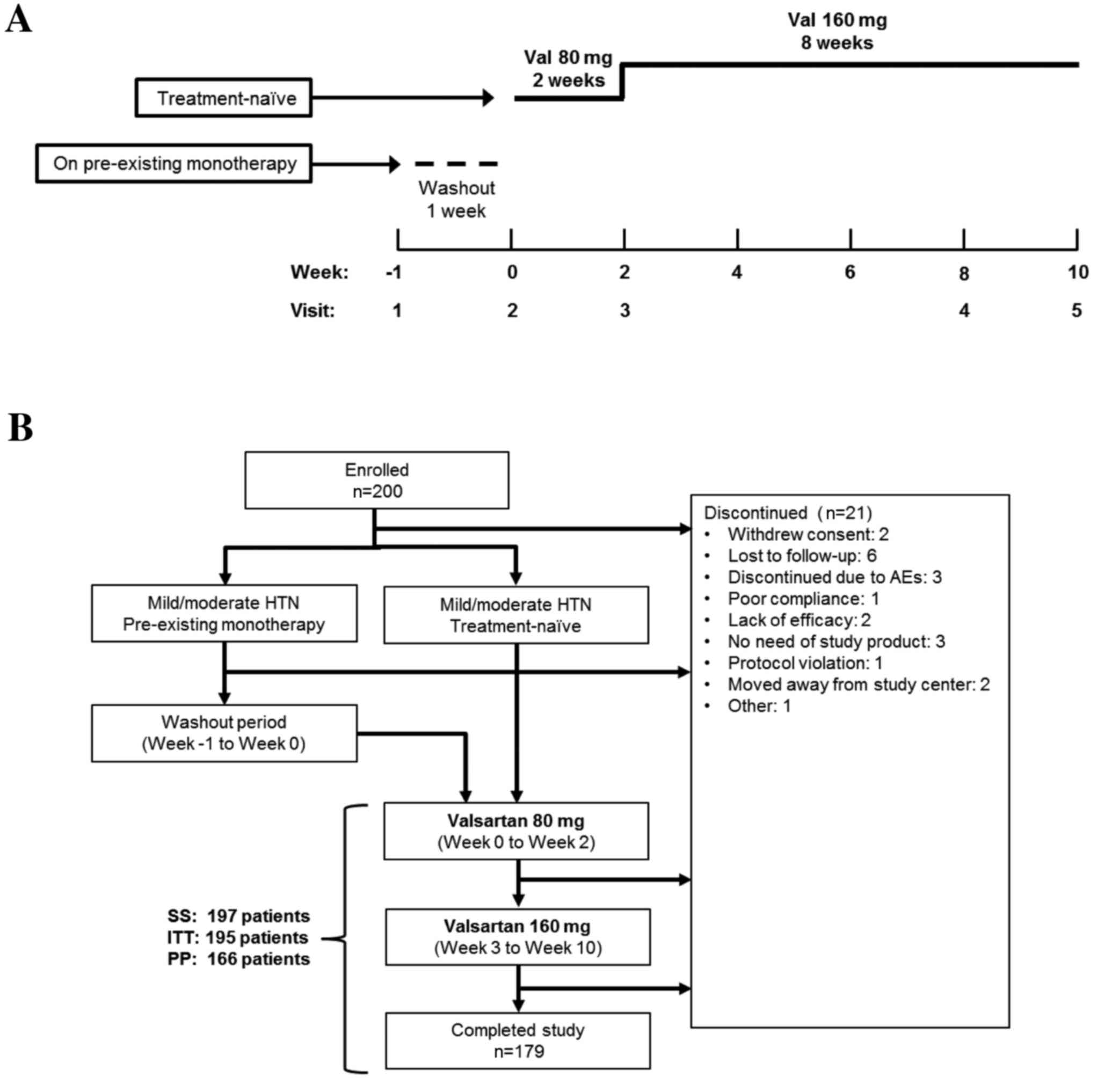
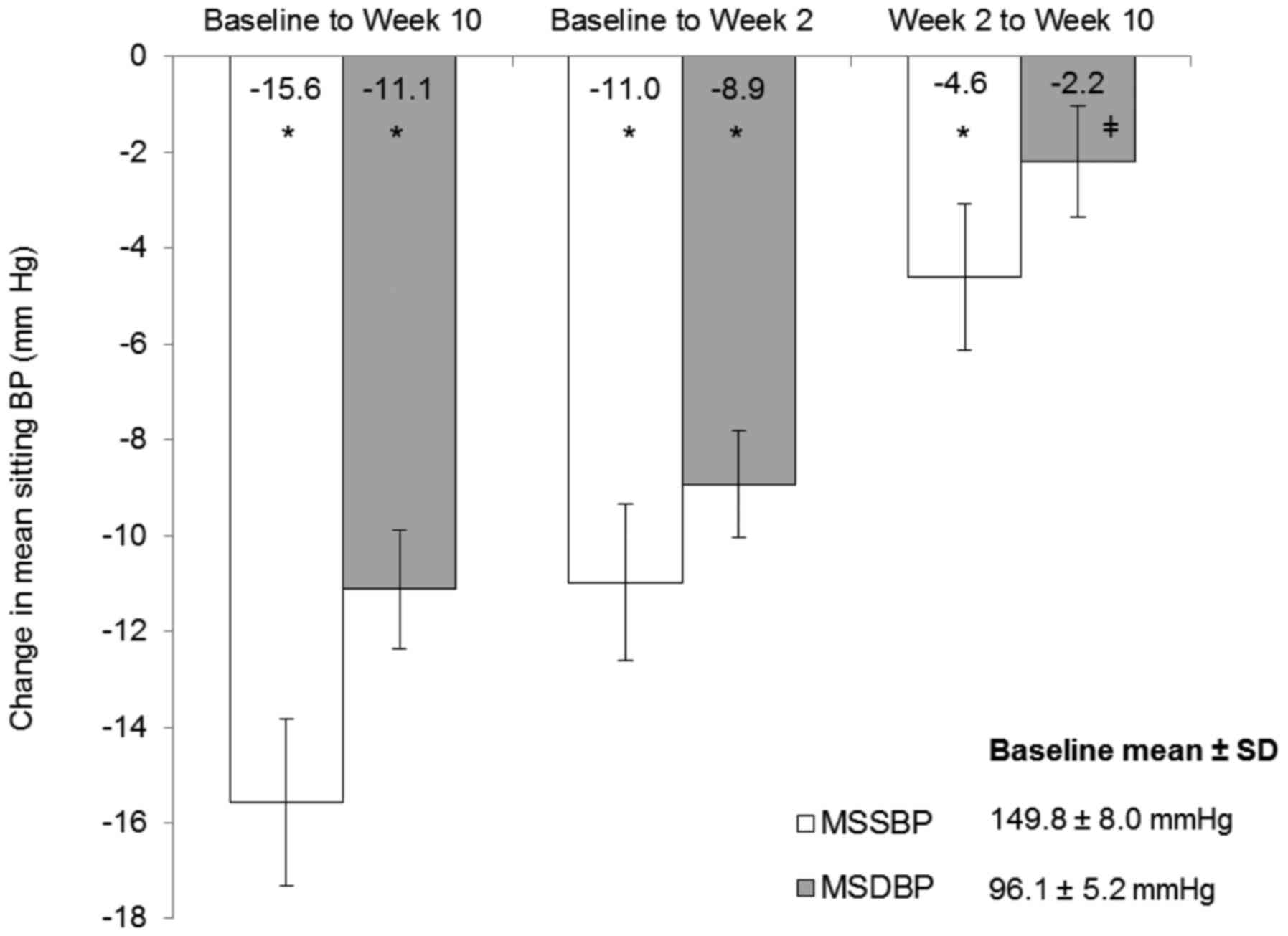
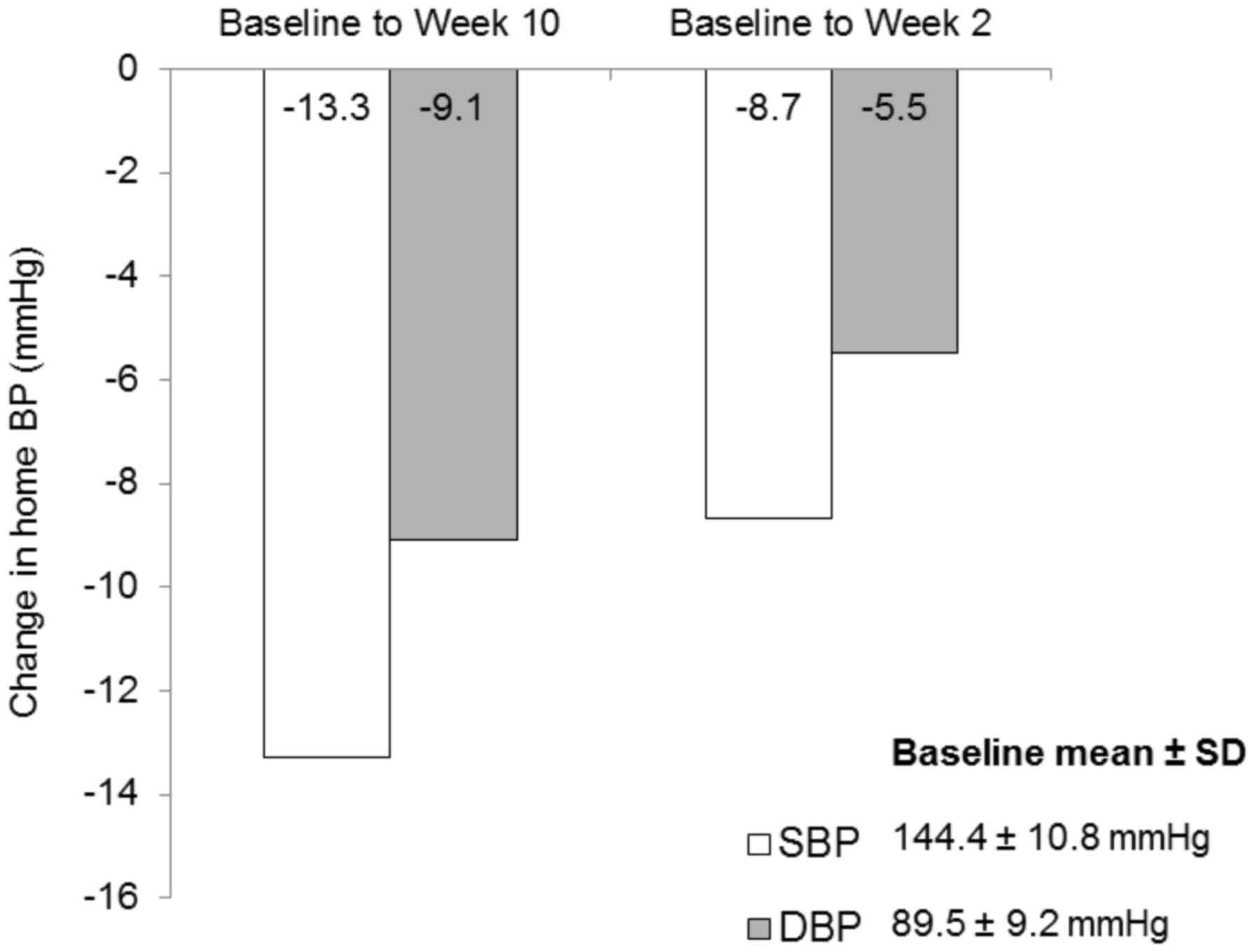
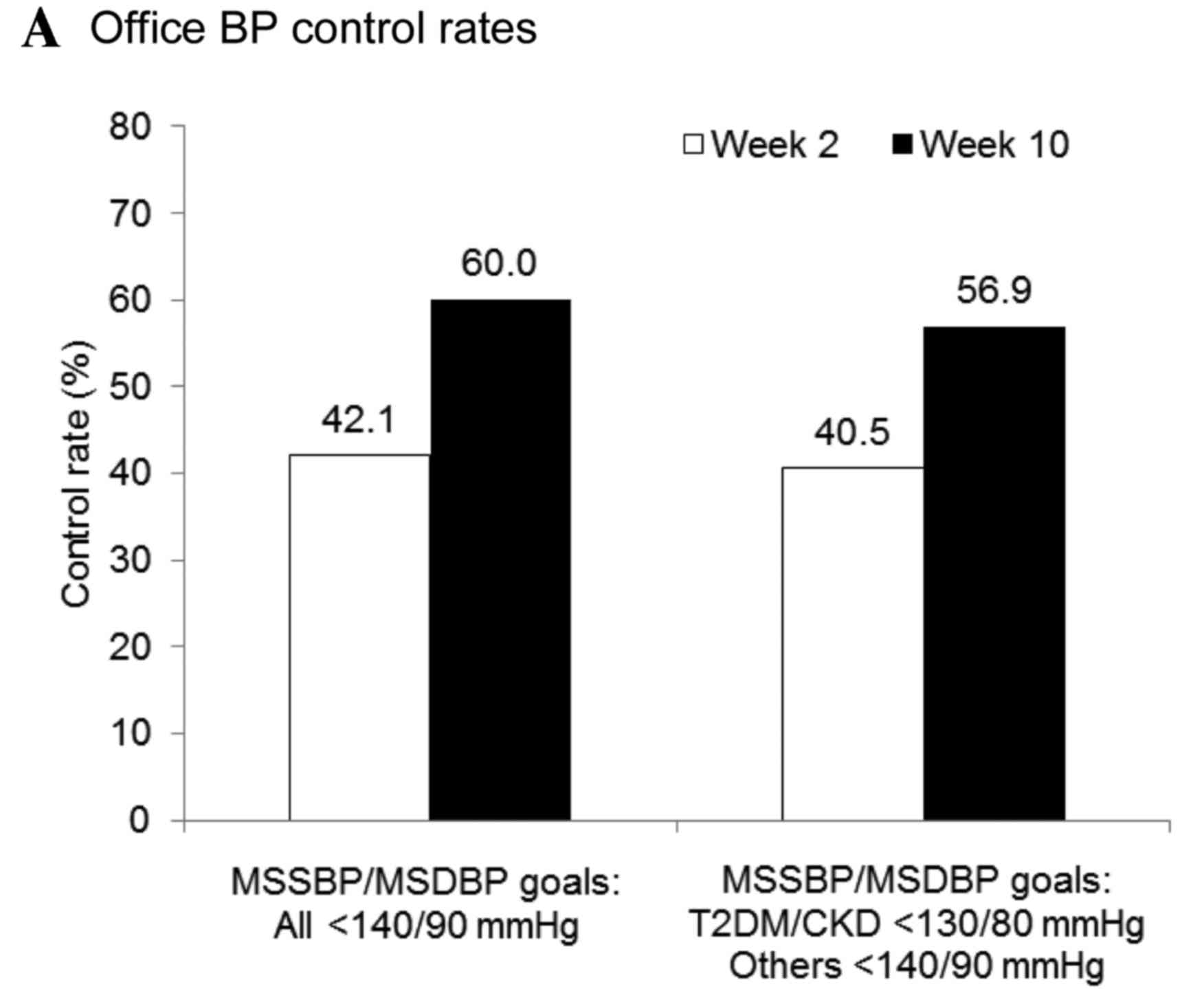
|
1
|
Chiang CE and Chen CH: Hypertension in the
Asia-Pacific region. J Hum Hypertens. 22:441–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kearney PM, Whelton M, Reynolds K, Whelton
PK and He J: Worldwide prevalence of hypertension: A systematic
review. J Hypertens. 22:11–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu DY, Liu LS, Yu JM and Yao CH: China
STATUS Study Group: National survey of blood pressure control rate
in Chinese hypertensive outpatients-China STATUS. Zhonghua Xin Xue
Guan Bing Za Zhi. 38:230–238. 2010.(In Chinese). PubMed/NCBI
|
|
4
|
Mancia G, Fagard R, Narkiewicz K, Redón J,
Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G,
Dominiczak A, et al: 2013 ESH/ESC Practice Guidelines for the
Management of Arterial Hypertension. Blood Press. 23:3–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verdecchia P and Angeli F: Assessment of
the optimal daily dose of valsartan in patients with hypertension,
heart failure, or both. Clin Ther. 26:460–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markham A and Goa KL: Valsartan. A review
of its pharmacology and therapeutic use in essential hypertension.
Drugs. 54:299–311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parati G, Asmar R, Bilo G, Kandra A, Di
Giovanni R and Mengden T: Effectiveness and safety of high-dose
valsartan monotherapy in hypertension treatment: The ValTop study.
Hypertens Res. 33:986–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pool JL, Glazer R, Chiang YT and Gatlin M:
Dose-response efficacy of valsartan, a new angiotensin II receptor
blocker. J Hum Hypertens. 13:275–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Julius S, Kjeldsen SE, Weber M, Brunner
HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, et
al: Outcomes in hypertensive patients at high cardiovascular risk
treated with regimens based on valsartan or amlodipine: The VALUE
randomised trial. Lancet. 363:2022–2031. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Study Group NAVIGATOR, McMurray JJ, Holman
RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell
M, Buse JB, et al: Effect of valsartan on the incidence of diabetes
and cardiovascular events. N Engl J Med. 362:1477–1490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ke YN, Dong YG, Ma SP, Yuan H, Ihm SH and
Baek SH: ADVISE study group: Improved blood pressure control with
nifedipine GITS/valsartan combination versus high-dose valsartan
monotherapy in mild-to-moderate hypertensive patients from Asia:
Results from the ADVISE study, a randomized trial. Cardiovasc Ther.
30:326–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu D, Yang K, Sun N, Gao P, Wang R,
Grosso A and Zhang Y: trial investigators: Amlodipine/valsartan
5/160 mg versus valsartan 160 mg in Chinese hypertensives. Int J
Cardiol. 167:2024–2030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stergiou GS, Nasothimiou E, Giovas P,
Kapoyiannis A and Vazeou A: Diagnosis of hypertension in children
and adolescents based on home versus ambulatory blood pressure
monitoring. J Hypertens. 26:1556–1562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clement DL, De Buyzere ML, De Bacquer DA,
de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun
JJ, Six RO, et al: Prognostic value of ambulatory blood-pressure
recordings in patients with treated hypertension. N Engl J Med.
348:2407–2415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perloff D, Sokolow M and Cowan R: The
prognostic value of ambulatory blood pressures. JAMA.
249:2792–2798. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verdecchia P, Porcellati C, Schillaci G,
Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C,
Zampi I, Santucci A, et al: Ambulatory blood pressure. An
independent predictor of prognosis in essential hypertension.
Hypertension. 24:793–801. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Revision Committee for the
Guidelines on Prevention and Control of Hypertension in China, .
Guidelines On Prevention And Control Of Hypertension In China 2010.
Chinese Journal of Hypertension. 19:701–741. 2011.
|
|
18
|
Oparil S, Dyke S, Harris F, Kief J, James
D, Hester A and Fitzsimmons S: The efficacy and safety of valsartan
compared with placebo in the treatment of patients with essential
hypertension. Clin Ther. 18:797–810. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benz JR, Black HR, Graff A, Reed A,
Fitzsimmons S and Shi Y: Valsartan and hydrochlorothiazide in
patients with essential hypertension. A multiple dose,
double-blind, placebo controlled trial comparing combination
therapy with monotherapy. J Hum Hypertens. 12:861–866. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Black HR, Graff A, Shute D, Stoltz R, Ruff
D, Levine J, Shi Y and Mallows S: Valsartan, a new angiotensin II
antagonist for the treatment of essential hypertension: Efficacy,
tolerability and safety compared to an angiotensin-converting
enzyme inhibitor, lisinopril. J Hum Hypertens. 11:483–489. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang J, Sun NL, Hao YM, Zhu JR, Tu Y,
Curt V and Zhang Y: Trial Investigators: Efficacy and tolerability
of a single-pill combination of amlodipine/valsartan in Asian
hypertensive patients not adequately controlled with valsartan
monotherapy. Clin Exp Hypertens. 33:179–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nash DT, Crikelair N and Zappe D:
Achieving BP goals with valsartan and HCTZ alone and in
combination: Pooled analysis of two randomized, double-blind,
placebo-controlled studies. Curr Med Res Opin. 24:2617–2626. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pool J, Oparil S, Hedner T, Glazer R,
Oddou-Stock P and Hester A: Dose-responsive antihypertensive
efficacy of valsartan, a new angiotensin II-receptor blocker. Clin
Ther. 20:1106–1114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weir MR, Levy D, Crikelair N, Rocha R,
Meng X and Glazer R: Time to achieve blood-pressure goal: Influence
of dose of valsartan monotherapy and valsartan and
hydrochlorothiazide combination therapy. Am J Hypertens.
20:807–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fogari R and Zoppi A: A drug safety
evaluation of valsartan. Expert Opin Drug Saf. 10:295–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|