Introduction
Ovarian clear cell carcinoma (OCCC) is a rare type
of epithelial ovarian cancer with high degree of malignancy, and
its incidence is 5–11% of ovarian epithelial tumors. OCCC is
reported to occur at an earlier age than serous ovarian cancer,
with a median age at diagnosis of 55 years compared with 64 years
(1). Although low-stage OCCC has a
relatively good prognosis, advanced-stage OCCC has a significantly
lower overall survival rate (2,3). The
treatment of OCCC mainly comprises surgery combined with
radiotherapy and chemotherapy in a comprehensive treatment program.
However, the resistance to platinum-based traditional chemotherapy
is very common in the clinic, so the prognosis is poor (4–7).
Chromatin remodeling, including the synthesis,
transcription and repair of DNA, is important in cell nuclear
activities. Genetic mutation of the chromatin remodeling complex
has been identified as a mechanism of tumor occurrence and
development (8). The AT-rich
interaction domain 1A (ARID1A) as a non-catalytic subunit of the
chromatin remodeling complex, has the ability to combine with DNA
or protein. Genetic mutations of ARID1A in various tumors are
considered to be associated with the biological behavior, treatment
and prognosis of the tumor (9,10). In
OCCC, the mutation rate of the ARID1A gene has been found to be
46–57% (11). However, whether such
a high mutation rate is associated with the resistance of OCCC to
chemotherapy remains unclear and requires investigation. Therefore,
the aim of the present study was to evaluate the sensitivity of the
OCCC cell line ES2 to cisplatin following silencing of the ARID1A
gene and to investigate the possible mechanism.
Materials and methods
Reagents and antibodies
Lipofectamine RNAi Max reagent was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Anti-ARID1A (ab50878) and anti-β-actin (ab134032) mouse monoclonal
antibodies, and anti-AKT (ab179463) rabbit monoclonal antibodies
were purchased from Abcam (Cambridge, UK).
Design and synthesis of small
interfering RNA (siRNA) sequences
The three pairs of ARID1A gene siRNA interference
fragments (siRNA-1, siRNA-2 and siRNA-3), and one pair of sequences
unrelated to ARID1A (negative control, NC) were designed and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
siRNA sequences used were as follows: siRNA-1 forward,
5′-GCCCUGAACAAUAACCUCATT-3′ and reverse,
5′-UGAGGUUAUUGUUCAGGGCTT-3′; siRNA-2 forward,
5′-CCAGUCCAAUGGAUCAGAUTT-3′ and reverse,
5′-AUCUGAUCCAUUGGACUGGTT-3′; siRNA-3 forward,
5′-CAGCUUGCCUGAUCUAUCUTT-3′ and reverse,
5′-AGAUAGAUCAGGCAAGCUGTT-3′; NC forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′.
Cell culture and transfection
A ES2 cell line was obtained from the Institute of
Basic Medical Sciences, Chinese Academy of Medical Sciences
(Beijing, China) and maintained in McCoy's 5A culture medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum, 100 µg/ml streptomycin and 100 U/ml penicillin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Cultured
cells were incubated in a humidity chamber (Thermo Fisher
Scientific, Inc.) containing 5% CO2 at 37°C. For
transfection, Lipofectamine RNAi Max was mixed with the
aforementioned siRNA, according to the manufacturer's instructions.
The solutions were subsequently combined with ES2 cells in 6-well
culture plates at a density of 3.0×105 cells/well. In
the blank control group, cells were treated with the transfection
reagent only (Lipofectamine RNAi Max); however, in the normal
control group, the cells did not undergo any treatment.
Western blot analysis
Cells were washed twice with PBS and lysed in a
buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 1 mM EDTA, 10%
glycerol and 0.5% NP-40 and protease inhibitor cocktail
(Sigma-Aldrich; Merck Millipore). Then, cells were centrifuged for
15 min at 4°C at 14,000 × g. Protein concentration was
determined using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology, Shanghai, China). Protein samples (50 µg) were
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto nitrocellulose membranes. The
membranes were blocked using 5% skimmed milk powder at room
temperature for 1 h. Then, the membranes were incubated with
anti-ARID1A (1:500), anti-AKT (1:1,000) and anti-β-actin (1:5,000)
primary antibodies at 4°C overnight, followed by incubation with a
horseradish peroxide-conjugated mouse IgG secondary antibody
(ab190475; 1:10,000; Abcam) or rabbit IgG secondary antibody
(ab190495; 1:10,000; Abcam) at room temperature for 2 h. Proteins
were visualized using an enhanced chemiluminescence kit (GE
Healthcare Life Sciences, Piscataway, NJ, USA), and the protein
band intensity was quantified via densitometric analysis using
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cisplatin treatment and cell viability
assay
Cell inhibition rate and cell viability
(IC50) values for cisplatin (Sigma-Aldrich; Merck
Millipore) were determined using the Cell Counting kit-8 (CCK-8;
Beyotime Institute of Biotechnology). Cells at a density of
3.0×103 cells/well were plated in 100 µl medium/well in
96-well culture plates. Following overnight incubation at 37°C and
5% CO2, cells were transfected with siRNA in the
presence of Lipofectamine RNAi Max. After 24 h of incubation, cells
were exposed to cisplatin at a range of concentrations (20, 40, 80,
120 and 160 µM). Each concentration of drug was added to duplicate
wells. Following 48 h of incubation, 10 µl/well CCK-8 reagent was
added directly to the medium and the plates were incubated for 1 h.
The absorbance values were read on a SpectraMax M3 microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA) and converted
to percentage cell viability in corresponding matched CCK-8-treated
cells, which were designated as 100% viable. IC50 values
(concentration of drug that results in a reduction of the
absorbance signal by 50% compared with the CCK-8-treated control)
were obtained from nonlinear regression analysis of
concentration-effect curves using SPSS software, version 18.0
(SPSS, Inc., Chicago, IL, USA). The IC50 value in the
negative control group was ~52 µM. Then, 50 µM cisplatin was added
to the plates after transfection, the cells were incubated for 48 h
and the cell survival rate of each group was calculated.
Flow cytometry assay
ES2 cells (3×105/well) were plated in 2
ml medium/well in 6-well culture plates. Following overnight
incubation at 37°C and 5% CO2, cells were transfected
with NC or siRNA-3 siRNA in the presence of Lipofectamine RNAi Max.
Following incubation for 24 h, 50 µM cisplatin was added to the
plates and the cells were incubated for a further 48 h to establish
the cisplatin and siRNA-3 plus cisplatin groups, respectively. The
single negative control group and single siRNA-3 group were
incubated for a further 48 h but not treated with cisplatin. The
cells of the four groups were then collected and labeled using a
fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection kit
(BD Biosciences, Franklin Lakes, NJ, USA). Apoptosis rates were
determined by flow cytometry (BD Biosciences) and analyzed with
FlowJo 10.0.8 software (FlowJo, LLC, Ashland, OR, USA).
Statistical analysis
Each experiment was performed in triplicate and
repeated a minimum of three times, with all data presented as the
mean ± standard deviation. Statistical analyses were performed
using SPSS software, version 18.0. Comparisons between groups were
conducted using the Student's t-test or analysis of variance, where
P<0.05 was considered to indicate a statistically significant
difference.
Results
ARID1A siRNA downregulates the protein
expression levels of ARID1A
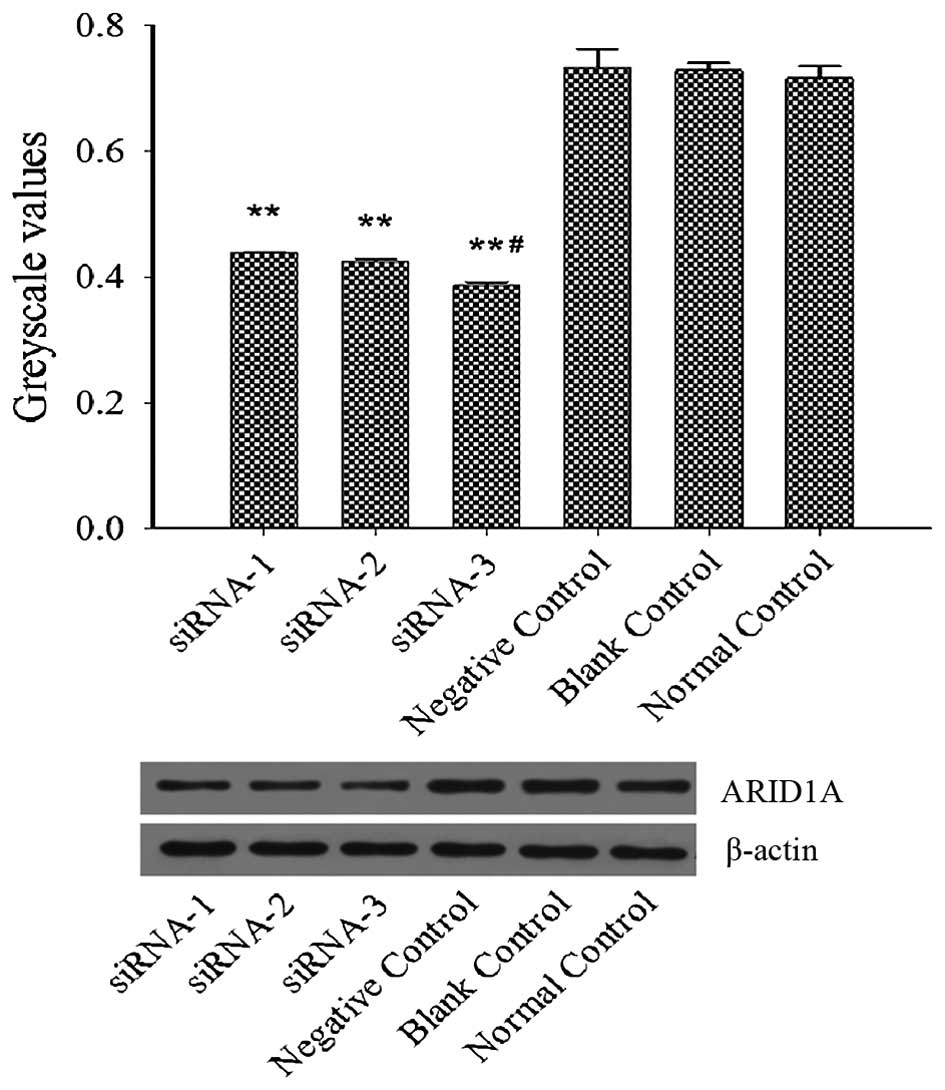
Expression levels of ARID1A were analyzed using
western blotting. ES2 cells were transfected with siRNA in the
presence of RNAi Max reagent for 6 h and then cultured in McCoy's
5A medium with 10% fetal bovine serum for 72 h. The results from
the western blot analysis revealed that the protein expression
levels of ARID1A in the cells of the siRNA-1, siRNA-2 and siRNA-3
groups were all markedly decreased and the level was the lowest in
the siRNA-3 group, as compared with the non-siRNA-transfected cells
(Fig. 1). Collectively, the results
demonstrated that ARID1A siRNA was able to silence ARID1A
expression and siRNA-3 interference was chosen for follow-up
experiments.
ARID1A siRNA reduces cell inhibition
rate
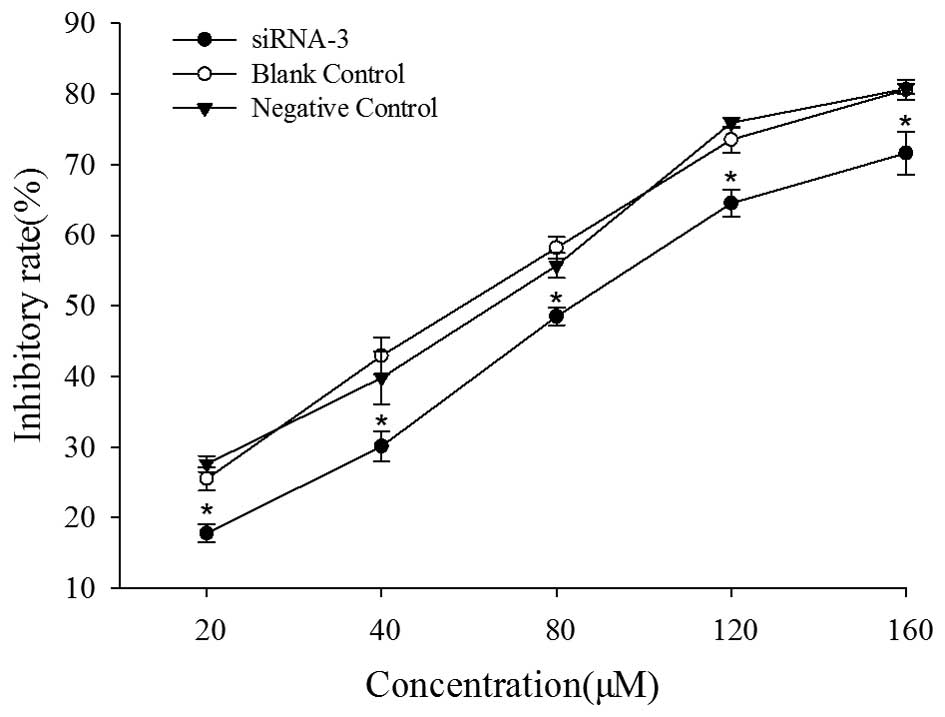
To elucidate the effects of ARID1A gene silencing on
the cisplatin sensitivity of ES2 cells, cells were exposed to
different concentrations of cisplatin. As shown in Fig. 2, at the same concentration of
cisplatin, the inhibitory rate of the siRNA-3 group was
significantly lower than that of blank control and negative control
groups (P<0.05). The IC50 value in the negative
control group was ~52 µM (Table I),
however, as shown in Table I, the
IC50 value in the siRNA-3 group was ~77 µM which was
significantly higher than that of the negative control and blank
control groups (P<0.05). Then, 50 µM cisplatin was added to the
96-well culture plates following transfection and the cell survival
rate of each group after 48 h incubation was calculated. The
results showed that the cell survival rate of the siRNA-3 group was
higher than that of the other two groups (Table I). These results indicate that ARID1A
siRNA decreased the sensitivity of ES2 cells to cisplatin.
 | Table I.IC50 of cisplatin and
survival rates of ES2 ovarian clear cell carcinoma cells. |
Table I.
IC50 of cisplatin and
survival rates of ES2 ovarian clear cell carcinoma cells.
| Group | IC50
(µM) | Survival
ratea (%) |
|---|
| Negative control | 52.55±3.62 | 52.67±2.30 |
| Blank control | 52.10±1.10 | 51.53±1.81 |
| siRNA-3 |
77.22±5.34b |
67.53±3.35b |
ARID1A siRNA decreases cell
apoptosis
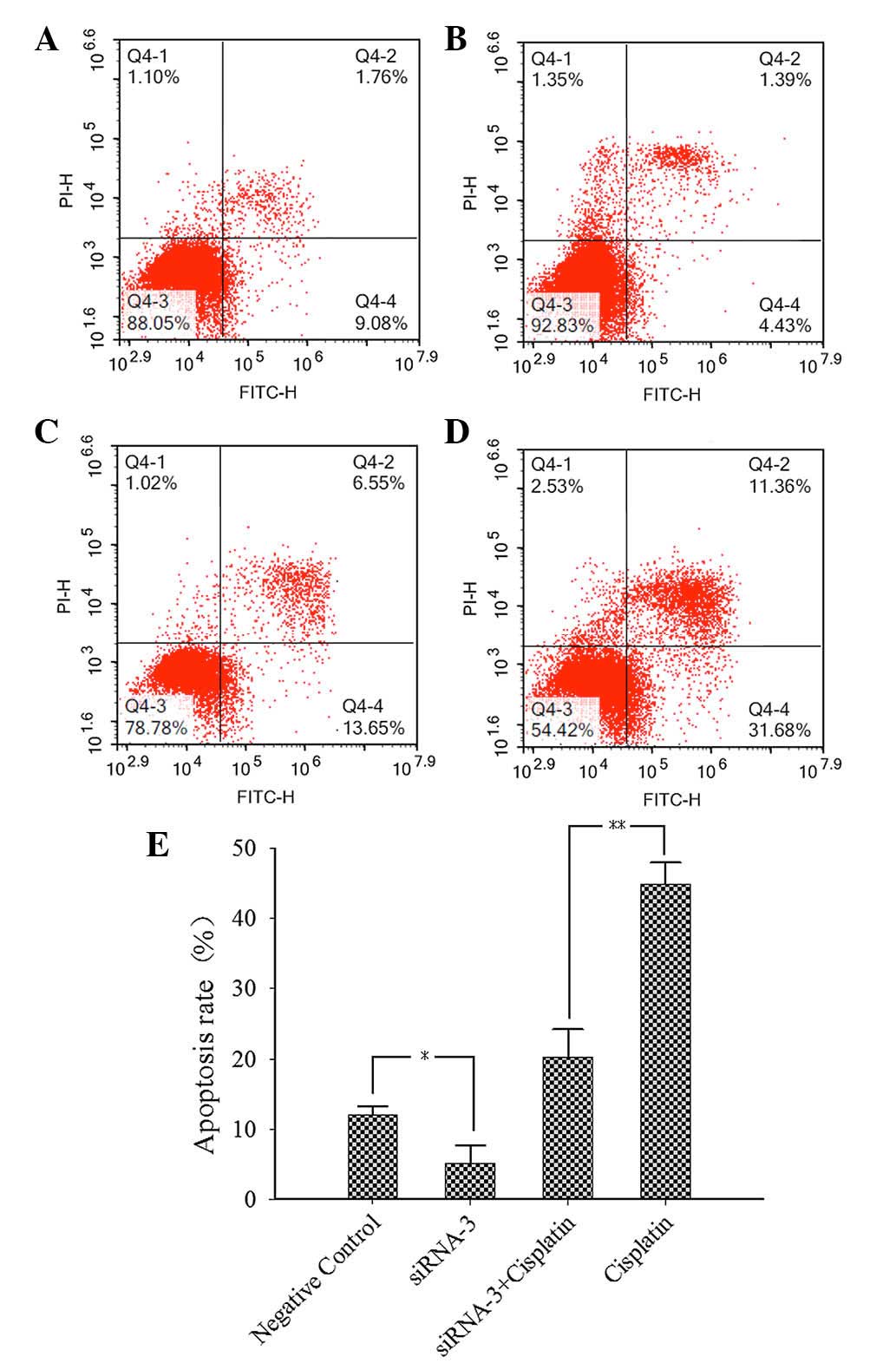
ES2 cells were transfected for 6 h and then 50 µM
cisplatin was added to the cells after incubation for 24 h.
Apoptotic rates were determined by flow cytometry. The results
(Fig. 3) indicate that the apoptotic
rate of the siRNA-3 plus cisplatin group (Fig. 3C) was significantly lower than that
of the cisplatin group (Fig. 3D). In
addition, the apoptotic rate of the siRNA-3 group (Fig. 3B) was lower than that of the negative
control group (Fig. 3A). These
results demonstrate that that cell apoptotic rate decreased when
the ARID1A gene was silenced.
ARID1A siRNA increases the expression
levels of AKT
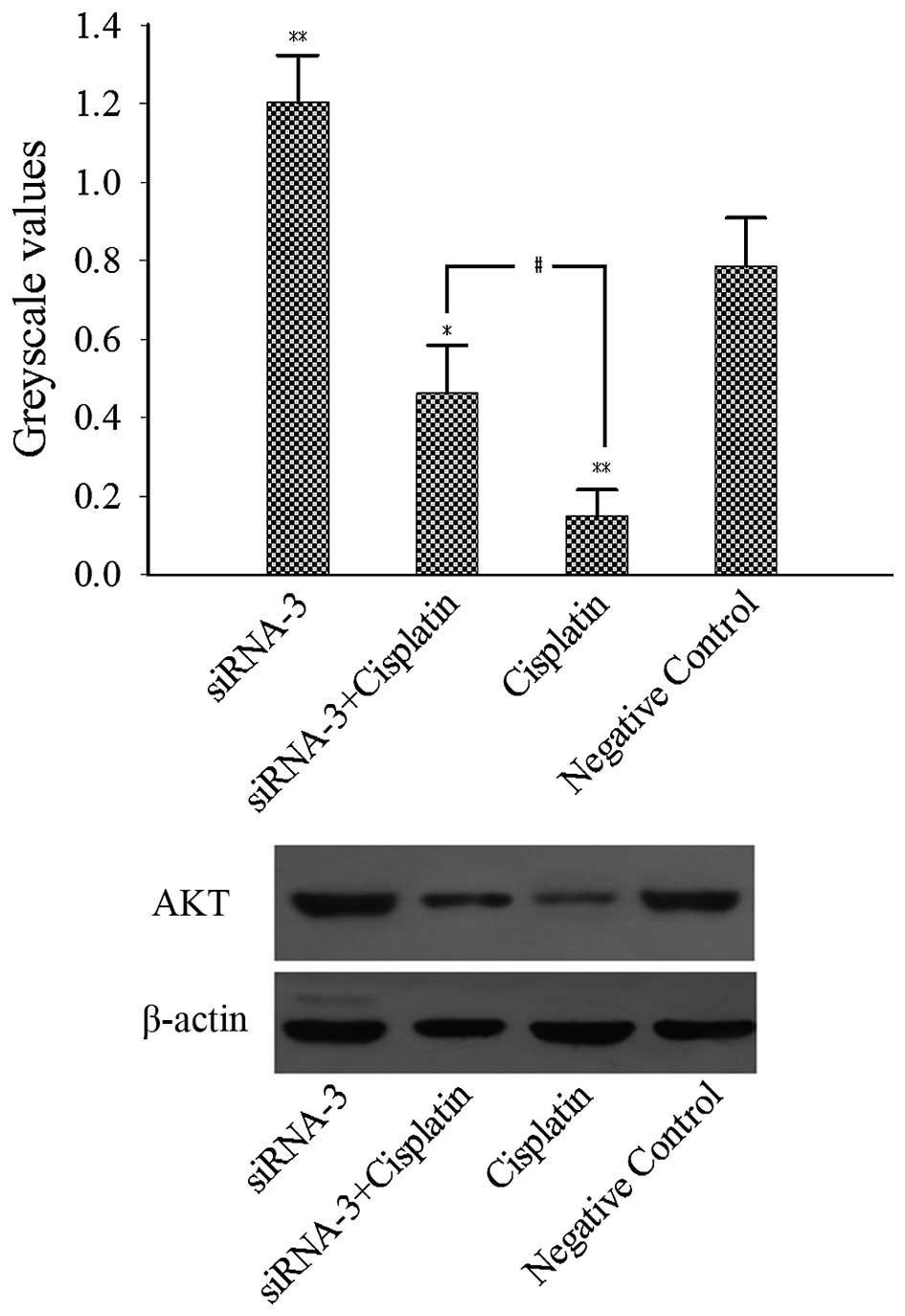
To elucidate the possible molecular mechanism by
which ARID1A siRNA decreased the sensitivity of ES2 cells to
cisplatin, the effect of ARID1A gene silencing on the AKT protein
level was investigated. The results of western blot analysis
(Fig. 4) demonstrated that AKT
expression was upregulated in the siRNA-3 group compared with the
negative control group. In addition, AKT expression in the
cisplatin group was significantly decreased compared with that in
the negative control group. Furthermore, AKT expression in the
siRNA-3 plus cisplatin group was significantly increased compared
with that in the cisplatin group. Thus, ARID1A gene silencing
appeared to attenuate the suppression of AKT expression by
cisplatin. These results indicate that ARID1A gene silencing
decreased the sensitivity of ES2 cells to cisplatin, in part via
the upregulation of AKT expression.
Discussion
OCCC is considered to be a unique clinical and
pathological type of ovarian cancer due to its typical histological
characteristics; the lesions coexist with ectopic endometrium, are
highly resistant to chemotherapy and have a poor prognosis
(2,4,12,13).
Although little is known about the molecular genetic changes of
tumor development, there is a widely accepted tumor hypothesis
which considers that OCCC originates from precancerous lesions
(such as endometriosis and benign clear cell adenofibroma) in the
development of the corresponding atypical lesions (such as atypical
endometriosis and borderline clear cell adenocarcinoma) (14,15). A
previous study found that there was a loss of ARID1A expression in
OCCC and adjacent to the ectopic endometrial epithelium, while
ARID1A expression was retained in the cystic epithelium distant
from the tumor (16). Therefore,
ARID1A is considered as a potential tumor suppressor gene, and its
expression is closely associated with tumor formation and prognosis
(17–19). Patients with advanced OCCC are
treated with cytoreductive surgery and chemotherapy with a
platinum-based regimen, but the problem of drug resistance has been
persistent in the clinic. The high mutation rate of ARID1A in OCCC
may be the cause of the resistance to platinum and other
chemotherapy drugs. In addition, a study found that after the
ARID1A gene was knocked out in Jurkat leukemia cells, there was
resistance to FAS-mediated apoptosis (20). The results of the present study also
suggest that ARID1A gene mutation may be associated with the drug
resistance of tumor cells.
The ES2 cell line is a typical OCCC cell line. In
the present study, the sensitivity of ES2 cells to the
chemotherapeutic drug cisplatin was detected with ARID1A gene
silencing, and the results indicated that the IC50 of
cisplatin was significantly increased compared with that of the
blank control group and negative control group when ARID1A gene
expression was reduced by specific siRNA. The cell survival rate of
the ARID1A siRNA group was significantly higher compared with that
of the negative control group following treatment with the same
concentration of cisplatin (50 µM). Collectively, these results
suggest that ARID1A gene silencing reduced the sensitivity of ES2
cells to cisplatin.
Further investigation into the apoptosis of ES2
cells with ARID1A gene silencing and cisplatin treatment was
conducted. The results showed that the cell apoptosis rate of the
ARID1A siRNA plus cisplatin group was significantly lower than that
of the cisplatin group. In addition, the cell apoptosis rate of the
ARID1A siRNA group was decreased compared with that of the negative
control group. Therefore, these results indicate that ARID1A gene
silencing decreased the apoptosis of ES2 cells and induced
resistance to the killing effect of cisplatin on cells to a certain
extent.
However, the drug resistance mechanism of tumor
cells caused by ARID1A gene mutation is not very clear. Previous
studies have reported that a variety of signaling pathways are
involved in the phosphatidylinositol 3-kinases (PI3K) family, which
regulates cell proliferation, differentiation and apoptosis
(21). The signaling pathway
constituted by PI3K type IA and its downstream molecular
serine/threonine protein kinase B (AKT) is closely associated with
the occurrence and development of tumors. Dysregulation of the
PI3K-AKT signaling pathway exists in many human tumors, and
mutations in certain components change the function of genes and
thereby cause the transformation of cells. This pathway is
associated with the proliferation, invasion and metastasis of tumor
cells (22,23). AKT acts on the anti-apoptotic pathway
via the phosphorylation of downstream target proteins, and affects
the survival of cells through acting on nuclear factor (NF)-κB and
P53. AKT activates inhibitor of NF-κB (IκB) kinase by
phosphorylation and results in the degradation of IκB, thereby
releasing NF-κB from the cytoplasm. The released NF-κB carries out
nuclear translocation, and then activates target genes to promote
survival of the cells (24,25). Previous studies have shown that
antisense oligonucleotide to AKT is able to inhibit the growth
ability of tumor cells in soft agar, induce apoptosis and increase
the sensitivity of tumor cells to chemotherapeutic agents (26). The results of the present study
revealed that the AKT expression level in the cisplatin group was
lower than that in the negative control group, and the AKT
expression in the ARID1A siRNA plus cisplatin group was higher than
that in the cisplatin group. Therefore, these results indicate that
cisplatin induces cell apoptosis by decreasing AKT expression;
however, ARID1A gene silencing caused AKT expression to increase,
resulting in resistance to the pro-apoptotic effect of cisplatin to
a certain extent, eventually leading to the ES2 cells becoming
resistant to cisplatin.
In conclusion, the results of the present study
demonstrated that ARID1A gene silencing reduced the sensitivity of
ES2 cells to cisplatin via the regulation of AKT expression. These
observations further our understanding of the association between
ARID1A gene mutation and drug resistance in OCCC and may provide a
novel therapeutic target for the treatment of OCCC.
References
|
1
|
Mackay HJ, Brady MF, Oza AM, Reuss A,
Pujade-Lauraine E, Swart AM, Siddiqui N, Colombo N, Bookman MA,
Pfisterer J, et al: Prognostic relevance of uncommon ovarian
histology in women with stage III/IV epithelial ovarian cancer. Int
J Gynecol Cancer. 20:945–952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan JK, Teoh D, Hu JM, Shin JY, Osann K
and Kapp DS: Do clear cell ovarian carcinomas have poorer prognosis
compared to other epithelial cell types? A study of 1411 clear cell
ovarian cancers. Gynecol Oncol. 109:370–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K,
Ueki M, Tsuda H, Suzuki M, Kigawa J, Takeuchi S, Tsuda H, et al:
Clear cell carcinoma of the ovary: A retrospective multicentre
experience of 254 patients with complete surgical staging. Br J
Cancer. 94:1369–1374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takano M, Tsuda H and Sugiyama T: Clear
cell carcinoma of the ovary: Is there a role of histology-specific
treatment? J Exp Clin Cancer Res. 31:532012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winter WE III, Maxwell GL, Tian C, Carlson
JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F and
McGuire WP: Gynecologic Oncology Group Study: Prognostic factors
for stage III epithelial ovarian cancer: A Gynecologic Oncology
Group Study. J Clin Oncol. 25:3621–3627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho CM, Huang YJ, Chen TC, Huang SH, Liu
FS, Chien CC Chang, Yu MH, Mao TL, Wang TY and Hsieh CY: Pure-type
clear cell carcinoma of the ovary as a distinct histological type
and improved survival in patients treated with
paclitaxel-platinum-based chemotherapy in pure-type advanced
disease. Gynecol Oncol. 94:197–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guan B, Wang TL and Shih IeM: ARID1A, a
factor that promotes formation of SWI/SNF-mediated chromatin
remodeling, is a tumor suppressor in gynecologic cancers. Cancer
Res. 71:6718–6727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lichner Z, Scorilas A, White NM, Girgis
AH, Rotstein L, Wiegand KC, Latif A, Chow C, Huntsman D and Yousef
GM: The chromatin remodeling gene ARID1A is a new prognostic marker
in clear cell renal cell carcinoma. Am J Pathol. 182:1163–1170.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozawa Y, Nakamura Y, Fujishima F, Felizola
SJ, Takeda K, Okamoto H, Ito K, Ishida H, Konno T, Kamei T, et al:
Decreased expression of ARID1A contributes to infiltrative growth
of esophageal squamous cell carcinoma. Tohoku J Exp Med.
235:185–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizuno M, Kikkawa F, Shibata K, Kajiyama
H, Ino K, Kawai M, Nagasaka T and Nomura S: Long-term follow-up and
prognostic factor analysis in clear cell adenocarcinoma of the
ovary. J Surg Oncol. 94:138–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Itamochi H, Kigawa J and Terakawa N:
Mechanisms of chemoresistance and poor prognosis in ovarian clear
cell carcinoma. Cancer Sci. 99:653–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Veras E, Mao TL, Ayhan A, Ueda S, Lai H,
Hayran M, Shih IeM and Kurman RJ: Cystic and adenofibromatous clear
cell carcinomas of the ovary: Distinctive tumors that differ in
their pathogenesis and behavior: A clinicopathologic analysis of
122 cases. Am J Surg Pathol. 33:844–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ayhan A, Mao TL, Seckin T, Wu CH, Guan B,
Ogawa H, Futagami M, Mizukami H, Yokoyama Y, Kurman RJ and Shih
IeM: Loss of ARID1A expression is an early molecular event in tumor
progression from ovarian endometriotic cyst to clear cell and
endometrioid carcinoma. Int J Gynecol Cancer. 22:1310–1315. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao TL, Ardighieri L, Ayhan A, Kuo KT, Wu
CH, Wang TL and Shih IeM: Loss of ARID1A expression correlates with
stages of tumor progression in uterine endometrioid carcinoma. Am J
Surg Pathol. 37:1342–1348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itamochi H, Oumi N, Oishi T, Shoji T,
Fujiwara H, Sugiyama T, Suzuki M, Kigawa J and Harada T: Loss of
ARID1A expression is associated with poor prognosis in patients
with stage I/II clear cell carcinoma of the ovary. Int J Clin
Oncol. 20:967–973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MJ, Gu MJ, Chang HK and Yu E: Loss of
ARID1A expression is associated with poor prognosis in small
intestinal carcinoma. Histopathology. 66:508–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo B, Cheung HW, Subramanian A, Sharifnia
T, Okamoto M, Yang X, Hinkle G, Boehm JS, Beroukhim R, Weir BA, et
al: Highly parallel identification of essential genes in cancer
cells. Proc Natl Acad Sci USA. 105:20380–20385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bosse T, ter Haar NT, Seeber LM, v Diest
PJ, Hes FJ, Vasen HF, Nout RA, Creutzberg CL, Morreau H and Smit
VT: Loss of ARID1A expression and its relationship with PI3K-Akt
pathway alterations, TP53 and microsatellite instability in
endometrial cancer. Mod Pathol. 26:1525–1535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ptak A, Hoffmann M, Gruca I and Barć J:
Bisphenol A induce ovarian cancer cell migration via the MAPK and
PI3K/Akt signalling pathways. Toxicol Lett. 229:357–365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong SJ, Pise-Masison CA, Radonovich MF,
Park HU and Brady JN: Activated AKT regulates NF-kappaB activation,
p53 inhibition and cell survival in HTLV-1-transformed cells.
Oncogene. 24:6719–6728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin P, Yi Y, Lu M, Wang M, Yang Y, Lu Y,
Song S, Zheng Z, Deng X and Zhang L: Heat shock protein 90
inhibitor mycoepoxydiene modulates kinase signaling in cervical
cancer cells and inhibits in-vivo tumor growth. Anticancer Drugs.
26:25–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|