Introduction
Hypertension is one of the most common and important
risk factors for cardiovascular disease worldwide and it has a high
prevalence in Asia (1). Despite the
availability and widespread use of antihypertensive drugs, control
rates of hypertension remain low (2). The most recent Chinese national survey
of blood pressure (BP) control reported a control rate of 30.6%
among hypertensive outpatients (3).
Pharmacological treatment for hypertension is conventionally
initiated with monotherapy. If BP control is not achieved, this may
be followed by up-titration or combination therapy with another
pharmacological agent. Although early introduction of combination
therapy is an increasingly favoured treatment approach (4), the use of multiple-drug combinations
may not be appropriate for all patients. For patients with less
severe forms of the disease, monotherapy with angiotensin II
receptor blockers such as valsartan, which has placebo-like
tolerability (5), remains a viable
option. Valsartan is widely used alone and in combination with
other antihypertensive drugs (6).
Dose-dependent antihypertensive efficacy has been demonstrated for
valsartan at doses up to 320 mg, with 80 or 160 mg as the
recommended starting dose in Europe and North America (7,8). The
antihypertensive efficacy of 160 mg valsartan has been demonstrated
in several large controlled clinical trials, including VALUE and
NAVIGATOR (9,10). However, clinicians in China typically
use a once-daily dose of 80 mg to initiate valsartan therapy.
Efficacy and safety data for 160 mg daily dosage of valsartan in
Chinese hypertensive patients remain insufficient (11,12).
Therefore, the present study was conducted to investigate the
potential beneficial effects of 160 mg valsartan, thereby providing
more evidence for its utilization in China.
Screening, diagnosis, and management of hypertension
are conventionally based on office BP measurements, although the
clinical relevance of out-of-office BP monitoring is also well
established (13). Out-of-office BP
monitoring, using home or ambulatory BP monitoring (HBPM or ABPM),
is recognised as an important adjunct to office BP for assessing
true BP status (4). There is
extensive evidence out-of-office BP, particularly ambulatory BP,
has a superior predictive value for cardiovascular outcomes and
hypertension-induced organ damage than office BP (14–16). The
objective of the Val-Perfect study was to evaluate the efficacy and
tolerability of 160 mg valsartan for treatment of mild to moderate
hypertension in Chinese patients. In parallel with office-based BP
measurements, the present study also evaluated the impact of
valsartan on ambulatory and home BP parameters.
Patients and methods
Study design
Val-Perfect was a multi-centre, prospective,
open-label, single treatment arm study conducted in the outpatient
clinics of 10 tertiary hospitals in China, including the Peking
University People's Hospital, Peking Union Medical College
Hospital, Peking University First Hospital, Beijing Chaoyang
Hospital, Chinese PLA General Hospital (all Beijing, China), Ruijin
Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China), The First Affiliated Hospital of Nanjing Medical
University (Nanjing, China), First Affiliated Hospital of Sun
Yat-sen University, Guangdong Province People's Hospital (both
Guangzhou, China) and West China Hospital, Sichuan University
(Nanchong, China). The study consisted of a one-week washout period
for patients on pre-existing antihypertensive monotherapy, followed
by a 10-week valsartan treatment period. During the 10-week
treatment period, all patients received 80 mg valsartan (Beijing
Novartis Pharma Ltd., Beijing, China) once daily for the first two
weeks, followed by 160 mg valsartan once daily for a further eight
weeks (Fig. 1A). Treatment was
discontinued if a patient withdrew informed consent, or if
continuation was judged by investigators to be detrimental to the
patient's well being. The present study was designed, conducted and
written-up in accordance with the International Conference on
Harmonisation (ICH) guidelines for good clinical practice (GCP),
with the applicable laws and regulations governing clinical
research in China, and with the ethical principles outlined in the
Declaration of Helsinki (clinicaltrials.gov; NCT01541189). The study protocol
was approved by the Ethics Committees of the participating
institutions.
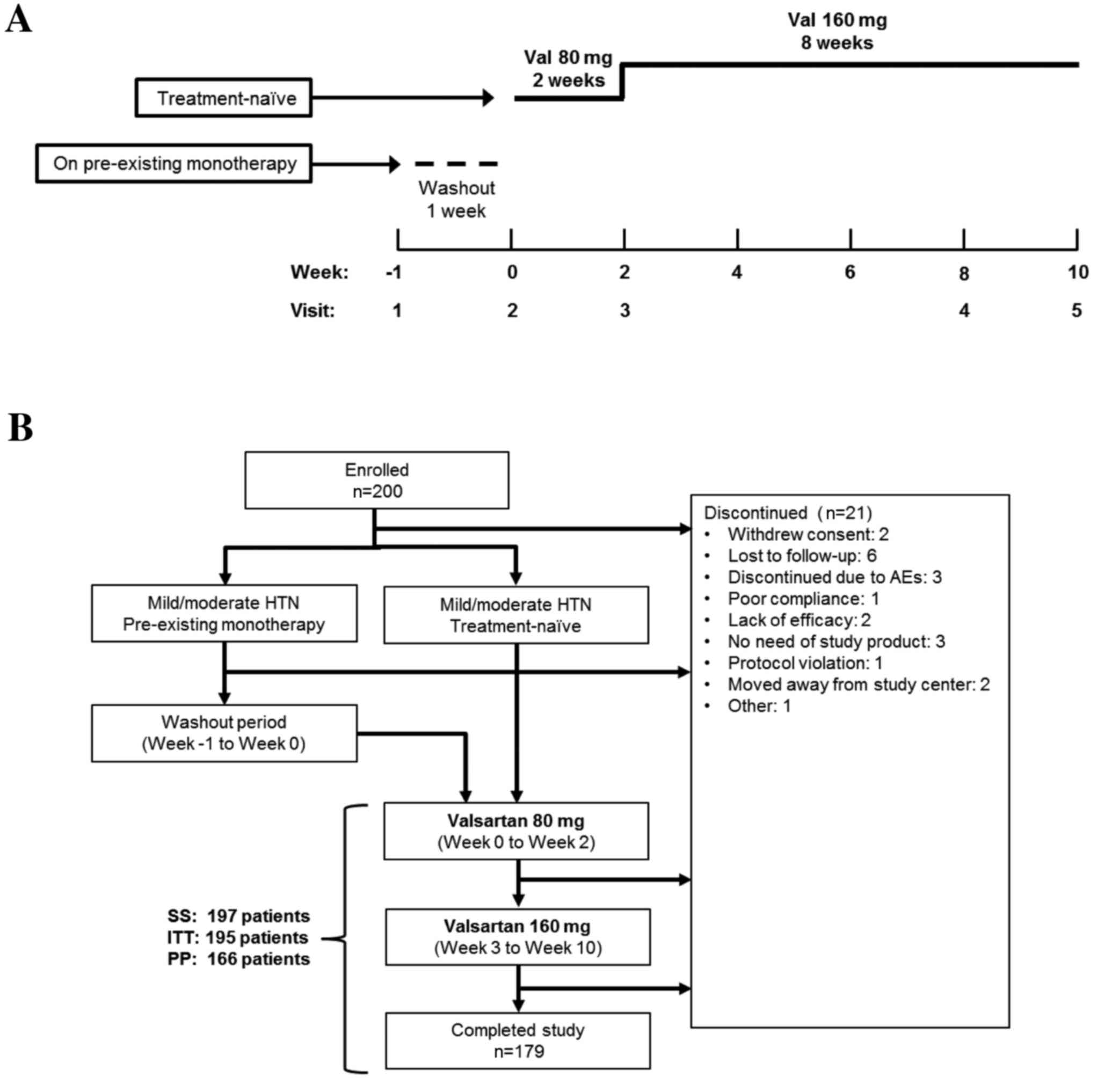 | Figure 1.(A) Study design and (B) patient
flow-chart. The present study consisted of a 10-week treatment
period (weeks 0–2, once-daily 80 mg valsartan; weeks 3–10,
once-daily 160 mg Val). For patients on pre-existing monotherapy,
antihypertensive medication was gradually removed over a one-week
washout period (week-1 to 0). Newly diagnosed (treatment-naïve)
patients entered the study at the treatment phase (week 0,
baseline). Val, valsartan; HTN, hypertension; AE, adverse event;
ITT, intent-to-treat; SS, safety set; PP, per-protocol. |
Patients
Patients eligible for inclusion in the present study
had to meet the following criteria: Aged 18–75 year exhibiting mild
to moderate primary hypertension; naïve to antihypertensive therapy
or on monotherapy; and able and willing to give informed consent to
participate in the study. Patients on pre-existing monotherapy were
required to have a mean sitting systolic BP/mean sitting diastolic
BP (MSSBP/MSDBP) of <160/100 mmHg at the beginning of the
washout phase (week 1). At the beginning of the open-label
treatment phase (week 0), all patients were required to have an
MSSBP of 140-<180 mmHg and an MSDBP of 90-<110 mmHg.
Key exclusion criteria were: Severe hypertension
(MSSBP ≥180 mmHg or MSDBP ≥110 mmHg) at the beginning of the
washout period, malignant hypertension, secondary hypertension,
type 1 diabetes mellitus, renal function impairment (serum
creatinine >2.0 mg/dl or 176.8 µmol/l), a history of significant
cardiovascular disease within the 6-month period prior to screening
and a known allergy to the study product.
Study product administration and BP
monitoring
The study product (valsartan) was supplied as an 80
mg film-coated tablet and was taken daily at 8:00 a.m. Patients
ingested one tablet per day in weeks 0–2 and two tablets per day in
weeks 3–10. At each study visit (weeks-1, 0, 1, 2, 8 and 10),
office BP was measured using an electronic sphygmomanometer
(HEM-7112; Omron Corp., Kyoto, Japan). This measurement was taken
~23 h after the last dose of the study product. BP was measured
with the patient in a seated position, with the cuff at heart
level. At the initial visit, BP was measured on both arms, and the
arm with the higher BP reading was used for all visits. The mean of
three BP readings (2/3 readings differing by <10 mmHg), taken at
2-min intervals, was used for analysis. Sitting heart rate was also
recorded.
Home BP measurements were obtained by patients using
a similar procedure and BP monitor (HEM-7112; Omron Corp.). BP was
measured in the morning (before ingestion of the study product) and
evening (12 h post-morning dose). HBPM was performed on the day
prior to the week 0 (baseline) visit, and on five consecutive days
before each follow-up visit (weeks 2, 6 and 10).
Ambulatory BP monitoring was performed over a 24-h
period prior to study visits at baseline and week 10, using a
validated ABP monitor (SpaceLabs 90207; SpaceLabs Healthcare,
Snoqualmie, WA, USA) worn on the non-dominant arm. BP was recorded
at 30-min intervals. For quality control, the monitoring time was
required to be ≥20 h, with a minimum of 14 valid awake-period
(8:00–22:00) readings and at least 10 valid sleep-period
(22:00–8:00) readings.
Efficacy and safety evaluation
Primary endpoints were the changes in office MSSBP
and MSDBP at week 10, relative to week 2 or 0 (baseline). Secondary
endpoints included changes in home BP and 24-h ambulatory BP at
weeks 2 and 10 relative to baseline, as well as the office BP and
24-h ambulatory BP control rates at week 10. The control rate for
home BP at week 10 was also determined. BP control rates were
determined according to the targets for office, home and ambulatory
BP published in the 2010 guidelines for the management of
hypertension in China (17). Two
sets of office BP goal definitions were used: i) MSSBP/MSDBP
<140/90 mmHg for all patientand ii) MSSBP/MSDBP <130/80 mmHg
for patients with type 2 diabetes mellitus (T2DM) or chronic kidney
disease (CKD), <140/90 mmHg for all other patients. Home BP
targets were SBP/DBP <135/85 mmHg for all patients. ABP targets
were 24-h mean SBP/DBP <130/80 mmHg, daytime SBP/DBP <135/85
mmHg, and nighttime SBP/DBP <120/70 mmHg, for all patients.
Efficacy analyses were performed for the
intent-to-treat (ITT) population, which included all enrolled
patients who received at least one dose of the study product,
undergone a baseline evaluation and at least one subsequent primary
or secondary efficacy evaluation. Analyses were repeated for the
per-protocol (PP) population, which included all patients who
completed the study without major deviations from the study
protocol.
ABPM analyses included only patients who exhibited
valid 24-h ABP recordings at baseline and at week 10, and whose
sleep-wake schedules were in line with that of the majority of the
study population. Nocturnal BP dipper status was determined from
24-h ABPM data. Patients whose nocturnal SBP showed a decrease of
<10% of mean daytime SBP [(mean daytime SBP-mean nighttime
SBP)/daytime SBP<10%] were classified as non-dippers (17).
Safety and compliance
Safety was evaluated in the safety set (SS; patients
who received at least one dose of the study product). Adverse
events (AEs) reported by patients or observed by investigators were
recorded, along with their severity and possible relationship to
the study product. Laboratory test results, including haematology,
blood chemistry and renal function, were recorded at each visit.
These were assessed by investigators for a possible relationship to
the study product and for clinical significance, based on local
laboratory reference ranges. Safety was assessed using AE frequency
and on the numbers of patients with laboratory values that were
outside normal ranges. Treatment compliance was assessed using
records of actual vs. prescribed numbers of tablets ingested by
patients.
Statistical methods
Sample size calculation was based on the change in
MSDBP from weeks 2–10. Assuming a standard deviation of 8 mmHg for
MSDBP and a dropout rate of 10%, it was calculated that a sample
size of 200 was required to detect a 2-mmHg change in MSDBP from
weeks 2–10, with 90% power and a significance level of 0.05
(two-sided). Paired t-tests were used to evaluate the significance
of BP changes at different time-points, relative to week 2 or
baseline, as applicable. Differences of paired samples were
analyzed using the McNemar paired samples non-parametric test with
an α=0.01. All significance tests were two-sided unless otherwise
stated. Analyses were performed using the SAS software package
(version 9.2; SAS Institute, Inc., Cary, NC, USA). Data are
presented as the mean ± standard deviation, unless indicated
otherwise.
Results
Characteristics of the study
population
The present study enrolled 200 patients diagnosed
with mild to moderate primary hypertension who were either naïve to
treatment or on antihypertensive monotherapy (Fig. 1A). Of these, 197 patients initiated
treatment with valsartan and were included in the SS (Fig. 1B). A total of 179 patients completed
the study, with a discontinuation rate of 10.5% (n=21) and 13
patients were excluded from the PP analysis due to protocol
violations. The SS, ITT and PP groups consisted of 197, 195 and 166
patients, respectively.
Demographics and baseline characteristics of the
study population are summarised in Table
I. A total of 115 males (59.0%) and 80 females (41.0%) were
included, and the mean age was 52.9±10.2 years. At baseline, the
mean SBP was 147.3±10.4 mmHg and DBP was 94.7±6.8 mmHg. Of the
total study population, 98 patients were newly diagnosed
(treatment-naïve) and 97 were on pre-existing antihypertensive
monotherapy (Table I). Median
disease duration was three years (range, 0–43 years).
 | Table I.ITT Patient demographics and baseline
characteristics. |
Table I.
ITT Patient demographics and baseline
characteristics.
| Characteristic | All (n=195) |
|---|
| Age, years |
52.9±10.2 |
| Gender |
|
| Male, n
(%) | 115 (59.0) |
| Female, n
(%) | 80
(41.0) |
| BMI
(kg/m2) | 25.6±3.3 |
| Ethnic group |
|
| Han
Chinese, n (%) | 192 (98.5) |
| Other, n
(%) | 3 (1.5) |
| SBP (mmHg) | 147.3±10.4 |
| DBP (mmHg) | 94.7±6.8 |
| Heart rate
(beats/min) | 71.5±8.3 |
| ALT (U/l) |
26.2±14.5 |
| AST (U/l) | 24.5±8.9 |
| BUN (mmol/l) |
5.2±1.5 |
| Cr (µmol/l) |
74.0±17.4 |
| TC (mmol/l) |
5.0±1.0 |
| TG (mmol/l) |
2.0±1.7 |
| HDL-C (mmol/l) |
1.2±0.3 |
| LDL-C (mmol/l) |
3.0±0.7 |
| Newly diagnosed
hypertension |
|
| Yes, n
(%) | 98
(50.3) |
| No, n
(%) | 97
(49.7) |
| Disease duration
(years) |
|
| Median
(range) | 3.0
(0–43) |
| Mean ±
SD |
6.3±7.5 |
| Concomitant
illnessa | 75
(38.5) |
| Yes, n
(%) | 75
(38.5) |
| No, n
(%) | 120 (61.5) |
| Present
cardiovascular risk factors/medical history, n (%) |
|
|
Dyslipidemia | 55
(28.2) |
|
Diabetes | 16 (8.2) |
| Kidney
disease | 3
(1.5) |
|
Clinically significant
laboratory findings (SS)b, n (%) |
|
Triglycerides | 55
(27.9) |
| Total
cholesterol | 28
(14.2) |
|
LDL-C | 26
(13.2) |
| Uric
acid | 23
(11.7) |
|
HDL-C | 20
(10.2) |
| Previous
antihypertensive drug classes, n (%) |
|
|
β-blockers | 3
(1.5) |
|
CCBs | 39
(20.0) |
|
ACEIs | 8
(4.1) |
|
ARBs | 38
(19.5) |
|
Other | 9
(4.6) |
Reductions in office and home BP
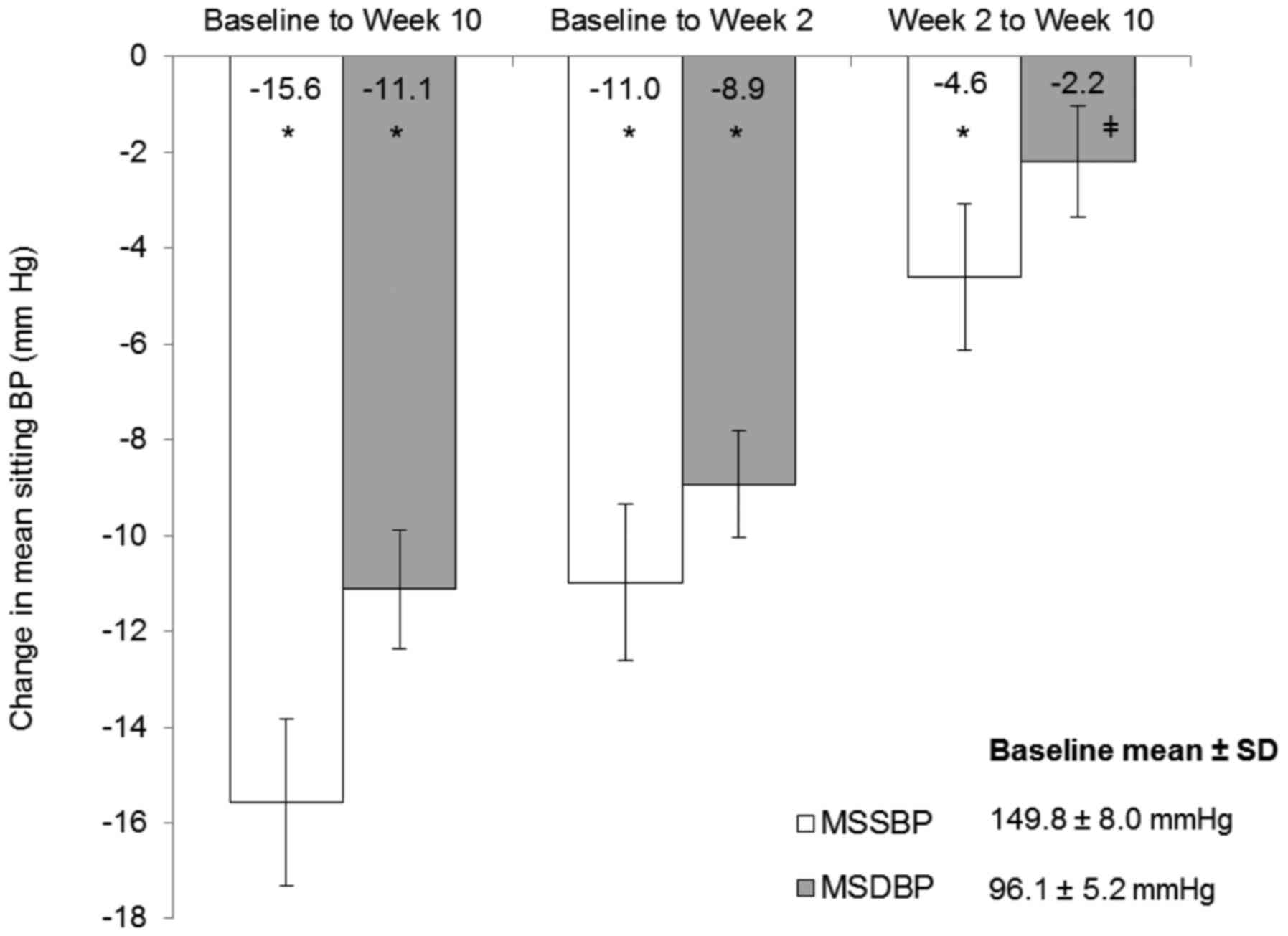
In the ITT population, mean reductions in office
MSSBP and MSDBP from baseline to week 10 were statistically
significant: 15.6 mmHg and 11.1 mmHg, respectively (both
P<0.0001; Fig. 2). Notably,
reductions in MSSBP and MSDBP from weeks 2–10, during the 160 mg
valsartan phase, were also statistically significant (MSSBP: 4.6
mmHg, P<0.0001; MSDBP: 2.2 mmHg, P=0.0003). Mean reductions in
office MSSBP and MSDBP from baseline to week 2 were 11.0 and 8.9
mmHg, respectively (both P<0.0001). Similar results were
obtained for the PP analyses (data not shown).
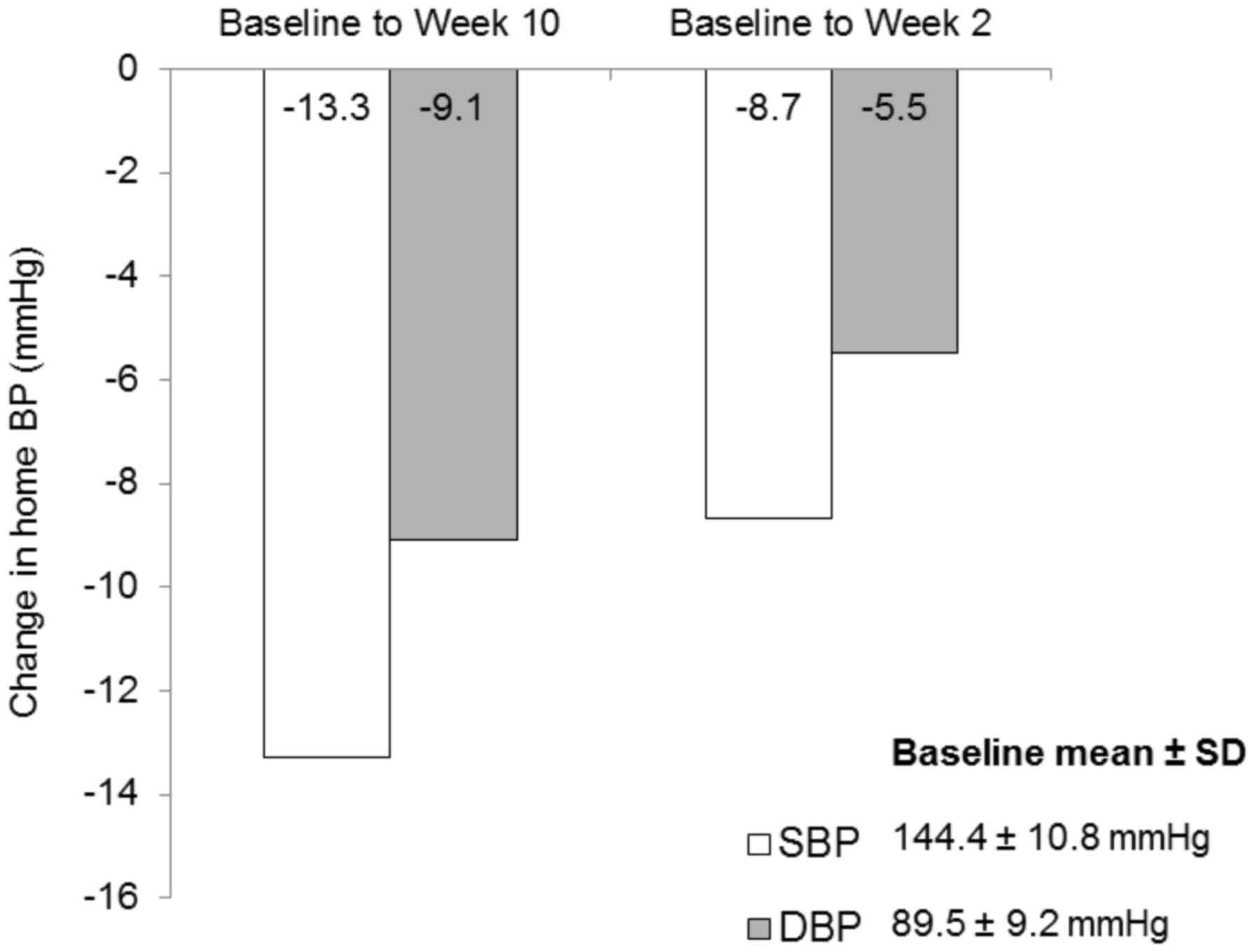
Home BP also decreased significantly following
10-week treatment. Mean overall reductions in SBP and DBP from
baseline to week 2 were 8.7 and 5.5 mmHg, respectively (both
P<0.0001; Fig. 3). Mean SBP and
DBP reductions from baseline to week 10 were 13.3 and 9.1 mmHg,
respectively (both P<0.0001). Similar results were obtained in
the PP analysis (data not shown).
Changes in ambulatory BP
parameters
At baseline, 24-h mean ambulatory SBP/DBP was
136.3/88.0 mmHg. ABPM revealed significant BP reductions at week
10, relative to baseline. Reductions in overall (24-h), daytime,
and nighttime mean SBP/DBP were all significant: 6.1/4.4, 5.6/4.0
and 7.8/5.4 mmHg, respectively (all P<0.0001; Table II). Significant BP reduction was
also observed during the final four h of the 24-h dosing interval
(SBP/DBP, −6.5/−4.3 mmHg; P<0.0001). The proportion of patients
with a nocturnal ‘dipper’ BP profile increased from 37.0% (n=57) at
baseline to 48.1% (n=74) at week 10. In addition, a significant
proportion (41.2%; n=40) of patients with a ‘non-dipper’ profile at
baseline exhibited a ‘dipper’ profile after 10 weeks of treatment
(P=0.0322; McNemar's test for paired samples).
 | Table II.Changes in ambulatory BP monitoring
parameters following 10 weeks of valsartan treatment. |
Table II.
Changes in ambulatory BP monitoring
parameters following 10 weeks of valsartan treatment.
| Ambulatory BP
parameters (mmHg) | SBP | DBP | P-value |
|---|
| 24-h average
ABP |
|
|
|
|
Baseline (Week 0) | 136.3±11.8 | 88.0±10.0 | – |
| Change
from baseline to week 10 −6.1±11.4 | −4.4±7.9 | <0.0001 |
|
| Daytime average
ABP |
|
|
|
|
Baseline (Week 0) | 139.4±12.7 | 90.5±11.1 | – |
| Change
from baseline to week 10 |
−5.6±12.8 | −4.0±9.2 | <0.0001 |
| Nighttime average
ABP |
|
|
|
|
Baseline (Week 0) | 129.0±13.5 | 82.3±10.0 | – |
| Change
from baseline to week 10 |
−7.8±12.8 | −5.4±9.2 | <0.0001 |
| Change in final 4 h
of dosing interval (baseline to week 10) |
−6.5±12.5 | −4.3±8.6 | <0.0001 |
| Patients with
nocturnal ‘dipper’ profile |
|
|
|
| At
baseline, n (%) | 57 (37.0) |
|
|
| At Week
10, n (%) | 74 (48.1) |
|
|
Improvements in office, home, and
ambulatory BP control rates
Office and home BP control rates were markedly
increased at the end of the treatment period. Following eight weeks
of treatment with once-daily 160 mg valsartan, office BP control
rates increased from 42.1% at week 2 to 60.0% at week 10 (BP goal
definition 1, MSSBP/MSDBP<140/90 mmHg for all patient; and
Fig. 4A). A similar increase in
control rate, from 40.5% at week 2 to 56.9% at week 10, was
observed using BP goal definition 2 (MSSBP/MSDBP <130/80 mmHg
for patients with T2DM or CKD, <140/90 mmHg for others). The
baseline home BP control rate was 26.1%. Consistent with the
improvement in office BP control, the home BP control rate (target
SBP/DBP <135/85 mmHg for all patients) increased from 57.2% at
week 2 to 66.7% at week 10 (Fig.
4B). Among patients who attained their office BP goals (SBP/DBP
<130/80 mmHg for patients with T2DM or CKD, SBP/DBP <140/90
mmHg for all other patients) by the completion of the study, the
home BP control rate was 80.4%. Overall control rates for 24-h
ambulatory BP markedly increased following 10 weeks of valsartan
treatment, from 11.0 to 23.4% (Fig.
4C).
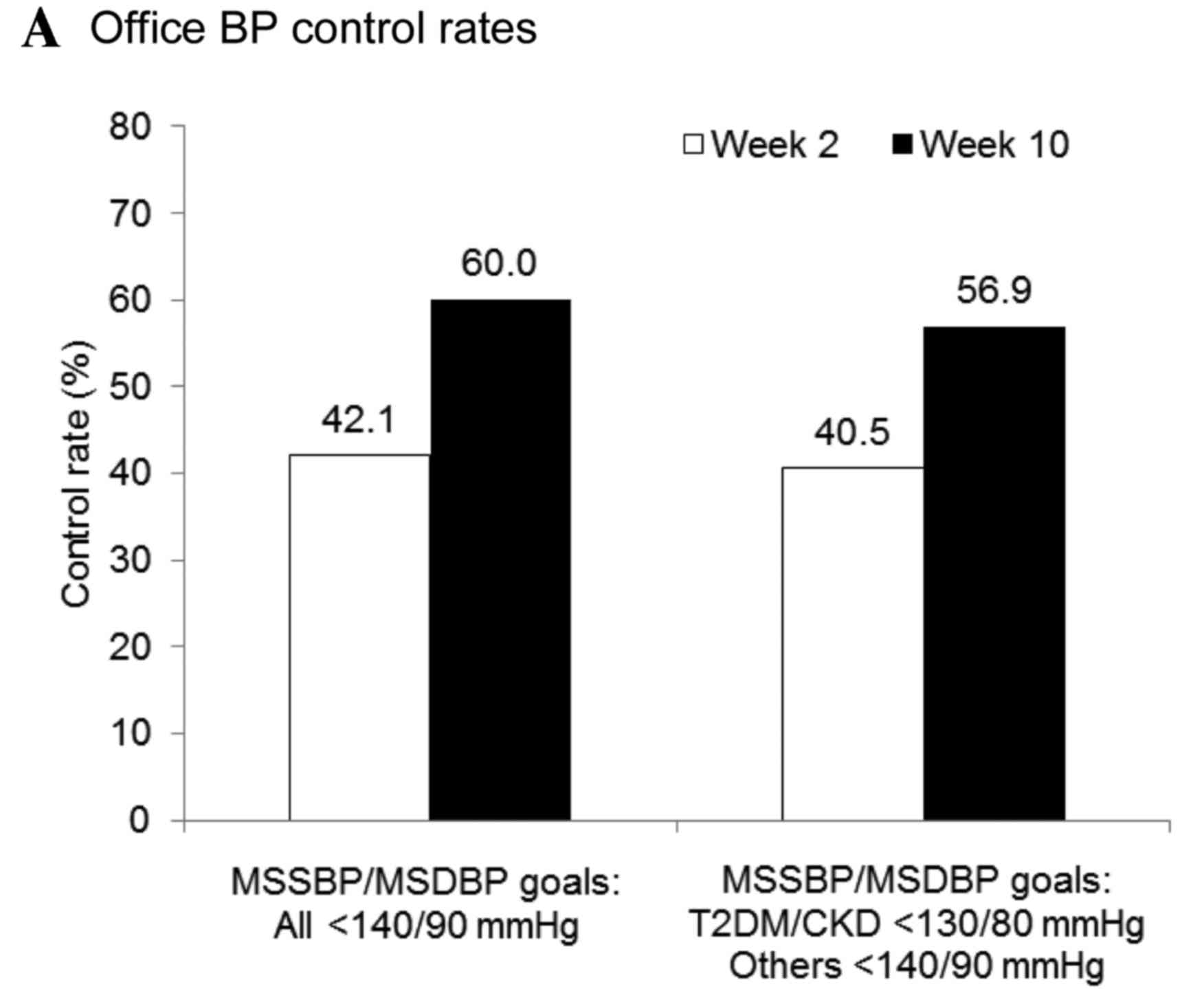 | Figure 4.Attainment of (A) office, (B) home,
and (C) ambulatory BP goals. Office BP targets: Definition 1,
MSSBP/MSDBP<140/90 mmHg for all patient; and definition 2,
MSSBP/MSDBP<130/80 mmHg for patients with T2DM or CKD,
<140/90 mmHg for all other patients. Home BP target:
SBP/DBP<135/85 mmHg for all patients. Ambulatory BP targets:
24-h mean SBP/DBP<130/80 mmHg, daytime SBP/DBP<135/85 mmHg,
and nighttime SBP/DBP<120/70 mmHg for all patients. BP, blood
pressur; and MSSBP, mean sitting systolic BP; MSDBP, mean sitting
diastolic BP; baseline, week 0; T2DM, type 2 diabetes mellitu; CKD,
chronic kidney disease. |
Safety and compliance
Of the 197 patients who received at least one dose
of the study product (the SS), 44 (22.3%) reported one or more AEs,
the majority of which were mild in severity. The incidence of both
AEs leading to discontinuation (1.5%; n=3) and study
product-related AEs (3.1%; n=6) was low. Table III presents the types of study
product-related AEs reported; of these, mild dizziness was the most
frequent (1.5%). There were no instances of mortality or study
product-related severe AEs. The number of patients with clinically
significant abnormalities in laboratory parameters (blood lipids
and uric acid) was similar at the beginning (Table I) and the end of the study period
(Table IV). Within the SS (n=197),
177 (98.3%) patients exhibited good treatment compliance (80–120%),
as assessed by pill counts.
 | Table III.Incidence of AEs. |
Table III.
Incidence of AEs.
| Variable | No. of patients (%)
SS: n=197 |
|---|
| Total AEs | 44 (22.3) |
| Study
product-related AEs | 6 (3.1) |
| Severe
AEs | 2 (1.0) |
| AEs
leading to discontinuation | 3 (1.5) |
| Study
product-related AEs by type |
|
|
Amaurosis | 1 (0.5) |
|
Dizziness | 3 (1.5) |
|
Headache | 1 (0.5) |
|
Hypotension | 1 (0.5) |
|
Pruritis | 1 (0.5) |
| Mucosal
and skin rash | 1 (0.5) |
|
Rash | 1 (0.5) |
 | Table IV.Clinically significant laboratory
findings following 10 weeks of valsartan treatment. |
Table IV.
Clinically significant laboratory
findings following 10 weeks of valsartan treatment.
| Variable | No. of patients (%)
SS: n=197 |
|---|
| Clinically
significant laboratory findingsa (%) |
|
|
Triglycerides | 40
(20.3) |
| Total
cholesterol | 21
(10.7) |
|
LDL-C | 19 (9.6) |
| Uric
acid | 15 (7.6) |
|
HDL-C | 11 (5.6) |
Discussion
Antihypertensive efficacy of valsartan has been
established over a range of doses, up to 320 mg/day, in North
American and European hypertensive patient populations (7,8,18). The results of the present study
demonstrated the antihypertensive efficacy of once-daily 160 mg
valsartan in Chinese patients with mild to moderate hypertension.
The blood pressure reductions observed in the current study
(15.6/11.1 mmHg for office SBP/DBP following 10 weeks treatment)
were comparable to those obtained in previous studies (7,11,19–23),
which examined single-drug or combination valsartan therapy in
patients with mild to moderate hypertension over a similar time
frame. In the latter studies, mean SBP reductions between 10.2 and
16.5 mmHg were observed, whereas mean DBP reductions were between
5.3 and 10.3 mmHg. In the present study, beyond the significant BP
reduction observed following the initial two-week treatment with
valsartan 80 mg, there was an additional, significant BP reduction
following up-titration to 160 mg for a further eight weeks.
Use of ABPM and HBPM facilitates assessment of
overall BP control and may contribute to improved BP management.
Studies have shown that out-of-office (ambulatory and home) BP is
able to predict cardiovascular events and hypertension-induced
organ damage more effectively than office BP (14–16). In
the present study, a significant antihypertensive effect of
valsartan was detected, regardless of the type of BP measurement
(office, home, or ambulatory BPM), indicating its effectiveness in
reducing out-of-office and office BP. In addition to reductions in
24-h mean SBP/DBP, significant BP reductions were also observed in
the final 4 h of the 24-h dosing interval, indicating that the
effects were sustained throughout the 24-h period.
Following 10 weeks of treatment, ~60% of patients
were able to achieve their office BP goal. This level is similar to
control rates achieved in a previous study that compared 80 and 160
mg valsartan monotherapy (up to 59%) with a single-pill combination
of 5/160 mg amlodipine/valsartan (~70%), in a group of patients who
had not responded to treatment with 80 mg valsartan (21). Therefore, although certain groups of
patients may require combination therapy, a substantial proportion
of patients are likely to be able to achieve adequate BP control on
higher-dose valsartan monotherapy (160 mg vs. the 80 mg dose
currently used in China).
One important limitation of the present study is the
open-label non-comparative design. A possible placebo effect cannot
be excluded without a comparative control group, which ultimately
weakens the reliability of the present conclusions. However, ABPM
is generally considered to reflect blood pressure levels more
objectively, thus potentially limiting the placebo effect.
Significant BP reductions were confirmed by ABPM analyses following
10-week valsartan treatment. In addition, the present design
corresponds more closely to real-world assessments of the 160 mg
dose, which does not permit formal evaluation of the efficacy of
this dose. Furthermore, the present results are consistent with the
known dose-dependent efficacy and safety profile of valsartan in
other patient populations (8,24).
Although the relatively short follow-up period did not permit
direct assessment of long-term effectiveness, the pattern of BP
reduction over the course of the study is consistent with previous
investigations of valsartan (18,20);
maximal reduction is typically detected within four weeks and
persists throughout long-term therapy. Consistent with existing
safety data (25), valsartan
exhibited a favourable safety profile in the present study, with a
low incidence of study product-related AEs and discontinuation due
to AEs (both <5%). There were no instances of mortality or study
product-related SAEs.
The present results provide further evidence of a
positive benefit-risk balance for the use of the 160 mg dose of
valsartan, compared with the 80 mg dose, in Chinese patients with
mild to moderate hypertension. Given the proven dose-dependent
efficacy of valsartan across a wide dose range and its favourable
safety profile, treatment with the higher dose of 160 mg may be a
reasonable therapeutic option, particularly for patients with less
severe hypertension.
Acknowledgements
The authors would like to thank Hongzhi Xie (Peking
Union Medical College Hospital, Beijing, China), Fang Zhou (The
First Affiliated Hospital, Nanjing Medical University), Hao Xue
(Academy of Military Medical Sciences, Beijing, China), and Tao Tao
(Novartis Pharmaceuticals, China) for their valuable contributions
to this study, as well as Patrick Brunel (Worldwide medical
affairs, Novartis Pharma AG), Rosemarie Kelly (Worldwide medical
affairs, Novartis Pharma AG) and Ashwani Kumar (Worldwide medical
affairs, Novartis Pharma AG) for critical review of the manuscript.
This study was sponsored by Novartis Pharmaceuticals (China).
References
|
1
|
Chiang CE and Chen CH: Hypertension in the
Asia-Pacific region. J Hum Hypertens. 22:441–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kearney PM, Whelton M, Reynolds K, Whelton
PK and He J: Worldwide prevalence of hypertension: A systematic
review. J Hypertens. 22:11–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu DY, Liu LS, Yu JM and Yao CH: China
STATUS Study Group: National survey of blood pressure control rate
in Chinese hypertensive outpatients-China STATUS. Zhonghua Xin Xue
Guan Bing Za Zhi. 38:230–238. 2010.(In Chinese). PubMed/NCBI
|
|
4
|
Mancia G, Fagard R, Narkiewicz K, Redón J,
Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G,
Dominiczak A, et al: 2013 ESH/ESC Practice Guidelines for the
Management of Arterial Hypertension. Blood Press. 23:3–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verdecchia P and Angeli F: Assessment of
the optimal daily dose of valsartan in patients with hypertension,
heart failure, or both. Clin Ther. 26:460–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markham A and Goa KL: Valsartan. A review
of its pharmacology and therapeutic use in essential hypertension.
Drugs. 54:299–311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parati G, Asmar R, Bilo G, Kandra A, Di
Giovanni R and Mengden T: Effectiveness and safety of high-dose
valsartan monotherapy in hypertension treatment: The ValTop study.
Hypertens Res. 33:986–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pool JL, Glazer R, Chiang YT and Gatlin M:
Dose-response efficacy of valsartan, a new angiotensin II receptor
blocker. J Hum Hypertens. 13:275–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Julius S, Kjeldsen SE, Weber M, Brunner
HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, et
al: Outcomes in hypertensive patients at high cardiovascular risk
treated with regimens based on valsartan or amlodipine: The VALUE
randomised trial. Lancet. 363:2022–2031. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Study Group NAVIGATOR, McMurray JJ, Holman
RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell
M, Buse JB, et al: Effect of valsartan on the incidence of diabetes
and cardiovascular events. N Engl J Med. 362:1477–1490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ke YN, Dong YG, Ma SP, Yuan H, Ihm SH and
Baek SH: ADVISE study group: Improved blood pressure control with
nifedipine GITS/valsartan combination versus high-dose valsartan
monotherapy in mild-to-moderate hypertensive patients from Asia:
Results from the ADVISE study, a randomized trial. Cardiovasc Ther.
30:326–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu D, Yang K, Sun N, Gao P, Wang R,
Grosso A and Zhang Y: trial investigators: Amlodipine/valsartan
5/160 mg versus valsartan 160 mg in Chinese hypertensives. Int J
Cardiol. 167:2024–2030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stergiou GS, Nasothimiou E, Giovas P,
Kapoyiannis A and Vazeou A: Diagnosis of hypertension in children
and adolescents based on home versus ambulatory blood pressure
monitoring. J Hypertens. 26:1556–1562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clement DL, De Buyzere ML, De Bacquer DA,
de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun
JJ, Six RO, et al: Prognostic value of ambulatory blood-pressure
recordings in patients with treated hypertension. N Engl J Med.
348:2407–2415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perloff D, Sokolow M and Cowan R: The
prognostic value of ambulatory blood pressures. JAMA.
249:2792–2798. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verdecchia P, Porcellati C, Schillaci G,
Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C,
Zampi I, Santucci A, et al: Ambulatory blood pressure. An
independent predictor of prognosis in essential hypertension.
Hypertension. 24:793–801. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Revision Committee for the
Guidelines on Prevention and Control of Hypertension in China, .
Guidelines On Prevention And Control Of Hypertension In China 2010.
Chinese Journal of Hypertension. 19:701–741. 2011.
|
|
18
|
Oparil S, Dyke S, Harris F, Kief J, James
D, Hester A and Fitzsimmons S: The efficacy and safety of valsartan
compared with placebo in the treatment of patients with essential
hypertension. Clin Ther. 18:797–810. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benz JR, Black HR, Graff A, Reed A,
Fitzsimmons S and Shi Y: Valsartan and hydrochlorothiazide in
patients with essential hypertension. A multiple dose,
double-blind, placebo controlled trial comparing combination
therapy with monotherapy. J Hum Hypertens. 12:861–866. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Black HR, Graff A, Shute D, Stoltz R, Ruff
D, Levine J, Shi Y and Mallows S: Valsartan, a new angiotensin II
antagonist for the treatment of essential hypertension: Efficacy,
tolerability and safety compared to an angiotensin-converting
enzyme inhibitor, lisinopril. J Hum Hypertens. 11:483–489. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang J, Sun NL, Hao YM, Zhu JR, Tu Y,
Curt V and Zhang Y: Trial Investigators: Efficacy and tolerability
of a single-pill combination of amlodipine/valsartan in Asian
hypertensive patients not adequately controlled with valsartan
monotherapy. Clin Exp Hypertens. 33:179–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nash DT, Crikelair N and Zappe D:
Achieving BP goals with valsartan and HCTZ alone and in
combination: Pooled analysis of two randomized, double-blind,
placebo-controlled studies. Curr Med Res Opin. 24:2617–2626. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pool J, Oparil S, Hedner T, Glazer R,
Oddou-Stock P and Hester A: Dose-responsive antihypertensive
efficacy of valsartan, a new angiotensin II-receptor blocker. Clin
Ther. 20:1106–1114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weir MR, Levy D, Crikelair N, Rocha R,
Meng X and Glazer R: Time to achieve blood-pressure goal: Influence
of dose of valsartan monotherapy and valsartan and
hydrochlorothiazide combination therapy. Am J Hypertens.
20:807–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fogari R and Zoppi A: A drug safety
evaluation of valsartan. Expert Opin Drug Saf. 10:295–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|