Introduction
Endogenous Cushing syndrome (ECS) results from
lengthy and inappropriate exposure to excessive levels of
glucocorticoid secretion, and is generally divided into
adrenocorticotropic hormone (ACTH)-dependent and ACTH-independent.
Ectopic ACTH syndrome (EAS) is characterized by hypercortisolemia
as a result of extra-pituitary ACTH secretion and accounts for 20%
of ACTH-dependent Cushing syndrome cases (1).
Nocardia spp., a gram-positive bacterium,
causes local or disseminated infection in humans and animals.
Patients with depressed cell-mediated immunity are at high risk for
infection, including those with solid-organ or hematopoietic stem
cell transplantation, human immunodeficiency virus infection,
long-term steroid use or malignancy (2). Although Nocardia has been
considered to be rare, a previous report has shown that its
incidence is increasing (3).
Extremely high glucocorticoid doses in patients with ECS affect
virtually every cell type involved in immunity and the inflammatory
response, particularly cell-mediated immunity, which causes such
patients to be a target of nocardiosis (4). However, the clinical features of few
cases of nocardiosis in EAS have been documented.
In the present study, a case of rare ectopic
ACTH-secreting paraganglioma in the mediastinum associated with
nocardiosis is presented. In addition, the clinical features of 11
published cases of EAS associated with Nocardia infection
were analyzed (5–11). The aim of this study was to provide
guidance for the clinical diagnosis and treatment of
Nocardia infection associated with EAS.
Case report
The patient and their family were informed that data
from the case would be submitted for publication and provided
consent accordingly. A 35-year-old male patient first presented to
the First Affiliated Hospital, Zhejiang University School of
Medicine (Hangzhou, China) in February 2012, with weakness,
polyuria and polydipsia for 7 years and had been diagnosed as
having hypertension with unsatisfactory drug control for 6 months.
Frequently, the patient suffered from blurred vision, headache and
limb numbness. One month prior to presentation, he had developed a
cough with dark yellow phlegm without fever and dyspnea. The
patient was transferred to this hospital to obtain a definite
diagnosis. Physical examination on admission found the patient to
have a blood pressure of 172/102 mmHg, heart rate of 80 beats/min
with occasional arrhythmia, body mass index of 25 kg/m2,
and normal body temperature. The patient exhibited a moon face,
buffalo hump, polycythemia, edema of the feet and central obesity.
Other positive findings included purple striae in the abdomen,
acne, and a dark skin color. There were no clinically palpable
nodules. Other systemic examination findings were normal.
The initial laboratory evaluation revealed
hypokalemia (K level, 2.48 mmol/l; reference, 3.50–5.20 mmol/l),
hyperglycemia (fasting plasma glucose, 10.97 mmol/l; reference,
3.9–7.8 mmol/l) and hyperlipidemia (triglycerides, 4.41 mmol/l;
reference, 0.3–1.7 mmol/l). Blood routine tests showed a normal
leukocyte count leukocyte count (8.1×109/L; reference,
4–10×109/L) but mildly increased neutrophil percentage
(83.6%; reference, 50–70%). A hormonal assessment panel was
performed, which revealed markedly increased urinary (3,118.08
µg/24 h; reference, 55.5–286.0 µg/24 h) and serum (>50 µg/dl;
reference, 5.0–25.0 µg/dl) cortisol and serum ACTH (372 pg/ml;
reference, 0.0–46.0 pg/ml) levels.
High-dose dexamethasone suppression test: The
measurement of serum cortisol between 8:00 and 9:00 AM before and
after a high-dose (8 mg) oral night intake of dexamethasone and is
considered suggestive of suppression when a reduction of >50% is
observed compared to the baseline value.
Low-dose dexamethasone suppression test was
performed after an overnight oral intake (between 11:00 PM and
12:00 AM) dexamethasone (1 mg), and blood collection for
measurement of serum cortisol should occur in the subsequent
morning between 8:00 and 9:00 AM. Cortisol values above 1.8 µg/dL
are considered abnormal. Cortisol levels were not suppressed by
high- or low-dose dexamethasone.
To investigate the symptoms of cough and
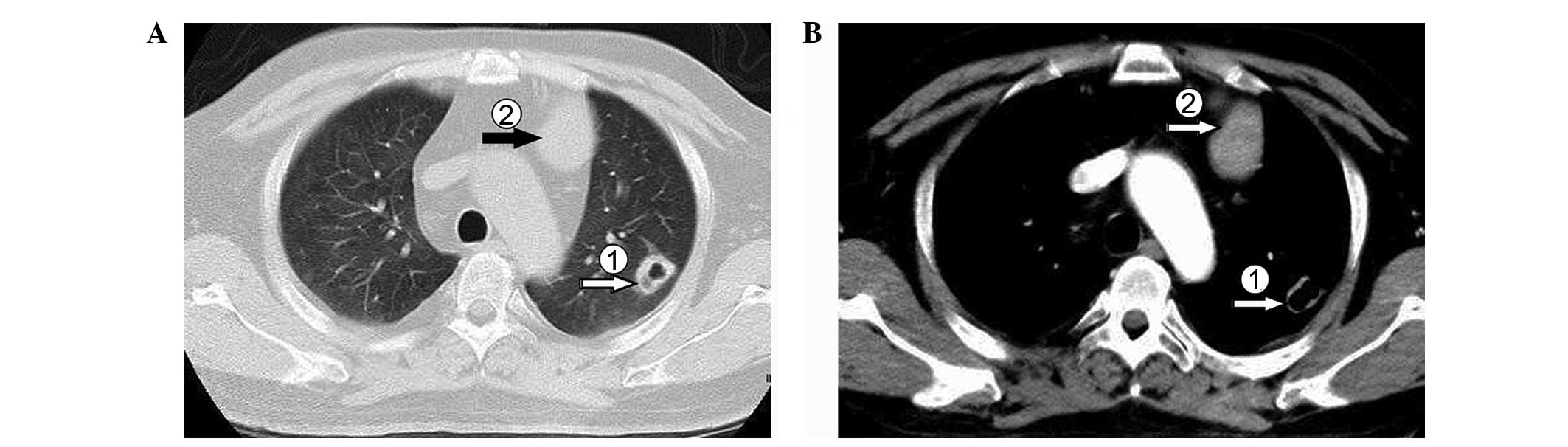
expectoration, chest computed tomography (CT; Fig. 1) scanning was performed. The
resulting image showed a soft tissue shadow in the anterior
mediastinum and multiple nodules with a partial cavity lesion in
bilateral lung fields. Brain magnetic resonance imaging (MRI)
revealed a slightly swollen pituitary gland, but this MRI result
was not able to explain the patient's clinical manifestations.
Laboratory tests, and the MRI and CT scans supported a preliminary
diagnosis of ectopic ACTH-secreting tumor. However, it was not
possible to determine whether the multiple lesions in the lung
constituted an infection focus or metastasis focus. For further
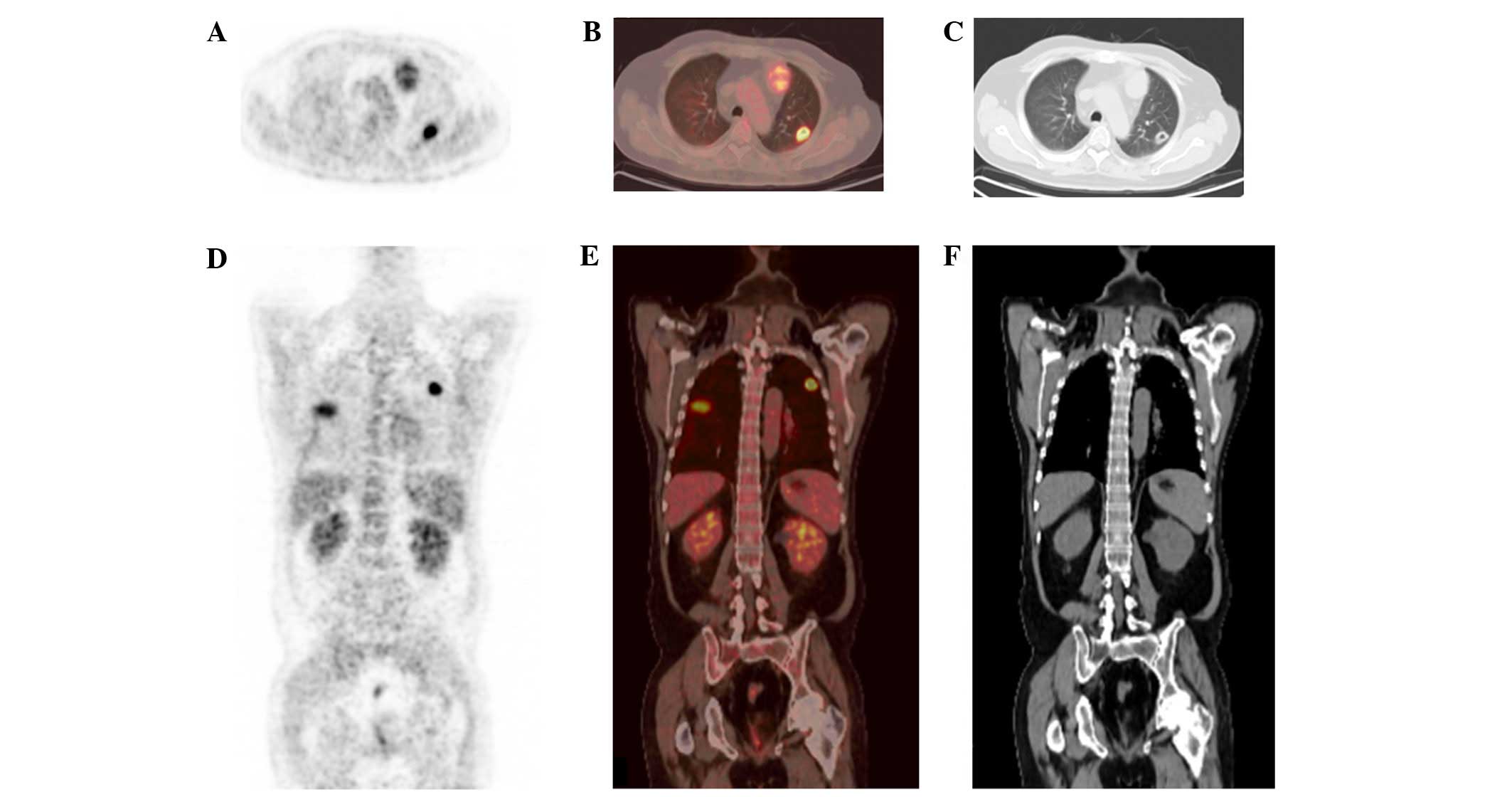
clarification, the patient underwent
18F-fluorodeoxyglucose (18F-FDG) positron
emission tomography (PET) combined with CT (Fig. 2). The resulting imaging showed a
tumor focus with mildly increased 18F-FDG metabolism in
the anterior mediastinum. Multiple clear-boundary nodular changes
in the bilateral pulmonary region suggested possible infection.
Histopathological analysis revealed tumor cells arranged in nests.
Immunohistochemistry showed that the periphery of the cell nests
was positive for chromogranin A and S-100. Immunohistochemistry
analysis was performed using a Dako Autostainer with Envision™
Detection Kit, chromogranin A (DAK-A3; 1:200) and polyclonal S-100
(1:300; Dako Denmark A/S, Glostrup, Denmark). Those microscopy
findings confirmed paraganglioma.
Treatment was initiated while the imaging and
laboratory examinations were ongoing. According to his
manifestations, the patient was initially treated with potassium
replacement (oral potassium chloride solution, 60 ml/day), insulin
(mixed protamine zinc recombinant human insulin injection adjusted
according to plasma glucose), intravenous fluids (Ringer's
solution; adjusted according to intake and output record) and
anti-hypertension medication (amlodipine, 5 mg/day; perindopril, 4
mg/day). As the patient had symptoms of cough and expectoration,
antibiotics (cefoperazone/sulbactam; dosage, 2 g; q8h) were
administered after admission, but there was no improvement in the
symptoms after 5 days. Following discussion with a doctor of
infectious diseases and consideration of the pulmonary CT scan
results, a possible diagnosis of pulmonary aspergillosis
could not be eliminated. Caspofungin (March 6, 2012; 20 days after
admission) at a dosage of 50 mg/day, combined with a formal
antibiotics regimen was recommended. Gram staining and sputum
culture were performed concurrently. Gram staining of the sputum on
March 9, 2012 (23 days after admission) revealed thin-beaded,
gram-positive branching rods and modified acid-fast staining was
then performed, according to previously described methods (12). The finding that these branching rods
were partially acid-fast positive was suggestive of infection with
Nocardia. The subsequent culture confirmed this result.
According to the clinical, radiological and etiological findings,
pulmonary nocardiosis was identified. In consideration of the
possibility of co-infection with Aspergillus, a new
antibiotic treatment was initiated with oral
trimethoprim-sulfamethoxazole (TMP-SMZ) at a dosage of 15 mg/kg/day
(960 mg/day) and intravenous infusion of caspofungin at a dosage of
50 mg/day. Approximately 1 week after starting the new regimen, the
patient's condition had improved. At 17 days after antibiotic
adjustment, a pulmonary CT scan showed a clear reduction of the
bilateral pulmonary foci, which strengthened the diagnosis of
infectious lesions in the lungs. Caspofungin was discontinued
because the rapid lung lesion shrinkage in the CT images did not
support aspergillosis. Furthermore, the Aspergillus
antigen levels were within the normal ranges and no evidence in the
microscopic examination indicated Aspergillus. Following
stabilization of hypokalemia, hyperglycemia, hypertension and
infection at 43 days after admission, the patient was transferred
to the Department of Thoracic Surgery. The mass in the anterior
mediastinum and a nodule in the left upper pulmonary lobe were
resected. Pathological evaluation suggested a chronic inflammatory
focus in left upper pulmonary lobe with no evidence of malignancy.
The pathological features and immunohistochemical observations for
the mass in the anterior mediastinum indicated paraganglioma and
corresponded with the result of formal biopsy.
At 3 days after the surgery, the plasma ACTH level
dropped shapely to 12.7 pg/ml, which was within the normal range.
The patient was discharged without any postoperative complications
and continued oral treatment with TMP-SMX for 6 months. A CT scan 4
months after surgery indicated the lung lesion had been replaced by
fibrous striped shadows without signs of recurrence. There was no
recurrence of either nocardiosis or paraganglioma during a 3-year
follow-up.
Discussion
The English-language literature published from
January 1980 to January 2014 (34 years) in the PubMed database,
Google Scholar and Web of Science database was searched using the
key words ‘Nocardia’, ‘Nocardia infection’,
‘Nocardiosis’ and ‘ACTH Syndrome, Ectopic’, ‘Ectopic ACTH
Syndrome’, ‘Ectopic ACTH Syndromes’. In addition, the references in
the articles that referred to nocardiosis and ectopic ACTH syndrome
were also examined. A total of 9 articles with full-text describing
11 cases were available (5–14). Including the present case, the
clinical characteristics of EAS with Nocardia infection in
12 cases were analyzed. Information concerning the demography and
clinical characteristics of the cases are summarized in xs I and
II.
The literature review found that EAS complicated
with nocardiosis was more common in men (9 cases, 75%) than in
women. This result is consistent with previous studies of
Nocardia spp. infection, which have reported that males are
more susceptible to infection than females (15,16).
However, a survey of cases of EAS indicated that men constitute
40–50% of patients (17). The mean
age at diagnosis was 48 years, which falls within the range of mean
ages reported previously (17,18).
Typical cushingoid features were readily observed in
these cases. The majority of patients exhibited skin changes and
muscle weakness, which are often observed in cases of exposure to
long-term and extra high-dose glucocorticoids. Those manifestations
may indicate that patients with EAS associated with nocardiosis
tend to have chronic disease. The 24-h free urine cortisol
concentration in the 12 cases examined in the present case was
apparently higher than in certain previous reports (19,20).
However, restricted by the limited number of cases, it is not
possible to determine the exact association of elevated 24-h free
urine cortisol concentration with nocardiosis. The level of
cortisol determines the degree of immunosuppression. The greater
the extent by which cortisol levels exceed the normal value, the
greater the possibility of opportunistic infections and the higher
the mortality rate (21). The
present review demonstrated that, of the 12 cases involving
Nocardia spp., the patients who had a fatal outcome as a
result of serious infection had comparatively higher urinary levels
of corticoids. In addition, patients with higher urine cortisol
concentrations tended to be co-infected with other opportunistic
pathogens, especially Aspergillus and Pneumocystis
carinii. Thus, the possibility of co-infection should be
considered for patients with high level of corticoids and a
combination antibiotic regimen applied as early as possible.
Radiography is a helpful diagnostic tool in
localizing primary tumors and identifying infection lesions. For
EAS patients, chest imaging is necessary to identify infection,
particularly for individuals without pulmonary symptoms. Imaging
results of the 12 cases showed the lung was the most common site of
nocardiosis and was involved in all cases included in this
literature review. Chest imaging is crucial in providing evidence
of Nocardia spp. infection. Although a variety of
radiological findings, such as cavities,
consolidations/infiltration, nodules/masses and pleural effusion
were present in the current case and previous patients in chest CT
or X-ray images, the main findings comprised cavity (6 cases, 50%)
and consolidation/infiltration (5 cases, 42%). Of note, the 4 cases
(33%) of mass/nodule tended to be multiple. In a previous study of
nocardiosis involving 51 cases performed by Blackmon et al,
it was shown that airspace consolidation and discrete nodules were
the most common manifestations while cavities were found in 21
cases (39.6%) (22). The high rate
of cavities in the present review may result from delayed
diagnosis, due to the low incidence and variable manifestations of
nocardiosis in EAS. Previous data indicated that cavity formation
mostly occurred within 2 weeks of infection (23). The mean time to establish a diagnosis
of nocardiosis may be as long as 42 days (24). Formation of a cavity may be
facilitated by a prolonged disease course. In conclusion, cavity
lesions and consolidation/infiltration were the major findings of
pulmonary nocardiosis for patients with EAS in the present study.
Accordingly, nocardiosis should be considered as a differential
diagnosis for EAS patients who have cavity formation or
consolidation nodules in the lung, particularly when these
manifestations are associated with brain and extra-pulmonary
lesions. It is noteworthy that the imaging features of pulmonary
nocardiosis in EAS patients are nonspecific and often mimic
malignancy or other infection such as fungal infection and
tuberculosis (19). In the present
case and another previous study (25), PET/CT imaging showed that nocardiosis
is often indicated by a high uptake of 18F-FDG, which is
similar to that in malignant lesions. In this situation, a
combination of PET/CT and image-guided biopsy can be a useful
supplementary method for making a differential diagnosis.
In general, patients from the literature with only
pulmonary infection exhibited sensitivity to TMP-SMZ monotherapy.
Considering the high incidence and poor prognosis of
Aspergillus co-infection, an antibiotic regimen containing
an antifungal drug is recommended for early application since it is
not possible to eliminate a diagnosis of fungal infection. However,
TMP-SMX monotherapy appears to be inadequate in severe or
disseminated cases (8–10,14).
Although optimal antimicrobial treatment regimens were not firmly
established, combined antibiotic therapy with a sulfa-containing
agent has been recommended for severe or systematic disease
(26). A combination of amikacin and
imipenem or broad-spectrum cephalosporin was more effective than
TMP-SMZ monotherapy in an experimental mouse model (27). However, a combination of imipenem and
amikacin was poorly tested in patients, which is inadequate for
drawing a conclusion. A three-drug regimen comprised of TMP-SMZ,
amikacin, and either ceftriaxone or imipenem has been recommended
when there is no evidence of resistance (28). Also, linezolid as an effective
alternative has been reported in several cases of nocardiosis
(29). Moreover, differing
antimicrobial susceptibility patterns for different Nocardia
species will induce varying therapeutic effects. Therefore,
susceptibility tests provide significant information for adjustment
of the antibiotic, particularly in cases insensitive to initial
empirical treatment. Generally, a treatment duration of 6 months is
recommended for EAS patients with pulmonary or cutaneous infection
and 12 months for those with central nervous system infection or
dissemination.
Controlling the plasma level of cortisol not only
can improve clinical manifestation but also help in the prevention
of Nocardia spp. infection. A study of opportunistic
infection indicated that 11 of 12 (92%) patients succumbed when
hypercortisolemia was not controlled, whereas 11 of 19 (58%)
survived with effective control of hypercortisolemia (30). Complete tumor resection is a direct
and effective curative method whenever possible, yet the success
rate is only 30–47% (19). Medical
therapy to control cortisol overproduction has been offered to
those who are not indicated for complete resection. Metyrapone,
ketoconazole and mitotane can all be used to lower plasma cortisol
by acting directly to inhibit synthesis and secretion in the
adrenal gland (31). These drugs are
not effective as solo long-term treatments and are mainly used as
preoperative preparations or as an adjunctive therapy after surgery
(31). When the cortisol level
cannot be controlled properly, when drug treatment is ineffective
or not well tolerated or the resource remains unclear after
long-term follow-up, total bilateral adrenalectomy will reduce
cortisol levels and rapidly resolve clinical features. Following
surgery, long-term treatment with glucocorticoids is necessary.
In conclusion, male patients are more vulnerable to
infection. Patients with EAS who have extra-high urine cortisol
levels and with a long disease course are at higher risk for
Nocardia spp. infection. CT presentations of pulmonary
nocardiosis in EAS at the time of diagnosis were heterogeneous.
Cavity (50%), consolidation/infiltration (42%) and nodule/mass
(33%) lesion were three common imaging findings in the present
review. Optimal antimicrobial treatment regimens have not been
firmly established. TMP-SMZ remains the first choice in EAS
associated with pulmonary nocardiosis empirically, while combined
antibiotic therapy with a sulfa-containing agent is recommended for
severe or disseminated cases.
References
|
1
|
Alexandraki KI and Grossman AB: The
ectopic ACTH syndrome. Rev Endocr Metab Disord. 11:117–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minero MV, Marín M, Cercenado E, Rabadán
PM, Bouza E and Muñoz P: Nocardiosis at the turn of the Century.
Medicine (Baltimore). 88:250–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beaman BL and Beaman L: Nocardia species:
Host-parasite relationships. Clin Microbiol Rev. 7:213–264. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lionakis MS and Kontoyiannis DP:
Glucocorticoids and invasive fungal infections. Lancet.
362:1828–1838. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rizwan A, Sarfaraz A, Jabbar A, Akhter J
and Islam N: Case report: Nocardia infection associated with
ectopic Cushings. BMC Endocr Disord. 14:512014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sutton BJ, Parks GE, Manavi CK, Palavecino
EL and Geisinger KR: Cushing's syndrome and nocardiosis associated
with a pulmonary carcinoid tumor: Report of a case and review of
the literature. Diagn Cytopathol. 39:359–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Momah N and Koroscil T: Occult ectopic
adrenocorticotropic hormone secretion: Diagnostic dilemma and
infective consequence. Clin Pract. 2:e822012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chrysanthidis T, Yavropoulou MP,
Metallidis S, Mpakaimi I, Zempekakis P, Yovos JG and Nikolaidis P:
Disseminated nocardiosis in ectopic adrenocorticotropic hormone
syndrome. Endocrinologist. 20:286–287. 2010. View Article : Google Scholar
|
|
9
|
Chowdry RP, Bhimani C, Delgado MA, Lee DJ,
Dayamani P, Sica GL and Owonikoko TK: Unusual suspects: Pulmonary
opportunistic infections masquerading as tumor metastasis in a
patient with adrenocorticotropic hormone-producing pancreatic
neuroendocrine cancer. Ther Adv Med Oncol. 4:295–300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beinart GA, Hollander H and Rao R: Ectopic
ACTH syndrome resulting in nocardiosis and acute respiratory
failure. Hosp Physician. 39:49–54. 2003.
|
|
11
|
Higgins TL, Calabrese LH and Sheeler LR:
Opportunistic infections in patients with ectopic ACTH-secreting
tumors. Cleve Clin Q. 49:43–49. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia LS: Clinical Microbiology
Procedures Handbook. 2. 3rd edition. ASM Press; Washington, DC:
2010
|
|
13
|
Natale RB, Yagoda A, Brown A, Singer C,
Stover D and Bajorunas D: Combined Pneumocystis carinii and
Nocardia asteroides pneumonitis in a patient with an ACTH-producing
carcinoid. Cancer. 47:2933–2935. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petersen DP and Wong LB: Nocardia
infection of the hand-case report. J Hand Surg Am. 6:502–505. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kageyama A, Yazawa K, Ishikawa J, Hotta K,
Nishimura K and Mikami Y: Nocardial infections in Japan from 1992
to 2001, including the first report of infection by Nocardia
transvalensis. Eur J Epidemiol. 19:383–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu X, Han F, Wu J, He Q, Peng W, Wang Y,
Huang H, Li H, Wang R and Chen J: Nocardia infection in kidney
transplant recipients: Case report and analysis of 66 published
cases. Transpl Infect Dis. 13:385–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ejaz S, Vassilopoulou-Sellin R, Busaidy
NL, Hu MI, Waguespack SG, Jimenez C, Ying AK, Cabanillas M, Abbara
M and Habra MA: Cushing syndrome secondary to ectopic ACTH
secretion: The University of Texas MD Anderson Cancer Center
experience. Cancer. 117:4381–4389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isidori AM, Kaltsas GA, Pozza C, Frajese
V, Newell-Price J, Reznek RH, Jenkins PJ, Monson JP, Grossman AB
and Besser GM: The ectopic adrenocorticotropin syndrome: Clinical
features, diagnosis, management and long-term follow-up. J Clin
Endocrinol Metab. 91:371–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ilias I, Torpy DJ, Pacak K, Mullen N,
Wesley RA and Nieman LK: Cushing's syndrome due to ectopic
corticotropin secretion: Twenty years' experience at the National
Institutes of Health. J Clin Endocrinol Metab. 90:4955–4962. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doi M, Sugiyama T, Izumiyama H, Yoshimoto
T and Hirata Y: Clinical features and management of ectopic ACTH
syndrome at a single institute in Japan. Endocr J. 57:1061–1069.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bakker RC, Gallas PR, Romijn JA and
Wiersinga WM: Cushing's syndrome complicated by multiple
opportunistic infections. J Endocrinol Invest. 21:329–333. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blackmon KN, Ravenel JG, Gomez JM, Ciolino
J and Wray DW: Pulmonary nocardiosis: Computed tomography features
at diagnosis. J Thorac Imaging. 26:224–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Zhou H, Xu P, Zhang P, Ma S and
Zhou J: Clinical and radiographic characteristics of pulmonary
nocardiosis: Clues to earlier diagnosis. PloS One. 9:e907242014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giraldi F Pecori, Moro M and Cavagnini F:
Study Group on the Hypothalamo-Pituitary-Adrenal Axis of the
Italian Society of Endocrinology: Gender-related differences in the
presentation and course of Cushing's disease. J Clin Endocr Metab.
88:1554–1558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao K, Dong MJ, Sheng ZK, Liu KF, Yang
SY, Liu ZF and Sheng JF: Elevated uptake of 18F-FDG in
PET/CT imaging of a nocardial pleural nodule. Clin Imaging.
36:383–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilson JW: Nocardiosis: Updates and
clinical overview. Mayo Clin Proc. 87:403–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gombert ME, Berkowitz LB, Aulicino TM and
duBouchet L: Therapy of pulmonary nocardiosis in immunocompromised
mice. Antimicrob Agents Chemother. 34:1766–1768. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ambrosioni J, Lew D and Garbino J:
Nocardiosis: Updated clinical review and experience at a tertiary
center. Infection. 38:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moylett EH, Pacheco SE, Brown-Elliott BA,
Perry TR, Buescher ES, Birmingham MC, Schentag JJ, Gimbel JF,
Apodaca A, Schwartz MA, et al: Clinical experience with linezolid
for the treatment of Nocardia infection. Clin Infect Dis.
36:313–318. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhansali A, Dutta P, Bhat MH, Sinha SK and
Nada R: Unusual opportunistic infection associated with endogenous
Cushing syndrome. Endocrinologist. 16:125–127. 2006. View Article : Google Scholar
|
|
31
|
Newell-Price J, Bertagna X, Grossman AB
and Nieman LK: Cushing's syndrome. Lancet. 367:1605–1617. 2006.
View Article : Google Scholar : PubMed/NCBI
|