Introduction
Nasopharyngeal carcinoma (NPC) is a type of
malignant epithelial cell tumor. The incidence and mortality of NPC
in China is amongst the highest in the world (1). NPC is typically poorly differentiated
and exhibits a tendency to metastasize and recur elsewhere, which
may lead to failures in treatment (2,3).
Additionally, invasion and metastasis lead to mortality in many
patients with NPC (4).
Histologically, the majority of cases of NPC (95%) are
undifferentiated or poorly differentiated, and these histological
types are able to spread and metastasize easily (5). It is therefore important to study the
mechanisms of invasion in NPC to elucidate the pathogenesis and
potential therapeutic treatments for this disease. Previous studies
have identified a number of molecular markers that are associated
with cell proliferation, invasion, differentiation and metastasis
in NPC (6,7); however, the molecular pathogenesis
underlying the development and progression of NPC remain to be
elucidated.
Rho-associated coiled coil-containing protein kinase
(ROCK) is a serine/threonine protein kinase and RhoA downstream
effector protein. In mammals, two isoforms of ROCK exist: ROCK1 and
ROCK2 (8). Overexpression of ROCK1
induces reorganization of the cytoskeleton, and promotes the
formation of stress fibers and dot matrix adhesion, thus regulating
cell movement and migration (9).
ROCK1 also serves an important role in the regulation and
maintenance of cell migration (8).
The phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit α (PIK3CA) gene encodes the p110α catalytic
subunit of phosphatidylinositol 3-kinase (PI3K), a member of the
PI3K/AKT pathway that is important for the regulation of cellular
functions including metabolism, proliferation, angiogenesis,
protein synthesis and apoptosis (10). It has previously been reported that
activating mutation of the PIK3CA oncogene negatively affects the
prognosis of non-small-cell lung cancer (11), which suggests that PIK3CA may act as
a potential prognostic biomarker.
However, to date, the association between ROCK1 and
PIK3CA in NPC has not been well characterized. In the present
study, the expression of these proteins in NPC tissue microarray
(TMA) sections was studied via immunohistochemistry (IHC) and
protein levels in cells were evaluated using western blot analysis.
The association between ROCK1 and PIK3CA protein expressions and
the clinicopathological features of patients were assessed to
determine the clinicopathological significance of ROCK1 and PIK3CA
in NPC.
Materials and methods
Patients and preparation of NPC
TMA
Archived formalin-fixed, paraffin-embedded
undifferentiated NPC tissue blocks were prepared from 81 patients
with NPC who underwent biopsy surgery between January 2012 and
November 2014 at the Radiation Oncology Department of Fudan
University Cancer Hospital (Shanghai, China). Firstly, a
pathologist marked the areas of tumor on the tissue block
subsequent to reviewing a hematoxylin and eosin-stained section
from each specimen. Single 2-mm diameter core tissues were taken
from each paraffin-embedded tissue and placed into a recipient
block. A total of 81 tissue sample cores were arrayed in each
single block. A retrospective chart review of all patients was
performed to collect data on clinical characteristics, including
age, sex, pathological features and disease stage (T and N)
according to the 2010 TNM classification of the American Joint
Committee on Cancer Staging system (12). The histological type of all samples
was undifferentiated. Approval was obtained from the Ethics
Committee of Fudan University Cancer Hospital and all patients
provided written prior informed consent.
IHC
For IHC analysis, TMA sections were deparaffinized
in xylene, and endogenous peroxidase activity was blocked with
methanol and 3% H2O2 for 15 min. For antigen
retrieval, 4-µm thick TMA sections were boiled in a pressure cooker
at ~120°C in citrate buffer (pH 6.0) (Beyotime Institute of
Biotechnology, Haimen, China) for 3 min. Tissues were incubated for
1 h at room temperature with the following primary antibodies:
Monoclonal rabbit anti-ROCK1 (cat. no. ab45171; 1:200; Abcam,
Cambridge, MA, USA) or monoclonal rabbit anti-PIK3CA (cat. no.
ab152155; 1:200; Abcam). The secondary antibody used was goat
anti-rabbit horseradish peroxidase (HRP) (cat. no. K500711;
ready-to-use; Dako; Aligent Technologies, Inc., Santa Clara, CA,
USA). Immunostaining of tissues was evaluated independently by two
trained pathologists who were unaware of the clinical background of
the samples. For each case, five random fields at 400x
magnification were captured by an Olympus IX73 microscope (Olympus
Corporation, Tokyo, Japan) and digital camera (Olympus Corporation)
using CellSens Dimension software (version 1.9; Olympus
Corporation).
ROCK1 and PIK3CA immunoreactivity were analyzed
using a semi-quantitative scoring system in which only cytoplasmic
membrane staining was considered. Based on the percentage of
positive cells, the level of ROCK1 and PIK3CA expression was
classified as (+++) if ≥76% of cells were stained, (++) if 26–75%
of cells were stained, (+) if ≤25% of cells were stained, and (−)
if cells completely lacked membranous staining. Thus, (−) and (+)
cases were classified as exhibiting low protein expression, whereas
(++) and (+++) cases were classified as exhibiting high protein
expression (Table I).
 | Table I.Association between ROCK1 and PIK3CA
immunoexpression and various clinicopathological parameters in
nasopharyngeal carcinoma. |
Table I.
Association between ROCK1 and PIK3CA
immunoexpression and various clinicopathological parameters in
nasopharyngeal carcinoma.
|
|
| ROCK1 | PIK3CA |
|---|
|
|
|
|
|
|---|
| Parameter | Number of cases
(n=81) (%) | Low (%) | High (%) | P-value | Low (%) | High (%) | P-value |
|---|
| Sex, n (%) |
|
|
| 0.490 |
|
| 0.553 |
| Male | 59 (72.8) | 41 (69.5) | 18 (30.5) |
| 23 (40.0) | 36 (60.0) |
|
|
Female | 22 (27.2) | 17 (77.3) | 5
(22.7) |
| 7
(31.8) | 15 (68.2) |
|
| Age, n (%) |
|
|
| 0.638 |
|
| 0.392 |
| ≥60 | 15 (18.5) | 10 (66.7) | 5
(33.3) |
| 7
(46.7) | 8
(53.3) |
|
|
<60 | 66 (81.5) | 48 (72.7) | 18 (27.3) |
| 23 (34.8) | 43 (65.2) |
|
| T status, n (%) |
|
|
| 0.242 |
|
| 0.458 |
| T1,
T2 | 47 (58.0) | 36 (76.6) | 11 (23.4) |
| 19 (40.4) | 28 (59.6) |
|
| T3,
T4 | 34 (42.0) | 22 (64.7) | 12 (35.3) |
| 11 (32.4) | 23 (67.6) |
|
| N status, n (%) |
|
|
| 0.032a |
|
| 0.027a |
| N0,
N1 | 41 (50.6) | 25 (61.0) | 16 (39.0) |
| 20 (48.8) | 21 (51.2) |
|
| N2,
N3 | 40 (49.4) | 33 (82.5) | 7
(17.5) |
| 10 (25.0) | 30 (75.0) |
|
| AJCC stage, n
(%) |
|
|
| 0.518 |
|
| 0.019a |
| I,
II | 16 (19.8) | 13 (81.3) | 3
(18.7) |
| 10 (62.5) | 6
(37.5) |
|
| III,
IV | 65 (80.2) | 45 (69.2) | 20 (30.8) |
| 20 (30.8) | 45 (69.2) |
|
Cell culture
The human nasopharyngeal epithelial cell line NP69
and the NPC cell line 5–8F were donated by the Sun Yat-sen
University Cancer Center (Guangzhou, China). Following passage,
cells were grown for 48 h in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific Inc.) at 37°C in an
atmosphere containing 5% CO2. Following passage, the
NP69 cell line was maintained in keratinocyte serum-free medium
supplemented with human epidermal growth factor and bovine
pituitary extract (cat. no. 17005-042; Gibco; Thermo Fisher
Scientific Inc.) in a humidified atmosphere containing 5%
CO2 at 37°C for 72 h.
Western blot analysis
Cells were washed twice with ice-cold PBS and
resuspended in 1 ml lysis buffer (25 mM Tris-HCl pH 7.6, 150 mM
NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) (Thermo Fisher
Scientific Inc.). Cell debris was removed by centrifugation (12,000
× g; 4°C; 10 min) and the protein concentration of the
supernatant was subsequently determined using the Bradford method
(13). Aliquots that contained 30 µg
total protein were separated by SDS-PAGE (6–10% gradient gels) and
proteins were transferred onto polyvinylidine diflouride membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5%
bovine serum albumin (Biosharp, Hefei, China) and incubated at 4°C
for 18 h with the following monoclonal antibodies: Rabbit
anti-ROCK1 (cat. no. ab45171; 1:2,000; Abcam), rabbit anti-PIK3CA
(cat. no. ab152155; 1:2,000; Abcam) and rabbit anti-β-actin (cat.
no. ab8227; 1:1,000; Abcam). Membranes were subsequently washed
three times with 0.1% Tween-20 in TBS and incubated for 2 h at room
temperature with HRP-conjugated secondary antibody (cat. no.
sc2301; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Immunoreactive protein bands were visualized using an
electrochemiluminescence system (GE Healthcare Life Sciences,
Chalfont, UK). To quantify the protein expression by Western
blotting analysis, densitometric analyses were performed using
ImageJ version 1.46 software (imagej.nih.gov/ij/). Experiments were performed at
least three times.
Statistical analysis
The associations between clinicopathologic variables
and ROCK1 and PIK3CA protein expression were examined using the
χ2 test. Continuous data were compared using a Student's
t-test if the distribution was normal, or a one-way analysis of
variance if distribution was asymmetrical. All data were analyzed
using SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference. All P-values were based on two-tailed tests.
Results
ROCK1 and PIK3CA expression in
NPC
Expressions of ROCK1 and PIK3CA in clinical NPC
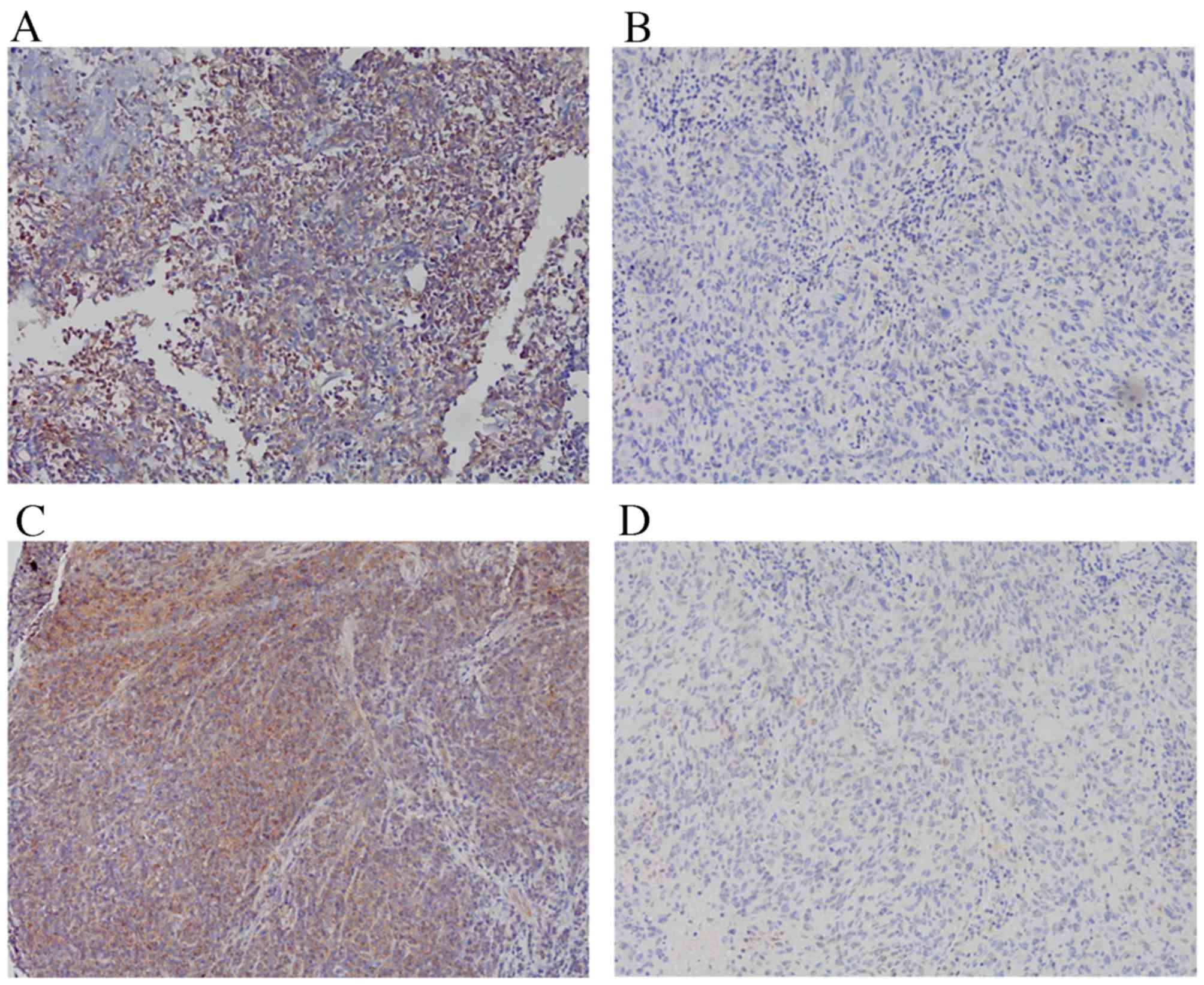
tissues were evaluated using IHC. Representative images of the
immunohistochemical staining patterns are displayed in Fig. 1. ROCK1 and PIK3CA expression was
observed in the cytoplasm, with high ROCK1 expression detected in
28.40% (23/81) of the NPC specimens and high PIK3CA expression
detected in 62.96% (51/81) of the NPC specimens.
Expression of ROCK1 and PI3KCA in NPC
cells
To assess the association between expressions of
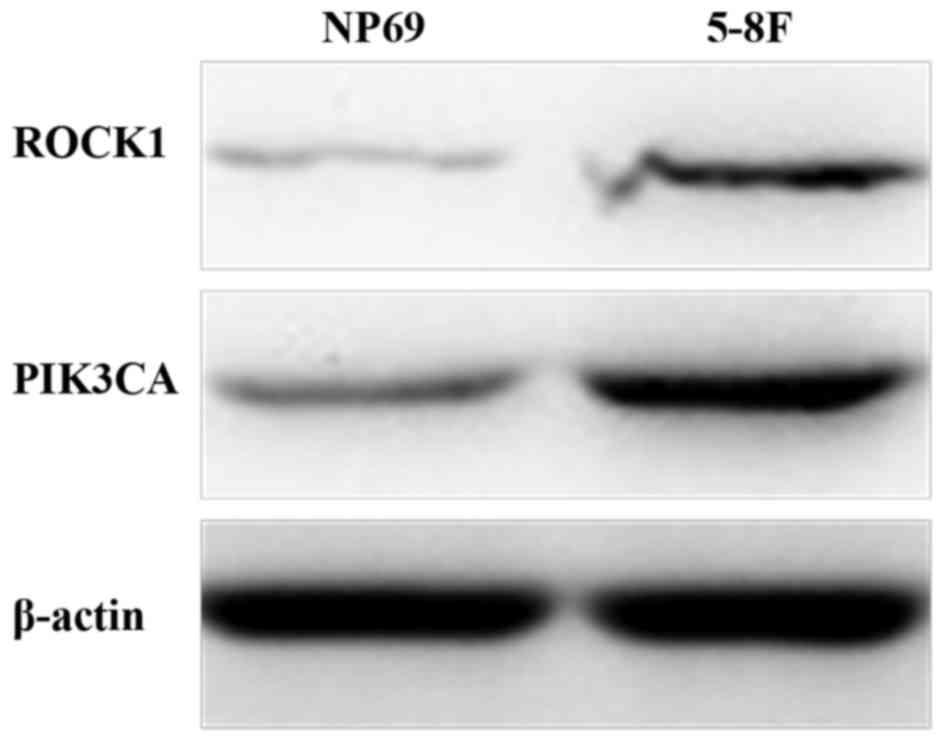
ROCK1 and PI3KCA and NPC, NP69 and 5–8F cells were used. ROCK1 and
PIK3CA protein levels were demonstrated to be significantly higher
in 5–8F cells compared with NP69 cells (Fig. 2). Expression of ROCK1 (relative to
β-actin) in NP69 and 5–8F cells was 0.326±0.078 and 1.191±0.114,
respectively (P<0.001). Expression of PIK3CA (relative to
β-actin) in NP69 and 5–8F cells was 0.696±0.134 and 1.159±0.144,
respectively (P=0.015). Similar results were obtained from at least
three independent western blots.
Association between ROCK1 and PIK3CA
protein expression and the clinicopathological features of NPC
The associations between ROCK1 and PIK3CA expression
and the clinicopathological features of NPC are displayed in
Table I. High expression of ROCK1
was significantly associated with advanced N stage (P=0.032). High
expression of PIK3CA was significantly associated with advanced N
stage (P=0.027) and TNM cancer stage (P=0.019). Furthermore,
χ2 and correlation analyses demonstrated that ROCK1
expression was significantly positively correlated with PIK3CA
expression, (P=0.01; r=0.313; Table
II). No statistically significant associations were observed
between ROCK1 and PIK3CA expression and patient gender, age or T
stage.
 | Table II.Correlation between the expression of
ROCK1 and PIK3CA in 81 cases of nasopharyngeal carcinoma. |
Table II.
Correlation between the expression of
ROCK1 and PIK3CA in 81 cases of nasopharyngeal carcinoma.
|
|
| PIK3CA |
|
|---|
|
|
|
|
|
|---|
| ROCK1 | Number of
cases | Low, n (% of
ROCK1) | High, n (% of
ROCK1) | P-value |
|---|
| Low | 58 | 27 (46.6) | 31 (53.4) | 0.01 |
| High | 23 | 3
(13.0) | 20 (87.0) |
|
Discussion
NPC is a heterogeneous tumor type and patients with
similar clinical and pathological features have varied outcomes,
highlighting the underlying diversity of this disease (14). Therefore, it is necessary to identify
effective prognostic factors that may represent potential molecular
targets to develop effective therapeutic treatments for NPC.
Previous studies have suggested that high level
expression of ROCK1 may promote cell invasion (15,16). The
ROCK pathway is one of the most important mechanisms of cellular
invasion (17). When activated, Rho
GTP binds to ROCK and the protein conformation of ROCK alters to
fully expose the catalytic domain, allowing ROCK to phosphorylate
downstream effector molecules (18).
Increased expression of ROCK1 has frequently been observed in
invasive and metastatic laryngeal squamous cell carcinoma, prostate
cancer and several other malignant carcinomas, and it has been
suggested that it serves a key role in promoting tumor invasion and
metastasis (19,20). In the present study, elevated
expression of ROCK1 was observed in 28.40% of NPC specimens, and
high ROCK1 expression was significantly associated with advanced N
stage. N stage is the most important predictor of survival in NPC
(21), reflecting the potential for
the tumor to invade and metastasize. To the best of our knowledge,
this is the first report detailing the association between ROCK1
and N category in NPC. The results of the present study suggest
that ROCK1 may be associated with tumor metastases.
PIK3CA is associated with cancer growth, invasion
and metastasis (22). PIK3CA
mutations are typically detected in a wide range of cancers,
including head and neck squamous cell carcinoma, colorectal cancer
and breast cancer (23–27). In the present study, high levels of
PIK3CA expression were detected in 62.96% of the NPC specimens.
This indicates that PIK3CA may function as a tumor promoter in NPC.
One of the aims of the present study was to assess the association
between PIK3CA expression and the clinicopathological factors of
NPC. Based on the results of the present study, it may be suggested
that high PIK3CA expression is associated with advanced N stage and
TNM stage.
Ehrenschwender et al (28) demonstrated that mutations in PIK3CA
enabled tumor necrosis factor-related apoptosis-inducing ligand and
Fas ligand to induce non-apoptotic, caspase-8-mediated ROCK1
activation. The present study demonstrated that high PIK3CA
expression was significantly correlated with high ROCK1 expression.
The results of the present study, together with those of
Ehrenschwender et al (28),
indicate that high expression of PIK3CA may promote tumor
proliferation, malignant transformation and invasion via the ROCK
pathway. Additionally, co-expression of these markers may have a
more significant prognostic value than expression of one protein
alone. A longer follow-up time is required to determine the overall
survival of patients in the present study. Future research may
include survival analysis between ROCK1 and PIK3CA expression and
the overall survival of patients with NPC.
In conclusion, the results of the present study
indicate that elevated expressions of ROCK1 and PIK3CA are
correlated with the progression of NPC. The present study may
provide a basis for future research into cancer therapy by
employing these proteins as potential molecular targets. Further
studies are required to elucidate the mechanisms by which ROCK1 and
PIK3CA contribute to NPC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81274141, 81450051,
81573656 and 81403232), the Plans of Colleges and Universities in
Jiangsu Province to Postgraduate Research and Innovation (grant no.
KYZZ15-0368), the Foundation of SuBei People's Hospital (grant no.
yzucms201409), and the Natural Science Foundation of Jiangsu
Province (grant no. SBK2014021480).
References
|
1
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perri F, Dell'Oca I, Muto P, Schiavone C,
Aversa C, Fulciniti F, Solla R, Scarpati GD, Buonerba C, Di Lorenzo
G and Caponigro F: Optimal management of a patient with recurrent
nasopharyngeal carcinoma. World J Clin Cases. 2:297–300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang T, Riaz N, Cheng S, Lu J and Lee N:
Intensity-modulated radiation therapy for nasopharyngeal carcinoma:
A review. J Radiat Oncol. 1:129–146. 2012. View Article : Google Scholar
|
|
4
|
Jin Y, Cai XY, Cai YC, Cao Y, Xia Q, Tan
YT, Jiang WQ and Shi YX: To build a prognostic score model
containing indispensible tumor markers for metastatic
nasopharyngeal carcinoma in an epidemic area. Eur J Cancer.
48:882–888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee AW, Ng WT, Chan YH, Sze H, Chan C and
Lam TH: The battle against nasopharyngeal cancer. Radiother Oncol.
104:272–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang W, Zeng Z, Wei F, Chen P, Schmitt
DC, Fan S, Guo X, Liang F, Shi L, Liu Z, et al: SPLUNC1 is
associated with nasopharyngeal carcinoma prognosis and plays an
important role in all-trans-retinoic acid-induced growth inhibition
and differentiation in nasopharyngeal cancer cells. FEBS J.
281:4815–4829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Li C, Li D, Xie Y, Shi J, Li G, Guan
Y, Li M, Zhang P, Peng F, et al: Periostin, a stroma-associated
protein, correlates with tumor invasiveness and progression in
nasopharyngeal carcinoma. Clin Exp Metastasis. 29:865–877. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newell-Litwa KA, Badoual M, Asmussen H,
Patel H, Whitmore L and Horwitz AR: ROCK1 and 2 differentially
regulate actomyosin organization to drive cell and synaptic
polarity. J Cell Biol. 210:225–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Y, Pontrello CG, DeFea KA, Reichardt
LF and Ethell IM: Focal adhesion kinase acts downstream of EphB
receptors to maintain mature dendritic spines by regulating cofilin
activity. J Neurosci. 29:8129–8142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai K, Killingsworth MC and Lee CS: Gene
of the month: PIK3CA. J Clin Pathol. 68:253–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Q, Zhang B, Shao Y, Chen L, Wang X,
Zhang Z, Shu Y and Guo R: Correlation between the expression levels
of miR-1 and PIK3CA in non-small-cell lung cancer and their
relationship with clinical characteristics and prognosis. Future
Oncol. 10:49–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A: Pharynx. AJCC Cancer Staging Manual. 7th.
Springer Science & Business Media LLC; NY: pp. 41–49. 2010
|
|
13
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng Z, Huang H, Zhang W, Xiang B, Zhou M,
Zhou Y, Ma J, Yi M, Li X, Li X, et al: Nasopharyngeal carcinoma:
Advances in genomics and molecular genetics. Sci China Life Sci.
54:966–975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vigil D, Kim TY, Plachco A, Garton AJ,
Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL and
Der CJ: ROCK1 and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Ye L, Zhang L and Jiang WG:
Placenta growth factor, PLGF, influences the motility of lung
cancer cells, the role of Rho associated kinase, Rock1. J Cell
Biochem. 105:313–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tripathi V, Popescu NC and Zimonjic DB:
DLC1 induces expression of E-cadherin in prostate cancer cells
through Rho pathway and suppresses invasion. Oncogene. 33:724–733.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Croft DR, Crighton D, Samuel MS, Lourenco
FC, Munro J, Wood J, Bensaad K, Vousden KH, Sansom OJ, Ryan KM and
Olson MF: p53-mediated transcriptional regulation and activation of
the actin cytoskeleton regulatory RhoC to LIMK2 signaling pathway
promotes cell survival. Cell Res. 21:666–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, He X, Ma Y, Liu Y, Shi H, Guo W
and Liu L: Overexpression of ROCK1 and ROCK2 inhibits human
laryngeal squamous cell carcinoma. Int J Clin Exp Pathol.
8:244–251. 2015.PubMed/NCBI
|
|
20
|
Zhang C, Zhang S, Zhang Z, He J, Xu Y and
Liu S: ROCK has a crucial role in regulating prostate tumor growth
through interaction with c-Myc. Oncogene. 33:5582–5591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen KW, Wang WY, Liang WM, Twu CW, Chao
JY, Liang KL, Wu CT, Jiang RS, Shih YT and Lin JC: The volume of
retropharyngeal nodes predicts distant metastasis in patients with
advanced nasopharyngeal carcinoma. Oral Oncol. 47:1171–1175. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Psyrri A, Seiwert TY and Jimeno A:
Molecular pathways in head and neck cancer: EGFR, PI3K and more. Am
Soc Clin Oncol Educ Book. 246–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang YS, Hsu HT, Ko YC, Yeh KT, Chang SJ,
Lin CY and Chang JG: Combined mutational analysis of RAS, BRAF,
PIK3CA, and TP53 genes in Taiwanese patients with oral squamous
cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol.
118:110–116.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu W, Tong GX, Manolidis S, Close LG,
Assaad AM and Su GH: Novel mutant-enriched sequencing identified
high frequency of PIK3CA mutations in pharyngeal cancer. Int J
Cancer. 122:1189–1194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barault L, Veyrie N, Jooste V, Lecorre D,
Chapusot C, Ferraz JM, Lièvre A, Cortet M, Bouvier AM, Rat P, et
al: Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH
kinase) signaling network correlate with poor survival in a
population-based series of colon cancers. Int J Cancer.
122:2255–2259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalinsky K, Jacks LM, Heguy A, Patil S,
Drobnjak M, Bhanot UK, Hedvat CV, Traina TA, Solit D, Gerald W and
Moynahan ME: PIK3CA mutation associates with improved outcome in
breast cancer. Clin Cancer Res. 15:5049–5059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ehrenschwender M, Siegmund D, Wicovsky A,
Kracht M, Dittrich-Breiholz O, Spindler V, Waschke J, Kalthoff H,
Trauzold A and Wajant H: Mutant PIK3CA licenses TRAIL and CD95L to
induce non-apoptotic caspase-8-mediated ROCK activation. Cell Death
Differ. 17:1435–1447. 2010. View Article : Google Scholar : PubMed/NCBI
|