Introduction
The health and homeostasis of blood vessels is
dependent on the integrity of the endothelial lining (1). Endothelial cells (ECs) have an
important role in vascular homeostasis and have been implicated in
various diseases; their dysfunction marks an early stage of
atherosclerosis (2). Cardiovascular
risk factors, including hyperlipidemia, insulin resistance and
vascular inflammation, may induce endothelial injury and apoptosis
during atherosclerosis (3).
Endothelial injury and dysfunction may be repaired by adjacent
viable ECs in a process involving cellular proliferation, migration
and apoptosis (4,5). An effective strategy for the prevention
or treatment of atherosclerosis is the maintenance of endothelial
barrier integrity, which is achieved by preserving the
proliferative capacity and inhibiting the apoptosis of ECs
(6). Oxidized low-density
lipoprotein (ox-LDL) is a critical marker of atherosclerosis
(7). Ox-LDL induces vascular EC
activation and dysfunction, which induces the pro-adhesive
properties of ECs and promotes the recruitment of monocytes,
leading to subsequent inflammation and the accumulation of
lipid-rich macrophages and proinflammatory lymphocytes within the
intima of artery walls (7). This
ultimately leads to the formation of atherosclerotic plaques
(7). Ox-LDL induces pro-inflammatory
responses, pro-oxidative conditions and EC apoptosis (8,9). Since
ox-LDL is a major risk factor for the onset and development of
atherosclerosis (7), the present
study used ox-LDL-treated human umbilical vein ECs (HUVECs) as an
experimental model to identify the specific microRNAs (miRNAs)
associated with endothelial dysfunction in atherosclerosis.
miRNAs are a family of highly conserved, small
non-coding RNAs that post-transcriptionally repress the expression
of their target genes by inducing the degradation of their target
mRNA or inhibiting their translation (10). miRNAs are involved in diverse
cellular functions, including differentiation, growth,
proliferation and apoptosis (10).
Some specific miRNAs have previously been implicated in the
pathogenesis of atherosclerosis by regulating various cellular
functions and the properties of vascular cells (11). In addition, miRNAs have been
demonstrated to be differentially expressed at the various stages
of atherosclerosis (12), and
abnormally expressed miRNAs have been closely associated with the
pathogenesis of atherosclerosis (12). For example, a previous study
demonstrated that miR-21 was involved in atherosclerosis by
regulating the function of arterial smooth muscle cells (13). Furthermore miR-133a was shown to
regulate the functions of arterial smooth muscle cells by targeting
RhoA, and was involved in the pathogenesis of arterial sclerosis
occlusion, which is a type of atherosclerosis affecting the lower
extremities (14). In our previous
study, a miRNA microarray analysis demonstrated that miR-98 was
downregulated in atherosclerotic tissue (13), thus suggesting that miR-98 may be
involved in the pathogenesis of atherosclerosis. Furthermore,
miR-98 was identified as a modulator of proliferation and apoptosis
in numerous types of cancer cells (15). miR-98 was demonstrated to suppress
the P53 tumor suppressor gene and has been suggested to be involved
in the potential therapeutic effect of
(−)-epigallocatechin-3-gallate for the treatment of various types
of non-small-cell lung cancers (16). Based on these results, the authors of
the present study hypothesized that miR-98 may be a regulator of
endothelial function.
Lectin-like oxidized low-density lipoprotein
receptor 1 (LOX-1), which has a C-type lectin-like extracellular
domain and a short cytoplasmic tail, is a novel type II membrane
protein receptor for ox-LDL (17).
In addition, LOX-1 is a key molecule involved in the pathogenesis
of atherosclerosis (17), and is a
critical receptor that induces the uptake of toxic ox-LDL by ECs,
which subsequently results in a series of vascular pathological
processes (18). Under certain
pathological conditions, high LOX-1 expression may be harmful to
vascular cells, in particular ECs, inducing vascular cell injury by
decreasing cellular viability, reducing cell growth and
proliferation and promoting cell death (19,20).
Therefore, LOX-1 may be considered a potential target for the
regulation of EC turnover. The present study aimed to investigate
the role of miR-98 in ox-LDL-induced dysfunction of ECs and the
underlying mechanism.
Materials and methods
Reagents
miR-98 mimics and inhibitor, and their control
oligonucleotides (oligos), were purchased from Guangzhou RiboBio,
Co., Ltd. (Guangzhou, China). Lipofectamine® RNAiMAX
transfection reagent was purchased from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Ox-LDL and
1,1′-dioctadecyl-3,3,3′3′-tetra-methylindocyanide perchlorate
(Dil)-labeled ox-LDL (Dil-ox-LDL) were obtained from Yiyuan
Biological Technology, Co., Ltd. (Guangzhou, China). The Cell
Counting kit-8 (CCK-8) Assay kit and the Annexin V/Propidium Iodide
(PI) Flow Cytometry Detection kit were purchased from Dojindo
Molecular Technologies, Inc. (Shanghai, China). The EdU Assay and
Hoechst 33342 Assay kits were purchased from Guangzhou RiboBio,
Co., Ltd. Rabbit anti-human LOX-1 monoclonal antibody (cat. no.
ab175922) was purchased from Abcam (Cambridge, UK). Rabbit
anti-human B-cell lymphoma-2 (Bcl-2) monoclonal antibody (cat. no.
2870S), rabbit anti-Bcl-2-associated X protein (Bax) polyclonal
antibody (cat. no. 2772S), rabbit anti-caspase-3 polyclonal
antibody (cat. no. 9662S) and rabbit anti-β-actin monoclonal
antibody (cat. no. 8457S) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Primers for LOX-1,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), miR-98 and U6
were obtained from Takara Biotechnology, Co., Ltd. (Dalian,
China).
Cell culture
HUVECs were purchased from Fuxiang Biotechnology,
Co., Ltd. (Shanghai, China) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) and 100 µg/ml penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.).
RNA interference
miR-98 mimics were used to imitate miR-98 and an
miR-98 inhibitor to deplete endogenous miR-98. HUVECs were seeded
into wells 24 h prior to transfection. The cells were transfected
with the miR-98 mimics, miR-98 inhibitor or their control oligos
(60 nmol/l) using Lipofectamine® RNAiMAX transfection
reagent, according to the manufacturer's protocol. The transfection
medium was replaced with fresh DMEM at 8 h following
transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
HUVECs (2×105 cells/well) were seeded
into 6-well plates 12 h prior to transfection. Ox-LDL was added to
the cells 24 h following transfection and the cells were incubated
for an additional 48 h. After washing the cells twice with
phosphate-buffered saline (PBS), total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Total RNA was
reverse-transcribed into cDNA using the PrimeScript RT Reagent kit
(Takara Biotechnology, Co., Ltd.). cDNA was amplified by qPCR using
SYBR Premix Ex Taq II (Takara Biotechnology, Co., Ltd.) on a
CFX96 thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
according to the manufacturer's protocol. For miR-98 amplification,
the PCR conditions were as follows: One cycle at 95°C for 10 sec;
39 cycles at 95°C for 5 sec and 60°C for 20 sec; one cycle at 95°C
for 15 sec and 65°C for 10 sec; and a final increase to 95°C. For
LOX-1 mRNA amplification, the PCR conditions were as follows: One
cycle at 95°C for 30 sec; 39 cycles at 95°C for 3 sec and 60°C for
30 sec, one cycle at 95°C for 15 sec and 60°C for 10 sec; and a
final increase to 95°C. The relative LOX-1 and miR-98 expression
levels were calculated using the 2−ΔΔCq method (21), following normalization to the GAPDH
and U6 small nuclear RNA internal controls, respectively. The
sequences of the primers were as follows: LOX-1 forward,
5′-GTCCATAGGGCACTTCCAGAAA-3′ and reverse,
5′-TGCTCGGAAGCTGAATGAGAA-3′; GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′;
miR-98, 5′-CCGAGGTAGTAAGTTGTATTGTT-3′; and U6,
5′-ACGCAAATTCGTGAAGCGTT-3′ (Takara Biotechnology, Co., Ltd.).
Western blot analysis
HUVECs (2×105 cells/well) were seeded
into 6-well plates 12 h prior to transfection. Ox-LDL was added to
the cells 24 h following transfection and the cells were incubated
for an additional 48 h. The cells were washed twice with PBS and
lysed in radioimmunoprecipitation assay lysis buffer (Cell
Signaling Technology, Inc.), supplemented with protease inhibitors
(Roche Diagnostics, Basel, Switzerland). The total protein
concentrations were determined using a BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). The protein lysates were
separated on an 8–12% gradient gel by SDS-PAGE and transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 5% non-fat dry milk in PBS
buffer for 1 h at room temperature, then incubated with rabbit
anti-human LOX-1, Bcl-2, Bax, β-actin, and caspase-3 primary
antibodies (dilutions, 1:1,000) overnight at 4°C. Subsequently, the
membrane was incubated with the secondary antibody for 1 h at room
temperature and the immunoblotting signal was developed using an
Enhanced Chemiluminescence Western Blotting Analysis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and exposed on X-ray
films (Kodak, Rochester, NY, USA). The relative protein expression
levels were normalized to β-actin and analyzed using ImageJ
software (https://imagej.nih.gov/ij/).
Cell proliferation
HUVECs (1×104 cells/well) were seeded
into 96-well plates in triplicate 12 h prior to transfection.
Ox-LDL (80 µg/ml) was added to the cells 24 h following
transfection and the cells were incubated for an additional 48 h.
For the CCK-8 assay, 10 µl CCK-8 solution was added to each well
and incubated for 1 h, after which the absorbance values were
measured at 450 nm using a spectrophotometer. For the EdU assay,
the cells were incubated with 50 µM EdU for 2 h and then fixed
using 4% paraformaldehyde for 15 min. The cells were subsequently
treated with 100 µl Apollo reaction cocktail for 30 min, followed
by 50 µl 1% Hoechst 33342 for 30 min for nuclei staining. Finally,
an inverted fluorescence microscope was used for visualization. A
total of 10 random microscope fields in each well were imaged, and
the number of Apollo-positive nuclei was divided by the total
number of nuclei stained with Hoechst 33342. The fluorescence
images were analyzed using Image-Pro® Plus software 6.0
(Media Cybernetics, Rockville, MD, USA).
Fluorescence microscopy analysis of
Dil-ox-LDL uptake and cellular apoptosis
HUVECs (1×104 cells/well) were seeded
into 96-well plates in triplicate 12 h prior to transfection.
Ox-LDL (80 µg/ml) was added to the cells 24 h following
transfection and the cells were incubated for an additional 48 h.
To visualize and assess ox-LDL uptake, the HUVECs were incubated
with 20 µg/ml Dil-ox-LDL for 3 h at 37°C. Following incubation, the
cells were gently washed three times with PBS and immediately
imaged using an inverted fluorescence microscope. To visualize the
morphological changes in the nuclear chromatin of apoptotic cells,
the cells were washed three times with PBS and fixed using 4%
paraformaldehyde for 15 min, followed by another three washes with
PBS. The fixed cells were incubated with 100 µl Hoechst 33342
solution (5 µg/ml dissolved in PBS) for 20 min in the dark. HUVEC
nuclear staining was examined using an inverted fluorescence
microscope. Viable HUVEC nuclei exhibited a pale blue color with
organized structures, whereas the nuclei of apoptotic HUVECs
exhibited a bright blue color and were reduced in size.
Flow cytometric analysis of cellular
apoptosis
HUVECs (2×105 cells/well) were seeded
into 6-well plates in triplicate 12 h prior to transfection. Ox-LDL
(80 µg/ml) was added to the cells 24 h following transfection and
the cells were incubated for an additional 48 h. The cells were
washed twice with PBS, digested with EDTA-free trypsin and washed
three times with PBS. Subsequently, the cells were double-stained
using the Annexin V/PI Flow Cytometry Detection kit, according to
the manufacturer's protocol. The percentages of viable cells
(Annexin V- and PI-negative), apoptotic cells (Annexin V-positive
and PI-negative) and necrotic cells (Annexin V- and PI-positive)
were detected using an EPICS XL-MCL™ Flow Cytometer (Beckman
Coulter, Inc., Fullerton, CA, USA). The results were analyzed using
Kaluza 1.2 software (Beckman Coulter, Inc.).
Luciferase assay
Using TargetScan 6.2, miR-98 was predicted a
potential target region in position 447–453 of LOX-1 mRNA
3′-untranslate dregion (UTR). The 3′-UTR miR-98 binding sites of
LOX-1 mRNA were amplified by PCR and cloned into the pLUC
luciferase reporter vector (Guangzhou Liang Zi Kang Biotechnology,
Co., Ltd., Guangzhou, China) to construct the fluorescent reporter
system. A pLUC vector containing the LOX-1 mRNA 3′-UTR with mutated
binding sites for miR-98 was also generated. HEK293T cells
(Guangzhou Liang Zi Kang Biotechnology, Guangzhou, China) were
cultured in 96-well plates in triplicate at 37°C until 70%
confluence was reached, after which the cells were co-transfected
with the wild-type or mutant LOX-1 3′-UTR vectors and the miR-98
mimics or control oligos (100 nmol/l) using FuGENE® HD
(Roche Diagnostics), according to the manufacturer's protocol.
After 48 h, the cells were lysed and the luciferase activity was
measured using the Dual-Glo™ Luciferase Assay kit (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three repeated experiments and were analyzed using
Student's t-test to compare two conditions. One-way analysis of
variance was used for comparing three or more conditions.
Statistical analyses were performed using GraphPad Prism software,
version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
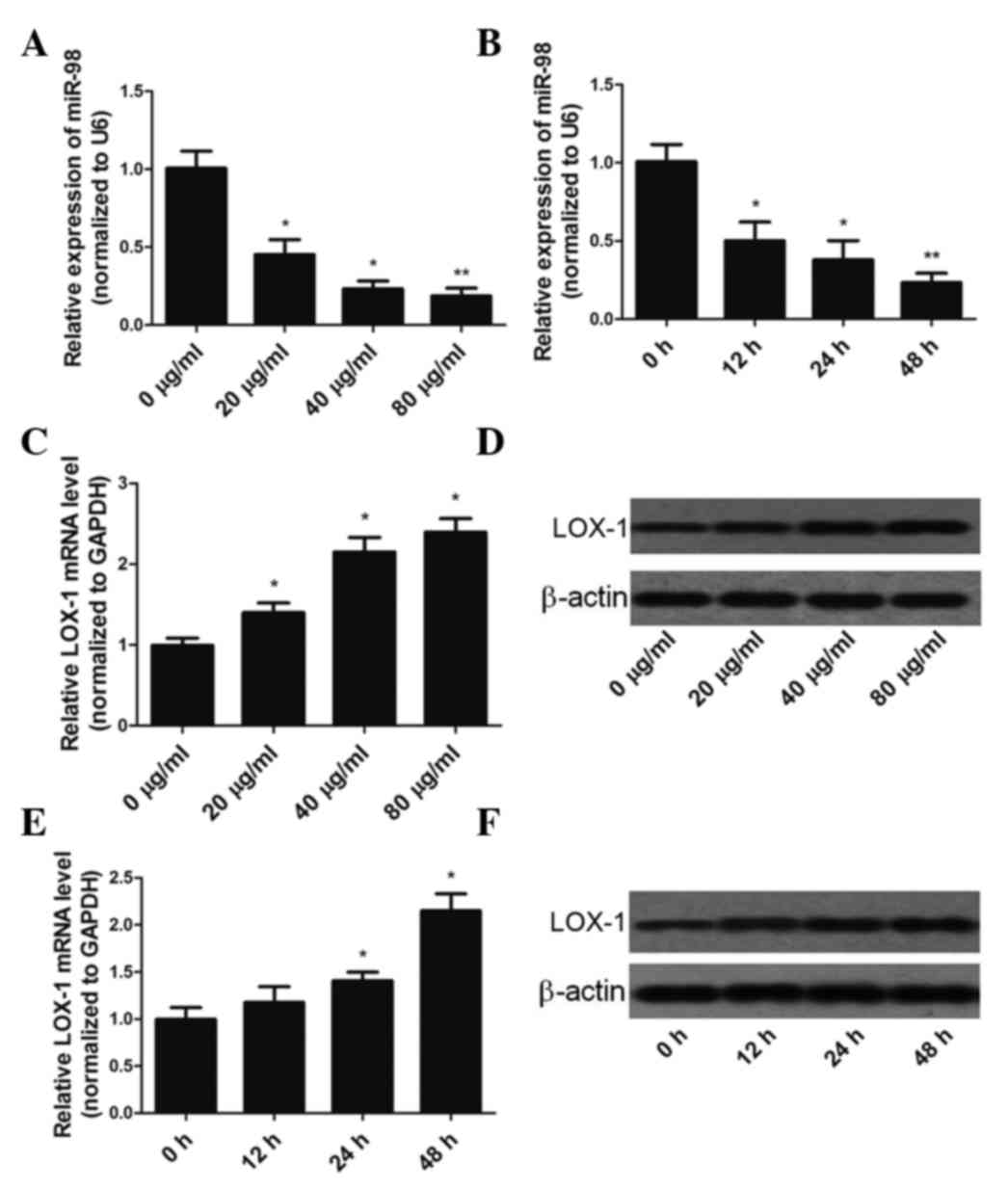
Downregulation of miR-98 expression
upregulates the expression of LOX-1 in ox-LDL-treated HUVECs
HUVECs were exposed to ox-LDL at various
concentrations (20–80 µg/ml) for 48 h or 40 µg/ml ox-LDL for
various durations (0–48 h), and the expression levels of miR-98 and
LOX-1 mRNA were determined by RT-qPCR. Incubation of HUVECs with
0–80 µg/ml ox-LDL for 48 h resulted in a dose-dependent
downregulation of miR-98 expression (Fig. 1A). In addition, miR-98 expression was
gradually downregulated during 40 µg/ml ox-LDL treatment,
decreasing by >75% at the 48 h time-point (Fig. 1B). In HUVECs treated with 0–80 µg/ml
ox-LDL, a dose-dependent increase in LOX-1 mRNA and protein
expression levels was observed (Fig. 1C
and D). Incubation of HUVECs with 40 µg/ml ox-LDL for 0–48 h
resulted in a time-dependent increase in LOX-1 mRNA and protein
expression levels (Figs. 1E and
F).
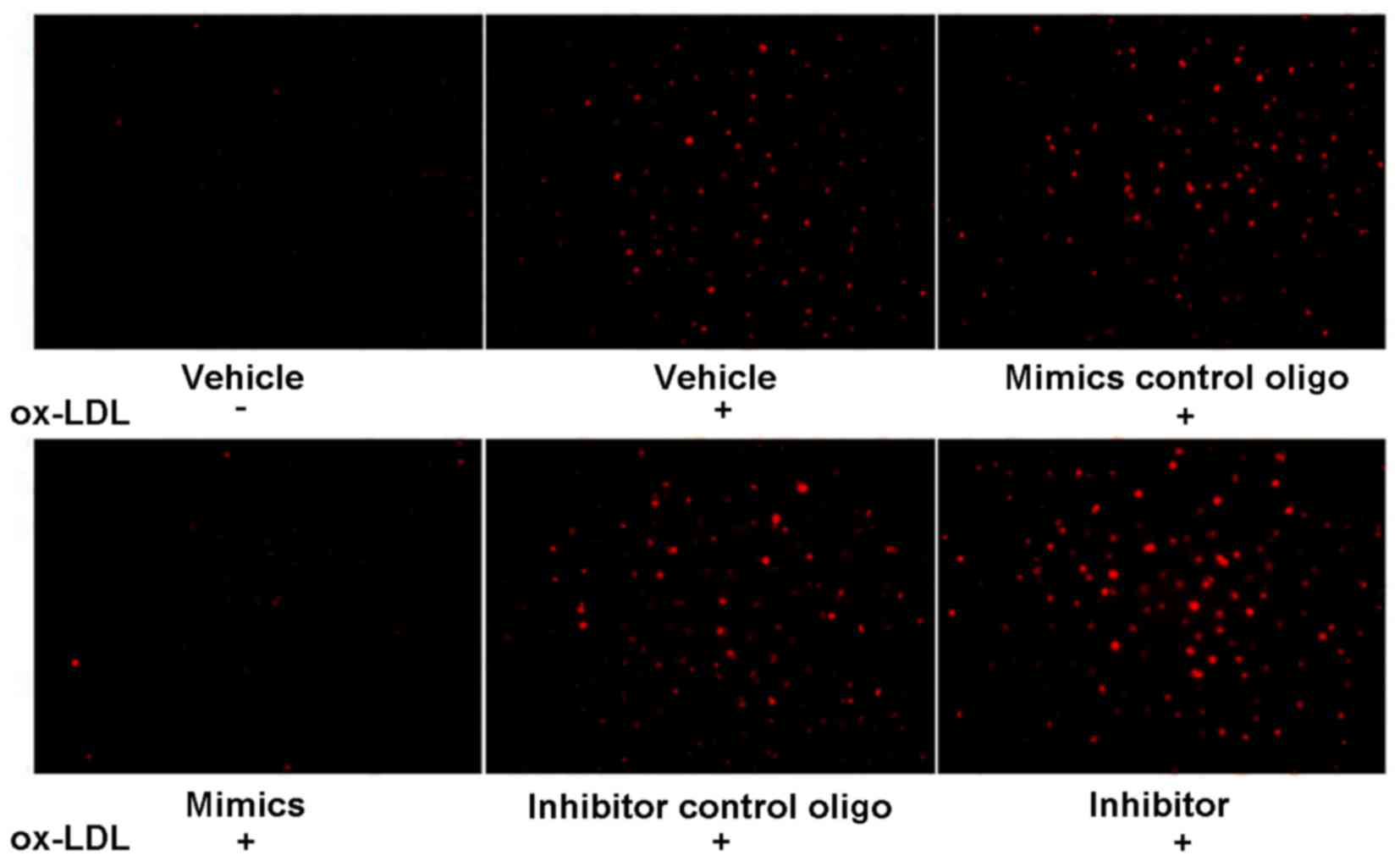
miR-98 regulates ox-LDL uptake by
HUVECs
To investigate the effects of miR-98 on the uptake
of ox-LDL by HUVECs, HUVECs were pretreated with ox-LDL (80 µg/ml)
for 48 h, followed by treatment with Dil-ox-LDL (20 µg/ml) for 3 h
at 37°C and visualization. As is shown in Fig. 2, ox-LDL enhanced the uptake of
Dil-ox-LDL. Furthermore, the miR-98 mimics blocked ox-LDL uptake,
whereas the miR-98 inhibitor increased ox-LDL uptake.
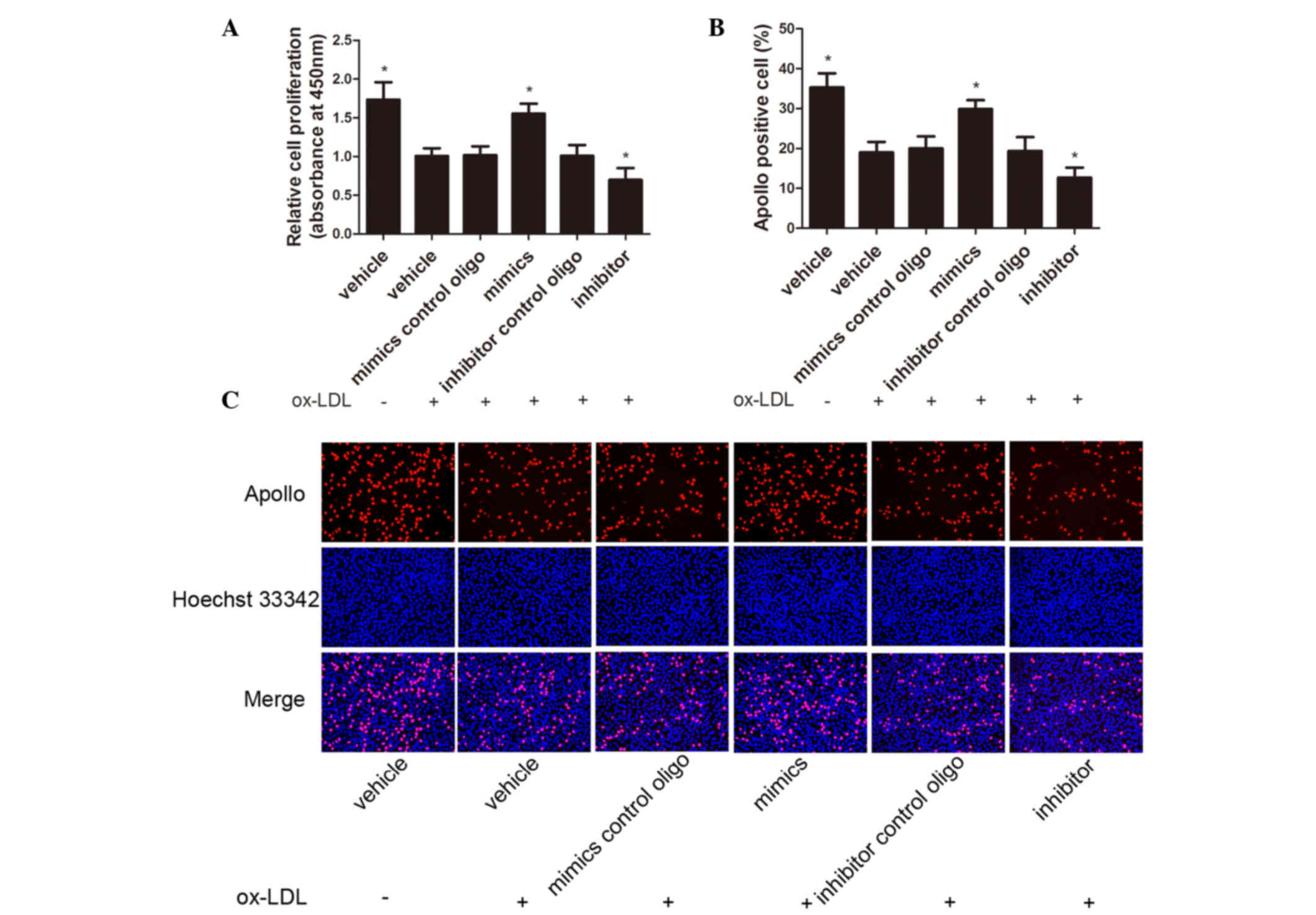
miR-98 regulates proliferation in
ox-LDL-treated HUVECs
To elucidate the role of miR-98 in HUVEC
proliferation, CCK-8 and EdU assays were performed. HUVECs were
transfected with the miR-98 mimics, miR-98 inhibitor or control
oligos and were then exposed to 80 µg/ml ox-LDL for 48 h. Exposure
of HUVECs to ox-LDL inhibited cell proliferation, which was
reversed by transfection with the miR-98 mimics. Conversely, the
miR-98 inhibitor suppressed the proliferation of HUVECs (Fig. 3A-C).
miR-98 inhibits apoptosis in
ox-LDL-treated HUVECs
To assess the effects of miR-98 on ox-LDL-induced
apoptosis of ECs, HUVECs were transfected with miR-98 mimics,
miR-98 inhibitor or control oligos and were then incubated with 80
µg/ml ox-LDL for 48 h. Subsequently, the levels of apoptosis were
assessed by determining the protein expression levels of LOX-1,
Bax, Bcl-2, caspase-3 and activated caspase-3 (p17). As is shown in
Fig. 4A, the expression levels of
LOX-1, Bax and activated caspase-3 (p17) were significantly
increased, and those of Bcl-2 were significantly decreased, in
HUVECs treated with 80 µg/ml ox-LDL for 48 h, as compared with the
vehicle group. In addition, the effects of ox-LDL on HUVECs were
attenuated by miR-98 mimics and aggravated by the inhibitor.
Subsequently, the levels of apoptosis were examined using
FITC-conjugated Annexin V/PI double-staining and flow cytometry. As
is shown in Fig. 4B and C, treatment
with ox-LDL induced apoptosis in HUVECs, and this was alleviated by
the miR-98 mimics and exacerbated by the miR-98 inhibitor. To
examine the morphological changes in nuclear chromatin, an inverted
fluorescence microscope was used to visualize Hoechst 33342
staining. The majority of nuclei in the vehicle group exhibited a
uniform pale blue color and had an organized structure, whereas
ox-LDL treatment resulted in nuclei with bright blue staining,
indicating pyknosis. The miR-98 mimics alleviated cell death,
whereas the miR-98 inhibitor enhanced apoptosis (Fig. 4D).
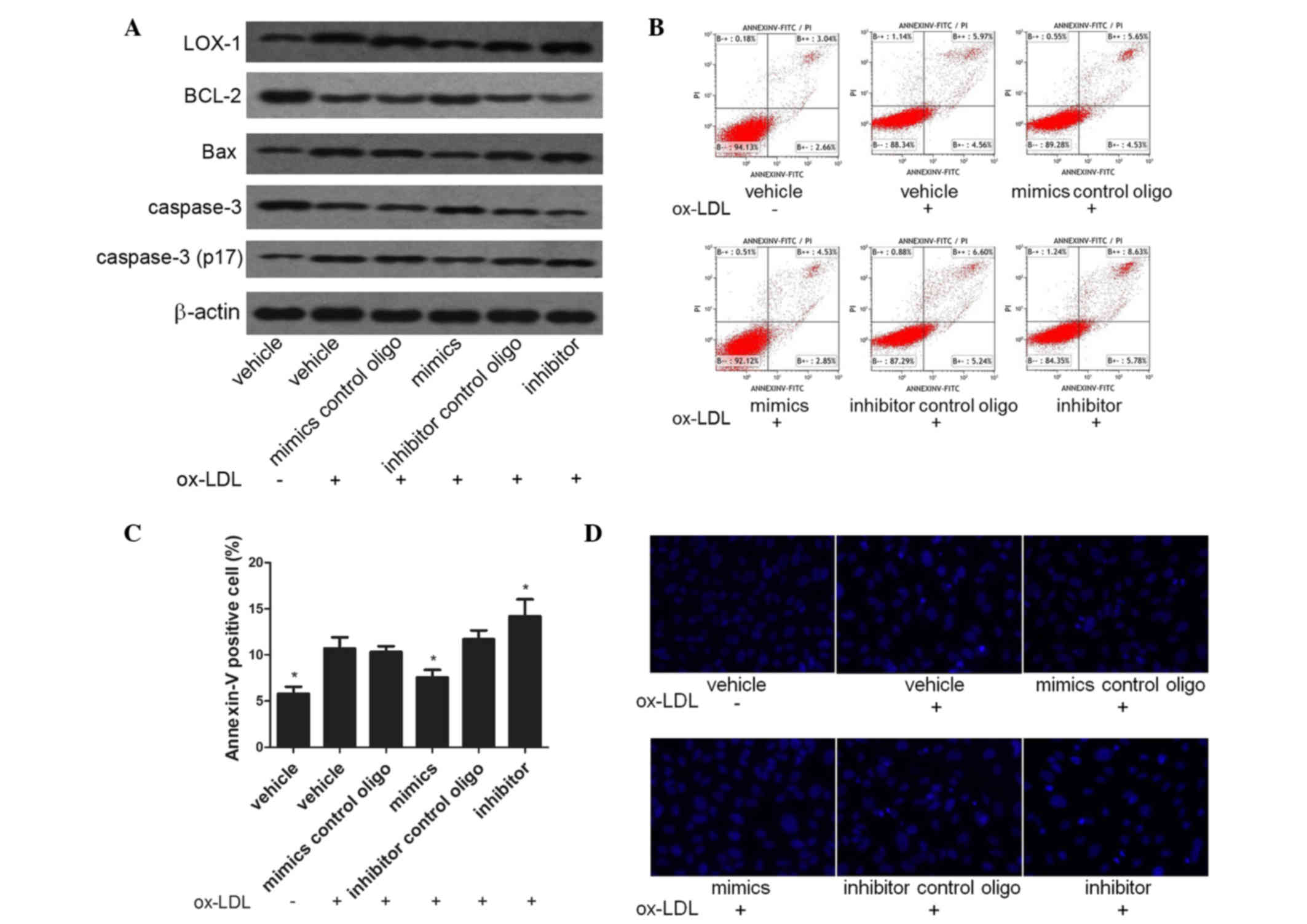 | Figure 4.Effects of miR-98 mimics and a miR-98
inhibitor on the apoptosis of ox-LDL-treated HUVECs. (A) The
protein expression levels of Bcl-2 were upregulated, and those of
LOX-1, Bax and cleaved caspase-3 were downregulated, in HUVECs
transfected with miR-98 mimics, as determined by western blotting.
(B and C) Ox-LDL-induced apoptosis levels were decreased in HUVECs
transfected with miR-98 mimics, whereas they were increased in
HUVECs transfected with a miR-98 inhibitor, as shown by an Annexin
V/PI dual-staining assay. (D) miR-98 mimics alleviated, whereas a
miR-98 inhibitor enhanced, cellular apoptosis and pyknosis in
ox-LDL-treated HUVECs, as demonstrated by the Hoechst 33342
staining assay. *P<0.05, vs. the vehicle and ox-LDL treatment
group. Oligo, oligonucleotide; miR-98, microRNA-98; ox-LDL,
oxidized low-density lipoprotein; HUVECs, human umbilical vein
endothelial cells; Bcl-2, B-cell lymphoma-2; LOX-1, lectin-like
oxidized low-density lipoprotein receptor 1; Bax, Bcl-2-associated
X protein; PI, propidium iodide. |
LOX-1 is a direct target of
miR-98
LOX-1 was selected as a potential target of miR-98,
since its mRNA 3′-UTR was complementary to miR-98 (predicted by
TargetScan 6.2) (Fig. 5A). The
protein expression levels of LOX-1 were decreased in HUVECs
transfected with miR-98 mimics, as compared with cells transfected
with control oligos (Fig. 5B).
Conversely, the protein expression levels of LOX-1 were increased
in HUVECs transfected with miR-98 inhibitor (Fig. 5C). Notably, the effect of the miR-98
mimics on LOX-1 mRNA expression levels was similar to that on LOX-1
protein expression levels (Fig. 5D).
These results suggest that miR-98 may bind to the 3′-UTR of LOX-1
mRNA and induce mRNA degradation, resulting in the downregulation
of LOX-1 expression at the mRNA and protein levels. In order to
determine whether miR-98 directly binds to the 3′-UTR of LOX-1 mRNA
a luciferase reporter assay was performed. The miR-98 mimics
significantly reduced luciferase activity in the presence of the
LOX-1 3′-UTR (wild-type 3′-UTR), as compared with the negative
control (Fig. 5E). However, when the
3′-UTR of the LOX-1 mRNA was mutated to disrupt the miR-98 binding
site (Fig. 5A), the inhibitory
effect of miR-98 on the relative luciferase activity was partially
reversed (Fig. 5E). These results
suggest that LOX-1 is a direct target of miR-98.
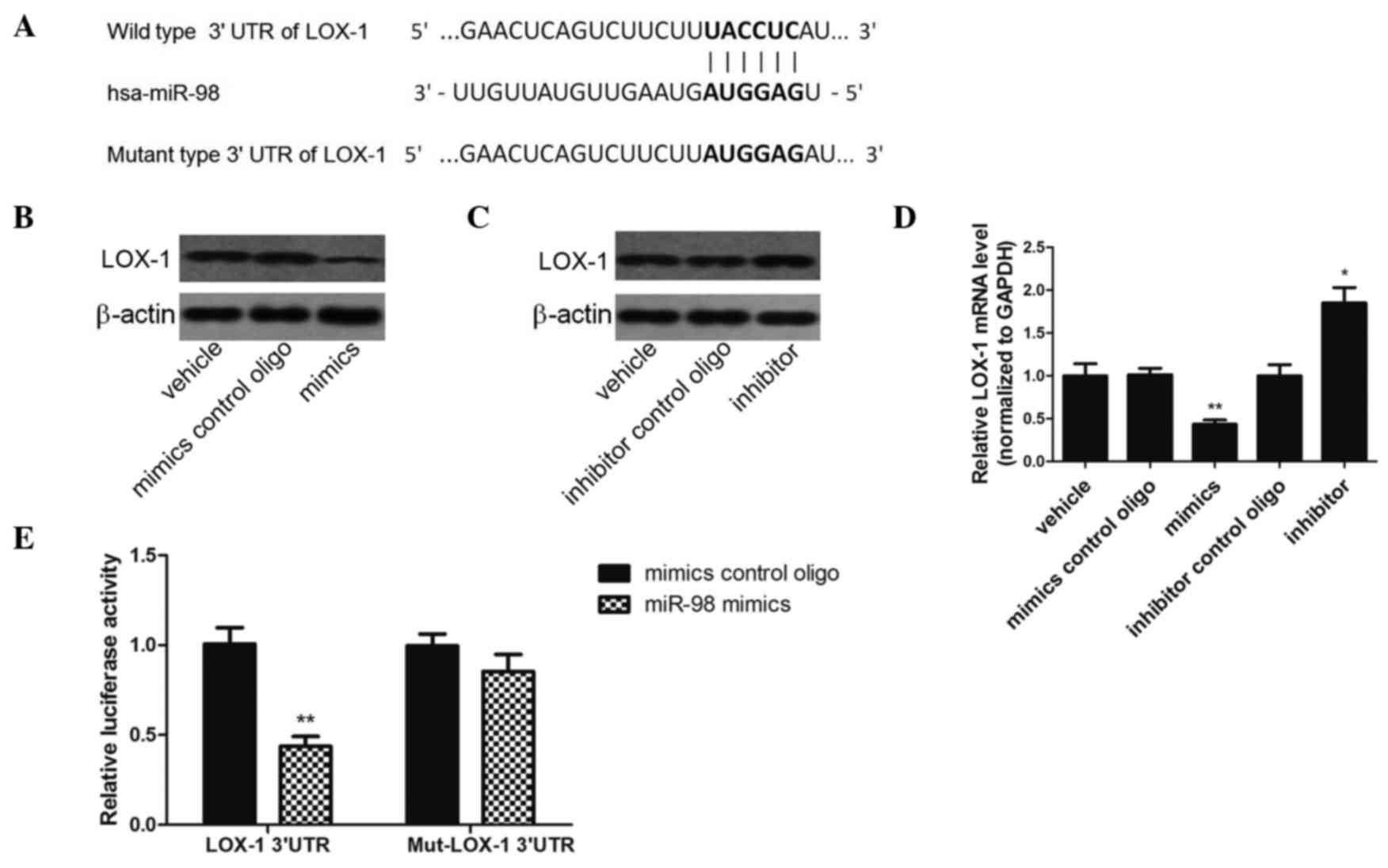 | Figure 5.LOX-1 is a direct target of miR-98.
(A) Depiction of the miR-98 seed sequence in the wild-type 3′-UTR
of LOX-1 and the mutated site. (B and C) The protein expression
levels of LOX-1 were downregulated by the miR-98 mimics (60 nmol/l)
and upregulated by the miR-98 inhibitor (60 nmol/l), as assessed by
western blotting. (D) The mRNA expression levels of LOX-1 were
downregulated by miR-98 mimics and upregulated by the miR-98
inhibitor (60 nmol/l), as assessed by reverse
transcription-quantitative polymerase chain reaction. *P<0.05,
**P<0.01, vs. the vehicle group. (E) Co-transfection of miR-98
with the wild-type LOX-1 3′-UTR in HEK293T cells led to a marked
decrease in luciferase activity, whereas co-transfection of miR-98
with the mutant LOX-1 3′-UTR had no effect on luciferase activity.
The luciferase activities were measured using the dual-luciferase
reporter assay. **P<0.01, vs. the mimics control group. LOX-1,
lectin-like oxidized low-density lipoprotein receptor 1; miR-98,
microRNA-98; 3′-UTR, 3′-untranslated region; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; oligo, oligonucleotide. |
Discussion
The present study demonstrated that miR-98
expression was essential for the replicative response of HUVECs to
ox-LDL injury. In addition, miR-98 was shown to be essential for
the protective effect against ox-LDL-induced apoptosis by targeting
the ox-LDL membrane receptor, LOX-1. The ability of miR-98 to
preserve EC proliferation and inhibit apoptosis may be responsible
for the reduction in lesion formation at regions where endothelial
permeability is increased due to hyperlipidemic stress.
Endothelial dysfunction, which includes cell
proliferation defects and aberrant apoptosis, is a key
characteristic of atherosclerosis (22). Hyperlipidemia in atherosclerosis
typically leads to EC injury and death, as well as detachment of
ECs from the vascular wall, loss of anti-adhesive properties toward
leukocytes and an increased permeability of the endothelial wall to
circulating lipids, all of which lead to atherosclerotic lesion
formation (23). Therefore, the
proliferative capacity of ECs is essential for maintaining
endothelial wall integrity by repairing the endothelial lining of
the vessel in response to injury. Elevated levels of ox-LDL as a
result of hyperlipidemia have been demonstrated to inhibit the
proliferation of ECs by suppressing basic fibroblast growth factor
expression, which is essential for endothelial proliferation
(24). The mitogen-activated protein
kinase/extracellular-signal-regulated kinase 1/2 signaling pathway
is involved in the cytotoxic effects of ox-LDL that inhibit the
proliferation of HUVECs (25). The
cytotoxicity of ox-LDL in HUVECs is dependent on the LOX-1 receptor
(26). Therefore rescuing EC
proliferation, which represents a potential therapeutic approach
for limiting the development of atherosclerosis (27), may be achieved by inhibiting the
expression of LOX-1.
LOX-1 is located at the EC membrane and is highly
expressed under specific pathological conditions, including during
the early stage of atherosclerosis (19). Under acute inflammatory conditions,
the lectin-like extracellular domain of LOX-1 may be released from
the cell membrane into circulation as the soluble LOX-1 (sLOX-1)
protein (28,29). Therefore, sLOX-1 may be considered a
potential diagnostic and prognostic marker for
atherosclerosis-associated events, including acute coronary
syndrome (28,29). LOX-1 is the major receptor for ox-LDL
uptake by ECs and induces a series of pathological processes in ECs
(30). A previous study demonstrated
that endothelial-specific LOX-1 overexpression enhanced aortic
ox-LDL levels, resulting in endothelial dysfunction, vascular
inflammation and plaque formation (31).
Stimulation of ECs with ox-LDL has previously been
demonstrated to promote the expression of LOX-1 (32), and this was consistent with the
present study; pretreatment of HUVECs with ox-LDL upregulated the
LOX-1 receptor at the transcriptional level in a time- and
concentration-dependent manner. In turn, elevated levels of LOX-1
facilitated ox-LDL uptake, resulting in pathological processes,
including uptake of ox-DL and HUVEC apoptosis. LOX-1 has a critical
role in the ox-LDL/LOX-1 positive feedback loop (33). In a previous study, ox-LDL was
demonstrated to be taken up by LOX-1 and subsequently induced
pathological changes in HUVECs, including senescence and apoptosis
(34). These results suggested that
LOX-1 may be a promising target for the treatment of vascular
endothelial dysfunction.
LOX-1 mediates ox-LDL-induced apoptosis in ECs
(35). Cellular apoptosis is induced
via two major signaling pathways involving death receptors or the
mitochondrial signaling pathway. In a previous study, LOX-1 was
shown to mediate the ox-LDL-induced increase in Fas-mediated
apoptosis in HUVECs via the death receptors pathway (36). Conversely, LOX-1 triggered the
apoptotic cascade in human coronary artery ECs via the
mitochondrial pathway (37). In the
mitochondrial apoptotic signaling pathway, the cell membrane
potential is altered, leading to the release of cytochrome c
and activation of the caspase signaling pathway (37). It has also been suggested that LOX-1
may mediate ox-LDL-induced apoptosis in ECs via the endoplasmic
reticulum stress signaling pathway (38). All apoptosis pathways eventually
converge on caspase-3 activation (39), and thus caspase-3 is considered a
critical factor or regulator in the apoptotic process. Caspase-3
can be cleaved to form two mature subunits, including p17 (17 kDa)
and p12 (12 kDa) (40). The level of
cleaved caspase-3 is an indicator of the level of activated
caspase-3 and, in turn, the level of cellular apoptosis (40). The present study measured the levels
of p17 (17 kDa) by western blotting in order to assess the
apoptosis of HUVECs. Apoptosis is regulated by several
apoptosis-associated proteins, including Bcl-2, which serves as an
anti-death factor (41). Bcl-2 is an
integral mitochondrial membrane protein that forms heterodimers
with Bax to prevent mitochondrial alterations and the subsequent
release of cytochrome c and other pro-apoptotic factors from
the mitochondria (41). Conversely,
polymerized Bax reduces the mitochondrial membrane potential,
thereby inducing cytochrome c release and caspase
activation, and leading to apoptosis (42). Therefore, the expression ratio of
Bax/Bcl-2 regulates the induction of caspase activation pathways by
ox-LDL (43). LOX-1 mediates
ox-LDL-induced apoptosis in ECs and thus may be a potential target
for blocking the atherosclerosis process at an early stage
(44).
Considering the role of LOX-1 in suppressing the
proliferation and inducing the apoptosis of ECs in response to
ox-LDL, the present study assessed whether miRNAs were able to
regulate LOX-1 expression and activity in ox-LDL-treated HUVECs. A
bioinformatics analysis suggested that LOX-1 was a potential target
of miR-98. Therefore, in order to investigate the association
between miR-98 and LOX-1, the present study used miR-98 mimics and
a miR-98 inhibitor to regulate LOX-1 expression in HUVECs. The
miR-98 mimics decreased the expression of LOX-1 at the mRNA and
protein levels, thus suggesting that miR-98 may bind to a seed site
in the 3′-UTR of LOX-1 and promote LOX-1 mRNA degradation. In order
to determine whether LOX-1 is a direct target of miR-98, a dual
luciferase reporter assay was performed, which confirmed that
miR-98 was able to directly bind to the 3-UTR seed sequence of
LOX-1 mRNA. These results suggested that, by suppressing LOX-1
expression, miR-98 blocked ox-LDL uptake in HUVECs and alleviated
LOX-1-mediated HUVEC apoptosis.
In the present study, as well as enhancing the
protein expression levels of LOX-1, ox-LDL increased the cleavage
and activation of caspase-3 (p17), decreased the expression levels
of the anti-apoptotic protein Bcl-2, and increased the expression
levels of the pro-apoptotic Bax in HUVECs. Conversely, the miR-98
mimics downregulated LOX-1 and Bax expression levels, elevated the
expression levels of Bcl-2 and inhibited the activation of
caspase-3. The miR-98 inhibitor produced the opposite effects.
As well as suppressing the uptake of ox-LDL by
HUVECs, the miR-98 mimics were able to attenuate ox-LDL-mediated
suppression of HUVEC proliferation. These results suggested that
miR-98 may be essential for the growth and proliferation of HUVECs,
as well as the replicative endothelial regeneration process
required to repair the damaged endothelial lining in
atherosclerosis.
In conclusion, the present study demonstrated that
miR-98 exerted a protective effect on proliferation and
anti-apoptotic functions on ECs by suppressing LOX-1 expression in
response to ox-LDL. The results of the present study may provide
novel insights into the molecular mechanisms underlying endothelial
injury and into the pathogenesis of atherosclerosis. The authors of
the present study hypothesize that, as the functional roles of
other miRNAs are established, miRNAs will emerge as novel targets
in therapeutic strategies for the treatment of endothelial
dysfunction in atherosclerosis.
Acknowledgements
The present study was supported by the China
National Natural Scientific Fund (grant no. 81170293).
References
|
1
|
Santoro MM, Samuel T, Mitchell T, Reed JC
and Stainier DY: Birc2 (cIap1) regulates endothelial cell integrity
and blood vessel homeostasis. Nat Genet. 39:1397–1402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mano T, Masuyama T, Yamamoto K, Naito J,
Kondo H, Nagano R, Tanouchi J, Hori M, Inoue M and Kamada T:
Endothelial dysfunction in the early stage of atherosclerosis
precedes appearance of intimal lesions assessable with
intravascular ultrasound. Am Heart J. 131:231–238. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pober JS, Min W and Bradley JR: Mechanisms
of endothelial dysfunction, injury and death. Annu Rev Pathol.
4:71–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Triggle CR, Samuel SM, Ravishankar S,
Marei I, Arunachalam G and Ding H: The endothelium: Influencing
vascular smooth muscle in many ways. Can J Physiol Pharmacol.
90:713–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Otsuka F, Finn AV, Yazdani SK, Nakano M,
Kolodgie FD and Virmani R: The importance of the endothelium in
atherothrombosis and coronary stenting. Nat Rev Cardiol. 9:439–453.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun C, Wu MH, Lee ES and Yuan SY: A
disintegrin and metalloproteinase 15 contributes to atherosclerosis
by mediating endothelial barrier dysfunction via Src family kinase
activity. Arterioscler Thromb Vasc Biol. 32:2444–2451. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Z, Subramanian P, Sevilmis G, Globke
B, Soehnlein O, Karshovska E, Megens R, Heyll K, Chun J,
Saulnier-Blache JS, et al: Lipoprotein-derived lysophosphatidic
acid promotes atherosclerosis by releasing CXCL1 from the
endothelium. Cell Metab. 13:592–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishigaki Y, Katagiri H, Gao J, Yamada T,
Imai J, Uno K, Hasegawa Y, Kaneko K, Ogihara T, Ishihara H, et al:
Impact of plasma oxidized low-density lipoprotein removal on
atherosclerosis. Circulation. 118:75–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galle J, Hansen-Hagge T, Wanner C and
Seibold S: Impact of oxidized low density lipoprotein on vascular
cells. Atherosclerosis. 185:219–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of MicroRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shan Z, Yao C, Li ZL, Teng Y, Li W, Wang
JS, Ye CS, Chang GQ, Huang XL, Li XX, et al: Differentially
expressed microRNAs at different stages of atherosclerosis in
ApoE-deficient mice. Chin Med J (Engl). 126:515–520.
2013.PubMed/NCBI
|
|
13
|
Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li
XX, Liu Y, Cheang TY, Huang XL and Wang SM: MicroRNA-21 regulates
vascular smooth muscle cell function via targeting tropomyosin 1 in
arteriosclerosis obliterans of lower extremities. Arterioscler
Thromb Vasc Biol. 31:2044–2053. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Ouyang M, Shan Z, Ma J, Li J, Yao C,
Zhu Z, Zhang L, Chen L, Chang G, et al: Involvement of
microRNA-133a in the development of arteriosclerosis obliterans of
the lower extremities via RhoA targeting. J Atheroscler Thromb.
22:424–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y and Lee CG: MicroRNA and
cancer-focus on apoptosis. J Cell Mol Med. 13:12–23. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou DH, Wang X and Feng Q: EGCG enhances
the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC
A549 cells. Nutr Cancer. 66:636–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu S, Ogura S, Chen J, Little PJ, Moss J
and Liu P: LOX-1 in atherosclerosis: Biological functions and
pharmacological modifiers. Cell Mol Life Sci. 70:2859–2872. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sawamura T, Wakabayashi I and Okamura T:
LOX-1 in atherosclerotic disease. Clin Chim Acta. 440:157–163.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehta JL, Chen J, Hermonat PL, Romeo F and
Novelli G: Lectin-like, oxidized low-density lipoprotein receptor-1
(LOX-1): A critical player in the development of atherosclerosis
and related disorders. Cardiovasc Res. 69:36–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Li XX, Ma Q and Cui J: The
variability of oxLDL-induced cytotoxicity on different types of
cell lines. Cell Biochem Biophys. 67:635–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gimbrone MA Jr, Topper JN, Nagel T,
Anderson KR and Garcia-Cardeña G: Endothelial dysfunction,
hemodynamic forces and atherogenesis. Ann N Y Acad Sci.
902:230–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grover-Páez F and Zavalza-Gómez AB:
Endothelial dysfunction and cardiovascular risk factors. Diabetes
Res Clin Pract. 84:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen CH, Jiang W, Via DP, Luo S, Li TR,
Lee YT and Henry PD: Oxidized low-density lipoproteins inhibit
endothelial cell proliferation by suppressing basic fibroblast
growth factor expression. Circulation. 101:171–177. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin G, Yang X, Li B, Yang M and Ren M:
Connexin43 siRNA promotes HUVEC proliferation and inhibits
apoptosis induced by ox-LDL: An involvement of ERK signaling
pathway. Mol Cell Biochem. 394:101–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong Q, Xiang R, Zhang DY and Qin S:
Ox-LDL increases OX40L in endothelial cells through a
LOX-1-dependent mechanism. Braz J Med Biol Res. 46:765–770. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schober A, Nazari-Jahantigh M, Wei Y,
Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H,
Hristov M, et al: MicroRNA-126-5p promotes endothelial
proliferation and limits atherosclerosis by suppressing Dlk1. Nat
Med. 20:368–376. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pirillo A and Catapano AL: Soluble
lectin-like oxidized low density lipoprotein receptor-1 as a
biochemical marker for atherosclerosis-related diseases. Dis
Markers. 35:413–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shaw DJ, Seese R, Ponnambalam S and Ajjan
R: The role of lectin-like oxidised low-density lipoprotein
receptor-1 in vascular pathology. Diab Vasc Dis Res. 11:410–418.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawamura T, Kume N, Aoyama T, Moriwaki H,
Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T and Masaki
T: An endothelial receptor for oxidized low-density lipoprotein.
Nature. 386:73–77. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akhmedov A, Rozenberg I, Paneni F, Camici
GG, Shi Y, Doerries C, Sledzinska A, Mocharla P, Breitenstein A,
Lohmann C, et al: Endothelial overexpression of LOX-1 increases
plaque formation and promotes atherosclerosis in vivo. Eur Heart J.
35:2839–2848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehta JL and Li DY: Identification and
autoregulation of receptor for OX-LDL in cultured human coronary
artery endothelial cells. Biochem Biophys Res Commun. 248:511–514.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hermonat PL, Zhu H, Cao M and Mehta JL:
LOX-1 transcription. Cardiovasc Drugs Ther. 25:393–400. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu J, Yang JH, Burns AR, Chen HH, Tang D,
Walterscheid JP, Suzuki S, Yang CY, Sawamura T and Chen CH:
Mediation of electronegative low-density lipoprotein signaling by
LOX-1: a possible mechanism of endothelial apoptosis. Circ Res.
104:619–627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mollace V, Gliozzi M, Musolino V, Carresi
C, Muscoli S, Mollace R, Tavernese A, Gratteri S, Palma E, Morabito
C, et al: Oxidized LDL attenuates protective autophagy and induces
apoptotic cell death of endothelial cells: Role of oxidative stress
and LOX-1 receptor expression. Int J Cardiol. 184:152–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imanishi T, Hano T, Sawamura T, Takarada S
and Nishio I: Oxidized low density lipoprotein potentiation of
Fas-induced apoptosis through lectin-like oxidized-low density
lipoprotein receptor-1 in human umbilical vascular endothelial
cells. Circ J. 66:1060–1064. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J, Mehta JL, Haider N, Zhang X,
Narula J and Li D: Role of caspases in Ox-LDL-induced apoptotic
cascade in human coronary artery endothelial cells. Circ Res.
94:370–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hong D, Bai YP, Gao HC, Wang X, Li LF,
Zhang GG and Hu CP: Ox-LDL induces endothelial cell apoptosis via
the LOX-1-dependent endoplasmic reticulum stress pathway.
Atherosclerosis. 235:310–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sugawara T, Fujimura M, Noshita N, Kim GW,
Saito A, Hayashi T, Narasimhan P, Maier CM and Chan PH: Neuronal
death/survival signaling pathways in cerebral ischemia. NeuroRx.
1:17–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han Z, Hendrickson EA, Bremner TA and
Wyche JH: A sequential two-step mechanism for the production of the
mature p17:p12 form of caspase-3 in vitro. J Biol Chem.
272:13–432, 13,436. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiang H, Hochman DW, Saya H, Fujiwara T,
Schwartzkroin PA and Morrison RS: Evidence for p53-mediated
modulation of neuronal viability. J Neurosci. 16:6753–6765.
1996.PubMed/NCBI
|
|
43
|
Kataoka H, Kume N, Miyamoto S, Minami M,
Morimoto M, Hayashida K, Hashimoto N and Kita T: Oxidized LDL
modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in
vascular smooth muscle cells. Arterioscler Thromb Vasc Biol.
21:955–960. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li DY, Chen HJ and Mehta JL: Statins
inhibit oxidized-LDL-mediated LOX-1 expression, uptake of
oxidized-LDL and reduction in PKB phosphorylation. Cardiovasc Res.
52:130–135. 2001. View Article : Google Scholar : PubMed/NCBI
|