Introduction
Delayed union and nonunion with or without defects
are common complications of traumatic fractures (1). Fortunately, mesenchymal stem cell
(MSC)-based therapy has recently emerged as an appealing and
potential therapeutic strategy for the treatment of delayed union,
nonunion or persistent bone defects (2). Bone marrow-derived MSCs (BMSCs), one
type of MSC cell, have the properties of plasticity and the ability
to differentiate into chondrocytes, osteocytes and adipocytes
(3). The transplantation of BMSCs in
damaged tissues has been an innovation used in tissue engineering,
particularly in the field of skeletal regenerative medicine
(4,5). Cultured BMSCs have been injected or
combined with biomaterials into fracture sits where the BMSCs
differentiate into osteoblasts to repair the fracture (2,6,7). Therefore, multiple stem cell-based
products and techniques to enhance the efficacy of local
implantation of BMSCs are currently being investigated to optimize
bone healing in various animal models and clinical trials (3,5).
The therapeutic application of BMSCs is limited due
to their susceptibility to oxidative stress which results in the
engrafted BMSCs' apoptosis in the injured bone area (8–10).
Previous studies have demonstrated that BMSCs injected into
fracture sites are confronted with its apoptosis within a few days
on account of harsh microenvironment conditions with oxidative
stress, which is a state of imbalance between reactibe oxygen
species (ROS) generation and intracellular antioxidants, resulting
in cellular damage, and eventually leading to cell death (11–13).
Therefore, strategies to protect the implantation of BMSCs from
apoptosis and to improve their survivability in oxidative stress
are therapeutically attractive in MSC-based therapy for delayed
union, nonunion and persistent bone defects.
Plants used in Traditional Chinese Medicine have
been regarded as a wide source of antioxidants with potential
pharmacological and biological effects (14). Berberine (BBR),
5,6-dihydro-9,10-dimethoxy-benzo(g)-1,3-benzodioxolo(5,6-a)
quinolizinium-, chloride, a natural isoquinoline quaternary
alkaloid, is a well-known constituent of the Chinese herb Huanglian
(15). The compound possesses a
variety of pharmacological and biochemical properties, such as
anti-inflammatory (16),
anti-microbial (17), anti-cancer
(18) and and antioxidant (19–21)
properties. A previous study demonstrated that BBR could attenuate
H2O2-induced oxidative injury in motor
neuron-like cells (22), smooth
muscle cells (19), endothelial
cells and mesangial cells (23), but
whether BBR exerts any protective effects against
H2O2 in rat (r)BMSCs is still unknown.
The present study, to the best of our knowledge,
demonstrated for the first time that BBR is capable of protecting
primary cultural rBMSCs against H2O2-induced
apoptosis via enhancing resistance to oxidative stress in
vitro, which could be a promising approach to improve stem cell
survival during transplantation in MSC-based therapy for traumatic
fractures.
Materials and methods
Animals and BBR
Male Sprague-Dawley (SD) rats, weight 80–90 g, were
obtained from the animal center of Guangzhou University of
Traditional Chinese Medicine (Guangzhou, China; certificate no.
44005900001722). Twenty-two rats were specific pathogen-free
animals housed with ad libitum access to food and water at a
constant temperature of 24±1°C in climate-controlled conditions
with a 12 h light/dark cycle and humidity of 55±5%. All animals
received human care in accordance with the guideline set by the
Care of Experiment Animals Committee of Guangzhou University of
Chinese Medicine. BBR (>99.0% purity) was provided by the
Department of Pharmacology & Toxicology of Sun Yat-Sen
University (Guangzhou, China). BBR was dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) and kept in −20°C away from the dark. The final
concentration of DMSO per well was 0.1%.
Isolation and culture of rBMSCs
MSCs were isolated from the bone marrow of rats as
previously reported with minor modification (24). Briefly, the femurs and tibias were
clipped from the SD rats under sterile conditions. After removing
all the connective tissues and cutting epiphyseal extremities, bone
marrow was flushed out with low (L-)glucose Dulbecco's modified
Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, YSA) containing 1% penicillin/streptomycin in a
sterile petri dish. The cells were centrifuged at 300 × g
for 8 min and resuspended in L-DMEM (1,000 mg/l glucose)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and a mixture of 1% penicillin/streptomycin. Bone
marrow was transferred to a plastic culture flask and incubated at
37°C with 5% CO2 in a humidified atmosphere. After 24 h,
the medium was changed to remove free-floating cells and replaced
every 3 days. At 80–90% confluence, the adherent cells were washed
with phosphate-buffered saline (PBS) twice, followed by digestion
in 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.) and
expanded at a 1:2 dilution.
Cell surface phenotype detection
Surface marker analysis was performed by flow
cytometry. rBMSCs were collected in passage three, cells were
resuspended in 100 µl flow cytometry staining buffer (Cyagen
Biosciences, Inc., Guangzhou, China) containing PBS and 0.1% bovine
serum albumin (Sigma-Aldrich; Merck Millipore) at a concentration
of 3×106 cells/ml and incubated for 30 min at 4°C with
the following specific primary antibodies: Anti-rat cluster of
differentiation (CD)90, anti-rat CD34 and anti-rat CD11b/c at a
concentration of 0.5 mg/ml, which were from the Cyagen Mesenchymal
Stem Cell Characterization kit (cat. no. RAXMX-09011; Cyagen
Bioscience Inc.) After incubation, the samples were washed twice in
1 ml flow cytometry staining buffer, then centrifuged at 250 ×
g for 5 min. Then, the supernatant was discarded. Following
incubation at 4°C with phycoerythrin-conjugated goat anti-rat at a
concentration of 1 mg/ml for 30 min in the dark, the samples were
washed twice with flow cytometry staining buffer, centrifuged at
250 × g for 5 min and resuspended in 400 µl of flow
cytometry staining buffer for cytometric analysis. Labeled cells
were analyzed by flow cytometry (Coulter EPICS XL; Beckman Coulter,
Inc., Brea, CA, USA) with standard software (FACSDiva; version
6.1.3; BD Biosciences, Franklin Lakes, NJ, USA).
Cell viability assay
Cell viability was assessed by detecting the optical
density (25). Briefly, rBMSCs were
seeded at a density of 1×105/ml in 96-well culture
plates for 24 h. Subsequently, the cells were pretreated with
different concentrations of BBR for 2 h and then exposed to
H2O2 for 24 h in 100 µl DMEM with 10% FBS and
1% penicillin/streptomycin. Then, 10 µl cell counting kit (CCK)-8
solution (Dojindo Laboratories, Kumamoto, Japan) was added into
each well. Following 2 h incubation, the absorbance at a wavelength
of 450 nm was detected using a Bio-kinetics reader (PE-1420;
Bio-Kinetics Corporation, Sioux Center, IA, USA). Cell viability
was presented as the percentage of the control culture value. The
results were calculated using three different batches of wells and
each experiment was performed in triplicate as independent
experiments.
Morphologic changes
rBMSCs were cultured in 12-well plates for 24 h and
then pretreated with BBR for 2 h with subsequent exposure to
H2O2 (Sigma-Aldrich; Merck Millipore) for 24
h. Then, cultured cells were washed twice with PBS and fixed with
4% paraformaldehyde for 10 min and stained with 2 µg/ml Hoechst
33258 (Sigma-Aldrich; Merck Millipore) for 20 min at 37°C in the
dark (26). Then, morphologic
changes were observed by phase contrast microscopy and cells images
were visualized through a fluorescence microscope.
Apoptosis analysis by flow
cytometry
The percentage of apoptotic cells were assessed
using Annexin V and propidium iodide (PI) staining (Annexin V-FITC
apoptosis detection kit; cat. no. KGA107; Jiancheng Biological
Engineering Research Institute, Nanjing, China) and flow cytometry.
In brief, rBMSCs were seeded at 5×104 cells/ml in 6-well
culture plates and incubated overnight. Following treatment, both
adherent and floating cells were harvested, washed twice in cold
PBS, and resuspended in 200 µl of binding buffer (Jiancheng
Biological Engineering Research Institute). Annexin V-FITC solution
(5 µl) was added and the cells were incubated for 30 min at 4°C in
the dark. Subsequently, 10 µl PI was added and the solution was
incubated for 15 min at the room temperature. The cell suspension
was immediately analyzed by flow cytometry (Coulter EPICS XL) with
standard software.
Measurement of ROS production
Production of intracellular ROS was evaluated using
a 2′7′-dichlorofluorescein (DCF) assay (27), which was conducted using
dichlorofluorescein diacetate (H2DCF-DA; Sigma-Aldrich;
Merck Millipore). Briefly, following treatment, rBMSCs were washed
and then incubated with 10 µM H2DCF-DA in serum-free
culture medium (Gibco; Thermo Fisher Scientific, Inc.) for 30 min
at 37°C in the dark. DCF fluorescence was illuminated by visual
effect of cell morphology through fluorescence microscopy or
analyzed using a fluorescence plate reader (Flex Station3;
Molecular Devices, LLC, Sunnyvale, CA, USA) at an excitation and
emission wavelength of 490 nm and 533 nm.
Detection of superoxide dismutase
(SOD)
SOD activity was tested with the xanthine oxidase
method as previously described (28). Briefly, rBMSCs were pretreated with
BBR for 2 h and incubated with 600 µM H2O2
for 24 h. Then, the cells were harvested and sonicated with cold
0.9% sodium chloride to obtain cell homogenates. Following
centrifugation at 3,500 × g at 4°C for 5 min, the
supernatants were obtained and then used for detecting
intracellular SOD. The level of SOD was calculated by measuring the
absorbance at 570 nm according to the instructions of a Superoxide
Dismutase Assay kit (cat. no. A001-2; Jiancheng Biological
Engineering Research Institute). The basal contents of SOD in
untreated control cells were taken as 100%.
Western blotting analysis
Western blotting analysis was performed as
previously described (29). Briefly,
at the end of the treatment period, cells were collected and lysed
with cold lysis buffer. Total protein concentration was determined
using a BCA assay kit (cat. no. KGET13; Keygen, Nanjing, China).
Protein samples (30 µg/kg) were separated by 10% SDS-PAGE and
transferred onto polyvinylidenedifluoride membranes. After being
blocked with 5% non-fat dry milk in Tris-buffered saline and
Tween-20 buffer, the membranes were incubated overnight at 4°C with
the following the primary antibodies: Rabbit polyclonal
anti-phosphorylated (p)-Akt (cat. no. 5012S), anti-B-cell lymphoma
2 (Bcl-2; cat. no. 3498) and anti-caspase-3 (Cat. no. 9661) (all
purchased from Cell Signaling Technology, Inc., Danvers, MA, USA;
1:1,000), rabbit polyclone anti-Bcl-2-associated X protein (cat.
no. sc-526; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1:500)
and mouse polyclonal anti-β-actin (cat. no. A1978; Sigma-Aldrich;
Merck Milliporel 1:10,000) at 4°C overnight. The next day, the
membranes were incubated for 1 h at room temperature with
horseradish peroxidase-conjugated anti-rabbit secondary antibody
(cat. no. sc-3836; Santa Cruz Biotechnology, Inc.; 1:5,000) and
anti-mouse secondary antibody (cat. no. W4021; Promega Corporation,
Madison, WI, USA; 1:10,000). The bands were detected with enhanced
chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK). The
blots were quantified using Quantity One software (version 4.62;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All quantified data represent an average of at least
three samples, and the results are presented as the mean ± standard
deviation. Differences among groups were tested by one-way analysis
of variance (ANOVA). Statistical analyses between two groups were
performed by unpaired Student's t-test. Following ANOVA analyses,
the Tukey's test was used. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using GraphPad version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Morphology and cell surface phenotype
detection of BMSCs
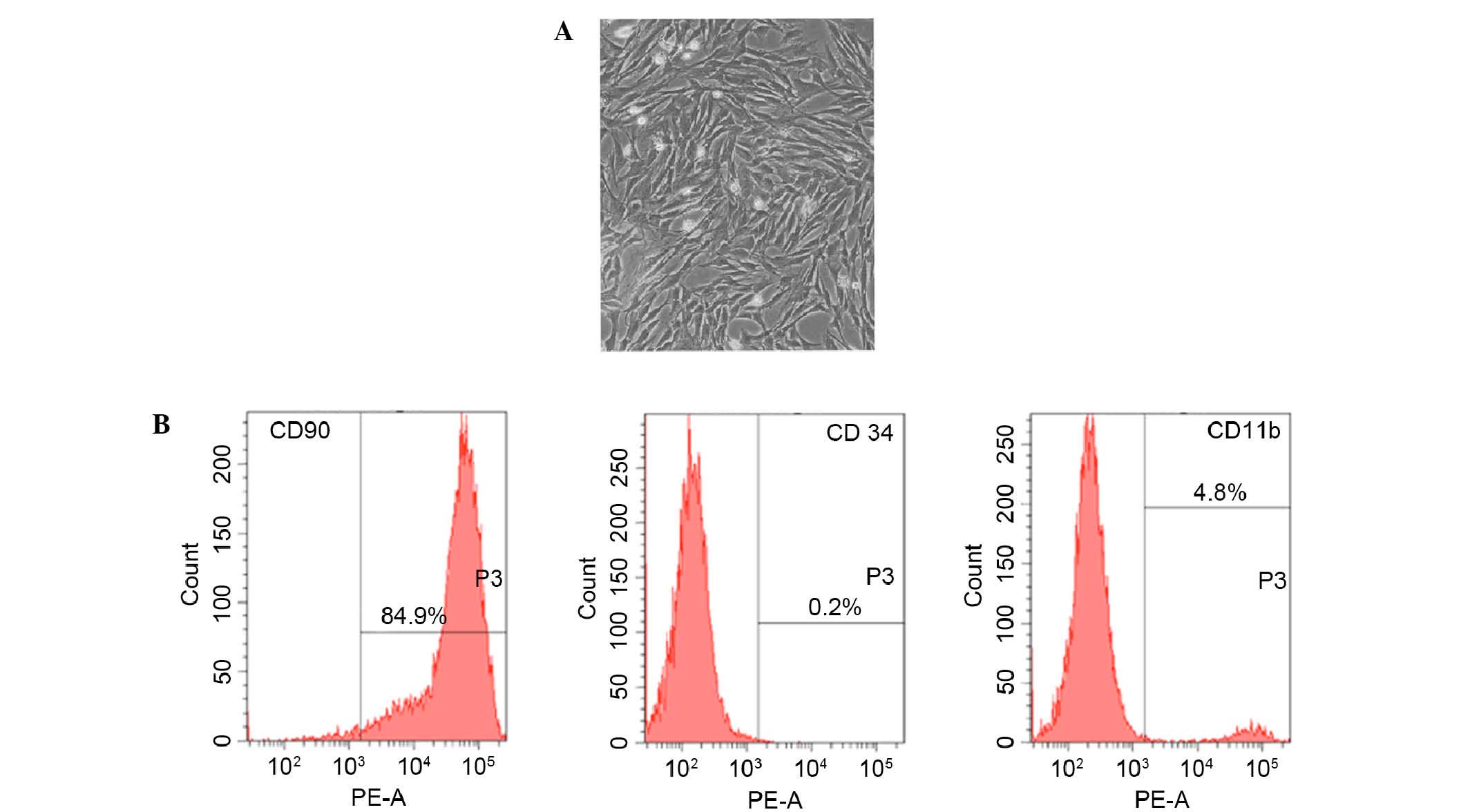
Fifteen days after being obtained from rat femur,
cultured rBMSCs were adherent to the dish and showed an elongated,
spindle-shaped and fibroblast-like morphology (Fig. 1A). Furthermore, the phenotype of
BMSC-related cell surface markers CD90, CD34 and CD11b were
detected. Results of flow cytometry demonstrated that 89.4% of the
cells expressed CD90, 4.8% of the cells expressed CD11b and 0.2% of
the cells expressed CD34 (Fig.
1B).
Effect of BBR and
H2O2 on rBMSC cell viability
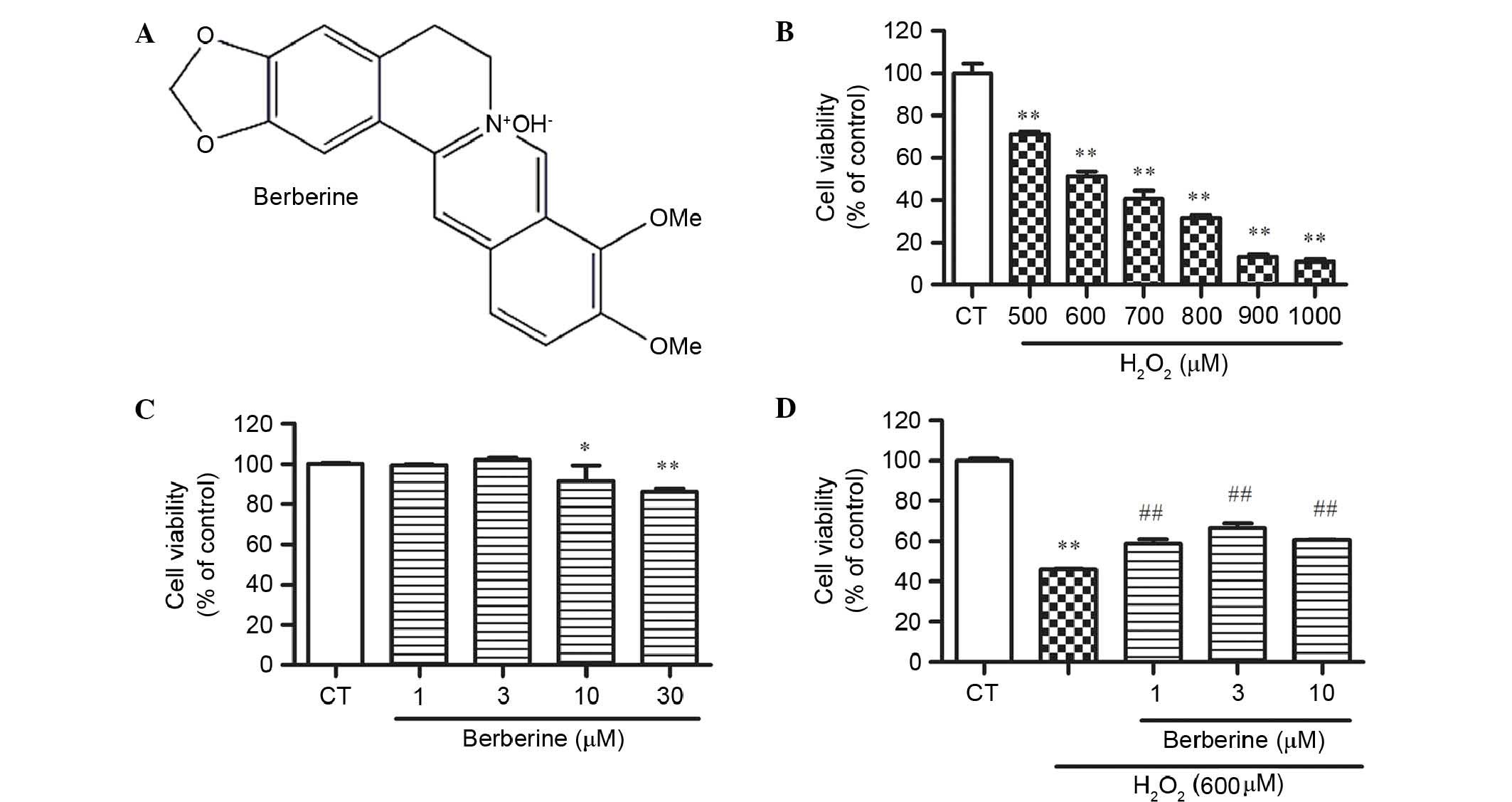
CCK-8 assay results demonstrated that BBR (Fig. 2A) did not cause cell death at a
concentration of 30 µM, but significantly reduced cell viability at
30 µM compared with the control (P<0.01; Fig. 2B). To determine the optimum
concentration of H2O2 for next experiment,
cells treated with 500, 600, 700, 800, 900 and 1000 µM for 24 h in
the complete medium were examined. H2O2
exhibited cytotoxicity dose-dependently, and 600 µM
H2O2 significantly reduced cell viability by
48.6% compared with the control (P<0.01; Fig. 2C). This concentration was used for
the following experiments.
BBR inhibited
H2O2-induced cell inhibition in rBMSCs
To examine whether BBR protects rBMSCs from
H2O2-induced cell death, cells were
pretreated with 1, 3 and 10 µM BBR for 2 h, then incubated with
H2O2 (600 µM) for 24 h. The results
demonstrated that the cell viability of BMSCs in the
H2O2 group decreased dramatically, while BBR
dose-dependently increases the cell viability. BBR at 3 µM showed
the best effect against H2O2 (P<0.01 vs.
the H2O2 group; Fig. 2D).
BBR reduced
H2O2-induced apoptosis-like cell death in
rBMSCs
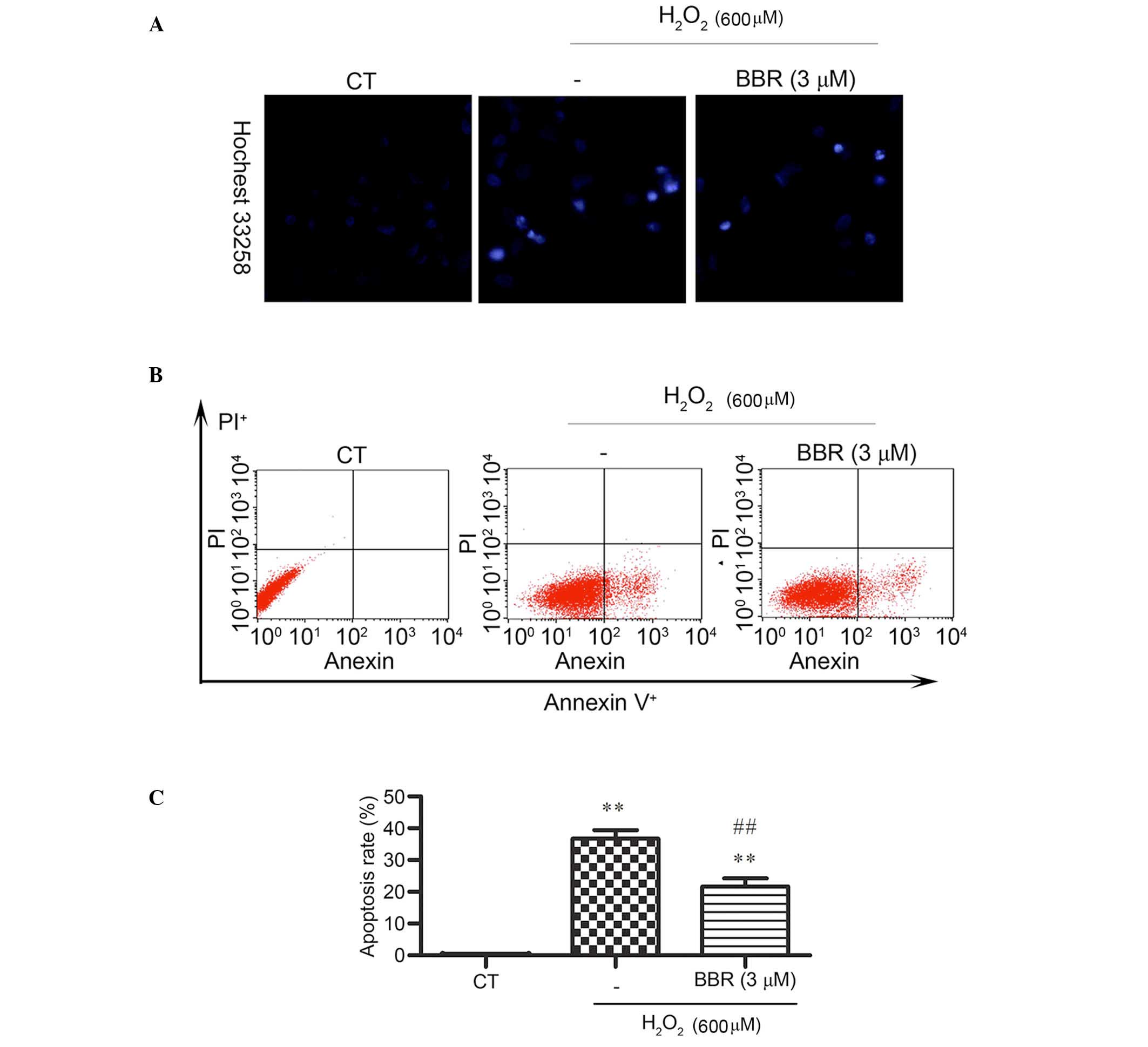
Hoechst 33258 staining was applied and observed by
fluorescence microscope to detect changes in cell nuclei. After
pretreatment with BBR, the number of cells with bright blue
fluorescence were reduced notably, indicating that BBR can markedly
decrease the number of apoptotic cells and nuclear condensation
(Fig. 3A) caused by
H2O2. The results of Annexin V-FITC/PI
staining detected by flow cytometery revealed (Fig. 3B and C) that the
H2O2 treatment group had a significantly
higher rate of apoptosis (36±3.39%) compared with the control group
(0.14± 0.02%) (P<0.01). However, the addition of BBR was able to
protect the cells against H2O2-induced
apoptosis and significantly reduce the rate of apoptotic cells
(P<0.01; 22± 4.21%).
BBR suppressed
H2O2-induced intracellular ROS formation
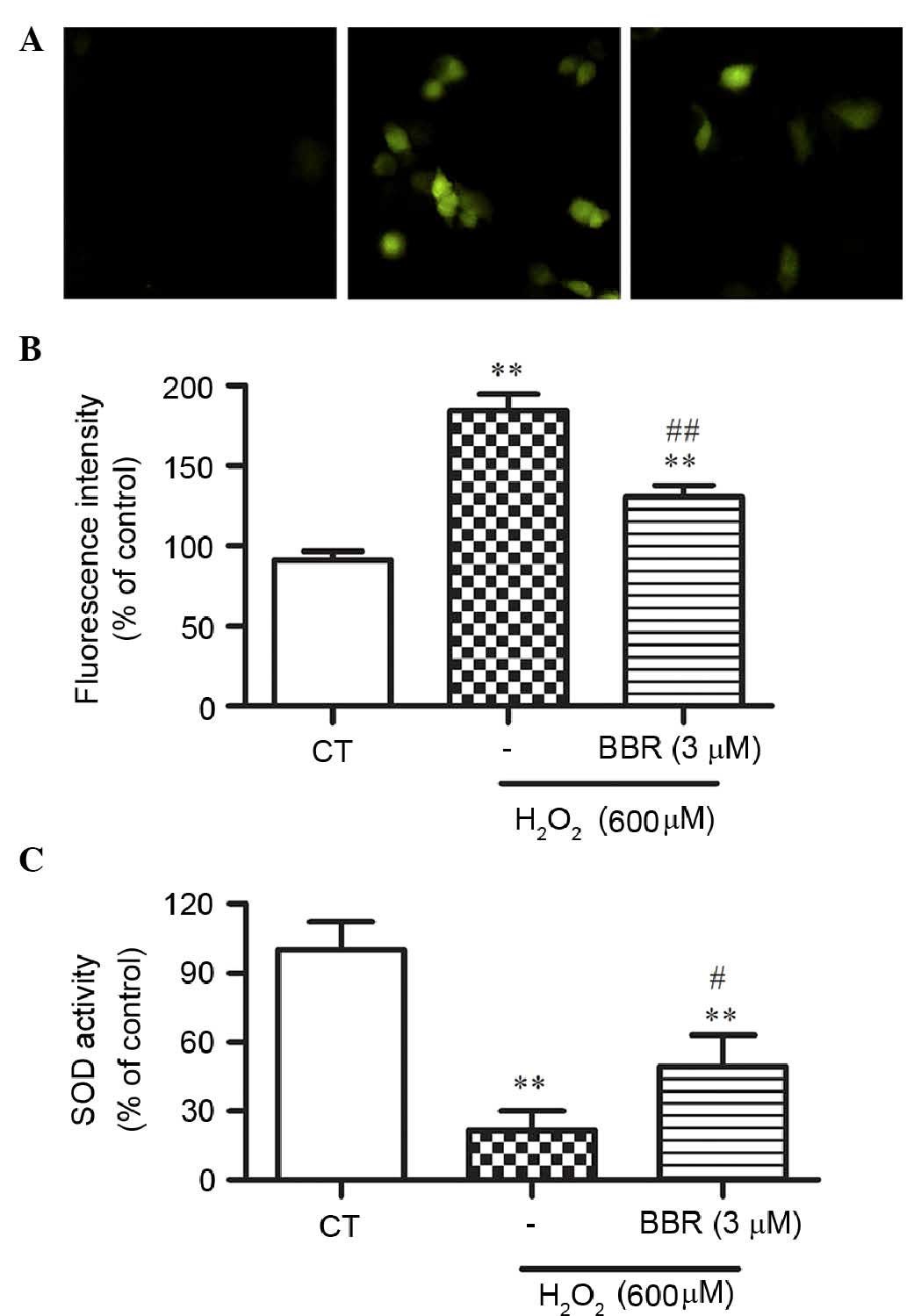
To elucidate the potential mechanisms underlying the
protection of BBR following H2O2 treatment,
H2DCF-DA staining, a ROS probe, was used to measure cellular
oxidative stress by DCF fluorescence. Treatment with 600 µM
H2O2 for 24 h significantly resulted in the
increase of DCF fluorescence compared with the control group
(P<0.01), which was significantly reversed by BBR (P<0.01;
Fig. 4A and B).
BBR alleviated
H2O2-induced decrease of the activity of
intracellular SOD
To further study whether BBR could alleviate
H2O2-induced oxidative stress, the activity
of SOD in rBMSCs was assessed. As shown in Fig. 4C, the activity of SOD was
significantly decreased in the H2O2-treated
group compared with that of the control group (P<0.01), while
pretreatment of BBR significantly restored this decrease in SOD
activity (P<0.05).
BBR protected rBMSCs from
H2O2-induced apoptosis via regulation of the
expression of apoptosis-related proteins p-Akt, Bcl-2, Bax and
caspase-3
To further explore the protective mechanisms of BBR,
the expression of anti-apoptotic protein Bcl-2, and the
pro-apoptotic proteins Bax and caspase-3, were investigated. As
shown in Fig. 5A-D, following
treatment with H2O2 (600 µM) for 12 h, it was
observed that the expression of Bcl-2 significantly decreased
(P<0.05), and the expression of Bax and caspase-3 significantly
increased (P<0.01), compared with the control group.
Pretreatment with BBR (3 µM) for 2 h significantly inhibited the
downregulation of anti-apoptotic proteins (P<0.01) and
significantly reduced the upregulation of pro-apoptotic proteins
(P<0.01). The phosphorylation of Akt had been reported to be
important in cell survival and apoptosis (30). In the current study (Fig. 5E), treatment with
H2O2 (600 µM) significantly decreased the
expression level of pSer473-Akt compared with the control group
(P<0.01), and this was significantly reversed with pretreatment
with BBR (3 µM; P<0.01). These results suggest that BBR can
protect rBMSCs from H2O2-induced apoptosis by
increasing the level of p-Akt.
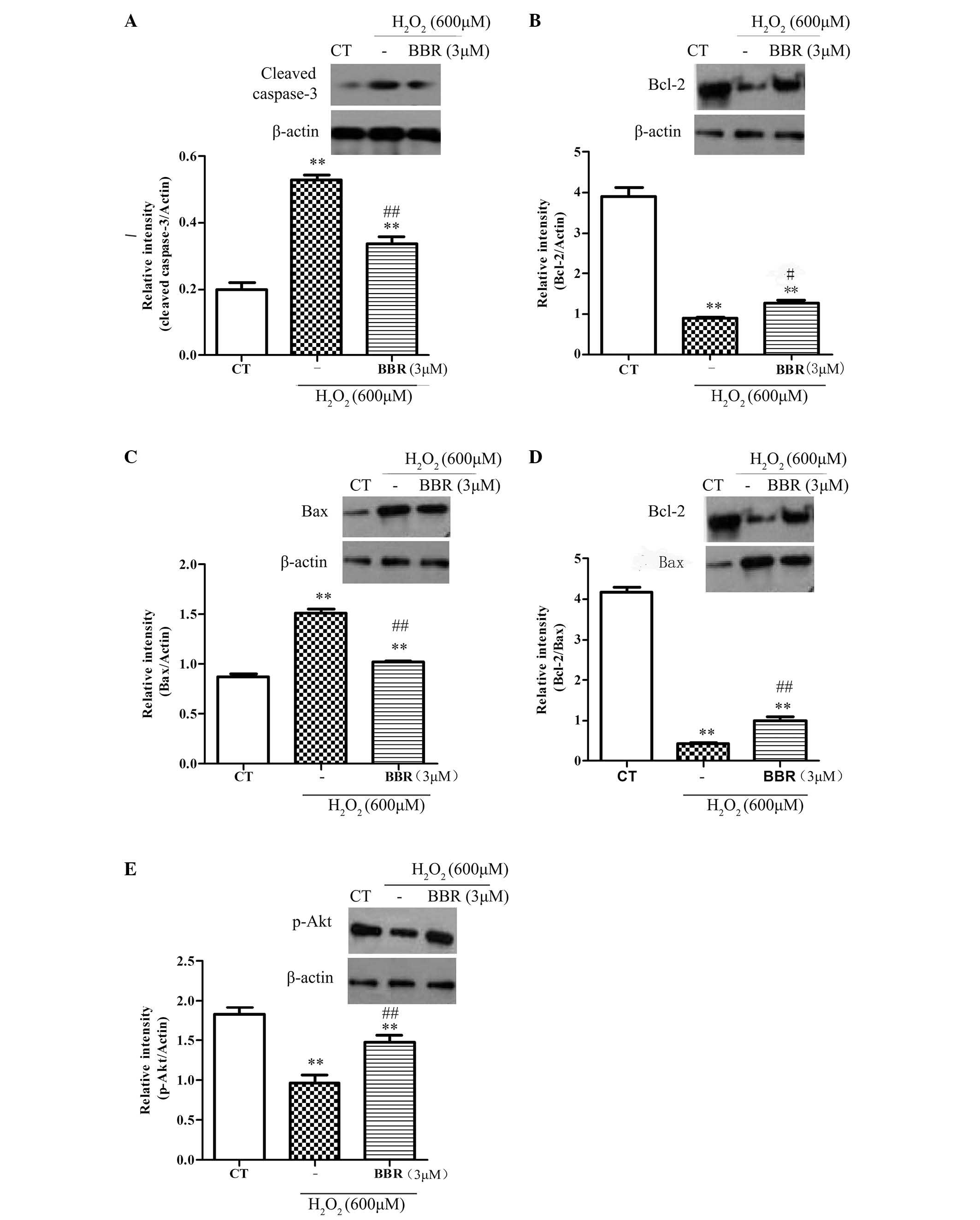 | Figure 5.Representative western blots showing
the effects of BBR on Bcl-2, Bax, caspase-3 and p-Akt expression in
rBMSCs. β-actin was used as a loading control. Cells were
pretreated with BBR for 2 h followed by treatment with
H2O2 (600 µM) for 12 h. (A) Following
pretreatment with BBR, the expression of caspase-3 significantly
reduced compared with the H2O2 group. (B) BBR
inhibited the H2O2-induced downregulation of
Bcl-2. (C) BBR suppressed the H2O2-induced
upregulation of Bax. (D) Increased ratio of Bcl-2/Bax was observed
when the cells were pretreated with BBR prior to incubation with
H2O2. (E) Treatment with
H2O2 significantly decreased the level of
p-Akt, which was blocked following pretreatment with BBR (3 µM).
**P<0.01 vs. the control group; #P<0.05,
##P<0.01 vs. the H2O2 group.
All data are presented as the mean plus standard deviation of three
independent experiments. BBR, berberine; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; CT, control; p-Akt, phosphorylated
Akt; rBMSCs, rat bone marrow-derived mesenchymal stem cells. |
Discussion
Treatment of delayed healing, nonunion or a
persistent bone defect represents a major challenge for
traumatology department (31). Owing
to the low immunogenicity and transplantability (32), BMSCs are an effective resource for
transplantation in clinical application, with the establishment of
cell banks for bone regenerative medicine (2,33).
Previously, much attention has been directed towards the poor
survival of transplantation in MSC-based therapy (5,9,34,35). For
basic and clinician scientists, it is necessary to utilize
strategies to improve low cell survival rates.
H2O2 has been extensively used
to imitate the microenvironment surrounding transplanted cells in
injured tissue in vitro (36). When exposed to
H2O2, cells are faced with high concentration
of ROS, which consequently damages the balance of oxidants and
antioxidants, resulting in apoptosis and eventually necrosis. In
the experiments in the current study, BBR significantly reduced the
level of apoptosis in H2O2-treated cells. In
addition, the results demonstrated that BBR exposure inhibited the
overproduction of intracellular ROS induced by exogenous
H2O2, and significantly enhanced the activity
of antioxidant enzyme SOD, which promotes a balance between the ROS
and anti-oxidative system in targeting oxidative stress.
Caspase-3 is the most crucial downstream apoptosis
protease in the caspase cascade, which executes apoptosis through
DNA degradation, chromatin condensation and nuclear fragmentation
(37). In the present study,
treatment of rBMSCs with 600 µM H2O2 induced
marked nuclear condensation and apoptotic death, and increased the
expression of cleaved caspase-3, indicating that the apoptosis of
the caspase cascade may be activated by H2O2.
However, following pretreatment with BBR (3 µM), the expression of
cleaved caspase-3 was significantly reduced, indicating the BBR
effectively attenuates oxidative injury by suppressing
H2O2-induced caspase activation.
To further explore the molecular mechanisms
underlying the moderation of apoptosis by BBR, the expression level
of vital apoptotic proteins was assessed. The Bcl-2 family of
proteins comprise the anti-apoptotic proteins and pro-apoptotic
proteins, of which the relative proportions control the fine
balance between cell survival and cell death via the intrinsic
apoptotic pathway (38). In the
present study, western blot indicated that BBR could reverse the
reduction of the anti-apoptotic protein Bcl-2, and could increase
the level of pro-apoptotic protein Bax, following treatment with
H2O2. In addition, pretreatment with BBR
prior to incubation with H2O2 significantly
increased the ratio of Bcl-2/Bax compared with cells treated with
H2O2 alone, showing that the Bcl-2 family has
a close association with the protective effects of BBR in
rBMSCs.
As a survival pathway, the Akt signaling pathway
mediating the anti-apoptosis mechanism is well understood (30). Exogenous H2O2
can influence Akt activation, which promotes cell survival
(39). The present study showed that
BBR pretreatment prevented the downregulation of p-Akt induced by
H2O2. This suggests that BBR caused an
increase of Akt phosphorylation in parallel with an increase of
protective effects. However, whether BBR can protect rBMSCs from
oxidative stress-induced apoptosis via the Akt pathway requires
further investigation.
In conclusion, the results of the present study
provide powerful evidence that pretreatment with BBR can alleviate
H2O2-induced apoptosis and enhance the
viability of rBMSCs via improvement of antioxidant activities and
regulation of apoptosis and the anti-apoptotic pathway. This may be
a useful strategy to improve low cell survival rates in treatment
of delayed union and nonunion with or without defects.
Acknowledgements
The authors would like to thank Miss Meihui Chen who
worked in Guangdong Provincial Hospital of Traditional Chinese
Medicine (Guangzhou, China) for assisting in the preparation of
this manuscript. The present study was supported by National
Natural Scientific Foundation of China (grant no. 81273783) and the
National Natural Scientific Foundation of China (grant no.
81473699).
References
|
1
|
Nauth A, Miclau T III, Li R and Schemitsch
EH: Gene therapy for fracture healing. J Orthop Trauma. 24:(Suppl
1). S17–S24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosset P, Deschaseaux F and Layrolle P:
Cell therapy for bone repair. Orthop Traumatol Surg Res. 100:(Suppl
1). S107–S112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asatrian G, Pham D, Hardy WR, James AW and
Peault B: Stem cell technology for bone regeneration: Current
status and potential applications. Stem Cells Cloning. 8:39–48.
2015.PubMed/NCBI
|
|
4
|
Qin Y, Guan J and Zhang C: Mesenchymal
stem cells: Mechanisms and role in bone regeneration. Postgrad Med
J. 90:643–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gómez-Barrena E, Rosset P, Lozano D,
Stanovici J, Ermthaller C and Gerbhard F: Bone fracture healing:
Cell therapy in delayed unions and nonunions. Bone. 70:93–101.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Devine MJ, Mierisch CM, Jang E, Anderson
PC and Balian G: Transplanted bone marrow cells localize to
fracture callus in a mouse model. J Orthop Res. 20:1232–1239. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taguchi K, Ogawa R, Migita M, Hanawa H,
Ito H and Orimo H: The role of bone marrow-derived cells in bone
fracture repair in a green fluorescent protein chimeric mouse
model. Biochem Biophys Res Commun. 331:31–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Majzunova M, Dovinova I, Barancik M and
Chan JY: Redox signaling in pathophysiology of hypertension. J
Biomed Sci. 20:692013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee S, Choi E, Cha MJ and Hwang KC: Cell
adhesion and long-term survival of transplanted mesenchymal stem
cells: A prerequisite for cell therapy. Oxid Med Cell Longev.
2015:6329022015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang W, Song BW, Moon JY, Cha MJ, Ham O,
Lee SY, Choi E, Choi E and Hwang KC: Anti-death strategies against
oxidative stress in grafted mesenchymal stem cells. Histol
Histopathol. 28:1529–1536. 2013.PubMed/NCBI
|
|
11
|
Halabian R, Tehrani HA,
Jahanian-Najafabadi A and Roudkenar M Habibi: Lipocalin-2-mediated
upregulation of various antioxidants and growth factors protects
bone marrow-derived mesenchymal stem cells against unfavorable
microenvironments. Cell Stress Chaperones. 18:785–800. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Ehnert S, Ihle C, Schyschka L,
Pscherer S, Nussler NC, Braun KF, Van Griensven M, Wang G, Burgkart
R, et al: Increased oxidative stress response in granulocytes from
older patients with a hip fracture may account for slow
regeneration. Oxid Med Cell Longev. 2014:8198472014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sies H: Oxidative stress: Oxidants and
antioxidants. Exp Physiol. 82:291–295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matkowski A, Jamiołkowska-Kozlowska W and
Nawrot I: Chinese medicinal herbs as source of antioxidant
compounds-where tradition meets the future. Curr Med Chem.
20:984–1004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen XW, Di YM, Zhang J, Zhou ZW, Li CG
and Zhou SF: Interaction of herbal compounds with biological
targets: A case study with berberine. Scientific World J.
2012:7082922012. View Article : Google Scholar
|
|
16
|
Mo C, Wang L, Zhang J, Numazawa S, Tang H,
Tang X, Han X, Li J, Yang M, Wang Z, et al: The crosstalk between
Nrf2 and AMPK signal pathways is important for the
anti-inflammatory effect of berberine in LPS-stimulated macrophages
and endotoxin-shocked mice. Antioxid Redox Signal. 20:574–588.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu YY, Tseng YT and Lo YC: Berberine, a
natural antidiabetes drug, attenuates glucose neurotoxicity and
promotes Nrf2-related neurite outgrowth. Toxicol Appl Pharmacol.
272:787–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi T, Zhuang L, Song G, Zhang B, Li G and
Hu T: Akt signaling is associated with the berberine-induced
apoptosis of human gastric cancer cells. Nutr Cancer. 67:523–531.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan Y, Tang Q, Hu BR and Xiang JZ:
Antioxidant properties of berberine on cultured rabbit corpus
cavernosum smooth muscle cells injured by hydrogen peroxide. Acta
Pharmacol Sin. 28:1914–1918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campisi A, Acquaviva R, Bonfanti R, Raciti
G, Amodeo A, Mastrojeni S, Ragusa S and Iauk L: Antioxidant
properties of Berberis aetnensis C. Presl (Berberidaceae) roots
extract and protective effects on astroglial cell cultures.
Scientific World Journal. 2014:3154732014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Geng YN, Jiang JD and Kong WJ:
Antioxidant and anti-inflammatory activities of berberine in the
treatment of diabetes mellitus. Evid Based Complement Alternat Med.
2014:2892642014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu YY, Chen CS, Wu SN, Jong YJ and Lo YC:
Berberine activates Nrf2 nuclear translocation and protects against
oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent
mechanism in NSC34 motor neuron-like cells. Eur J Pharm Sci.
46:415–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Su X, Gao Y, Sun B, Yu Y, Wang X
and Zhang F: Berberine protects mesenchymal stem cells against
hypoxia-induced apoptosis in vitro. Biol Pharm Bull. 32:1335–1342.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Zhang Y and Qi G: Evaluation of
isolation methods and culture conditions for rat bone marrow
mesenchymal stem cells. Cytotechnology. 65:323–334. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishitsuka K, Hideshima T, Hamasaki M, Raje
N, Kumar S, Hideshima H, Shiraishi N, Yasui H, Roccaro AM,
Richardson P, et al: Honokiol overcomes conventional drug
resistance in human multiple myeloma by induction of
caspase-dependent and-independent apoptosis. Blood. 106:1794–1800.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Wang D, Li Y and Zhang X:
Protective effects of Verapamil against H2O2-induced apoptosis in
human lens epithelial cells. Biomol Ther (Seoul). 22:553–557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tetz LM, Kamau PW, Cheng AA, Meeker JD and
Loch-Caruso R: Troubleshooting the dichlorofluorescein assay to
avoid artifacts in measurement of toxicant-stimulated cellular
production of reactive oxidant species. J Pharmacol Toxicol
Methods. 67:56–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G,
Wang G and Zhong J: Lycium barbarum polysaccharides protect human
lens epithelial cells against oxidative stress-induced apoptosis
and senescence. PloS One. 9:e1102752014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun B, Feng M, Tian X, Lu X, Zhang Y, Ke
X, Huang S, Cao J and Ding X: DL-3-n-Butylphthalide protects rat
bone marrow stem cells against hydrogen peroxide-induced cell death
through antioxidation and activation of PI3K-Akt pathway. Neurosci
Lett. 516:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuda S, Nakanishi A, Wada Y and
Kitagishi Y: Roles of PI3K/AKT/PTEN pathway as a target for
pharmaceutical therapy. Open Med Chem J. 7:23–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Panteli M, Pountos I, Jones E and
Giannoudis PV: Biological and molecular profile of fracture
non-union tissue: Current insights. J Cell Mol Med. 19:685–713.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sotiropoulou PA and Papamichail M: Immune
properties of mesenchymal stem cells. Methods Mol Biol.
407:225–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pountos I, Georgouli T, Kontakis G and
Giannoudis PV: Efficacy of minimally invasive techniques for
enhancement of fracture healing: Evidence today. Int Orthop.
34:3–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng
R, Liu D and Lin F: Effects of transplantation with bone
marrow-derived mesenchymal stem cells modified by Survivin on
experimental stroke in rats. J Transl Med. 9:1052011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ilavenil S, Kim da H, Jeong YI, Arasu MV,
Vijayakumar M, Prabhu PN, Srigopalram S and Choi KC: Trigonelline
protects the cardiocyte from hydrogen peroxide induced apoptosis in
H9c2 cells. Asian Pac J Trop Med. 8:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Ann Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen J, Crawford R, Chen C and Xiao Y: The
key regulatory roles of the PI3K/Akt signaling pathway in the
functionalities of mesenchymal stem cells and applications in
tissue regeneration. Tissue Eng Part B Rev. 19:516–528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang P, Peairs JJ, Tano R and Jaffe GJ:
Oxidant-mediated Akt activation in human RPE cells. Invest
Ophthalmol Vis Sci. 47:4598–4606. 2006. View Article : Google Scholar : PubMed/NCBI
|