Introduction
Cancer is a major cause of mortality worldwide. In
clinical practice, metastasis is the primary challenge in cancer
treatment as it is the most malignant facet of cancer progression
and leads to large numbers of mortality. Metastasis is a
complicated multi-step process, which includes loss of cellular
adhesion, increased tumor motility and invasiveness, intravasation
transport, survival in the circulatory system, extravasation and
metastatic colonization in distant organs (1). Examining the impact of metastasis in
vivo remains an important tool in preclinical studies for
evaluating novel therapies and studying cancer metastasis (2). However, it is difficult to optimally
evaluate metastasis in animal models. For example, only a limited
number of studies have developed spontaneous lung metastases in
transgenic mouse models (3,4). A common limitations of these studies
was that metastasis status was assessed in sacrificed mice;
therefore, the tumor burden could not be accurately assessed in a
living model (2,5). However, molecular imaging of reporter
gene expression in cancer cells may provide a rapid, sensitive and
non-invasive method of monitoring tumor behavior. The aim of the
present study was to establish a non-invasive method to measure
tumor size and distribution in vivo.
Andrographolide, a bicyclic diterpenoid lactone, is
the primary ingredient of the medicinal herb Andrographis
paniculata. Andrographolide exhibits a broad spectrum of
pharmacological activities including anti-oxidation,
anti-inflammation, anti-viral and anti-cancer effects (6). Furthermore, andrographolide has
demonstrated radiosensitization in vitro and in vivo
(7), which was associated with the
downregulation of protein kinase B (Akt) and nuclear factor
kappa-light-chain-enhancer of activated B cells (NF-κB) activity.
Akt and NF-κB activity have exhibited an association with tumor
cell invasion and epithelial-mesenchymal transition (8,9). These
results imply that andrographolide may inhibit metastatic lesions.
In the current study, the effect of andrographolide on cancer
metastasis was investigated, using a novel imaging system.
Materials and methods
Reagents and antibodies
Andrographolide (98% purity; Merck Millipore,
Darmstadt, Germany) was dissolved in dimethyl sulfoxide (DMSO) as a
concentrated stock solution. Primary monoclonal antibodies against
anti-Ras (DCABH-9932) were purchased from Upstate Biotechnology
Inc. (Lake Placid, NY, USA); phospho-p44/42 ErK1/2 (Thr202/Tyr204;
9101) and p44/42 p44/42 MAPK (Erk1/2; 4695) were purchased from
Cell Signaling Technology, Inc., (Beverly, MA, USA). Horseradish
peroxidase (HRP)-conjugated anti-rabbit IgG (A0545) and anti-mouse
IgG (A9044) monoclonal secondary antibodies, and isoflurane and
luciferin were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany).
Cell culture
Rat kidney (RK3E/tv-a) cells were infected with a
retrovirus carrying the oncogene H-Ras or control gene
puromycin-N-acetyl-transferase (puro) as described in a previous
study (10). Rat kidney (RK3E/tv-a)
cells were kindly provided by Dr. Fu's laboratory (National
Yang-Ming University, Taipei, Taiwan). Ras-expressing cells, but
not puro-expressing cells, underwent malignant transformation in
vitro and tumorigenesis in vivo. Cells were maintained
as described previously (10).
Subsequently, Ras-transformed cells were stably transfected with
plasmids containing luciferase gene (Luc), and cells were
designated as Ras/Luc. The oncogenicity, luciferase activity and
Ras expression levels of this cell line were analyzed and
validated.
Soft agar assays
Prior to use, 6-well plates were overlaid with 0.6%
nutrient agar that contained Sea Plaque agar in Dulbecco's modified
Eagle medium supplemented with 3% fetal bovine serum (FBS), 100
units/ml penicillin, 100 µg/ml streptomycin and 3 µg/ml puromycin.
Ras/Luc cells (104) were mixed well with 0.3% nutrient
agar, seeded into prepared 6-well plates and incubated at 37°C for
two weeks. Every 3–4 days, the cells were replenished with 0.3%
nutrient agar. Following two weeks of incubation, colonies were
stained with crystal violet, photographed, and counted using a
light microscope.
Luciferase assay
Ras/Luc cells (103) were plated in a
96-well plate and cultured overnight at room temperature. For
transfection, 0.1 mg pGL3-reporter vectors and 10 ng pRL-TK renilla
internal control (Promega Corp., Madison, WI, USA) per well were
co-transfected using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. At 48 h post-transfection, cells were
lysed in 100 ml 6X passive lysis buffer at room temperature for 20
min. For the luciferase assay, 30 ml lysate was aliquoted into a
96-well plate to measure firefly luciferase and renilla luciferase
activity. Fluorescence intensity was read with a luminometer by
mixture cell lysate and luciferin (Beckman Coulter, Inc., Brea, CA,
USA).
Western blot analysis
Cells (106) were seeded into 100-mm
plates, incubated overnight, and collected by scraping and
centrifugation at 1,200 rpm for 5 min at room temperature. Cell
pellets were subsequently lysed in radioimmune precipitation assay
buffer (RIPA) supplemented with protease inhibitors. RIPA buffer
was prepared with trisaminomethane (50 mM; pH 7.4), NaCl (150 mM),
1% Nonidet P-40, 0.25% sodium deoxycholate, EDTA (5 mM; pH 8.0) and
EGTA (1 mM; pH 8.0). Cell suspensions were subsequently centrifuged
at 13,000 rpm for 20 min at 4°C to collect clear lysates, and the
protein concentration was measured using the Bio-Rad protein assay
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total protein
(50 µg lysate proteins) was separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes. Following
blocking with 5% non-fat milk/Tris-Buffered Saline-Tween-20 (TBST),
membranes were incubated overnight with primary antibody including
anti-Ras (1:1,000), anti-p-Erk (1:1,000) and anti-Erk (1:1,000) at
4°C for 1 h. Secondary HRP-conjugated anti-rabbit IgG (A0545) and
anti-mouse IgG antibodies diluted in 1% BSA/TBS/0.1% Tween 20
(1:20,000) were sequentially incubated with the membranes for 1 h
each at room temperature. Protein bands were visualized by
chemiluminescence using an enhanced chemiluminescence detection
system (Amersham Biosciences, Uppsala, Sweden). ImageJ software
(version 1.40; National Institutes of Health, Bethesda, MA, USA)
was used to quantify the intensity of the images.
In vivo tumorigenesis assays
The Ras/Luc cell line, which is a Ras-transformed
cell line constitutively expressing the luciferase gene, was used
in the present study. Briefly, 106 Ras/Luc cells in 100
µl phosphate-buffered saline were injected into the back or tail
vein of nude mice. A total of 59 male (aged 6–8 weeks-old) nude
mice (BALB/cAnN.Cg-Foxn1nu/CrlNarl) were obtained from the National
Laboratory Animal Center (Tainan, Taiwan).
BALB/cAnN-Foxn1nu/CrlNarl mice were maintained under 12-h
light-dark cycle at room temperature (24±1°C) and 60±5% humidity.
They were provided with a standard diet and had ad libitum
access to water during experimentation. In vivo
bioluminescence images were captured every five days following
tumor injection. Subsequently, mice were administered 150 mg/kg
D-luciferin via intra-peritoneal injection 15 min prior to image
acquisition. Mice were gas anesthetized with 1–3% isoflurane,
relocated to the warmed stage in the chamber and continuously
exposed to 1–2% isoflurane for sustained sedation during imaging.
The isoflurane level was adjusted according to the number of and
weight of the animals in the chamber. Mice were in the chamber for
5–10 min and operators adjusted the gas flow to ensure mice were
completely anesthetized. Photons emitted from mice were detected
using the IVIS50 imaging system (Caliper Life Sciences, Inc.,
Alameda, USA) and regions of interest (ROIs) from displayed images
were quantified as photons/second (ph/s) using the Living Image
software (version 2; PerkinElmer Inc., Houston, TX, USA).
Following establishment of an imaging system to
monitor tumor formation in vivo, mice were divided into four
groups according to the various treatments administered: Group 1
(n=15), andrographolide (10 µM); group 2 (n=15), radiation (2 Gy)
and vehicle (100 µl DMSO); group 3 (n=14), andrographolide (10 µM)
plus radiation (2 Gy); and group 4 (n=15), vehicle (100 µl DMSO).
Andrographolide dosage was selected based on a previous in
vivo study (7). Ras-transformed
cells were injected into the tail vein and were traced periodically
using the in vitro imaging system (IVIS). Andrographolide
was administered concurrently with radiation. Cobalt-60 gamma rays
(Atomic Energy of Canada Limited, Chalk River, Ontario, Canada)
were used for radiation. Mice body weights were recorded every
week. ROIs from displayed images were drawn around the tumor and
the size of interested organs and their ph/s were quantified. All
animal protocols were performed according to the instructions
issued by the Institutional Animal Care and Use Committee of
National Yang-Ming University (Taipei, China). Following IVIS
examination, the mice were sacrificed by cervical dislocation, and
their tissues were subsequently examined.
Determination of
H2O2 level
Cells (2×105) were seeded into 6-well
plates and cultured overnight in a CO2 incubator at
37°C. The medium was removed 24 h later and replaced with fresh
medium with or without andrographolide. H2O2
levels were measured following exposure of Ras-transformed cells to
10 µM andrographolide for 3 h, with or without radiation treatment.
Following another 3-h interval, cells were collected. The effects
of andrographolide under various conditions on the generation of
intracellular H2O2 were determined via
2′,7′-dichlorofluorescin diactetate (DCFH2-DA)
treatment. Flow cytometry (wavelength, 505/535 nm) was used to
determine dichlorodihydrofluoroscein (DCF) fluorescence intensity
(Becton Dickinson, Lincoln Park, NJ, USA). For control experiments,
5 µM N-acetylcysteine and 0.03% H2O2 were
added to the culture 30 min prior to adding
DCFH2-DA.
Statistical analysis
SPSS software (version 21; IBM SPSS, Armonk, NY,
USA) was used for statistical analyses. Data are presented as mean
± standard deviation of three independent, duplicated experiments.
Statistical analyses were performed using two-tail unpaired
Student's t-test to compare two groups of data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of a non-invasive method
to measure tumor size and distribution in vivo
Initially, Ras-transformed cells stably transfected
with plasmids containing Luc were established, designated as
Ras/Luc, and the oncogenicity, luciferase activity and Ras
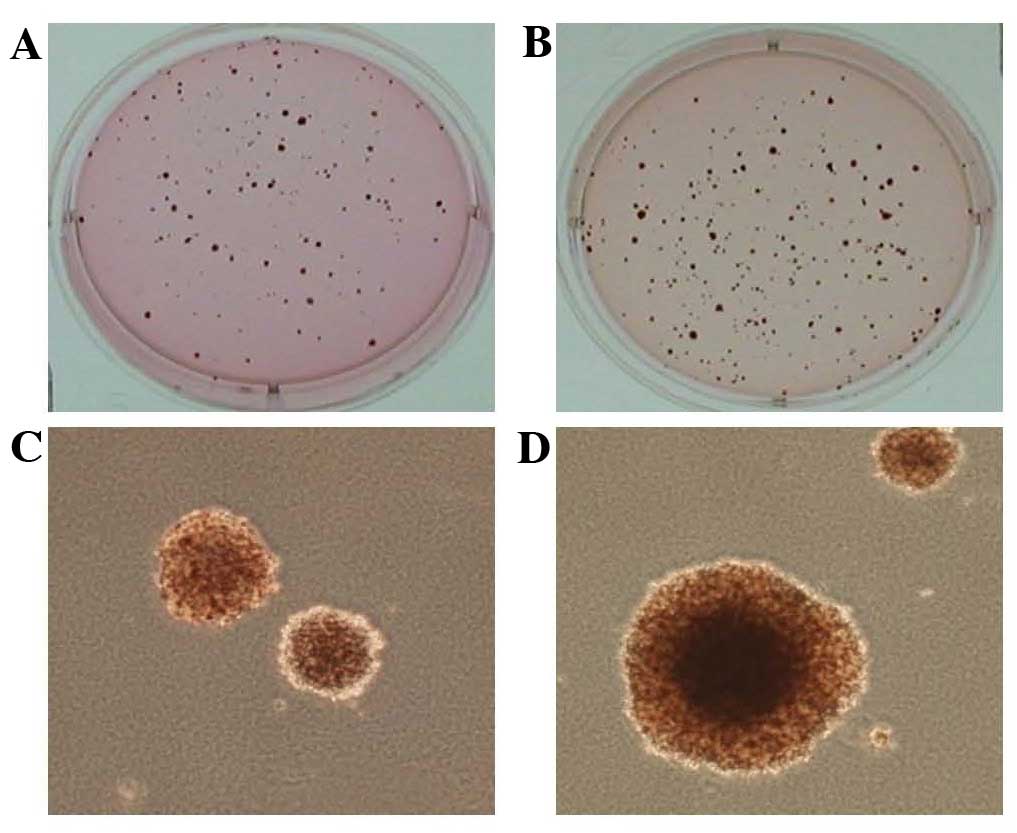
expression of this cell line were determined. As shown in Fig. 1, Ras/Luc cells successfully grew in
soft agar, which is one of the hallmark characteristics of cellular
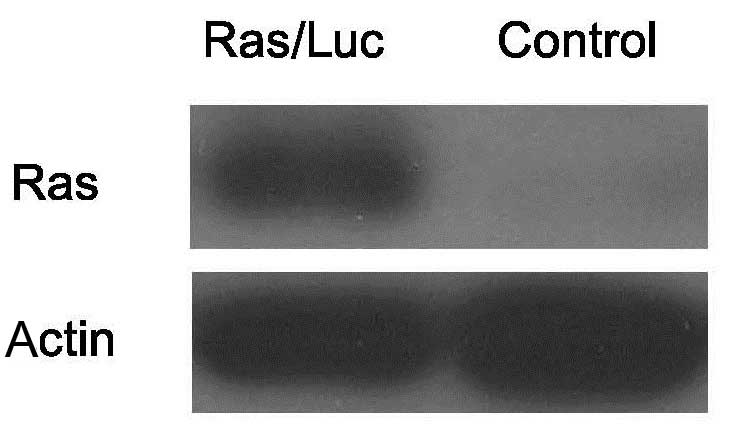
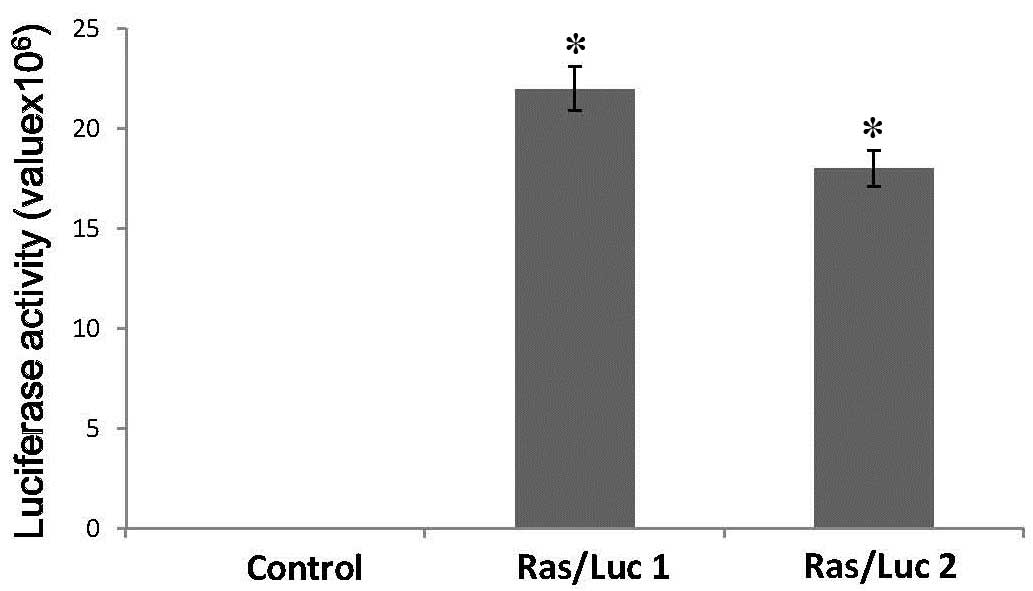
transformation and uncontrolled cell growth. As indicated in
Figs. 2 and 3, the Ras/Luc cell line exhibited
luciferase activity and Ras expression.
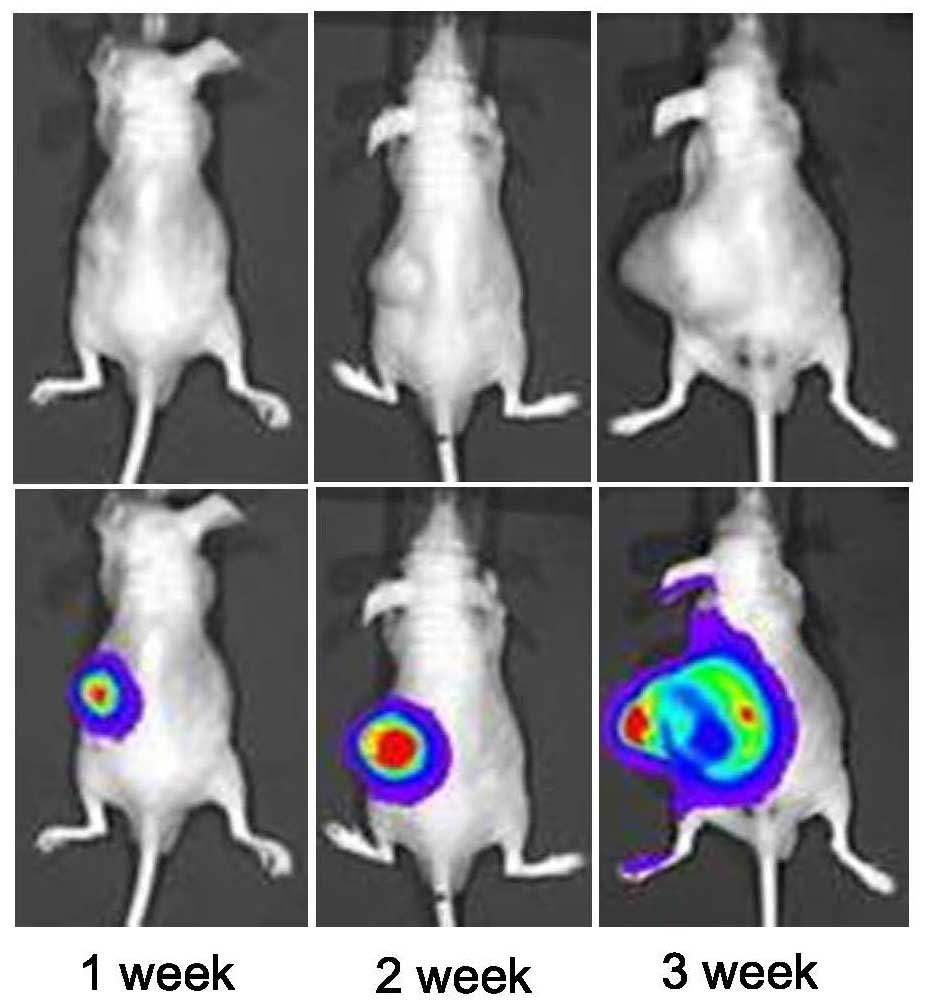
Tumorigenesis was tested in vivo. Ras/Luc
cells were subcutaneously transplanted into the back of nude mice
and subsequently, the presence of tumors was detected via
bioluminescence imaging using IVIS following luciferin injection
(Fig. 4). In addition, the
metastatic activity of Ras/Luc cells in vivo was also
examined using IVIS. Ras-transformed cells were injected into the
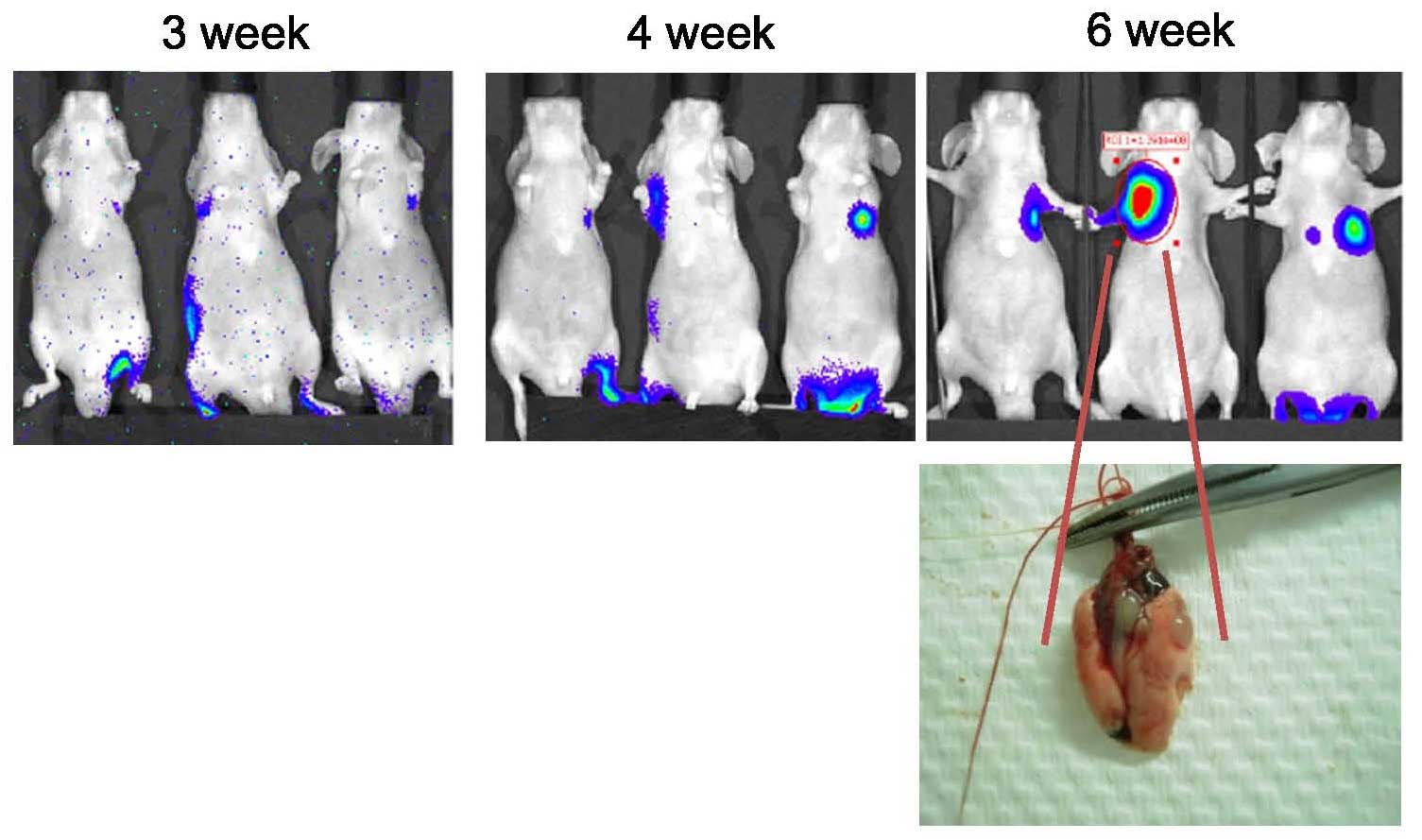
tail vein and traced periodically using IVIS. As shown in Fig. 5, lung metastases were detected by
IVIS, as photons emitted from the mice were detected and ROIs were
quantified as ph/s. IVIS can detect and quantify absolute signals.
These results are reproducible (11). Following the experimental stage of
the present study, the tumor burden was measured in the sacrificed
mice. Lung tissue was dissected and the anatomical location was
detected and confirmed (Fig. 5).
However, the tumor size was difficult to accurately assess due to
their small sizes and metastatic conditions. Despite this
limitation, the results of the present study were unlikely to have
been compromised. The successful establishment of IVIS with a
Ras/Luc mouse xenograft model facilitated evaluation of tumor
kinetics and the metastatic spread of tumors.
Effects of combined treatment with
andrographolide and radiation on cancer metastasis
Various treatments were examined among the four
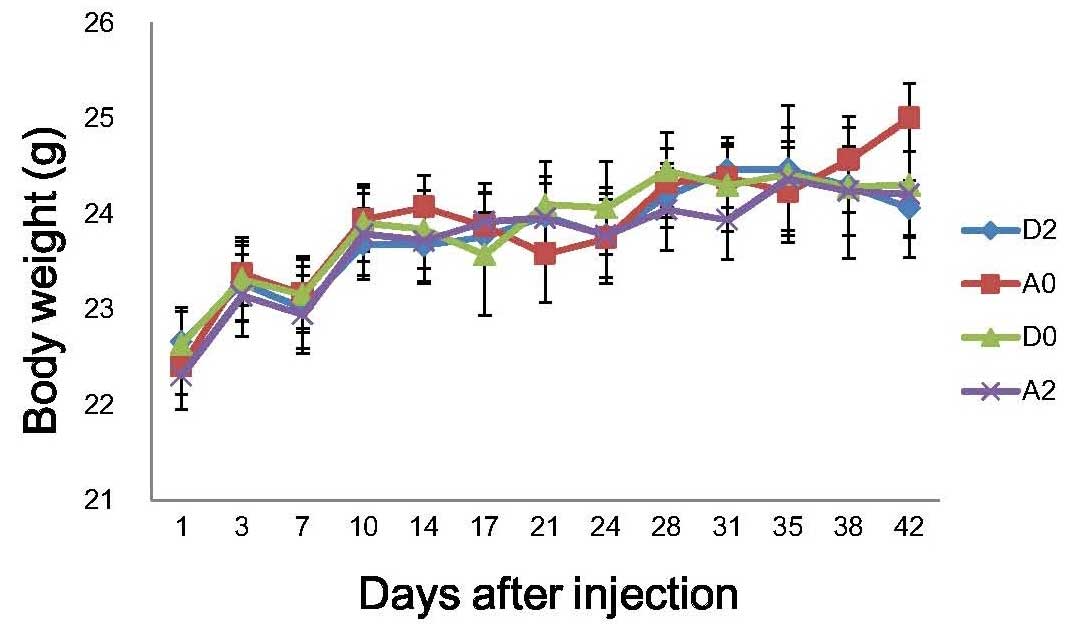
groups. No significant alterations in body weight were detected
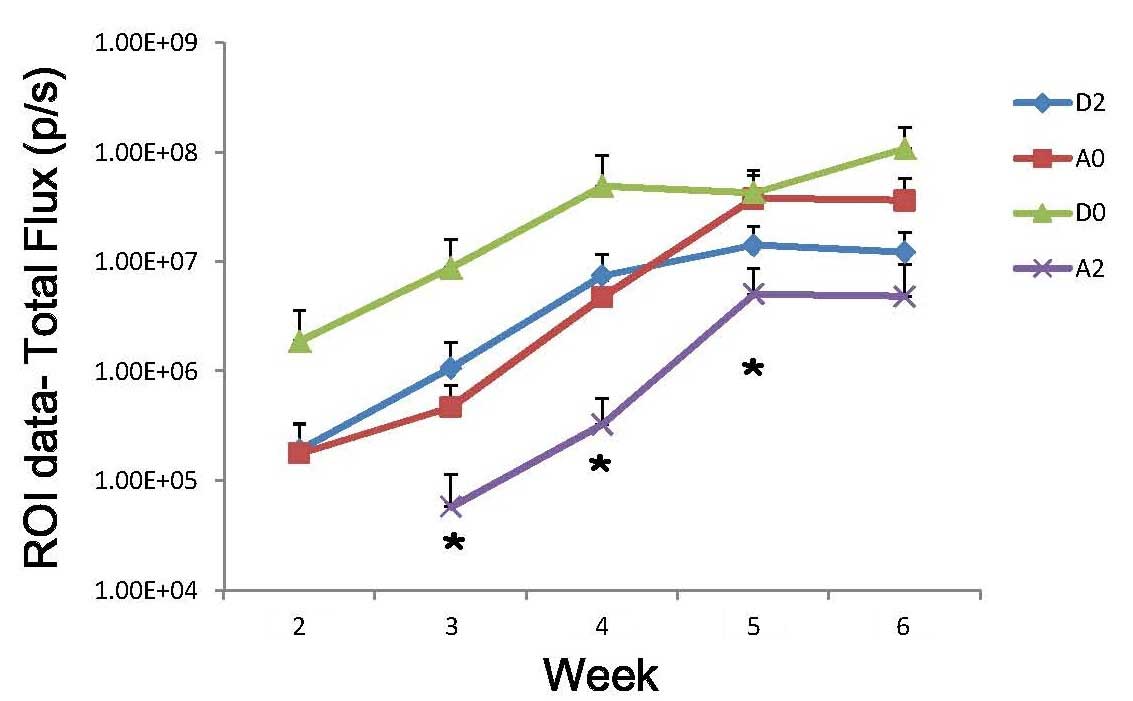
following andrographolide and/or radiation treatment (Fig. 6). As shown in Fig. 7, andrographolide treatment with
radiation significantly inhibited cancer metastasis (P<0.05).
Mice lung metastatic rates are presented in Table I. The ratio among vehicle,
andrographolide (10 µM), radiation (2 Gy) and andrographolide (10
µM) plus radiation (2 Gy) was 73.3, 53.3, 53.3 and 28.6,
respectively. The preliminary results indicated that
andrographolide had an inhibitive effect on cancer metastasis.
 | Table I.Lung metastatic rate in mice. |
Table I.
Lung metastatic rate in mice.
| Tumorigenesis in
lung area |
|---|
|
|---|
| Group | Mice with
tumors/mice injected | Ratio (%) |
|---|
| D0 | 11/15 | 73.3 |
| A0 | 8/15 | 53.3 |
| D2 | 8/15 | 53.3 |
| A2 | 4/14 | 28.6 |
Activated Erk protein expression
levels are significantly increased by andrographolide
Whether the anti-cancer effect of andrographolide
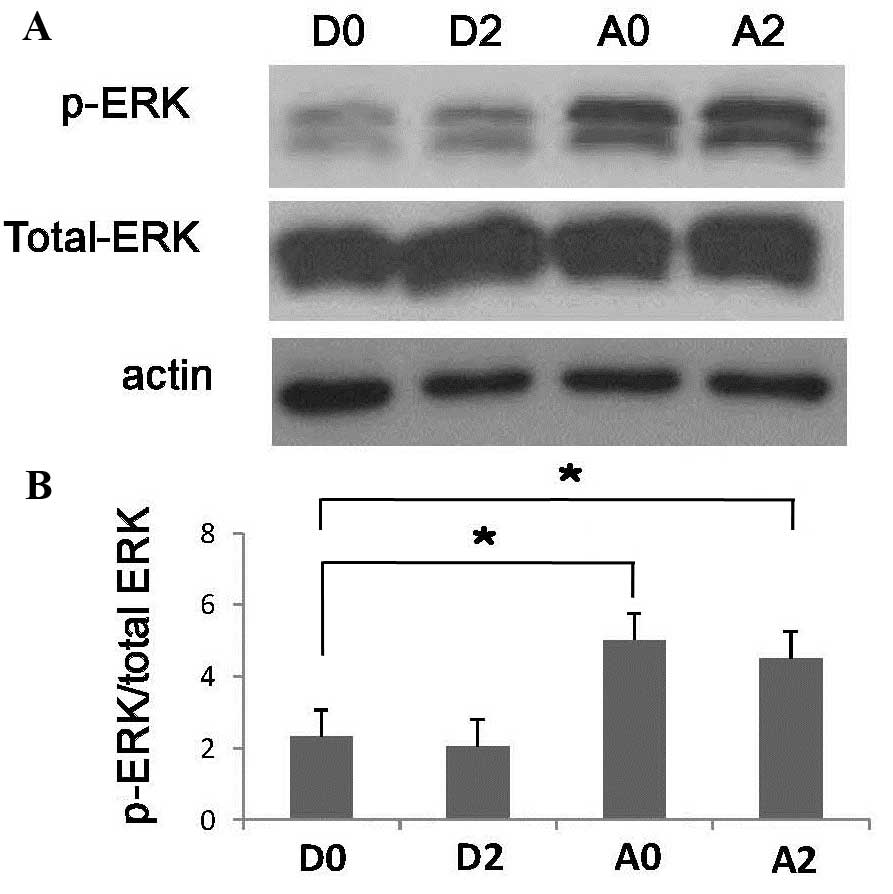
was associated with the Erk signaling pathway was investigated.
Notably, western blot analysis showed significant
andrographolide-induced phosphorylation of Erk (Thr202/Tyr204) with
or without radiation (P<0.05), indicating that andrographolide
treatment may lead to Erk activation (Fig. 8).
Andrographolide induces
H2O2 production
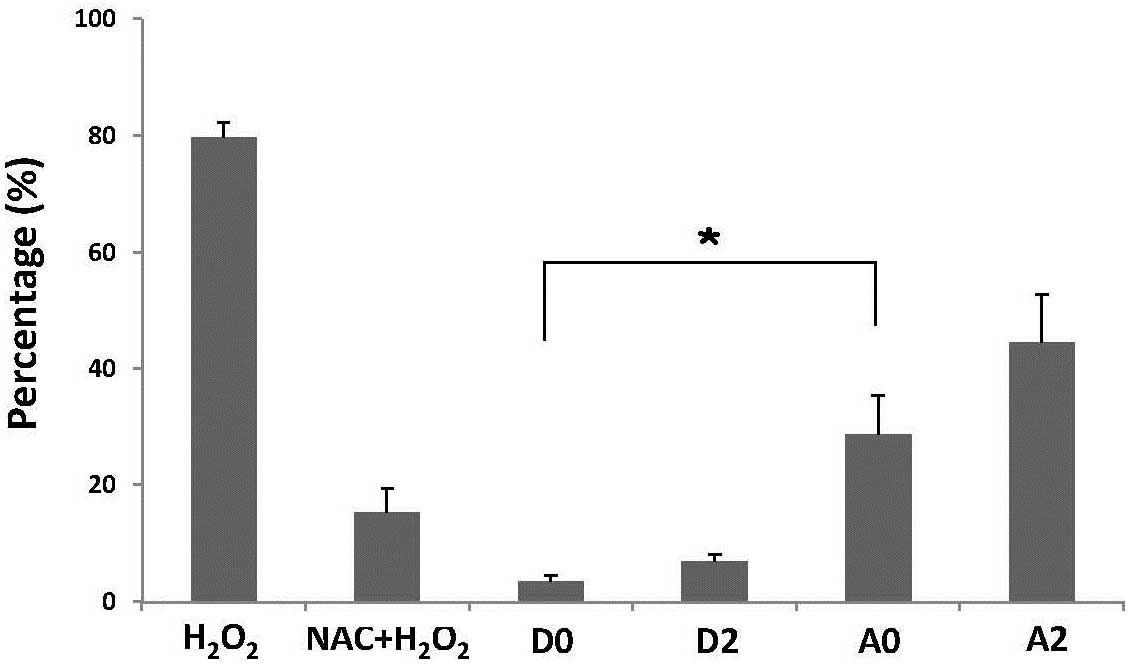
Fig. 9 demonstrates
that the intensity of DCF fluorescence increased following
andrographolide treatment, indicating an overproduction of
H2O2 in Ras-transformed cells, compared with
the control cells. *P<0.05.
Discussion
Cancer is characterized by uncontrolled tumor
growth, invasion, and metastasis (12). Metastasis is a complicated multi-step
process that provides a major challenge for cancer treatment.
Furthermore, it is difficult to utilize a living animal model to
optimally evaluate metastasis status in vivo. The aim of the
present study, was to establish a non-invasive method to measure
tumor size and distribution in vivo. Tumorigenesis and
metastasis were successfully detected by IVIS; therefore, a
non-invasive method to measure tumor size and distribution in
vivo was established. Furthermore, the animal model established
was used to examine the kinetics of tumor growth and metastasis
in vivo.
Andrographolide has various pharmacological
properties, including anti-oxidative, anti-inflammatory, antiviral
and anti-cancer effects (13–15). The
authors of the present study have previously demonstrated that
andrographolide is able to sensitize Ras-transformed cells to
radiation in vitro and in vivo (7). In the present study, the role of
combined radiation and andrographolide treatment in reducing
metastatic activity of Ras-transformed cells in vivo was
investigated via this model. Andrographolide combined with
radiation significantly inhibited cancer metastasis.
Multiple signaling pathways, including PI3K/Akt, and
NF-κB, are highly associated with metastatic activity. The
PI3K/Akt/mTOR pathway regulates VEGF expression in various tumors
to control angiogenesis (16).
Another study reported that activation of the PI3K/Akt/mTOR/p70S6K
pathway promoted the viability and migration of colorectal cancer
cells (17). Furthermore,
overexpression of the NF-κB pathway was demonstrated to be
sufficient to increase colorectal cancer cell proliferation,
motility and metastasis (18). These
results indicate that the PI3K/Akt and NF-κB signaling pathways
have an important role in cancer cell metastatic activity (19). The authors of the present study have
previously demonstrated that NF-κB activity was elevated by
radiation and significantly reduced by andrographolide combined
with radiation, and that the level of activated Akt protein was
also significantly reduced by andrographolide (7). These findings indicated that
andrographolide may exhibit reductive effects on cancer metastasis.
However, the cellular mechanisms responsible for this phenomenon
are yet to be elucidated.
Active Erk regulates various cytoplasmic and nuclear
targets that perform important cellular functions, including
proliferation, migration, differentiation and death (20,21).
Nevertheless, previous studies have indicated that Erk-signaling
pathways increase cell survival, primarily by promoting the
activity of anti-apoptotic proteins and repressing pro-apoptotic
proteins (22,23). However, an increasing number of
studies have demonstrated that Erk is associated with two
apparently opposing processes; aberrant Erk activation has been
demonstrated to promote cell death, and Erk activity has reportedly
been implicated in cell death induced by various anticancer drugs
(23–25). Notably, Erk activation requires ROS
production to induce cell death (26). In the present study, andrographolide
was able to induce Erk activation and H2O2
production. Therefore, the Erk pathway may be involved in
andrographolide-mediated anti-cancer effects.
In vivo bioluminescence imaging utilizes the
light emitted by luciferases to produce a functional image. The
photons emitted are able to penetrate the whole body and can be
applied to monitor deep tumors. This type of analysis enables
studies of gene expression, tumor growth, and cell migration over
time in live animals (27). For
studies of the mechanisms of novel drugs or radiation, this system
is valuable for pre-clinical in vivo evaluation and
accelerates the experimental process to efficiently generate
results. Notably, tumor cell death, necrosis and metastasis can be
detected earlier due to sensitive signal changes (11). However, this method has several
limitations. Firstly, activity is restricted to the intracellular
environment (27). Secondly, this
system may only be used for pre-clinical in vivo evaluations
and cannot be used in humans as luciferin is required to activate a
reporter gene; therefore, the procedure still raises ethical
questions. Thirdly, this system forms a type of biological image;
therefore the quantification signals require a baseline for
comparison. Although it may be influenced by several orders of
signal magnitude, it is not compromising the quantitative result
(25).
In the present study, a Ras/Luc cell line was
constructed. Activating Ras mutations are frequently found in human
tumors and the H-Ras oncogene is correlated with radiation
resistance (7). The novel imaging
method used in the present study may provide a rapid, sensitive and
non-invasive technique for assessing therapies, and studying cancer
metastasis and radiotherapy.
In conclusion, the present study established a
non-invasive method to measure tumor size and distribution in
vivo. Furthermore, andrographolide combined with radiation
demonstrated an ability to inhibit cancer metastasis, which merits
further study to elucidate the mechanisms involved.
Acknowledgements
This study was supported by the Buddhist Dalin Tzu
Chi General Hospital [grant no. DTCRD101(2)-I-13].
References
|
1
|
Meyer T and Hart IR: Mechanisms of tumour
metastasis. Eur J Cancer. 34:214–221. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Gaskins K, Yu Z, Xiong Y, Merino
MJ and Kebebew E: An in vivo mouse model of metastatic human
thyroid cancer. Thyroid. 24:695–704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin Y, Li F, Zheng C, Wang Y, Fang Z, Guo
C, Wang X, Liu H, Deng L, Li C, et al: NEDD9 promotes lung cancer
metastasis through epithelial-mesenchymal transition. Int J Cancer.
134:2294–2304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu XG, Zhao L, Willingham MC and Cheng
SY: Thyroid hormone receptors are tumor suppressors in a mouse
model of metastatic follicular thyroid carcinoma. Oncogene.
29:1909–1919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim WG, Guigon CJ, Fozzatti L, Park JW, Lu
C, Willingham MC and Cheng SY: SKI-606, an Src inhibitor, reduces
tumor growth, invasion, and distant metastasis in a mouse model of
thyroid cancer. Clin Cancer Res. 18:1281–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim JC, Chan TK, Ng DS, Sagineedu SR,
Stanslas J and Wong WS: Andrographolide and its analogues:
Versatile bioactive molecules for combating inflammation and
cancer. Clin Exp Pharmacol Physiol. 39:300–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hung SK, Hung LC, Kuo CD, Lee KY, Lee MS,
Lin HY, Chen YJ and Fu SL: Andrographolide sensitizes
Ras-transformed cells to radiation in vitro and in vivo. Int J
Radiat Oncol Biol Phys. 77:1232–1239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiao M, Sheng S and Pardee AB: Metastasis
and AKT activation. Cell Cycle. 7:2991–2996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu SL, Huang YJ, Liang FP, Huang YF,
Chuang CF, Wang SW and Yao JW: Malignant transformation of an
epithelial cell by v-Src via tv-a-mediated retroviral infection: A
new cell model for studying carcinogenesis. Biochem Biophys Res
Commun. 338:830–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim E, Modi KD and Kim J: In vivo
bioluminescent imaging of mammary tumors using IVIS spectrum. J Vis
Exp. pii:12102009.
|
|
12
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji LL, Wang Z, Dong F, Zhang WB and Wang
ZT: Andrograpanin, a compound isolated from anti-inflammatory
traditional Chinese medicine Andrographis paniculata, enhances
chemokine SDF-1alpha-induced leukocytes chemotaxis. J Cell Biochem.
95:970–978. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiart C, Kumar K, Yusof MY, Hamimah H,
Fauzi ZM and Sulaiman M: Antiviral properties of ent-labdene
diterpenes of Andrographis paniculata nees, inhibitors of herpes
simplex virus type 1. Phytother Res. 19:1069–1070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang FP, Lin CH, Kuo CD, Chao HP and Fu
SL: Suppression of v-Src transformation by andrographolide via
degradation of the v-Src protein and attenuation of the Erk
signaling pathway. J Biol Chem. 283:5023–5033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie SR, Wang Y, Liu CW, Luo K and Cai YQ:
Liquiritigenin inhibits serum-induced HIF-1α and VEGF expression
via the AKT/mTOR-p70S6K signalling pathway in HeLa cells. Phytother
Res. 26:1133–1141. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Duan L, Zou Z, Li H, Yuan S, Chen
X, Zhang Y, Li X, Sun H, Zha H, et al: Activation of the
PI3K/Akt/mTOR/p70S6K pathway is involved in S100A4-induced
viability and migration in colorectal cancer cells. Int J Med Sci.
11:841–849. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gavert N, Ben-Shmuel A, Lemmon V, Brabletz
T and Ben-Ze'ev A: Nuclear factor-kappaB signaling and ezrin are
essential for L1-mediated metastasis of colon cancer cells. J Cell
Sci. 123:2135–2143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramos JW: The regulation of extracellular
signal-regulated kinase (ERK) in mammalian cells. Int J Biochem
Cell Biol. 40:2707–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin P and Pognonec P: ERK and cell
death: Cadmium toxicity, sustained ERK activation and cell death.
FEBS J. 277:39–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watabe M, Masuda Y, Nakajo S, Yoshida T,
Kuroiwa Y and Nakaya K: The cooperative interaction of two
different signaling pathways in response to bufalin induces
apoptosis in human leukemia U937 cells. J Biol Chem.
271:14067–14072. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YH, Lee DH, Jeong JH, Guo ZS and Lee
YJ: Quercetin augments TRAIL-induced apoptotic death: Involvement
of the ERK signal transduction pathway. Biochem Pharmacol.
75:1946–1958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sinha D, Bannergee S, Schwartz JH,
Lieberthal W and Levine JS: Inhibition of ligand-independent ERK1/2
activity in kidney proximal tubular cells deprived of soluble
survival factors up-regulates Akt and prevents apoptosis. J Biol
Chem. 279:10962–10972. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
von Degenfeld G, Wehrman TS and Blau HM:
Imaging beta-galactosidase activity in vivo using sequential
reporter-enzyme luminescence. Methods Mol Biol. 574:249–259. 2009.
View Article : Google Scholar : PubMed/NCBI
|