Introduction
Dentigerous cyst is an odontogenic lesion that
represents the second most common odontogenic cyst after radicular
cyst, accounting for ~24% of all true cysts in the jaw (1). A typical dentigerous cyst presents
clinically as an asymptomatic unilocular radiolucency enclosing the
crown of an unerupted or impacted tooth (2). However, dentigerous cyst can cause
local destruction, bony expansion, root resorption, or displacement
of teeth, which occurs more commonly with long-standing lesions
(3).
Ameloblastoma represents the second most common
odontogenic tumor. It is slowly growing, locally invasive and has a
high rate of recurrence if not treated adequately (4). It accounts for ~1% of all tumors and
cysts of the jaws (5). There are
three clinicoradiographic variants of this tumor, namely the solid
or multi-cystic variant (86%), the unicystic variant (13%), and the
peripheral variant (1%) (6).
The lining of a dentigerous cyst develops from
reduced enamel epithelium that envelops the crown prior to eruption
(7), whereas the tissues from which
ameloblastoma may arise involve dental lamina rests, the developing
enamel organ, the epithelial lining of an odontogenic cyst, or the
basal cells of the oral mucosa (6).
However, the etiology and the precise histogenesis of dentigerous
cyst and ameloblastoma remain unclear (8,9).
The Ki-67 protein is a nuclear and nucleolar
protein. It is tightly associated with somatic cell proliferation
(10). Ki-67 is a widely used
proliferating marker. It is expressed in all stages of the cell
cycle, with the exception of the G0 phase (11).
Cyclooxygenase (COX)-2 is a cytokine-inducible
enzyme which is present in the nuclear membrane and luminal side of
the endoplasmic reticulum (12,13). It
converts free arachidonic acid into prostanoids, including
prostaglandins (PGs) and thromboxanes (14). COX-2 is involved in multiple
physiological functions and triggers key pathological processes,
such as inflammation and tumorigenesis (14,15).
Upregulation of COX-2 and an increased prostaglandin E2 (PGE2)
level are frequently detected in premalignant and malignant tissues
of epithelial origin (16). COX-2
may play a role in different steps of tumor progression by
increasing the proliferation of mutated cells (17). Previous studies have shown that COX-2
has a positive correlation with the expression of Ki-67 proteins in
various types of tumor (12,18–22).
The expression and role of COX-2 in odontogenic
lesions have not been thoroughly elucidated. Studies of COX-2 in
dentigerous cyst and ameloblastoma may help to improve
understanding of the nature and behavior of odontogenic cysts and
tumors, and eventually may provide a definitive target for a
pharmacological approach in the management of those lesions. The
aim of the current study was to evaluate COX-2 expression and its
correlation with the proliferation of odontogenic epithelium in
dentigerous cyst and ameloblastoma.
Material and methods
Patient samples
A total of 33 samples were collected, 29 of which
were retrieved from the archive of the Oral Pathology Laboratory,
Tongji Hospital, Huazhong University of Science and Technology
(Wuhan, China) over the 3 years from 2008 to 2010 as formalin-fixed
paraffin-embedded (FFPE) samples. The remaining 4 samples were
surgically removed from patients attending the Department of
Maxillofacial Surgery of Tongji Hospital, Huazhong University of
Science and Technology, and collected as frozen tissue samples.
These samples were distributed into two categories which were:
Dentigerous cyst (n=16; 13 FFPE and 3 frozen) and ameloblastomas
(n=17; 16 FFPE and 1 frozen). Dentigerous cyst and ameloblastoma
represent odontogenic lesions that encompass reactive tissues and
tumors, respectively, which replace the healthy bone; there is no
true tissue equivalent to serve as a negative control. The protocol
followed the criteria established by the World Medical Association
Declaration of Helsinki. This study was approved by the
Institutional Review Board of Tongji Medical College, Huazhong
University of Science and Technology.
Immunohistochemical analysis
Antibodies
Immunohistochemical analysis was performed using the
following primary polyclonal antibodies: Rabbit anti-human Ki-67
antigen, corresponding to a sequence mapping at the C-terminal of
Ki-67 (BA1508; Wuhan Boster Biological Technology, Ltd., Wuhan,
China; dilution 1:100) and rabbit anti-human COX-2 antigen raised
against a synthetic peptide corresponding to a sequence at the
N-terminal of human COX-2 (BA0738; Wuhan Boster Biological
Technology, Ltd.; dilution 1:100).
Immunostaining method
Immunostaining was performed using the standard
streptavidin-biotin peroxidase complex assay method (Wuhan Boster
Biological Technology, Ltd.). FFPE samples were cut into 5-µm
tissue sections and then were dewaxed and rehydrated. Endogenous
peroxidase activity was quenched using 3%
H2O2 solution before the antigen unmasking
step in 0.01 M citrate buffer that had been heated to boiling point
in a microwave. The frozen samples were cut into 5-µm sections in a
cryostat chamber (Leica Biosystems Nussloch GmbH, Nussloch,
Germany), then dried at room temperature and fixed in 4%
paraformaldehyde. Endogenous peroxidase activity was blocked using
0.03% H2O2 in absolute methanol. After that,
both FFPE and frozen samples were treated equally with normal goat
serum (Wuhan Boster Biological Technology, Ltd.) for 50 min at room
temperature and then incubated with the aforementioned primary
antibodies at 4°C overnight. Following treatment with 10 µg/ml
biotinylated secondary antibody (goat anti rabbit IgG; BA1003;
Wuhan Biological Technology, Ltd.) for 2 h at room temperature, the
slides were stained with 20 µg/ml streptavidin-biotin-peroxidase
complex. Finally, the sections were developed with
3,3′-diaminobenzidine substrate and slightly counterstained with
Mayer's hematoxylin. Negative controls were incubated with
phosphate-buffered saline instead of the primary antibodies.
Immunohistochemical scoring
Standard light microscopy was used to
semiquantitatively score the staining by counting the percentage of
positive cells and scoring the intensities in ≥10 continuous and
representative high power (x400) fields. Ki-67 expression was
identified as a yellowish-brown nuclear stain that was scored as
follows: i) Absent, when there was no identified staining of the
odontogenic epithelium or when the staining was questionable; ii)
weak for ≤20%; iii) mild for 21–40% and iv) strong for >40%
positivity rate of the odontogenic epithelium. COX-2 expression was
recognized as granular yellowish-brown staining of different
intensities that was mainly located in the nuclear membrane and the
cytoplasm of the positive cells. For COX-2 scoring, firstly the
average COX-2 staining intensity was rated on a scale from 0 to 3
as follows: 0, no staining at all; 1, weak staining; 2, moderate
staining; and 3, strong staining. Then, the percentage of
positively stained cells in ≥10 continuous and representative high
power (x400) fields was determined and scored as follows: 0, no
identified staining of the odontogenic epithelium or unnoticeable
staining; 1, ≤20% staining; 2, 21–40% staining; and 3, >40%
staining. The final score was calculated by adding the score
obtained from the staining intensity to that derived from the
percentage of positive cells, with a maximum score of 6. A final
score of 0 was regarded as negative, 2 as weak, 3 or 4 as mild, and
5 or 6 was considered as strong immunoreactivity.
Statistical analysis
The Statistical Package for the Social Sciences
(SPSS) 19.0 software (IBM SPSS, Armonk, NY, USA) was used for
analyzing the results. Comparisons and correlations between the
immunohistochemical results for protein expression were
statistically analyzed using Mann-Whitney U test and Spearman's
rank correlation coefficient, respectively. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical features
The most relevant clinical features of the patients
in this study are summarized in Table
I. A total of 11 cases were females (33.3%) and 22 cases were
males (66.7%). The patients' ages ranged from 12 to 74 years, with
a mean of 36.2 years. The mandible was the most common site of
involvement (20 cases; 60.6%), whereas the maxilla was involved in
the remainder 13 cases (39.4%).
 | Table I.Clinicopathological characteristics
of the involved patients (n=33). |
Table I.
Clinicopathological characteristics
of the involved patients (n=33).
| Variable | Total | Dentigerous
cyst | Ameloblastoma |
|---|
| Patients (n) | 33 | 16 | 17 |
| Age (years) |
|
|
|
|
Mean | 36.2 | 36.7 | 32.8 |
|
Range | 12–74 | 12–74 | 12–70 |
| Gender, n
(%)a |
|
|
|
|
Male | 22 (66.7) | 13 (39.4) | 9 (27.3) |
|
Female | 11 (33.3) | 3 (9.1) | 8 (24.2) |
| Location, n
(%)a |
|
|
|
|
Maxilla | 13 (39.4) | 12 (36.4) | 1 (3.0) |
|
Mandible | 20 (60.6) | 4 (12.1) | 16 (48.5) |
Ki-67 protein expression
Ki-67 protein expression was detected as
yellowish-brown nuclear staining in the odontogenic epithelial
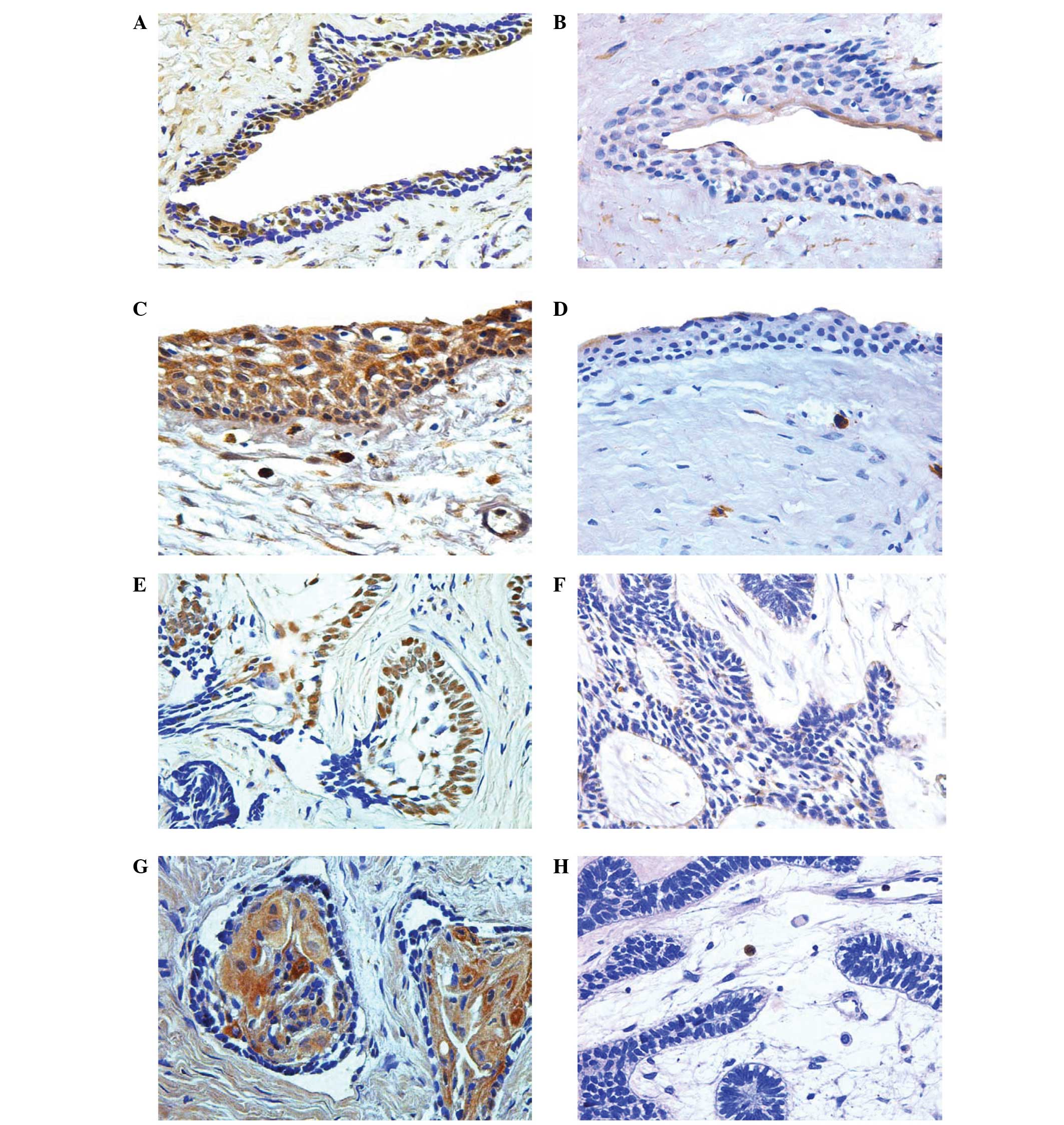
cells of dentigerous cysts and ameloblastomas (Fig. 1). In the dentigerous cysts, positive
cells were distributed throughout the cystic wall. Ki-67 expression
was absent in 25%, weak in 50%, mild in 12.5%, and strong in 12.5%
of odontogenic epithelial cells in dentigerous cyst samples
(Table II). The distribution of
positive Ki-67 stained cells in ameloblastomas was variable; some
samples showed an equal distribution of Ki-67 staining in the
central stellate reticulum-like cells and the peripheral columnar
cells; other samples showed predominant staining of central cells,
while the remaining samples expressed a predominant staining of
peripheral cells. Ki-67 expression was absent in 23.52%, weak in
41.17%, mild in 17.64%, and strong in 17.64% of odontogenic
epithelial cells in ameloblastoma samples (Table II).
 | Table II.Expression of Ki-67 and COX-2
proteins in dentigerous cyst and ameloblastoma. |
Table II.
Expression of Ki-67 and COX-2
proteins in dentigerous cyst and ameloblastoma.
|
| N (%) |
|---|
|
|
|
|---|
| Protein | Dentigerous cyst
(n=16) | Ameloblastoma
(n=17) |
|---|
| Ki-67 |
|
|
|
Positive | 12 (75.0) | 13 (76.5) |
|
Weak | 8 (50.0) | 7 (41.2) |
|
Mild | 2 (12.5) | 3 (17.6) |
|
Strong | 2 (12.5) | 3 (17.6) |
| COX-2 |
|
|
|
Positive | 13 (81.3) | 16 (94.1) |
|
Weak | 3 (18.8) | 5 (29.4) |
|
Mild | 7 (43.7) | 9 (52.9) |
|
Strong | 3 (18.8) | 2 (11.8) |
COX-2 expression
COX-2 expression was demonstrated as a
yellowish-brown granular stain of different intensities that was
expressed in the cytoplasm and nuclear membrane of the positive
odontogenic epithelial cells in dentigerous cysts and
ameloblastomas (Fig. 1). In the
dentigerous cysts, positive cells were observed throughout the
cystic wall. COX-2 was absent in 18.75%, weak in 18.75%, mild in
43.75%, and strong in 18.75% of odontogenic epithelial cells in
dentigerous cyst samples (Table
II). In ameloblastoma samples, a predominant and high intensity
of COX-2 staining was identified in the central stellate
reticulum-like cells compared with the peripheral columnar cells.
COX-2 expression was absent in 5.89%, weak in 29.41%, mild in
52.94%, and strong in 11.76% of odontogenic epithelial cells in the
ameloblastoma samples (Table
II).
Correlation analysis
Although the numerical mean values of Ki-67 and
COX-2 expression levels were higher in ameloblastomas than in
dentigerous cysts, analysis using the Mann-Whitney U test showed
that the differences in the expression of Ki-67 and COX-2 between
dentigerous cysts and ameloblastomas were not significant
(P>0.05).
By contrast, analysis using Spearman's rank
correlation coefficient showed that there was a positive
correlation between the expression of Ki-67 and COX-2 in the
odontogenic epithelium of dentigerous cysts (σ=0.582; P=0.018;
Table III) and a strong positive
correlation between the expression of Ki-67 and COX-2 in the
odontogenic epithelium of ameloblastomas (σ=0.656; P=0.004;
Table III).
 | Table III.Spearman's rank correlation analysis
for odontogenic epithelial proliferation with Ki-67 and COX-2 in
dentigerous cyst and ameloblastoma. |
Table III.
Spearman's rank correlation analysis
for odontogenic epithelial proliferation with Ki-67 and COX-2 in
dentigerous cyst and ameloblastoma.
|
| Correlation of
Ki-67 and COX-2 |
|---|
|
|
|
|---|
| Sample type | σ | P-value |
|---|
| Dentigerous
cyst | 0.582 | 0.018 |
| Ameloblastoma | 0.656 | 0.004 |
Discussion
COX-2 is a cytokine-inducible enzyme that converts
free arachidonic acid into prostanoids including PGs and
thromboxanes (12,14). It is involved in multiple
physiological functions and triggers key pathological processes
such as inflammation and tumorigenesis (14,15). The
current study demonstrated the expression of COX-2 in the
odontogenic epithelium of dentigerous cyst and ameloblastoma. COX-2
is upregulated in a wide variety of human tumors including oral
cancer (12,23–25). The
precise contribution of COX-2 to neoplastic growth has not been
elucidated (26). However, the
induction of COX-2 has been shown to inhibit apoptosis, promote
cell growth, and enhance cell motility and adhesion (27). In addition, epidemiological studies
indicate that the use of nonsteroidal anti-inflammatory drugs
(NSAIDs) is associated with a reduced risk of malignancies,
particularly in the digestive tract (28). Studies have indicated an association
of COX-2 expression with the initiation of tumorigenesis (29), malignant transformation (19), local invasion (30), lymph node metastasis (23), and recurrence (28,31). The
expression of COX-2 in precancerous lesions has been reported to be
higher than that in malignancies in various human organs, including
bronchial (32), colonic (33), esophageal (34), as well as head and neck tumors
(25,35,36).
These findings suggest that COX-2 may be involved in the early
stages of carcinogenesis (34). The
COX-2 gene is most commonly elevated by growth factors and
mediators of inflammation such as lipopolysaccharide, tumor
necrosis factor α, interleukin-1, and 12-O-tetradecanoylphorbol
13-acetate, whilst anti-inflammatory cytokines and glucocorticoids
suppress COX-2 expression (13). The
mechanism of COX-2 upregulation in tumors is unknown. One
possibility is that the cancer cells become intrinsically more
active in expressing COX-2 than do the non-neoplastic cells.
Furthermore, the activation of oncogenes such as HER-2/neu, and
inactivation of tumor suppressor genes such as p53, have been
implicated in the induction of COX-2 expression (37).
Little is known about the expression and role of
COX-2 in odontogenic cysts and tumors. Mendes et al showed a
distinct overexpression of COX-2 in keratocytic odontogenic tumor,
and they hypothesized that they would be likely to observe a
certain degree of COX-2 expression in developmental cysts such as
dentigerous cysts (38,39). In addition, a previous study of 30
radicular cyst specimens demonstrated the expression of COX-2 in
the lining epithelium of all 30 specimens, and reached the
conclusion that COX-2 may be involved in a possible mechanism for
radicular cyst pathogenesis and expansion through its effect on the
production of PG and matrix metalloproteinase (40).
Sonic hedgehog (SHH) signaling molecules have been
demonstrated to be expressed in dentigerous cyst (41) and ameloblastoma (42). Concordance between the SHH pathway
and the induction of COX-2 expression has been concluded to proceed
through the effect of SHH on the upregulation of proliferative
markers such as p53 leading to the activation of the Ras/Raf/ERK
cascade, which, in turn, induces the expression of COX-2 (38,43). The
primary requirement for any lesion to expand within the bone is the
ability to resorb the dense crystalline environment (44). COX-2 is upregulated during bone
repair and under pathological conditions such as inflammation and
neoplasia. Thus, the skeleton is supplied with high levels of PGs,
particularly PGE2, which plays either a stimulatory or inhibitory
role in bone metabolism. PGs, mainly PGE2, are able to stimulate
bone resorption by increasing the numbers and functional activity
of osteoclasts (45).
According to the results of the current study, the
proliferation rate of the odontogenic epithelium, which is
indicated by the Ki-67 protein expression level, was not
significantly different between dentigerous cysts and
ameloblastomas. Ki-67 is a widely used proliferating marker. It is
expressed in all stages of the cell cycle with the exception of the
G0 phase (11). Studies
comparing the proliferation rate between dentigerous cysts and
ameloblastomas have generated conflicted results, which may reflect
differences in the markers and criteria used for the cellular
proliferation assessment. Gadbail et al found a
significantly higher Ki-67 labeling index in ameloblastomas than in
dentigerous cysts (46). Also, in
another study, Ki-67 expression was observed to be higher in
ameloblastomas that arise from dentigerous cysts than in the
dentigerous cysts (47). Piattelli
et al showed that ameloblastoma had higher proliferating
cell nuclear antigen-positive cell counts than dentigerous cyst
(48). Similarly, when IPO-38 was
used as a proliferation marker, it was found that the labeling
index of IPO-38 was higher in ameloblastomas than in dentigerous
cysts (49). However, another study
conducted by de Vicente et al demonstrated that Ki-67
expression was higher in dentigerous cysts than in ameloblastomas
(50). Furthermore, dentigerous cyst
have been found to have significantly higher counts for the
proliferative activity marker nucleolar organizer regions than
unicystic ameloblastoma (51).
Bôas et al found that there was no
correlation between the immunoexpression of COX-2 and Ki-67 in oral
squamous cell carcinoma (52).
Similar results were observed in ovarian tumors (53). These studies concluded that COX-2 may
be required for carcinogenesis in pathways other than those
affecting the proliferation of cells in oral squamous cell
carcinoma or ovarian tumor (52,53),
stating that the interference of COX-2 with tumor growth and
dissemination is not limited to the stimulation of mitogenesis
(53). Another study did not find a
correlation between COX-2 and Ki-67 expression in keratocytic
odontogenic tumor (39). The current
study results demonstrated that proliferation activity and COX-2
expression were positively correlated in the odontogenic epithelium
of dentigerous cyst and ameloblastoma, respectively. These results
are in accordance with other studies that revealed such a
correlation in other different tumor types, for example, in
mucosa-associated lymphoid tissue lymphoma (20), colorectal adenoma (19), cervical adenocarcinomas (12), renal cell carcinoma (18), esophageal adenocarcinoma (21), and esophageal squamous cell carcinoma
(22). It has been shown that COX-2
may play a role in different steps of tumor progression by
increasing the proliferation of mutated cells, thus, favoring tumor
promotion (17). In addition,
overexpression of COX-2 gene alters the response to growth
regulatory signals and inhibits apoptosis (54). The inhibition of apoptosis by COX-2
includes effects on the intrinsic and extrinsic pathways of
apoptosis (55). This action
prolongs the survival of abnormal cells, which in turn favors the
accumulation of sequential genetic changes that increase the risk
of tumorigenesis (56). Furthermore,
COX-2 induction or overexpression is associated with an increased
production of PGE2, which is known to modulate cell proliferation,
cell death and tumor invasion in different cancer types (55). NSAIDs have been demonstrated to
inhibit the proliferation of different cancer cell types expressing
COX-2, supporting the evidence that PGs produced by COX-2 intervene
in tumor cell proliferation (17).
Ki-67 and COX-2 exhibited higher scores and stronger
correlations in their expression levels in ameloblastoma samples
than in dentigerous cyst samples in the present study. However, the
results of the current study reflect a similarity in the expression
levels and correlation of Ki-67 and COX-2 in the odontogenic
epithelium of the two odontogenic lesions dentigerous cyst and
ameloblastoma. However, it has been demonstrated that the functions
of COX-2 gene are complex and may involve different mechanisms
depending on the cell types and the conditions studied (29). Therefore, it is necessary to further
elucidate other effects of COX-2 in the epithelium and connective
tissue of odontogenic lesions and to compare dentigerous cyst with
ameloblastoma. Previous studies have shown that each of
ameloblastoma and dentigerous cyst presents a distinct evolution
and biological behavior, and in addition to their clinical and
behavioral differences (57), they
reflect diversities in various features such as gene expression
(58), pattern of expression of
cytokines (3), interaction of
different proteins, and collagen components (57).
In conclusion, COX-2 was found to be expressed in
the odontogenic epithelium of dentigerous cyst and ameloblastoma.
Furthermore, it may contribute to local extension of dentigerous
cyst and ameloblastoma by increasing the proliferation of their
odontogenic epithelial cells. This study presents COX-2 as a
possible target in the management of dentigerous cyst and
ameloblastoma.
Acknowledgements
The authors would like to thank Professor Wei Min
Chen and Professor Xue Jin Tao for help in clinical sample
collection.
References
|
1
|
Daley TD, Wysocki GP and Pringle GA:
Relative incidence of odontogenic tumors and oral and jaw cysts in
a Canadian population. Oral Surg Oral Med Oral Pathol. 77:276–280.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang LL, Yang R, Zhang L, Li W,
MacDonald-Jankowski D and Poh CF: Dentigerous cyst: A retrospective
clinicopathological analysis of 2082 dentigerous cysts in British
Columbia, Canada. Int J Oral Maxillofac Surg. 39:878–882. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolokythas A, Karas M, Sarna T, Flick W
and Miloro M: Does cytokine profiling of aspirate from jaw cysts
and tumors have a role in diagnosis? J Oral Maxillofac Surg.
70:1070–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: World Health Organization Classification of
TumorsPathology and Genetics of Head and Neck Tumors. IARC
Publishing Group; Lyon: 2005
|
|
5
|
Small IA and Waldron CA: Ameloblastomas of
the jaws. Oral Surg Oral Med Oral Pathol. 8:281–297. 1955.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neville BW, Damm DD, Allen CM and Bouquot
JE: Oral and Maxillofacial Pathology. 2nd. WB Saunders;
Philadelphia: pp. 589–642. 2002
|
|
7
|
Pavelić B, Levanat S, Crnić I, Kobler P,
Anić I, Manojlović S and Sutalo J: PTCH gene altered in dentigerous
cysts. J Oral Pathol Med. 30:569–576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benn A and Altini M: Dentigerous cysts of
inflammatory origin. A clinicopathologic study. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 81:203–209. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Namin AK, Azad TM, Eslami B, Sarkarat F,
Shahrokhi M and Kashanian F: A study of the relationship between
ameloblastoma and human papilloma virus. J Oral Maxillofac Surg.
61:467–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Endl E and Gerdes J: The Ki-67 protein:
Fascinating forms and an unknown function. Exp Cell Res.
257:231–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JS, Choi YD, Lee JH, Nam JH, Choi C,
Lee MC, Park CS, Juhng SW, Kim HS and Min KW: Expression of
cyclooxygenase-2 in adenocarcinomas of the uterine cervix and its
relation to angiogenesis and tumor growth. Gynecol Oncol.
95:523–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chandrasekharan NV and Simmons DL: The
cyclooxygenases. Genome Biol. 5:2412004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D and Dubois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: Structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rasmuson A, Kock A, Fuskevåg OM, Kruspig
B, Simón-Santamaría J, Gogvadze V, Johnsen JI, Kogner P and
Sveinbjörnsson B: Autocrine prostaglandin E2 signaling promotes
tumor cell survival and proliferation in childhood neuroblastoma.
PLoS One. 7:e293312012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao Y and Prescott SM: Many actions of
cyclooxygenase-2 in cellular dynamics and in cancer. J Cell
Physiol. 190:279–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyata Y, Koga S, Kanda S, Nishikido M,
Hayashi T and Kanetake H: Expression of cyclooxygenase-2 in renal
cell carcinoma: Correlation with tumor cell proliferation,
apoptosis, angiogenesis, expression of matrix metalloproteinase-2
and survival. Clin Cancer Res. 9:1741–1749. 2003.PubMed/NCBI
|
|
19
|
Sato T, Yoshinaga K, Okabe S, Okawa T,
Higuchi T, Enomoto M, Takizawa T and Sugihara K: Cyclooxygenase-2
expression and its relationship with proliferation of colorectal
adenomas. Jpn J Clin Oncol. 33:631–635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li HL, Sun BZ and Ma FC: Expression of
COX-2, iNOS, p53 and Ki-67 in gastric mucosa-associated lymphoid
tissue lymphoma. World J Gastroenterol. 10:1862–1866. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang TJ and Cho MY: Cyclooxygenase-2
expression and cell proliferation are increased in MUC2-positive
area of columnar-lined esophagus. Pathol Int. 55:546–549. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang JX, Xiao W, Chen WC, Lin MS, Song
ZX, Chen P, Zhang YL, Li FY, Qian RY and Salminen E: Relationship
between COX-2 and cell cycle-regulatory proteins in patients with
esophageal squamous cell carcinoma. World J Gastroenterol.
16:5975–5981. 2010.PubMed/NCBI
|
|
23
|
Itoh S, Matsui K, Furuta I and Takano Y:
Immunohistochemical study on over expression of cyclooxygenase-2 in
squamous cell carcinoma of the oral cavity: Its importance as a
prognostic predictor. Oral Oncol. 39:829–835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakurai K, Urade M, Noguchi K, Hashitani
S, Takaoka K, Segawa E and Kishimoto H: Prognostic significance of
cyclooxygenase-2 and DNA topoisomerase IIalpha expression in oral
carcinoma. Head Neck. 29:1002–1009. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Renkonen J, Wolff H and Paavonen T:
Expression of cyclo-oxygenase-2 in human tongue carcinoma and its
precursor lesions. Virchows Arch. 440:594–597. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu WK, Sung JJ, Lee CW, Yu J and Cho CH:
Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: An
update on the molecular mechanisms. Cancer Lett. 295:7–16. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal anti-inflammatory drugs as anticancer agents:
Mechanistic, pharmacologic, and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhi H, Wang L, Zhang J, Zhou C, Ding F,
Luo A, Wu M, Zhan Q and Liu Z: Significance of COX-2 expression in
human esophageal squamous cell carcinoma. Carcinogenesis.
27:1214–1221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shirahama T, Arima J, Akiba S and Sakakura
C: Relation between cyclooxygenase-2 expression and tumor
invasiveness and patient survival in transitional cell carcinoma of
the urinary bladder. Cancer. 92:188–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohen BL, Gomez P, Omori Y, Duncan RC,
Civantos F, Soloway MS, Lokeshwar VB and Lokeshwar BL:
Cyclooxygenase-2 (COX-2) expression is an independent predictor of
prostate cancer recurrence. Int J Cancer. 119:1082–1087. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wolff H, Saukkonen K, Anttila S,
Karjalainen A, Vainio H and Ristimäki A: Expression of
cyclooxygenase-2 in human lung carcinoma. Cancer Res. 58:4997–5001.
1998.PubMed/NCBI
|
|
33
|
Eberhart CE, Coffey RJ, Radhika A,
Giardiello FM, Ferrenbach S and DuBois RN: Up-reguration of
cyclooxygenase 2 gene expression in human colorectal adenomas and
adenocarcinomas. Gastroenterology. 107:1183–1188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shamma A, Yamamoto H, Doki Y, Okami J,
Kondo M, Fujiwara Y, Yano M, Inoue M, Matsuura N, Shiozaki H and
Monden M: Up-regulation of cyclooxygenase-2 in squamous
carcinogenesis of the esophagus. Clin Cancer Res. 6:1229–1238.
2000.PubMed/NCBI
|
|
35
|
Banerjee AG, Gopalakrishnan VK,
Bhattacharya I and Vishwanatha JK: Deregulated cyclooxygenase-2
expression in oral premalignant tissues. Mol Cancer Ther.
1:1265–1271. 2002.PubMed/NCBI
|
|
36
|
Shibata M, Kodani I, Osaki M, Araki K,
Adachi H, Ryoke K and Ito H: Cyclo-oxygenase-1 and −2 expression in
human oral mucosa, dysplasias and squamous cell carcinomas and
their pathological significance. Oral Oncol. 41:304–312. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ristimäki A: Cyclooxygenase 2: From
inflammation to carcinogenesis. Novartis Found Symp. 256:215–226,
259–269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mendes RA, Carvalho JF and van der Waal I:
Potential relevance of cyclooxygenase-2 expression in keratocystic
odontogenic tumours-an immunohistochemical study. J Oral Pathol
Med. 40:497–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mendes RA, Carvalho JF and van der Waal I:
A comparative immunohistochemical analysis of COX-2, p53, and Ki-67
expression in keratocystic odontogenic tumors. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 111:333–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsai CH, Huang FM, Yang LC, Chou MY and
Chang YC: Immunohistochemical localization of cyclooxygenase-2 in
radicular cysts. Int Endod J. 35:854–858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang L, Sun ZJ, Chen XM and Chen Z:
Immunohistochemical expression of SHH, PTC, SMO and GLI1 in
glandular odontogenic cysts and dentigerous cysts and dentigerous
cysts. Oral Dis. 16:818–822. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kumamoto H, Ohki K and Ooya K: Expression
of Sonic hedgehog (SHH) signaling molecules in ameloblastomas. J
Oral Pathol Med. 33:185–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han JA, Kim JI, Ongusaha PP, Hwang DH,
Ballou LR, Mahale A, Aaronson SA and Lee SW: P53-mediated induction
of Cox-2 counteracts p53- or genotoxic stress-induced apoptosis.
EMBO J. 21:5635–5644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Harris M: Odontogenic cyst growth and
prostaglandin-induced bone resorption. Ann R Coll Surg Engl.
60:85–91. 1978.PubMed/NCBI
|
|
45
|
Fracon RN, Teófilo JM, Satin RB and Lamano
T: Prostaglandins and bone: Potential risks and benefits related to
the use of nonsteroidal antiinflammatory drugs in clinical
dentistry. J Oral Sci. 50:247–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gadbail AR, Patil R and Chaudhary M:
Co-expression of Ki-67 and p53 protein in ameloblastoma and
keratocystic odontogenic tumor. Acta Odontol Scand. 70:529–535.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Piattelli A, Lezzi G, Fioroni M,
Santinelli A and Rubini C: Ki-67 expression in dentigerous cysts,
unicystic ameloblastomas, and ameloblastomas arising from dental
cysts. J Endod. 28:55–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Piattelli A, Fioroni M, Santinelli A and
Rubini C: Expression of proliferating cell nuclear antigen in
ameloblastomas and odontogenic cysts. Oral Oncol. 34:408–412. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thosaporn W, Iamaroon A, Pongsiriwet S and
Ng KH: A comparative study of epithelial cell proliferation between
the odontogenic keratocyst, orthokeratinized odontogenic cyst,
dentigerous cyst and ameloblastoma. Oral Dis. 10:22–26. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
de Vicente JC, Torre-Iturraspe A,
Gutiérrez AM and Lequerica-Fernández P: Immunohistochemical
comparative study of the odontogenic keratocysts and other
odontogenic lesions. Med Oral Patol Oral Cir Bucal. 15:e709–e715.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coleman HG, Altini M and Groeneveld HT:
Nucleolar organizer regions (AgNORs) in odontogenic cysts and
ameloblastomas. J Oral Pathol Med. 25:436–440. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bôas DS, Takiya CM, Coelho-Sampaio TL,
Monção-Ribeiro LC, Ramos EA, Cabral MG and dos Santos JN:
Immunohistochemical detection of Ki-67 is not associated with
tumor-infiltrating macrophages and cyclooxygenase-2 in oral
squamous cell carcinoma. J Oral Pathol Med. 39:565–570. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yoshida A, Sarian LO, Andrade LA,
Pignataro F, Pinto GA and Derchain SF: Cell proliferation activity
unrelated to COX-2 expression in ovarian tumors. Int J Gynecol
Cancer. 17:607–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tsujii M and DuBois RN: Alteration in
cellular adhesion and apoptosis in epithelial cells overexpressing
prostaglandin endoperoxide synthase-2. Cell. 83:493–501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sobolewski C, Cerella C, Dicato M,
Ghibelli L and Diederich M: The role of cyclooxygenase-2 in cell
proliferation and cell death in human malignancies. Int J Cell
Biol. 2010:2151582010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin DT, Subbaramaiah K, Shah JP,
Dannenberg AJ and Boyle JO: Cyclooxygenase-2: A novel molecular
target for the prevention and treatment of head and neck cancer.
Head Neck. 24:792–799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Henriques ÁC, Vasconcelos MG, Galvão HC,
de Souza LB and de Almeida Freitas R: Comparative analysis of the
immunohistochemical expression of collagen IV, MMP-9 and TIMP-2 in
odontogenic cysts and tumors. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 112:468–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lim J, Ahn H, Min S, Hong SD, Lee JI and
Hong SP: Oligonucleotide microarray analysis of ameloblastoma
compared with dentigerous cyst. J Oral Pathol Med. 35:278–285.
2006. View Article : Google Scholar : PubMed/NCBI
|