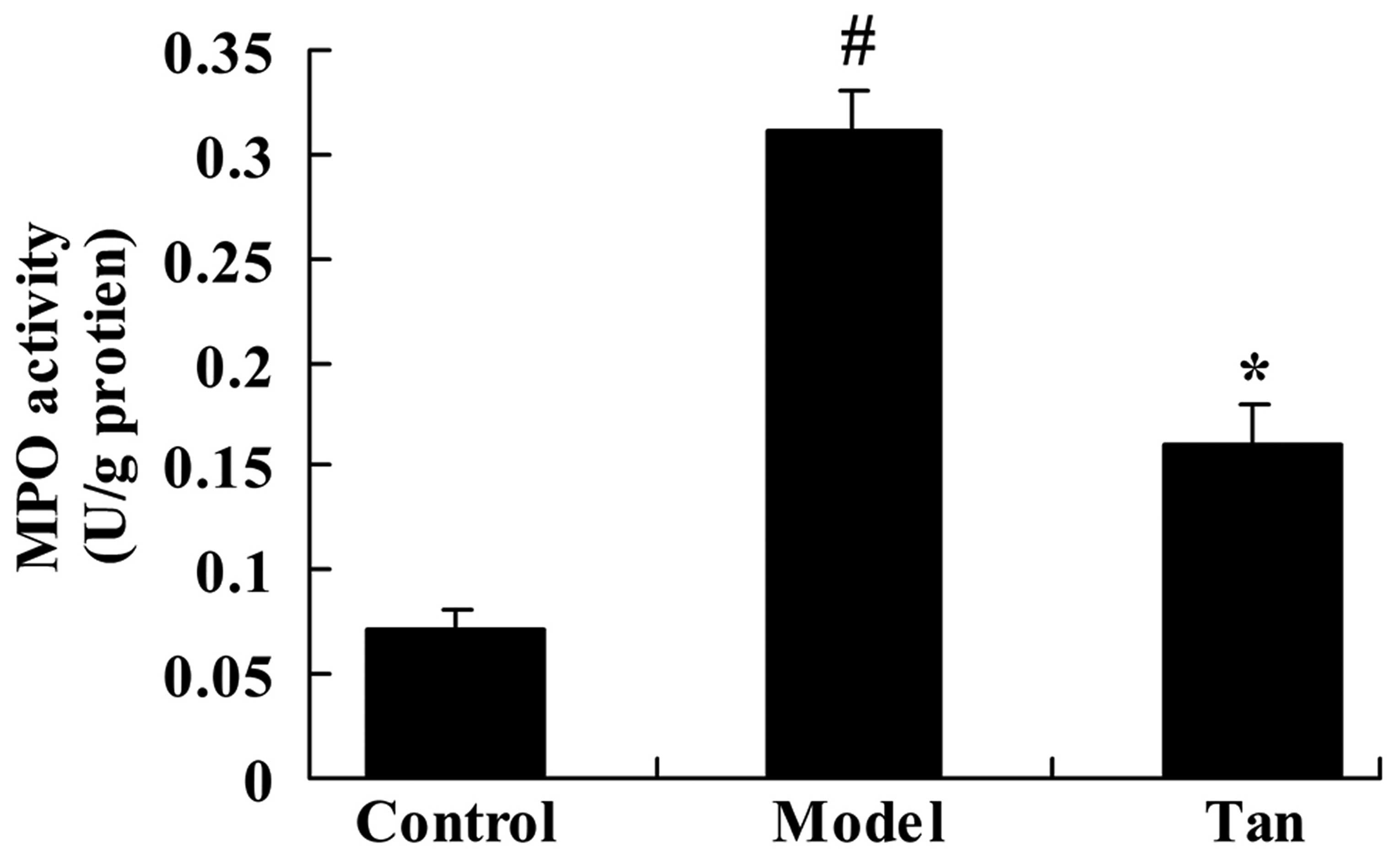
As shown in Fig. 3,
in the I/RIRI rat group, MPO activity was significantly increased
compared with that of control group. The promotion of MPO activity
in I/RIRI rats was significantly inhibited by the administration of
tanshinone IIA, compared with the I/RIRI rat group (Fig. 3).
Effect of tanshinone IIA on
proinflammatory cytokines
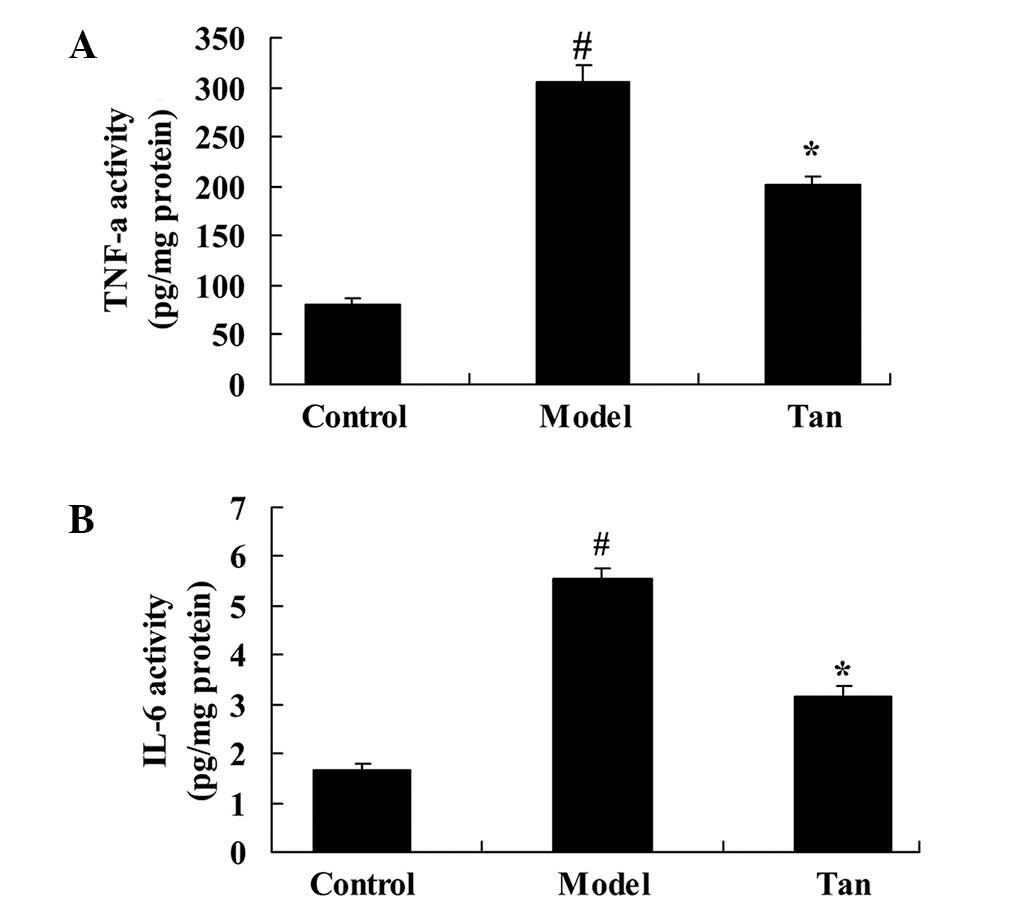
There was a significant increase in TNF-α and IL-6
activities were observed in I/RIRI rat group, compared with those
of control group (Fig. 4). However,
pretreatment with tanshinone IIA significantly mitigated the
increase TNF-α and IL-6 activities in I/RIRI rats, compared with
I/RIRI rat group (Fig. 4).
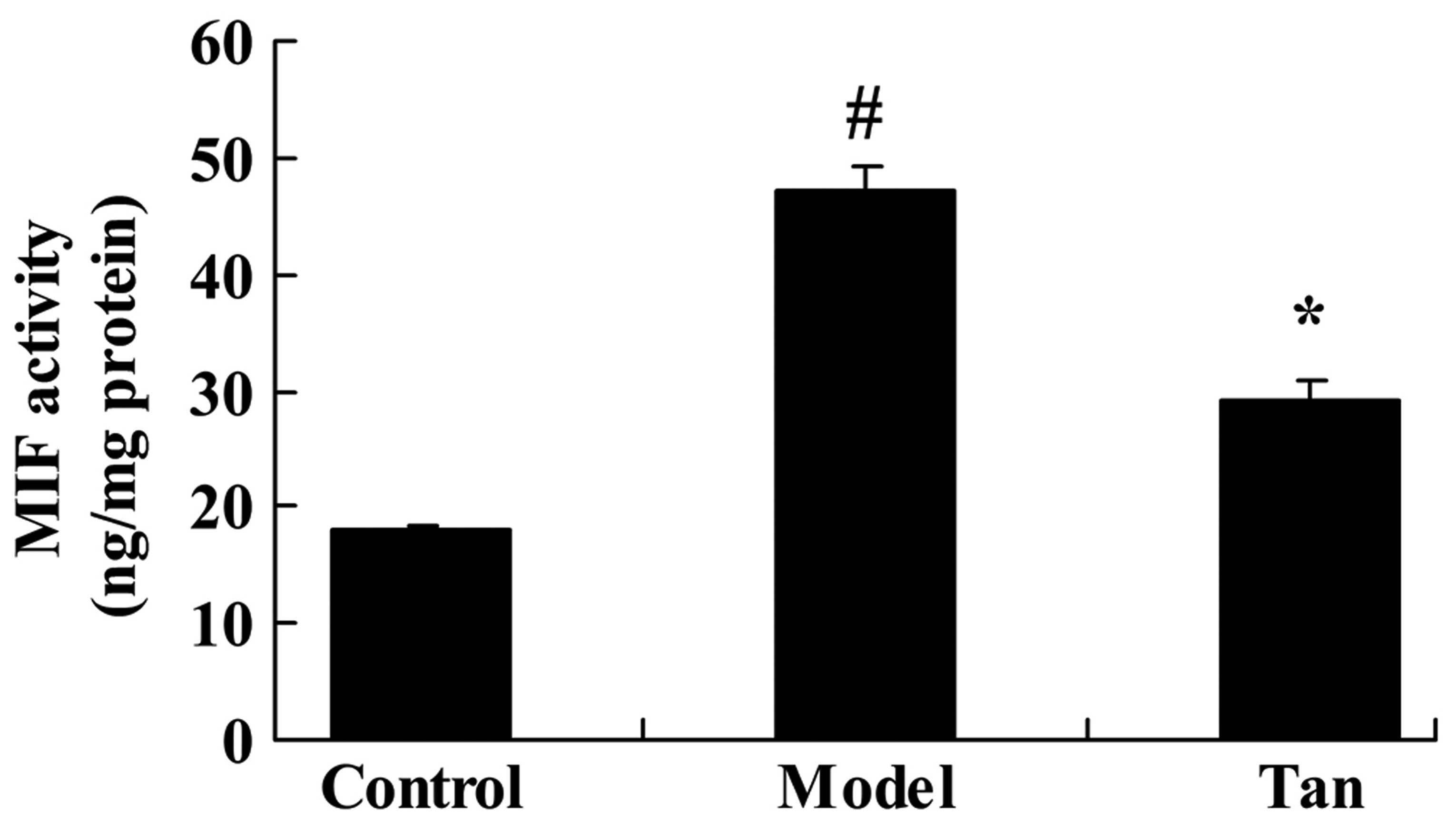
Effect of tanshinone IIA on MIF
activity
As shown in Fig. 5,
MIF activity of I/RIRI rat was significantly higher than the
control group. Relative to the I/RIRI rat group, tanshinone IIA
significantly reduced MIF activity in I/RIRI rats (Fig. 5).
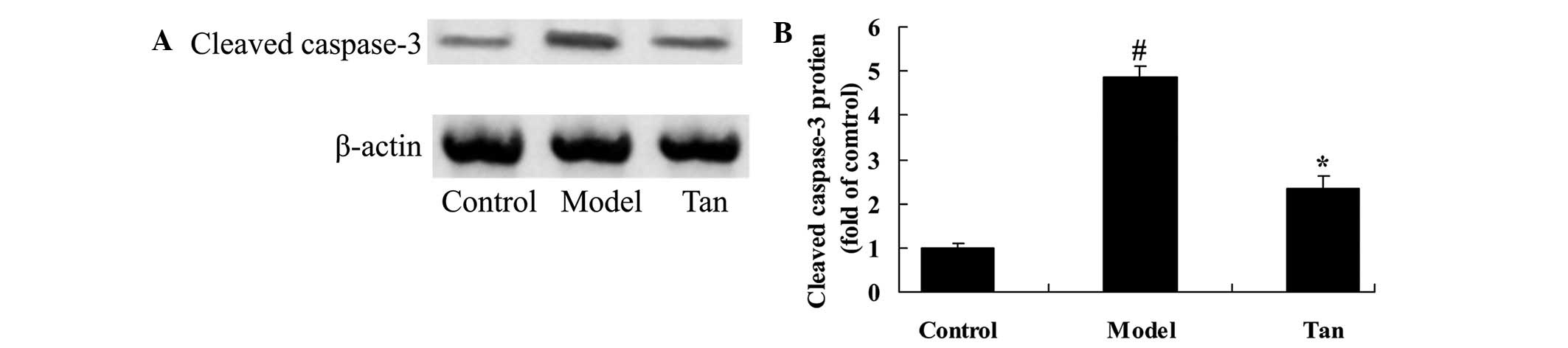
Effect of tanshinone IIA on cleaved
caspase-3
In order to investigate the effect of tanshinone IIA
on apoptosis in I/RIRI rats, cleaved caspase-3 protein expression
was analyzed using western blot analysis. There was a significant
increase in cleaved caspase-3 protein expression of I/RIRI rat,
compared with those of control group (Fig. 6). However, the increase cleaved
caspase-3 protein expression of I/RIRI rat was significantly
inhibited by pretreatment with tanshinone IIA, compared with the
I/RIRI rat group (Fig. 6).
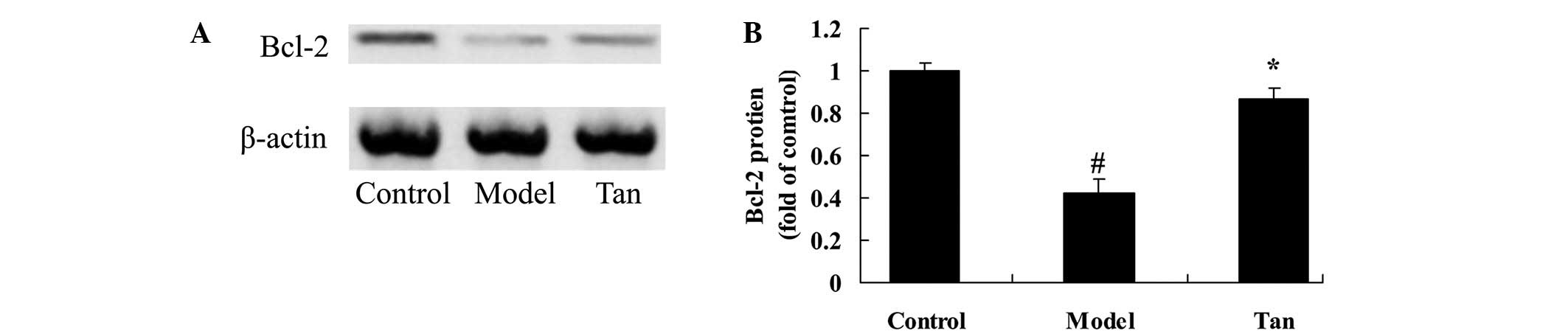
Effect of tanshinone IIA on Bcl-2
protein expression
To further explore the effect of tanshinone IIA on
apoptosis of I/RIRI rat, Bcl-2 protein expression was analyzed
using western blot analysis. These results of western blot analysis
showed that I/RIRI significantly suppressed the Bcl-2 protein
expression in rat, compared with those of control group (Fig. 7). As shown in Fig. 7, the suppression of Bcl-2 protein
expression was significantly increased by supplementation with
tanshinone IIA, compared with the I/RIRI rat group (Fig. 7).
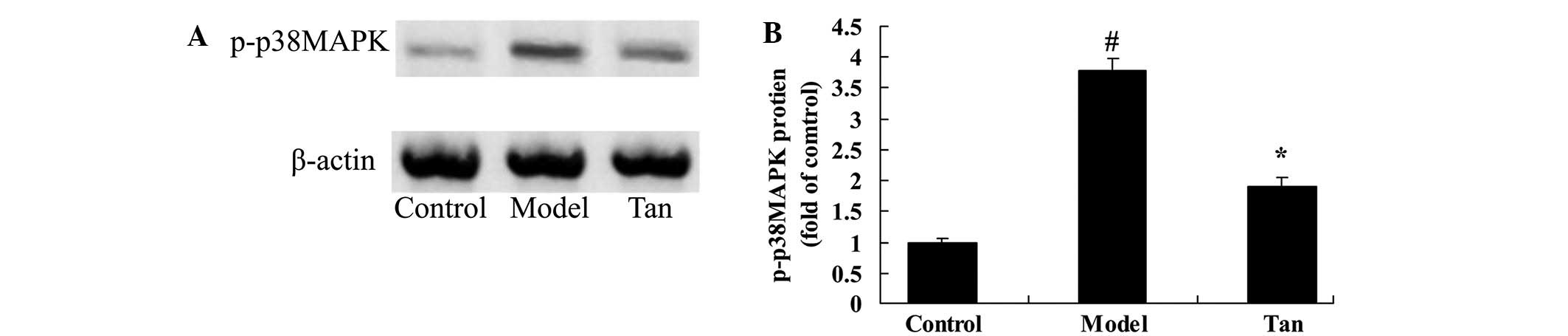
Effect of tanshinone IIA on p-p38 MAPK
protein expression
To further research the effect of tanshinone IIA on
apoptosis of I/RIRI rat, p-p38 MAPK protein expression was analyzed
using western blot analysis. Western blot analysis showed that
p-p38 MAPK protein expression was significantly higher than that of
the control group (Fig. 8).
Following surgery, tanshinone IIA significantly reduced the
elevation of p-p38 MAPK protein expression in I/RIRI rat, compared
with the I/RIRI rat group (Fig.
8).
Discussion
Ischemia/reperfusion is a common pathological
process in clinical practice. It can be detected following acute
hemorrhage, cardio-pulmonary resuscitation, cardiopulmonary bypass
heart surgery and organ transplantation surgery (2). However, taking kidney transplantation
as an example, ischemia/reperfusion may cause delayed recovery of
transplant renal function, and in cases of inducing acute rejection
may result in acute heart failure of the transplanted kidney at an
early stage (5). With the
application of anti-rejection drugs in clinical contexts, the
occurrence rate of acute rejection has decreased to 80% (19). The year survival rate of transplant
kidney has increased from 45 to 90% (19). Chronic organ damage induced by RIRI
has attracted increasing attention (20). The present results indicate that
tanshinone IIA pretreatment normalizes markers of renal function,
and reduced BUN and creatinine levels in the I/RIRI-induced rats.
These findings are consistent with previous results demonstrating
that tanshinone IIA attenuates hepatic (21) and cerebral ischemia/reperfusion
injury (14).
MPO primarily exists in the azurophilic granule of
neutrophile granulocytes. Its activity can indicates the
infiltration degree of neutrophile granulocytes in brain tissue
(22). MPO activity was
significantly increased in the ischemic brain tissue of rats at 24
h after I/RIRI, which indicates that I/RIRI is inflammatory
reaction participated by neutrophile granulocyte (23). Previous studies reported that after
reduction of I/RIRI, the expression level of intercellular adhesion
molecule 1 (ICAM-1) or reduction of neutrophile granulocyte which
can reduce I/RIRI and protective effect to renal (24). In present study, it was found that
tanshinone IIA significantly reduced the I/RIRI-induced MPO
activity in I/RIRI rats. Chen et al reported that tanshinone
IIA reduced cerebral ischemia/reperfusion injury via the inhibition
of MPO activity (25).
Inflammatory reaction is closely associated with
I/RIRI. ICAM-1 serves an important role in the key event of
inflammatory reaction and accumulation of neutrophile granulocytes
(8). A large number of cytokines
generated by I/RIRI, including TNF-α, may promote the expression of
ICAM-1 and induce adhesion, aggregation of neutrophile granulocytes
on microvascular endothelial cells (9). It is an indicator of I/RIRI
inflammatory reactions in vascular endothelial cells (10). In the present study, tanshinone IIA
significantly mitigated the TNF-α and IL-6 activities in I/RIRI
rats. Hu et al demonstrated that tanshinone IIA protects
myocardial ischemia reperfusion injury via reducing inflammatory
reactions (16). Yin et al
clearly show that tanshinone IIA attenuates the inflammatory
response after traumatic injury of the spinal cord in adult rats
(26).
The start action of immune-inflammatory responses
induced by I/RIRI on multiple organ dysfunction has been gradually
recognized (23). In recent years,
it has been detected through investigation that MIF plays a central
role in inflammatory reactions, including activation in T
lymphocytes (20). MIF is generated
by a variety of tissues and cells, including pituitary gland
tissues, monocyte/macrophages and T lymphocytes (18). MIF is a proinflammatory mediator and
a hormone derived from hypophysis (20). It can be used as a negative feedback
regulator of glucocorticoid physiological activities. The present
results demonstrate that tanshinone IIA significantly reduced MIF
activity in I/RIRI rats. Chen et al reported that tanshinone
IIA reduced cerebral ischemia/reperfusion injury via the inhibition
of MIF activity (25). Furthermore,
Zhang et al suggested that tanshinone IIA attenuates
seawater aspiration-induced lung injury via the suppression of MIF
(12).
Neuronal death following I/RIRI predominantly
present as necrosis and apoptosis (6). Cell apoptosis is an active process of
death under physiological and pathological conditions, and is
regulated by internal and external factors (19). During I/RIRI process, necrosis of
neurons and apoptosis are both observed (27). Necrocytosis is located at central
area of ischemia, while cell apoptosis is mainly observed at
ischemic penumbra (28). Previous
studies have indicated that I/RIRI apoptosis is regulated by a
variety of genes (29). Caspase-3 is
the most critical apoptotic protease in caspases cascade reaction
(30). Caspase-3 plays a role in
apoptosis process started by various procedures (19). It can be activated by splitting of
DNA cyclin-dependent kinases to change its structure and promote
cell apoptosis (29). The present
data suggest that supplementation with tanshinone IIA significantly
inhibited the increase cleaved caspase-3 protein expression in
I/RIRI rats. Zhang et al reported that tanshinone IIA
protects against ischemia-reperfusion injury by reducing caspase-3
activity in rats (31). Zhou et
al suggested that tanshinone IIA attenuates the cerebral
ischemic injury via the suppression of caspases-3 (32).
Bcl-2 is an important antiapoptosis gene,
particularly playing a prominent role in ischemia reperfusion
injury (11). The results of this
prior study showed that a large number of apoptotic cells occur at
distal convoluted renal tubular after renal ischemic reperfusion.
Consequently, the distal convoluted tubule after I/RIRI may play a
role in anti-apoptosis via Bcl-2 overexpression and thus reduce
cell damage (11). Previous studies
also indicate that the antioxygenation of Bcl-2 is indirect
(11). Namely, it may inhibit the
generation of superoxide anions, but not the removal of active
oxygen directly (33). The
superoxide anion inhibiting effect of Bcl-2 is associated with
inhibiting the release of cytochrome C (34). The present results suggest that
supplementation of tanshinone IIA markedly increased Bcl-2 protein
expression in I/RIRI rats. Zhou et al showed that tanshinone
IIA protects against methylglyoxal-induced injury through Bcl-2 and
p38 in human brain microvascular endothelial cells (35). Zhang et al reported that
tanshinone IIA protects against Bcl-2 and Bax expression of spinal
ischemia/reperfusion injury rats (18).
MAPK family is a signal conditioning enzyme
connecting AFP receptor and dominant gene expression (36). It may be activated in cases of
external stimulation, such as hypoxic-ischemic, inflammation, low
oxygen and aglycemia. It controls the process of adaptation,
proliferation, differentiation and apoptosis of cells. P38 MAPK is
present within the cytoplasm, and when activated it is rapidly
transferred into the nucleus and takes action on corresponding
targets within cells (37). It can
activate inferior kinase or a variety of transcriptional regulatory
factors, such as ATF-2, Elk-1, CHOP10 and MEF-2C (36,37).
Furthermore, it can activate MAPK activator protein kinase 2 and 3,
and caspase family members (37).
The combination of activated transcription factor and
cis-regulatory elements may result in substantial genetic
expression associated with apoptosis and is closely related to
delayed neuronal death (36). The
present results showed tanshinone IIA significantly reduced the
elevation of p-p38 MAPK protein expression in I/RIRI rats. Zhou
et al showed that tanshinone IIA protects against
methylglyoxal-induced injury through Bcl-2 and p38 in human brain
microvascular endothelial cells (35).
In conclusion, tanshinone IIA pretreatment
attenuates I/RIRI, and this effect is mediated partly via he
suppression of MPO, inflammatory reaction, MIF, and
apoptosis-mediating caspase-3 and p-p38 MAPK in I/RIRI rats.
References
|
1
|
Hoff U, Lukitsch I, Chaykovska L, Ladwig
M, Arnold C, Manthati VL, Fuller TF, Schneider W, Gollasch M,
Muller DN, et al: Inhibition of 20-HETE synthesis and action
protects the kidney from ischemia/reperfusion injury. Kidney Int.
79:57–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Yao Y, Xiao F, Lan X, Yu C, Zhang
Y, Jiang C, Yang J, Pei G, Li Y, et al: Administration of
dexamethasone protects mice against ischemia/reperfusion induced
renal injury by suppressing PI3K/AKT signaling. Int J Clin Exp
Pathol. 6:2366–2375. 2013.PubMed/NCBI
|
|
3
|
Jo SK, Ko GJ, Boo CS, Cho WY and Kim HK:
Heat preconditioning attenuates renal injury in ischemic ARF in
rats: Role of heat-shock protein 70 on NF-kappaB-mediated
inflammation and on tubular cell injury. J Am Soc Nephrol.
17:3082–3092. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan S, Wang G, Guo Y, Gui D and Wang N:
Preventive effects of a natural anti-inflammatory agent,
astragalosideIV, on ischemic acute kidney injury in rats. Evid
Based Complement Alternat Med. 2013:2840252013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei Q, Xiao X, Fogle P and Dong Z: Changes
in metabolic profiles during acute kidney injury and recovery
following ischemia/reperfusion. PLoS One. 9:e1066472014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grossini E, Molinari C, Pollesello P,
Bellomo G, Valente G, Mary D, Vacca G and Caimmi P: Levosimendan
protection against kidney ischemia/reperfusion injuries in
anesthetized pigs. J Pharmacol Exp Ther. 342:376–388. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin Y, Alderliesten MC, Stokman G,
Pennekamp P, Bonventre JV, de Heer E, Ichimura T, de Graauw M,
Price LS and van de Water B: Focal adhesion kinase signaling
mediates acute renal injury induced by ischemia/reperfusion. Am J
Pathol. 179:2766–2778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collino M, Benetti E, Miglio G, Castiglia
S, Rosa AC, Aragno M, Thiemermann C and Fantozzi R: Peroxisome
proliferator-activated receptor β/δ agonism protects the kidney
against ischemia/reperfusion injury in diabetic rats. Free Radic
Biol Med. 50:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Paola R, Genovese T, Impellizzeri D,
Ahmad A, Cuzzocrea S and Esposito E: The renal injury and
inflammation caused by ischemia-reperfusion are reduced by genetic
inhibition of TNF-αR1: A comparison with infliximab treatment. Eur
J Pharmacol. 700:134–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizutani A, Okajima K, Uchiba M, Isobe H,
Harada N, Mizutani S and Noguchi T: Antithrombin reduces
ischemia/reperfusion-induced renal injury in rats by inhibiting
leukocyte activation through promotion of prostacyclin production.
Blood. 101:3029–3036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isaka Y, Suzuki C, Abe T, Okumi M,
Ichimaru N, Imamura R, Kakuta Y, Matsui I, Takabatake Y, Rakugi H,
et al: Bcl-2 protects tubular epithelial cells from
ischemia/reperfusion injury by dual mechanisms. Transplant Proc.
41:52–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Zhang B, Xu DQ, Li WP, Xu M, Li
JH, Xie XY, Fan QX, Liu W, Mu DG, et al: Tanshinone IIA attenuates
seawater aspiration-induced lung injury by inhibiting macrophage
migration inhibitory factor. Biol Pharm Bull. 34:1052–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Poppel PC, Breedveld P, Abbink EJ,
Roelofs H, van Heerde W, Smits P, Lin W, Tan AH, Russel FG, Donders
R, et al: Salvia miltiorrhiza root water-extract (Danshen) has no
beneficial effect on cardiovascular risk factors. A randomized
double-blind cross-over trial. PLoS One. 10:e01286952015.
|
|
14
|
Chen Y, Wu X, Yu S, Fauzee NJ, Wu J, Li L,
Zhao J and Zhao Y: Neuroprotective capabilities of tanshinone IIA
against cerebral ischemia/reperfusion injury via anti-apoptotic
pathway in rats. Biol Pharm Bull. 35:164–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wen PY, Li J, Lu BL, Liu J, Yang FZ, Zhou
L, Luo H, Li WW and Zhou J: Tanshinone IIA increases levels of
NeuN, protein disulfide isomerase, and
Na+/K+-ATPase and decreases evidence of
microglial activation after cerebral ischemic injury. Neuroreport.
27:435–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu H, Zhai C, Qian G, Gu A, Liu J, Ying F,
Xu W, Jin D, Wang H, Hu H, et al: Protective effects of tanshinone
IIA on myocardial ischemia reperfusion injury by reducing oxidative
stress, HMGB1 expression and inflammatory reaction. Pharm Biol.
53:1752–1758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Gan W and An G: Influence of
tanshinone IIA on heat shock protein 70, Bcl-2 and Bax expression
in rats with spinal ischemia/reperfusion injury. Neural Regen Res.
7:2882–2888. 2012.PubMed/NCBI
|
|
18
|
Wang S, Zang W, Yang Y, Zhang Q, Zhao M,
Gao Z, Li G, Meng Q, Liu Q and Zheng X: Tanshinone IIA and Baicalin
inhibiting the formation of benzo[a]pyrene and benzo[a]pyrene
induced cytotoxicity: Correlation with scavenging free radical.
Environ Toxicol Pharmacol. 36:403–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El Morsy EM, Ahmed MA and Ahmed AA:
Attenuation of renal ischemia/reperfusion injury by açaí extract
preconditioning in a rat model. Life Sci. 123:35–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Yu G, Liu SY, Li JB, Wang JF, Bo
LL, Qian LR, Sun XJ and Deng XM: Hydrogen-rich saline protects
against renal ischemia/reperfusion injury in rats. J Surg Res.
167:e339–e344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi YY, Xiao L, Zhang LD, Song SH, Mei Y,
Chen T, Tang JM, Liu F, Ding GS, Shi YZ and Wang QX: Tanshinone IIA
pretreatment attenuates hepatic ischemia-reperfusion. Front Biosci
(Elite Ed). 4:1303–1313. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Xu L, Zhao J, Wang D, Guo R, Wang
J, Gong W, Liu T, Zhang Y and Dong L: Regulatory mechanism of
pyrrolidine dithiocarbamate is mediated by nuclear factor-κB and
inhibits neutrophil accumulation in ARDS mice. Exp Ther Med.
8:614–622. 2014.PubMed/NCBI
|
|
23
|
Kurcer Z, Oguz E, Ozbilge H, Baba F, Aksoy
N, Celik H, Cakir H and Gezen MR: Melatonin protects from
ischemia/reperfusion-induced renal injury in rats: This effect is
not mediated by proinflammatory cytokines. J Pineal Res.
43:172–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ragab D, Abdallah DM and El-Abhar HS:
Cilostazol renoprotective effect: Modulation of PPAR-γ, NGAL, KIM-1
and IL-18 underlies its novel effect in a model of
ischemia-reperfusion. PLoS One. 9:e953132014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Wu X, Yu S, Lin X, Wu J, Li L,
Zhao J and Zhao Y: Neuroprotection of tanshinone IIA against
cerebral ischemia/reperfusion injury through inhibition of
macrophage migration inhibitory factor in rats. PLoS One.
7:e401652012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou
WG, Sun SK and Luo ZJ: Tanshinone IIA attenuates the inflammatory
response and apoptosis after traumatic injury of the spinal cord in
adult rats. PLoS One. 7:e383812012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang CC, Lin LC, Wu MS, Chien CT and Lai
MK: Repetitive hypoxic preconditioning attenuates renal
ischemia/reperfusion induced oxidative injury via upregulating
HIF-1 alpha-dependent bcl-2 signaling. Transplantation.
88:1251–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan XG, Cheng BH, Wang X, Ding LC, Liu HQ,
Chen J and Bai B: Lateral intracerebroventricular injection of
Apelin-13 inhibits apoptosis after cerebral ischemia/reperfusion
injury. Neural Regen Res. 10:766–771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ben Mkaddem S, Pedruzzi E, Werts C, Coant
N, Bens M, Cluzeaud F, Goujon JM, Ogier-Denis E and Vandewalle A:
Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in
Toll-like receptor 4-activated apoptosis during renal
ischemia/reperfusion injury. Cell Death Differ. 17:1474–1485. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao WY, Han S, Zhang L, Zhu YH, Wang LM
and Zeng L: Mitochondria-targeted antioxidant peptide SS31 prevents
hypoxia/reoxygenation-induced apoptosis by down-regulating p66Shc
in renal tubular epithelial cells. Cell Physiol Biochem.
32:591–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang MQ, Zheng YL, Chen H, Tu JF, Shen Y,
Guo JP, Yang XH, Yuan SR, Chen LZ, Chai JJ, et al: Sodium
tanshinone IIA sulfonate protects rat myocardium against
ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim
pathway. Acta Pharmacol Sin. 34:1386–1396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou L, Bondy SC, Jian L, Wen P, Yang F,
Luo H, Li W and Zhou J: Tanshinone IIA attenuates the cerebral
ischemic injury-induced increase in levels of GFAP and of
caspases−3 and −8. Neuroscience. 288:105–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chien CT, Shyue SK and Lai MK: Bcl-xL
augmentation potentially reduces ischemia/reperfusion induced
proximal and distal tubular apoptosis and autophagy.
Transplantation. 84:1183–1190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chien CT, Chang TC, Tsai CY, Shyue SK and
Lai MK: Adenovirus-mediated bcl-2 gene transfer inhibits renal
ischemia/reperfusion induced tubular oxidative stress and
apoptosis. Am J Transplant. 5:1194–1203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou WJ, Gui QF, Wu Y and Yang YM:
Tanshinone IIA protects against methylglyoxal-induced injury in
human brain microvascular endothelial cells. Int J Clin Exp Med.
8:1985–1992. 2015.PubMed/NCBI
|
|
36
|
Lempiäinen J, Finckenberg P, Mervaala EE,
Storvik M, Kaivola J, Lindstedt K, Levijoki J and Mervaala EM:
Dexmedetomidine preconditioning ameliorates kidney
ischemia-reperfusion injury. Pharmacol Res Perspect. 2:e000452014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park KM, Kramers C, Vayssier-Taussat M,
Chen A and Bonventre JV: Prevention of kidney
ischemia/reperfusion-induced functional injury, MAPK and MAPK
kinase activation and inflammation by remote transient ureteral
obstruction. J Biol Chem. 277:2040–2049. 2002. View Article : Google Scholar : PubMed/NCBI
|