Introduction
Neuropathic pain is a common chronic pain, and
includes spontaneous pain, allodynia, hyperalgesia and
hypersensitivity, and pain disorders (1). Clinically, sciatica and chronic back
pain are the most common types of neuropathic pain, and the
pathogenesis is complicated (2). The
primary causes are the degeneration of the corresponding organs,
lumbar disc herniation caused by trauma, nerve damage,
calcification of ligaments, bone hyperplasia, inflammation and
tumors (3–5). Due to the long period of clinical
treatment, disease recurrence and poor prognosis, the quality of
life of patients with neuropathic pain is greatly affected.
Therefore, it is important to investigate the mechanisms underlying
neuropathic pain at molecular level.
miRNA are a type of non-coding RNA molecules (18–22
nt in length) in eukaryotes, which can regulate the expression of
proteins at the mRNA level (6–8). Studies
have shown that miRNA molecules serve important biological
functions in the development, apoptosis and synaptic release of the
nervous system (9–11). miR-145 is an important miRNA that is
associated with numerous diseases, including hypertension, cancer
and inflammation. For example, miR-145 regulates the effect of
aspirin on endothelial cell proliferation and inflammation
(12). Moreover, miR-145 is reported
to be closely associated with airway smooth muscle inflammation in
chronic obstructive pulmonary disease (13). In addition, it has been demonstrated
that the expression of miRNA-145 (miR-145) is significantly
decreased in damaged nerve tissues (14,15),
suggesting that miR-145 may serve an important role in neuropathic
pain caused by nerve damage. Ras responsive element binding protein
1 (RREB1) is an important transcription factor, which can initiate
the transcription of many downstream genes once activated. It has
been reported that RREB1 is associated with type 2 diabetes and
tumor (16,17). However, the expression pattern and
role of RREB1 in neuropathic pain remains unknown.
At present, the expression of miR-145 and its
function in chronic constriction sciatica (CCI), one type of
neuropathic pain, is unclear. In the present study, the role of
miR-145 in CCI is investigated using a CCI model in rats.
Intrathecal injection of agomiRNA (agomiR)-145 and in vivo
transfection of small hairpin (sh) RNA-ras responsive element
binding protein 1 (RREB1) was performed, and the pain behaviors of
PWMT and PWTL were measured. In addition, the expression levels of
miR-145, RREB1 and phosphorylated protein kinase B (p-AKT) are
detected.
Materials and methods
Animals
A total of 155 healthy Sprague-Dawley (SD) rats
(specific-pathogen-free; weight, 200–250 g; male) were purchased
from Chengdu Dashuo Biotech Co. (Chengdu, China). The mice were
maintained in standard conditions at room temperature with 50%
humidity, under 12 h/12 h light/dark cycle and with free access to
food and water. All animal experiments were conducted according to
the ethical guidelines of the First Hospital of Tsinghua University
(Beijing, China).
Reagents
TRIzol Total RNA Extraction kit was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
PrimeScript RT Reagent kit and SYBR PrimeScript RT-PCR kit were
purchased from Takara Biotechnology Co., Ltd. (Dalian, China). The
plasmids of shRNA-normal control (NC), shRNA-RREB1, agomiR-NC and
agomiR-145 were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). EntransterTM-in vivo transfection
reagent was purchased from Engreen Biosystem Co., Ltd. (Beijing,
China). Goat anti-rat RREB1 (St. Louis Park, MN, USA), rabbit
anti-rat p-AKT (Cell Signaling Technology, Inc., Danvers, MA, USA),
mouse anti-rat GAPDH and horseradish peroxidase (HRP)-conjugated
secondary antibodies were purchased from Abcam (Burlingame, CA,
USA). Dual Luciferase Reporter Assay kit was purchased from Promega
Corporation (Madison, WI, USA).
Establishment of CCI model and animal
grouping
A total of 125 rats were randomly divided into the
control, sham, CCI model, agomiR-145 and agomiR-NC groups, with 25
rats in each group. For the CCI group, the left sciatic nerve of
rats was exposed by surgery and ligated. For the sham group, the
left sciatic nerve of rats was exposed by surgery, but without
ligation. Following the establishment of the CCI model, intrathecal
injection of agomiR-145 was performed in the agomiR-145 group once
daily for 7 consecutive days. For the agomiR-NC group, intrathecal
injection of agomiR-NC was performed once daily for 7 consecutive
days. For the control group, no surgery or treatment was
performed.
Following the establishment of the CCI model, an
additional 30 healthy male SD rats were randomly divided into
shRNA-NC and shRNA-RREB1 groups. In vivo transfection of
shRNA-RREB1 and shRNA-NC plasmids was performed by
EntransterTM-in vivo transfection reagent, according to the
manufacturer's instructions. The transfection was performed once
daily for 7 consecutive days following the establishment of
CCI.
At 1 day prior to, and 1, 3, 5 and 7 days following
surgery, the PWTL and PWMT of rats in each group were detected. At
each time point, 5 rats in each group were used. In addition, at
each time point, 5 rats in each group were sacrificed by cervical
dislocation following anesthesia using 1% pentobarbital (EMD
Millipore, Billerica, MA, USA). The lumbar spinal cord tissues were
collected from these rats for miRNA and protein detection.
PWTL
Rats were placed on a 7370 Planter Test machine (Ugo
Basile S.R.L., Varese, Italy) and allowed to acclimatize for 3 days
prior to testing. A radiant thermal stimulator was focused onto the
plantar surface of the hind paw for 15 sec. The nociceptive
endpoints in the radiant heat test were the characteristic lifting
or licking of the hind paw, and the time to the endpoint was
recorded as the PWTL. To avoid tissue damage, a cut-off time of 30
sec was used. There were 3 repeats of the trial per rat, and 5 min
intervals between trials. The mean PWTL was obtained from the three
recordings.
PWMT
A 2390 Electronic von-Frey Anesthesiometer
(Naturegene Life Science, Cranbury, NJ, USA) was used. PWMT was
performed according to the method described by Hargreaves (18). Brisk withdrawal or paw flinching were
considered as positive responses. There were 3 repeats of the trial
per rat, and 5 min intervals between trials. The mean PWMT was
calculated from the three recordings.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from lumbar spinal cord
tissues using the TRIzol Total RNA Extraction kit, according to the
manufacturer's instructions. RNA was reverse transcribed into cDNA
using a PrimeScript RT Reagent kit. The expression level of miR-145
was detected by RT-qPCR. The forward primer sequence for miR-145
was 5′-CCAGTTTTCCCAGGAATC-3′; the reverse primer sequence was a
universal primer provided by the kit. RT-qPCR was performed using a
SYBR Green PrimeScript RT-PCR kit, according to the manufacturer's
instructions. The volume of the PCR reaction system was 20 µl,
which consisted of 10 µl qRT-PCR mix, 0.5 µl forward primer and 0.5
µl reverse primer, 2 µl cDNA and 7 µl ddH2O. The
following thermal cycling conditions were used: Pre-denaturation at
95°C for 1 min; 40 cycles of 95°C for 15 sec; and 60°C for 30 sec.
The 2−ΔΔCt method was used to calculate the relative
expression levels of miR-145 (19).
Western blotting analysis
Tissues were ground into a powder in liquid
nitrogen, and were lysed on ice in radioimmunoprecipitation assay
buffer (RIPA) lysis buffer (Beyotime Institute of Biotechnology,
Beijing, China) with 1 mM PMSF for 30 min. Total proteins were
extracted by centrifugation at 14,000 × g for 10 min at 4°C.
Total proteins were extracted (15 µg) from lumbar spinal cord
tissues and separated by a 10% SDS-PAGE. Next, proteins were
transferred onto a polyvinylidene fluoride membrane. After blocking
with non-fat milk, the membrane was incubated with the following
rabbit primary antibodies at 4°C overnight: Anti-RREB1 (ab64168,
1:2,000), anti-p-AKT (ab38449, 1:2,000) and anti-GAPDH (ab8245,
1:5,000, all Abcam). After washing, the membrane using 0.1%
phosphate-buffered saline and Tween 20 for 5 mins each time and in
total three times, the membrane was incubated with HRP-conjugated
secondary antibodies [goat anti-rabbit IgG (ab6721), and goat
anti-mouse IgG H&L (ab6789, both 1:10,000, both Abcam)] at room
temperature for 1 h. Finally, the membrane was developed by
enhanced chemiluminescence plus reagent (Hanbio Biotechnology Co.
Ltd., Shanghai, China). The developed film was scanned using
AlphaImager gel imaging systems (AlphaImager, Santa Clara, CUA,
USA), and the western blot images were analyzed using Quantity One
software version 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). GAPDH was used as an internal control.
Dual luciferase reporter assay
The dual luciferase reporter assay was performed
using a Dual Luciferase Reporter Assay kit. Briefly, the wild type
and mutant 3′-untranslated region (UTR) of RREB1 mRNA were cloned
into a pMIR-REPORT vector. Then, these plasmids, together with
miR-145 mimics (100 nM), were co-transfected into 293T cells (Cell
Bank of the Chinese Academy of Sciences, Shanghai, China). After
incubation at 37°C for 24 h, cells were lysed by washing with cold
PBS, the cells were incubated with RIPA lysis buffer with 1% PMSF
on ice for 20 min and fluorescence was detected using GloMax 20/20
Luminometer (Promega Corporation). Cells without miR-145 mimic
transfection were used as control cells. The fluorescence intensity
of Renilla was used as an internal reference.
Statistical analysis
Data was expressed as the mean ± standard deviation
and was analyzed using SPSS statistical analysis software (version
17.0; SPSS, Inc., Chicago, IL, USA). A paired t-test was used to
analyze the difference between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
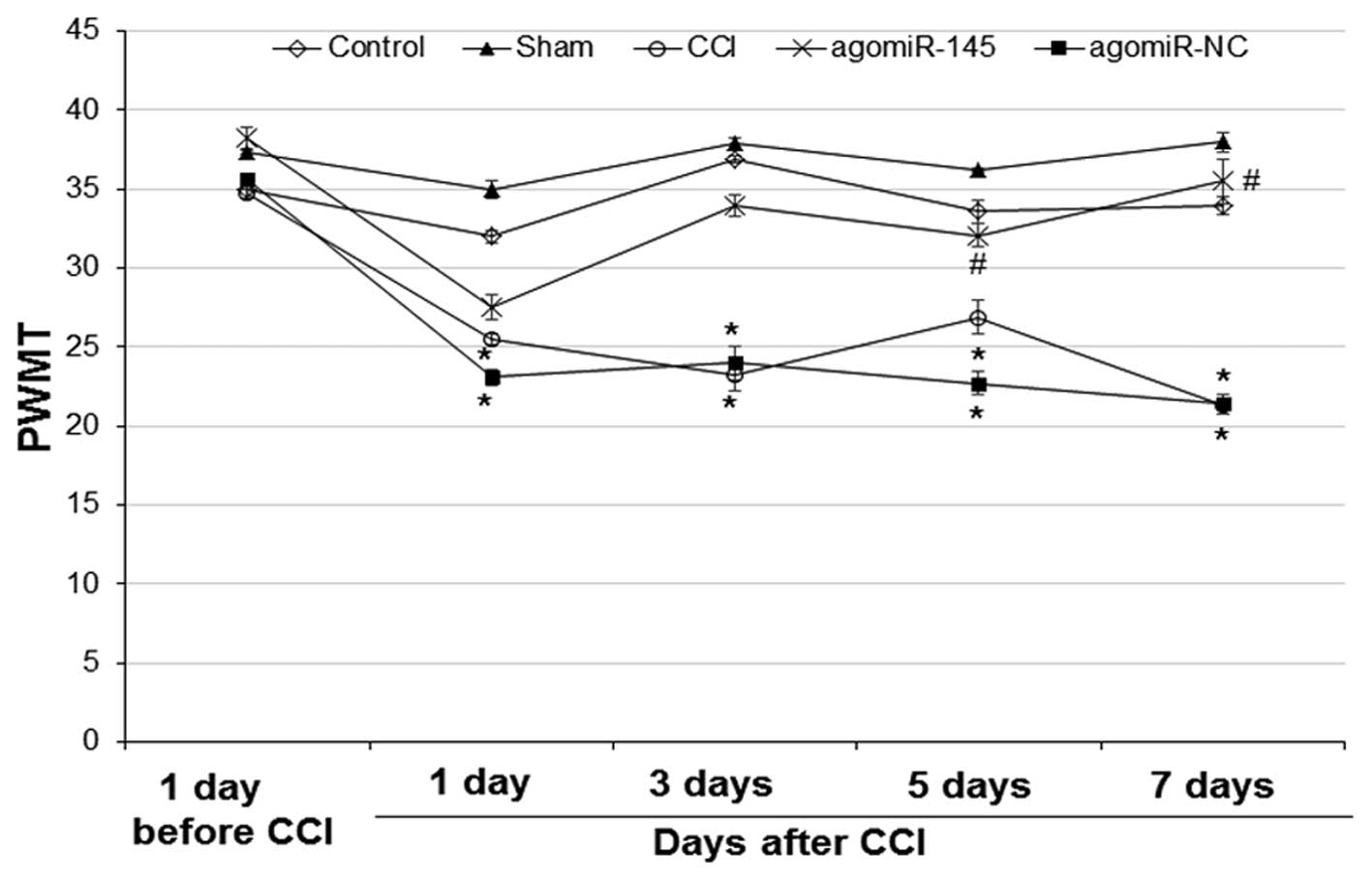
Effect of miR-145 on PWMT in CCI rat
models
To investigate the effect of miR-145 on the
mechanical threshold of rats, PWMT was performed 1 day prior to,
and 1, 3, 5 and 7 days after CCI. As shown in Fig. 1, at 1 day prior to CCI, there was no
significant difference in mechanical threshold among the 5 groups.
In the control and sham group, the mechanical threshold was not
significantly different at any time points. However, the mechanical
threshold in the CCI and agomiR-NC group at 1, 3, 5 and 7 days
after CCI was significantly decreased compared with 1 day prior to
CCI (CCI: 1 day, P=0.034; 3 day, P=0.024; 5 day, P=0.042; 7 day,
P=0.020 and agomiR-NC: 1 day, P=0.023; 3 day, P=0.029; 5 day,
P=0.022; 7 day, P=0.019). At 5 and 7 days after CCI, the mechanical
threshold in the agomiR-145 group was significantly increased
compared with the CCI group (5 day, P=0.033; 7 day, P=0.027). No
significant difference was identified between the CCI and agomiR-NC
groups at any time points. These results suggest that miR-145
treatment alleviates the mechanical threshold decrease induced by
CCI.
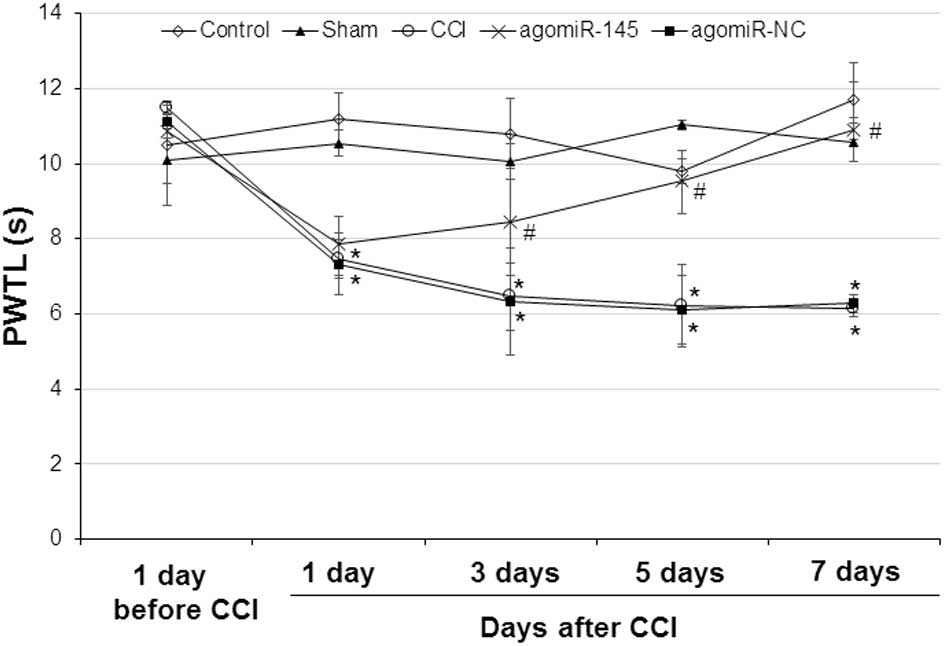
Effect of miR-145 on PWMT in CCI rat
models
To determine the effect of miR-145 on thermal
latency, PWTL was performed 1 day prior to, and 1, 3, 5 and 7 days
after CCI. Similar to PWMT, no significant difference was found 1
day prior to CCI among the 5 groups (Fig. 2). In the control and sham group,
there was no significant difference in thermal latency at any time
points. However, at 1, 3, 5 and 7 days after CCI, the thermal
latency in the CCI and agomiR-NC groups was significantly lower
compared with the thermal latency 1 day prior to CCI (CCI: 1 day,
P=0.036; 3 day, P=0.023; 5 day, P=0.040; 7 day, P=0.022 and
agomiR-NC: 1 day, P=0.032; 3 day, P=0.027; 5 day, P=0.037; 7 day,
P=0.025). After miR-145 treatment, the thermal latency was elevated
in the agomiR-145 group. Compared with the CCI group, the thermal
latency in the agomiR-145 group was significantly higher at 3, 5
and 7 days after CCI (3 day, P=0.039; 5 day, P=0.027; 7 day,
P=0.017). There was no significant difference in the thermal
latency between the CCI and agomiR-NC groups at any time points.
This data indicates that miR-145 treatment improves the thermal
latency decrease induced by CCI.
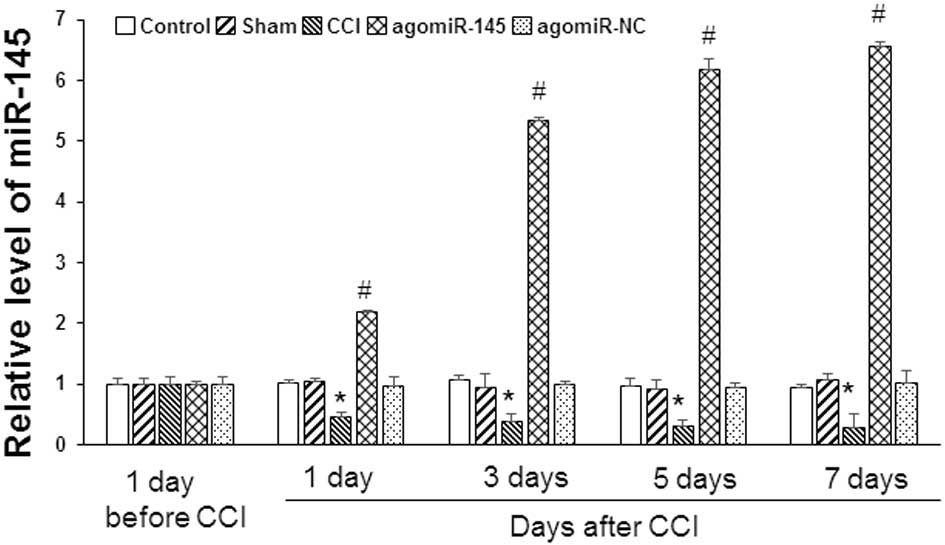
Expression of miR-145 in CCI rat
models
To detect the expression of miR-145 in lumbar spinal
cord tissues, RT-qPCR was conducted 1 day prior to, and 1, 3, 5 and
7 days after CCI. Results are presented in Fig. 3. There was no significant difference
in miR-145 expression levels between the control and sham groups,
or between the CCI and agomiR-NC groups, at any time points.
Compared with the control group, miR-145 expression levels in the
CCI group was significantly lower at 1, 3, 5 and 7 days after CCI
(1 day, P=0.043; 3 day. P=0.038; 5 day, P=0.002; 7 day, P=0.001).
After treatment with agomiR-145, miR-145 expression was increased
in the agomiR-145 group. The expression level of miR-145 1, 3, 5
and 7 days after CCI was 2.18±0.43, 5.34±0.57, 6.18±0.81 and
6.56±1.16, respectively, in the agomiR-145 group, which was
significantly higher compared with the CCI group (1 day, P=0.027; 3
day, P=0.007; 5 day, P=0.001; 7 day, P=0.001). Thus, the results
demonstrate that miR-145 expression is decreased in CCI rats, and
that agomiR-145 treatment can increase miR-145 expression levels in
CCI rats.
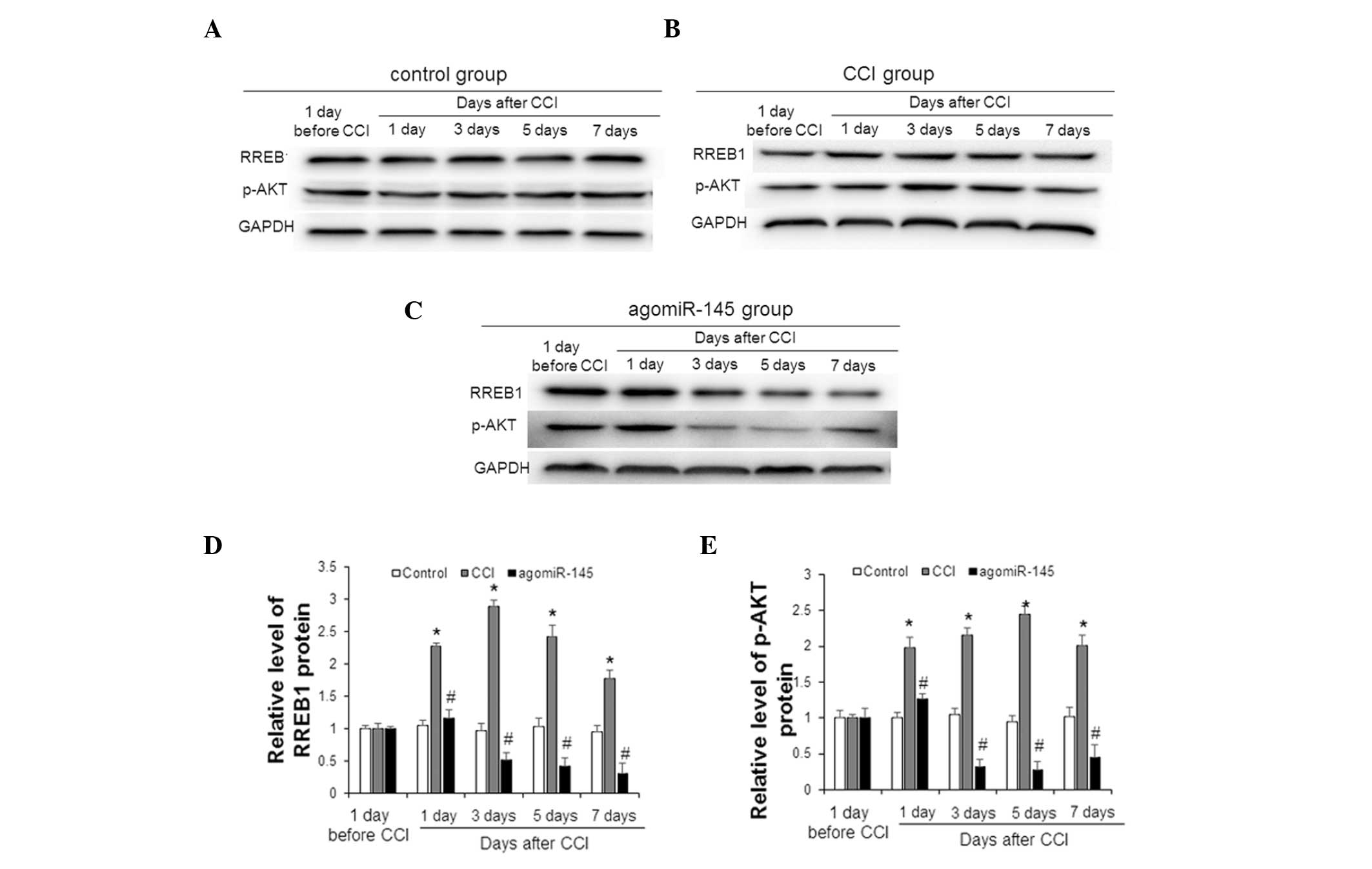
Effect of miR-145 on the expression
level of RREB1 and p-AKT in CCI rat models
To measure the expression of RREB1 and p-AKT in the
lumbar spinal cord tissue of CCI rats 1 day prior to, and 1, 3, 5
and 7 days after CCI, western blot analysis was performed;
representative and quantitative western blot results are presented
in Fig. 4. In the control group,
there was no significant difference in the expression of RREB1 or
p-AKT at any time points (Fig. 4A).
In the CCI group, the expression levels of RREB1 and p-AKT were
elevated (Fig. 4B). As shown in
Fig. 4C, following treatment with
agomiR-145, the expression levels of RREB1 and p-AKT in the
agomiR-145 group were decreased at 3, 5 and 7 days after CCI.
Statistically, the expression levels of RREB1 (1 day; P=0.039; 3
day, P=0.021; 5 day, P=0.027; 7 day, P=0.036; Fig. 4D) and p-AKT (1 day; P=0.031; 3 day,
P=0.027; 5 day, P=0.021; 7 day, P=0.033; Fig. 4E) in the CCI group were significantly
higher compared with the control group at 1, 3, 5 and 7 days after
CCI. In addition, at 1, 3, 5 and 7 days after CCI, the agomiR-145
group had significantly lower expression levels of RREB1 (1 day;
P=0.048; 3 day, P=0.032; 5 day, P=0.015; 7 day, P=0.018; Fig. 4D) and p-AKT (1 day; P=0.046; 3 day,
P=0.016; 5 day, P=0.011; 7 day, P=0.023; Fig. 4E) compared with the CCI group. The
changes in expression levels of RREB1 and p-AKT in the sham and
agomiR-NC groups were similar to those in the control and CCI
groups, respectively (data not shown). These results indicate that
CCI increases the expression levels of RREB1 and p-AKT in lumbar
spinal cord tissues, whereas miR-145 decreases the increase induced
by CCI.
Effect of RREB1 protein on pain
behavior, protein expression and miR-145 expression levels in CCI
rat models
To determine the effect of RREB1 in CCI rat models,
in vivo transfection of shRNA-RREB1 and shRNA-NC plasmids
was performed following the establishment of CCI in rats. The pain
behavior of PWMT and PWTL, protein expression of RREB1 and p-AKT,
and expression level of miR-145 were detected. As shown in Fig. 5A, the mechanical threshold of CCI
rats in the shRNA-NC group 1, 3, 5 and 7 days after CCI was
decreased compared with that at 1 day prior to CCI. However, in the
shRNA-RREB1 group, the mechanical threshold of CCI rats was
increased compared with the shRNA-NC group; this difference was
significant at 3, 5 and 7 days after CCI (3 day, P=0.032; 5 day,
P=0.370; 7 day, P=0.041). Similar results were observed in the
thermal latency of CCI rats (Fig.
5B). Thermal latency in the shRNA-RREB1 group was significantly
increased compared with the shRNA-NC group 1, 3, 5 and 7 days after
CCI (1 day, P=0.021; 3 day, P=0.026; 5 day, P=0.031; 7 day,
P=0.043). As shown in Fig. 5C, the
expression levels of RREB1 and p-AKT 7 days after CCI were
decreased following shRNA-RREB1 transfection. In addition, compared
with the shRNA-NC group, the shRNA-RREB1 group had significantly
higher levels of miR-145 7 days after CCI (P=0.023; Fig. 5D). Collectively, these results
suggest that downregulation of RREB1 protein expression levels in
CCI rats may improve pain behavior, decrease p-AKT protein
expression and increase miR-145 expression levels.
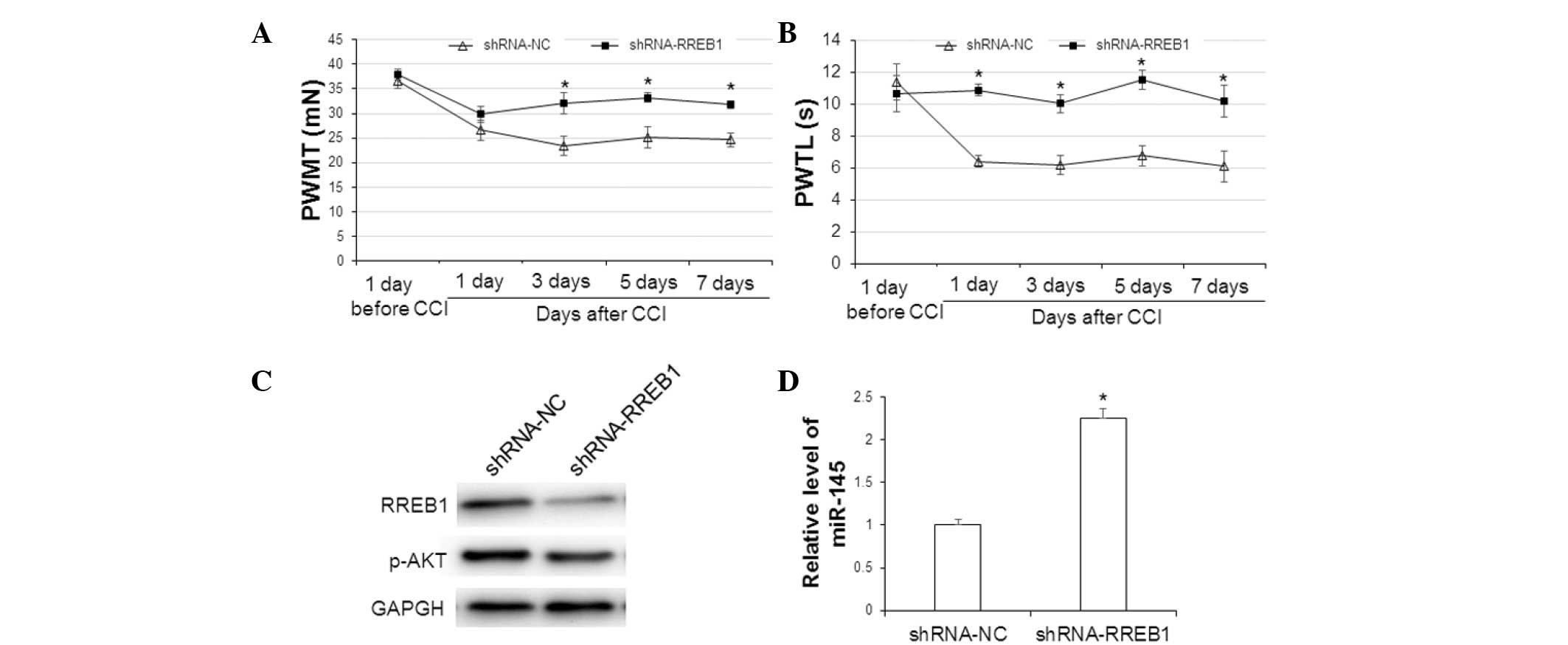 | Figure 5.Analysis of pain behavior, protein
expression and miR-145 expression level in CCI rats. Rats with CCI
were divided into shRNA-RREB1 and shRNA-NC groups (n=15 rats in
each group). Lumbar spinal cord tissues were collected 7 days after
CCI. (A) PWMT and (B) PWTL was performed 1 day before and 1, 3, 5
and 7 days after CCI. (C) Expression levels of RREB1 and p-AKT were
detected by western blotting analysis. (D) Expression level of
miR-145 was detected by reverse transcription-quantitative
polymerase chain reaction. *P<0.05 vs. the shRNA-NC group. The
error bars show the mean±standard deviation. miR-145, microRNA-145;
CCI, chronic constriction injury; shRNA, small hairpin RNA; RREB1,
ras responsive element binding protein 1; NC, normal control; PWMT,
paw withdrawal mechanical threshold; PWTL, paw withdrawal thermal
latency; p-AKT, phosphorylated protein kinase B. |
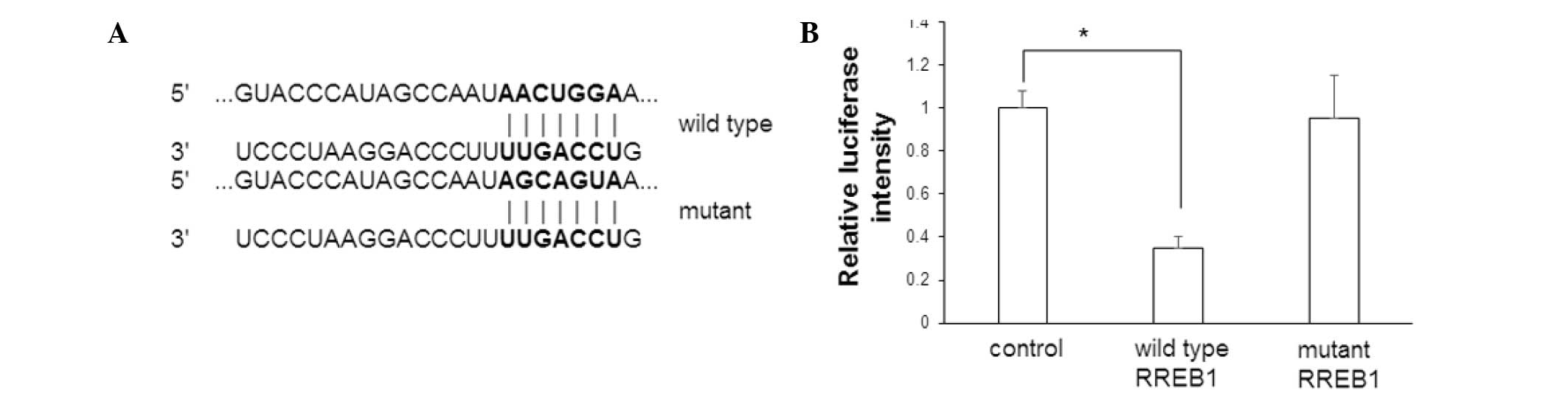
Effect of miR-145 on RREB1
To determine whether RREB1 mRNA is one of the
targets of miR-145, a dual luciferase reporter assay was performed
following transfection of miR-145 mimics and wild type or mutant
RREB1 mRNA. Cells without miR-145 mimic transfection were used as
control cells. The sequences of the wild type and mutant 3′-UTR
region of RREB1 mRNA are presented in Fig. 6A. The luciferase intensity is
presented in Fig. 6B. In cells
transfected with miR-145 mimics and wild type RREB1, the luciferase
intensity was significantly decreased compared with control cells
(P=0.017). However, there was no significant difference in
luciferase intensity between control cells and cells transfected
with miR-145 mimics and mutant RREB1. This data suggests that RREB1
mRNA is a target of miR-145.
Discussion
Due to the lack of effective treatment methods,
neuropathic pain severely threatens the health of patients
(20). At present, the molecular
mechanisms underlying neuropathic pain are unclear. In addition,
effective diagnosis and treatment targets for neuropathic pain are
lacking. It is reported that miRNA molecules are important
regulatory factors in the nervous system and are extensively
involved in various physiological and pathological activities of
the nervous system, such as memory, neural remodeling and
neurodegenerative changes (21).
In the present study, the role of miR-145 in
neuropathic pain is investigated in CCI rat models. The values of
PWMT and PWTL in the CCI group were significantly decreased,
suggesting that the CCI model was successfully established in rats.
Consistent with the results presented by Zhang et al
(22), miR-145 expression levels in
spinal cord tissue were significantly decreased in CCI rats. To
further elucidate the role of miR-145 in CCI development,
intrathecal injection of agomiR-145 was performed. The results
demonstrate that the values of PWMT and PWTL in the agomiR-145
group were significantly higher compared with the CCI group. This
indicates that miR-145 improves pain behaviors of CCI rats and may
serve a role in the development of CCI. Bioinformatical analysis
using the online software called Targetscan (www.targetscan.com.cn) was used for prediction, and it
demonstrates that agomiR-145 may regulate the expression of the
transcription factor RREB1. However, Kent et al (23) observed that RREB1 can inhibit the
expression of miR-145. In addition, studies have demonstrated that
miR-145 can inhibit the phosphatidylinositol 3-kinase (PI3K)/AKT
signaling pathway through regulating the expression of
integrin-linked kinase (24,25).
The PI3K/AKT signaling pathway serves an important
role in vascular endothelial growth factor (VEGF)-induced
hyperalgesia (26). VEGF is involved
in the occurrence of neuropathic pain by activating the PI3K/AKT
signaling pathway in neurons, and inducing the expression of
transient receptor potential vanilloid subfamily member 1 (25). In the current study, the protein
expression levels of RREB1 and p-AKT were examined in spinal cord
tissue of rats. It was identified that the protein expression
levels of RREB1 and p-AKT were significantly increased in CCI rats.
However, following intrathecal injection of agomiR-145, the protein
expression levels of RREB1 and p-AKT were significantly decreased.
In order to further determine the association between miR-145 and
RREB1, RREB1 expression was interfered by in vivo
transfection of shRNA-RREB1. Following the downregulation of RREB1
expression, PWTL and PWMT values in CCI rats were significantly
increased. In addition, the miR-145 expression level was
upregulated, and the p-AKT protein expression level was decreased.
The in vitro dual luciferase reporter assay demonstrated
that miR-145 can bind with the 3′-UTR region of RREB1. These
findings demonstrate that miR-145 can regulate RREB1 expression and
affect the PI3K/AKT signaling pathway.
In conclusion, the results in the present study
suggest that miR-145 serves an important role in the development of
neuropathic pain through regulating RREB1 expression and the
PI3K/AKT signaling pathway. In addition, the results indicate that
miR-145 may be a potential target in the diagnosis and treatment of
neuropathic pain.
Acknowledgements
The authors thank Dr. Jiaxiang Ni from the
Department of Pain Management, Xuanwu Hospital of Capital Medical
University, for his valuable help during the preparation of the
manuscript.
References
|
1
|
Gilron I, Baron R and Jensen T:
Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin
Proc. 90:532–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fontaine D, Blond S, Mertens P and
Lanteri-Minet M: Neurosurgical treatment of chronic pain.
Neurochirurgie. 61:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fallon MT, Storey DJ, Krishan A, Weir CJ,
Mitchell R, Fleetwood-Walker SM, Scott AC and Colvin LA: Cancer
treatment-related neuropathic pain: Proof of concept study with
menthol-a TRPM8 agonist. Support Care Cancer. 23:2769–2777. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Helfert SM, Reimer M, Höper J and Baron R:
Individualized pharmacological treatment of neuropathic pain. Clin
Pharmacol Ther. 97:135–142. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rice AS and Smith MT: Angiotensin II type
2-receptor: New clinically validated target in the treatment of
neuropathic pain. Clin Pharmacol Ther. 97:128–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jamali Z, Asl Aminabadi N, Attaran R,
Pournagiazar F, Oskouei S Ghertasi and Ahmadpour F: MicroRNAs as
prognostic molecular signatures in human head and neck squamous
cell carcinoma: A systematic review and meta-analysis. Oral Oncol.
51:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bienertova-Vasku J, Novak J and Vasku A:
MicroRNAs in pulmonary arterial hypertension: Pathogenesis,
diagnosis and treatment. J Am Soc Hypertens. 9:221–234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greco S, Gorospe M and Martelli F:
Noncoding RNA in age-related cardiovascular diseases. J Mol Cell
Cardiol. 83:142–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saab BJ and Mansuy IM: Neuroepigenetics of
memory formation and impairment: The role of microRNAs.
Neuropharmacology. 80:61–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akerblom M and Jakobsson J: MicroRNAs as
neuronal fate determinants. Neuroscientist. 20:235–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lutz BM, Bekker A and Tao YX: Noncoding
RNAs: New players in chronic pain. Anesthesiology. 121:409–417.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo X, Yu L, Chen M, Wu T, Peng X, Guo R
and Zhang B: miR-145 mediated the role of aspirin in resisting
VSMCs proliferation and anti-inflammation through CD40. J Transl
Med. 14:2112016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Leary L, Sevinç K, Papazoglou IM, Tildy
B, Detillieux K, Halayko AJ, Chung KF and Perry MM: Airway smooth
muscle inflammation is regulated by microRNA-145 in COPD. FEBS
Lett. 590:1324–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu A, Huang Z, Zhang C, Zhang X, Zhao J,
Zhang H, Zhang Q, Wu S and Yi X: Differential expression of
microRNAs in dorsal root ganglia after sciatic nerve injury. Neural
Regen Res. 9:1031–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Norcini M, Sideris A, Hernandez LA Martin,
Zhang J, Blanck TJ and Recio-Pinto E: An approach to identify
microRNAs involved in neuropathic pain following a peripheral nerve
injury. Front Neurosci. 8:2662014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Massi D, Cesinaro AM, Tomasini C, et al:
Atypical Spitzoid melanocytic tumors: a morphological, mutational,
and FISH analysis. J Am Acad Dermatol. 64:919–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franklin RB, Zou J and Costello LC: The
cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human
pancreatic adenocarcinoma. Cancer Biol Ther. 15:1431–1437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hargreaves KM: Capsicum and local
anesthetic cocktails for trigeminal pain. Pain. 150:32010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gilron I, Baron R and Jensen T:
Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin
Proc. 90:532–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aksoy-Aksel A, Zampa F and Schratt G:
MicroRNAs and synaptic plasticity-a mutual relationship. Philos
Trans R Soc Lond B Biol Sci. 369:201305152014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang HY, Zheng SJ, Zhao JH, Zhao W, Zheng
LF, Zhao D, Li JM, Zhang XF, Chen ZB and Yi XN: MicroRNAs 144, 145,
and 214 are down-regulated in primary neurons responding to sciatic
nerve transection. Brain Res. 1383:62–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kent OA, Fox-Talbot K and Halushka MK:
RREB1 repressed miR-143/145 modulates KRAS signaling through
downregulation of multiple targets. Oncogene. 32:2576–2585. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boufraqech M, Zhang L, Jain M, Patel D,
Ellis R, Xiong Y, He M, Nilubol N, Merino MJ and Kebebew E: miR-145
suppresses thyroid cancer growth and metastasis and targets AKT3.
Endocr Relat Cancer. 21:517–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noguchi S, Yasui Y, Iwasaki J, Kumazaki M,
Yamada N, Naito S and Akao Y: Replacement treatment with
microRNA-143 and-145 induces synergistic inhibition of the growth
of human bladder cancer cells by regulating PI3K/Akt and MAPK
signaling pathways. Cancer Lett. 328:353–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stein AT, Ufret-Vincenty CA, Hua L,
Santana LF and Gordon SE: Phosphoinositide 3-kinase binds to TRPV1
and mediates NGF-stimulated TRPV1 trafficking to the plasma
membrane. J Gen Physiol. 128:509–522. 2006. View Article : Google Scholar : PubMed/NCBI
|