Introduction
Sheehan syndrome is characterized by
hypopituitarism, which is due to ischemic necrosis of the pituitary
gland secondary to postpartum hemorrhage (1). Major manifestations include failure to
lactate, breast atrophy, secondary amenorrhea, genital and axillary
hair loss, dry skin, hypopigmentation and other evidence of
hypopituitarism. It can also present acutely with circulatory
collapse, congestive cardiac failure and hypotension. Hypotension
can be quickly corrected under hormone and volume replacement
therapy. The present study reported on a case of Sheehan syndrome
in a 48-year-old Chinese woman who had refractory hypotension and
required longstanding vasopressor blood pressure support and
hormone replacement therapy, which is rarely reported in the
literature. The causes of refractory hypotension have been
attributed to decreased cardiac output owing to cardiomyopathy and
hypovolemia arising from hypoproteinemia. After three months, her
blood pressure remained at levels of ~110/70 mmHg and her cardiac
function partly reversed with hydrocortisone and levothyroxine
replacement therapy.
Case report
A 48-year-old Chinese woman was admitted to the
emergency department of Qilu Hospital of Shandong University
(Jinan, China) with progressive chest distress and dyspnea for two
weeks after a common cold. She had been suffering from long-term
chronic symptoms, including fatigue, anorexia and light-headedness
with a history of Sheehan syndrome without therapy. The patient had
no history of cardiac disease or diabetes mellitus. In addition,
the patient stopped menstruating after her second pregnancy 20
years previously.
Physical examination revealed the following: Mild
hypothermia (axillary temperature, 35.8°C), low blood pressure
(74/49 mmHg) and a pulse rate of 40 beats/min. Pale skin, facial
edema and cool extremities with significant pitting edema of lower
limbs were observed. Breast atrophy and sparse axillary and pubic
hair were striking features. Lung examination revealed a moist rale
in the bilateral lung bases with a respiratory rate of 20
breaths/min, muffled heart sounds and bradycardia were found in the
cardiac auscultation area. Neurologically, the patient was without
any focal signs.
Postpartum hypopituitarism had been identified and
endocrine examination was performed (Table I). In addition to insulin-like growth
factor-1 (IGF-1) deficiency, the patient's pituitary-thyroid,
pituitary-gonadal and pituitary adrenal axes were dysfunctional and
magnetic resonance imaging of the pituitary gland revealed an empty
sella, indicating anterior hypopituitarism resulting from
postpartum hemorrhage. Biochemical parameters at baseline and
during follow-up are presented in Table
II. A chest computed tomography (CT) scan showed ill-defined
ground glass opacity in the left lower lobe due to pulmonary
consolidation. The abdominal CT scan was normal. An echocardiogram
showed left ventricular (LV) global hypokinesis with decreased LV
ejection fraction (LVEF), a medium amount of pericardial effusion
and enlarged left atrium and right ventricle (Table III). Mask oxygen inhalation and
anti-infective treatment (intravenous drip of meropenem and
levofloxacin for two weeks) were initiated to improve respiratory
symptoms. Along with exacerbation of congestive heart failure and
renal and hepatic injuries, the patient was treated with volume
replacement, digoxin (0.125 mg/dat) and dopamine (3–5 µg/min/kg) to
raise the blood pressure, albumin infusion to increase colloid
osmotic pressure, furosemide injection to relieve edema as well as
further symptomatic treatments. Initially, the patient was given a
stress dose of intravenous hydrocortisone (50 mg/6 h) with a
gradual reduction to 100 mg/day, followed by a low dose of oral
levothyroxine (25 µg/day), which was increased to 100 µg/day. Due
to the patient's low and erratic blood pressure, she was fully
weaned off vasopressor blood pressure support over 20 days, with
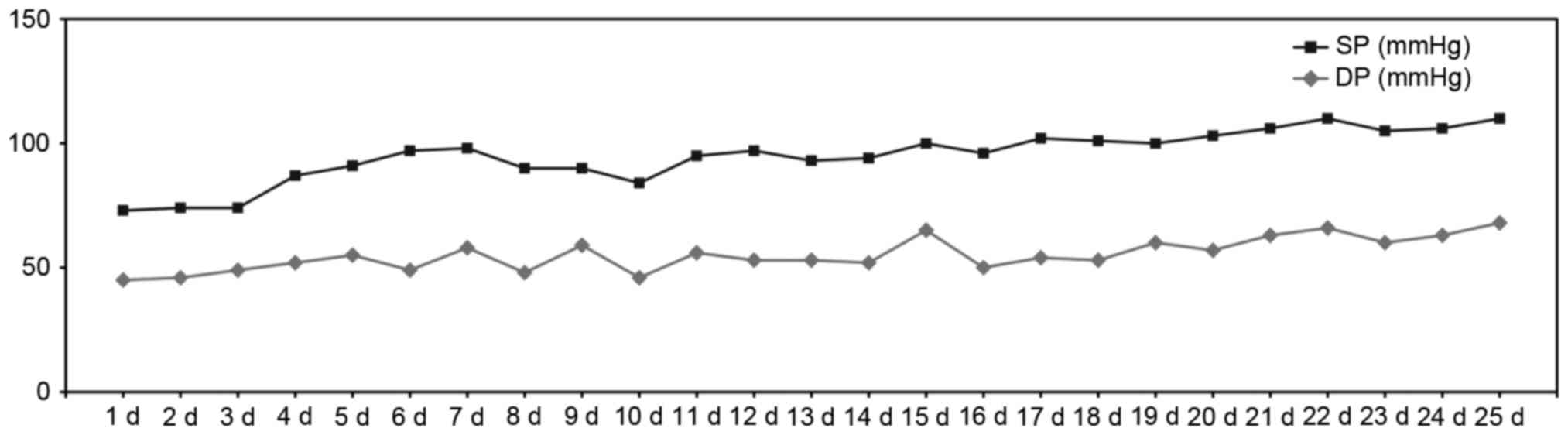
blood pressure remaining at levels of around 90/60 mmHg (Fig. 1). Moreover, the patient's general
health condition markedly improved, dyspnea eased, chest distress
disappeared, edema decreased and fatigue alleviated. Abnormal
biochemical indexes were almost restored to normal levels. A later
echocardiography showed dramatic changes in LVEF from 30 to 55%,
with a decrease of pericardial effusion and mild tricuspid
regurgitation (Table III). The
patient was discharged on hydrocortisone (40 mg/day) and
levothyroxine (100 µg/day) 28 days after being admitted.
 | Table I.Endocrine levels at baseline and
follow-up. |
Table I.
Endocrine levels at baseline and
follow-up.
|
| During hospital | After discharge |
|
|---|
|
|
|
|
|
|---|
| Parameter | Baseline | Day 21 | 3 months | Normal range |
|---|
| Free triiodothyronine
(pmol/l) | <1.54 | 2.40 | 4.12 | 2.63–5.63 |
| Free thyroxine
(pmol/l) | <5.15 | 10.41 | 27.06 | 9.01–19.01 |
| Thyroid-stimulating
hormone (µIU/ml) | 0.36 | 0.26 | 0.04 |
0.35–4.35 |
| Cortisol (µg/dl) | 1.3 |
|
| 8.7–22.7 |
| Adrenocorticotrophic
hormone (pg/ml) | 1.6 |
|
|
4.7–48.7 |
| Luteinizing hormone
(mIU/ml) | 0.55 |
|
| 7.7–58.7 |
| Follicle-stimulating
hormone (mIU/ml) | 0.34 |
|
| 25.8–134.8 |
| Estradiol
(pg/ml) | 12.17 |
|
| 10-39.5 |
| Prolactin
(ng/ml) | 0.31 |
|
| 3.4–24.4 |
| Insulin-like growth
factor-1 (ng/ml) | 5.2 |
|
|
60–350 |
| Growth hormone
(ng/ml) | 0.027 |
|
| 0.01–5.01 |
 | Table II.Biochemical parameters at baseline and
follow-up. |
Table II.
Biochemical parameters at baseline and
follow-up.
|
| During hospital | After discharge |
|
|---|
|
|
|
|
|
|---|
| Factors | Day 1 | Day 5 | Day 8 | Day 15 | Day 17 | Day 25 | 1 month | Normal range |
|---|
| White blood cells
(x109/l) | 7.75 | 15.61 | 13.99 | 10.87 | 9.40 | 6.60 | 6.92 | 3.5–9.5 |
| Neutrophils (%) | 74.1 | 93.7 | 93.9 | 91.1 | 89.8 | 78.2 | 75.6 | 40–75 |
| Red blood cells
(x1012/l) | 3.43 | 3.24 | 4.31 | 3.74 | 3.62 | 3.55 | 3.42 | 3.8–5.8 |
| Hemoglobin
(g/dl) | 103 | 101 | 137 | 117 | 112 | 110 | 108 | 115–150 |
| Platelets
(x109/l) | 209 | 118 | 121 | 175 | 193 | 172 | 224 | 125–350 |
| Alanine transaminase
(U/l) | 39 | 308 | 356 | 53 | 42 | 18 | 13 | 7–40 |
| Aspartate
transaminase (U/l) | 68 | 399 | 297 | 44 | 41 | 19 | 18 | 13–35 |
| Total protein
(g/l) | 72.0 | 46.0 | − | 47.6 | 48.1 | 50.6 | 65.7 | 60–85 |
| Albumin (g/l) | 45.0 | 30.4 | 28.8 | 32.6 | 30.7 | 32.9 | 43.2 | 40–55 |
| Blood urea nitrogen
(mmol/l) | 13.3 | 8.9 | 10.0 | 11.4 | 10.9 | 14.1 | 11.1 | 2.3–7.3 |
| Creatinine
(µmol/l) | 119 | 125 | 129 | 121 | 114 | 99 | 99 | 53–97 |
| Natrium (mmol/l) | 136 | 144 | 143 | 144 | 143 | 139 | 141 | 137–147 |
| Potassium
(mmol/l) | 2.60 | 3.85 | 3.78 | 3.78 | 2.57 | 4.96 | 3.07 | 3.5–5.5 |
| Fasting blood glucose
(mmol/l) | 4.70 | 9.15 | – | 8.27 | – | 6.80 | 4.43 | 3.9–6.9 |
| Cholesterol
(mmol/l) | 4.18 | – | – | – | – | – | 5.03 | 2.80–6.80 |
| Triglyceride
(mmol/l) | 1.5 | – | – | – | – | – | 0.64 | 0.30–1.30 |
| LDL-C (mmol/l) | 2.45 | – | – | – | – | – | 2.95 | 1.00–3.00 |
| HDL-C (mmol/l) | 0.83 | – | – | – | – | – | 1.78 | 0.80–2.80 |
 | Table III.Indexes for cardiac function at
baseline and follow-up. |
Table III.
Indexes for cardiac function at
baseline and follow-up.
|
| During hospital | After discharge |
|
|---|
|
|
|
|
|
|---|
| Factor | Day 1 | Day 5 | Day 17 | Day 25 | 1 month | 3 months | Normal range |
|---|
| Cardiac
enzymes |
|
|
|
|
|
|
|
|
Troponin-I (ng/ml) |
0.01 |
0.23 |
0.11 |
0.05 |
0.02 | – |
0-0.06 |
| CK-MB
(ng/ml) | 8.0 | 4.7 | 4.6 | 2.1 | 2.2 | – | 0.3–4.3 |
|
Nt-proBNP (pg/ml) | 548 | – | – | – | – | – |
<125 |
|
Electrocardiogram |
|
|
|
|
|
|
|
| Heart
rate (beats/min) | 40 | 111 | 63 | 65 | 65 | 77 | 60–100 |
| PR
interval | 200 | 130 | 196 | 160 | 196 | 190 | 120–200 |
| QRS
duration (msec) | 78 | 61 | 83 | 68 | 80 | 78 |
<120 |
| T
wave | Flat | Flat | Flat | Flat | Flat | Normal | Normal |
| QT/QTc
(msec) | 600/490 | 292/358 | 362/402 | 180/225 | 494/405 | 352/384 |
Variable/350–440 |
| Low
voltage | Limb leads | Limb leads | Limb leads | Limb leads | Limb leads | None | None |
|
Echocardiography |
|
|
|
|
|
|
|
| LA
dimension (mm) | 40 | – | – | 35 | – | 34 | <35 |
| RV
dimension (mm) | 30 | – | – | 26 | – | 23 | <25 |
| LVEF
(%) | 30 | – | – | 55 | – | 64 | >50 |
| LV
motion | Abnormal diastolic
hypokinesis | – | – | Abnormal diastolic
filling | – | Abnormal diastolic
filling | Normal |
|
Tricuspid regurgitation |
Severe-moderate | – | – | Moderate | – | Mild | Normal |
|
Pericardial effusion | Medium amount | – | – | Medium amount | – | Small amount | None |
At follow-up 3 months after discharge, a repeat
echocardiogram showed LV filling disturbance with a continuous
increase in LVEF (64%) as well as normal cardiac size, and blood
pressure was increased to 110/70 mmHg with glucocorticoid and
levothyroxine replacement therapy. The dose of levothyroxine was
decreased from 100 to 75 µg as a result of a higher free thyroxine
level.
Discussion
Acute pituitary insufficiency, also called pituitary
crisis, is a life-threatening condition following a period of
non-specific symptoms due to chronic pituitary insufficiency, the
causes of which involve electrolyte imbalance, infection, trauma or
other forms of stress. Volume depletion and low cardiac output are
common in acute pituitary deficiency and recovery of normal
cardiovascular status is rapidly achieved under hormone and volume
replacement therapy. Vesely et al (2) reported a case of post-herpes
encephalitic anterior pituitary insufficiency (dysfunction of
pituitary-thyroid and pituitary-gonadal axis) with hypothermia and
hypotension in a 49-year-old man, and his blood pressure of 90/60
mmHg quickly returned to normal after thyroid hormone replacement
therapy. However, the patient of the present study experienced
refractory hypotension and required longstanding vasopressor blood
pressure support and hormone replacement therapy, which was rarely
reported in the literature. Retrospective analysis of 77 retrieved
cases of Sheehan syndrome at Qilu Hospital (Jinan, China) from 1999
to 2015 revealed that patients with hypotension accounted for 29.9%
(23 cases), whose normalization of blood pressure almost generally
occurred on the third or fourth day of treatment, after hormone
replacement and correction of hyponatremia.
In the present case, one cause of severe hypotension
was closely associated with hypoproteinemia owing to chronic
malnutrition and liver damage. Albumin can expand fluid and
maintain a stable plasma colloid osmotic pressure, while
hypoproteinemia impairs water balance, increasing the likelihood of
hypovolemia. Moreover, albumin infusion combined with diuretic
injection helped to ease myocardial edema and pericardial effusion,
which are beneficial for cardiac function.
Initially, septic shock resulting in hypotension
could not be excluded, as indicated by an increased white blood
cell count and neutrophils, respiratory symptoms and chest
radiological findings. After potent fluoroquinolones (levofloxacin)
and cabapenems (meropenem) had been administered for two weeks,
respiratory symptoms were obviously remitted. However, the white
blood cell count did not decrease, which was associated with the
intravenous infusion of hydrocortisone (50 mg/6 h). With the
extenuation of hydrocortisone, the white blood cell count returned
to normal. Infectious factors were no longer considered, as the
blood pressure was not markedly elevated.
During the evaluation, symptomatic heart failure and
elevated cardiac enzymes raised the suspicion of myocardial
ischemia or an acute infraction resulting in hypotension. It has
been reported that endocrine disorders lead to abnormal lipid and
glucose metabolism and are therefore implicated in the genesis of
acute coronary syndrome (ACS). However, this possibility was
quickly ruled out based on the following points: i) Cardiac markers
are not specific for ACS and only resemble a manifestation of
myocardial damage to a certain extent; ii) serum lipid levels, a
risky factor for assessing cardiovascular disease, were in the
normal range; iii) nonspecific and abnormal electrocardiogram (ECG)
patterns, such as sinus bradycardia, prolonged QT intervals, low
limb lead voltage and abnormal T wave, suggested a correlation with
metabolic disease; iv) compared with the original ECG, there were
no dynamic changes indicating ACS; v) the patient had no history of
cardiac disease or diabetes mellitus; vi) an echocardiogram showed
LV global hypokinesis with decreased LVEF, which was consistent
with cardiomyopathy (Table III).
Therefore, the patient's refractory hypotension was attributed to
cardiomyopathy.
Of all endocrine hormone deficiencies linked to
cardiomyopathy, glucocorticoid, thyroid hormone and growth hormone
deficiencies have major roles. The mechanisms responsible for the
development of cardiomyopathy are varied: i) Thyroid hormone
deficiency has a significant impact on myocardial injury, resulting
in weakening of myocardial contraction and relaxation, decrease in
cardiac output, and rhythm disturbances, through genomic and
non-genomic effects (3–5). ii) Catecholamine overproduction during
stress may be toxic to the myocardium, which is unprotected by
inadequate glucocorticoids, impairing cardiac function;
glucocorticoid deficiency disturbs the transport function of the
membrane calcium pump, affecting myocardial contractility (6,7). iii)
Numerous experimental studies have demonstrated that growth
hormone/IGF-1 deficiency has a deleterious influence on cardiac
growth, myocardial contractility and vascular system (8,9).
However, certain case studies have indicated that growth hormone
deficiency has a minimal role in pathogenic mechanisms of
cardiomyopathy (10,11). Laway et al (10) described a case of cardiomyopathy
linked to Sheehan syndrome and pulmonary tuberculosis in a
25-year-old women, whose cardiac function was completely restored
after replacement therapy with glucocorticoid and
levothyroxine.
On the basis of the abovementioned pathogenesis, the
patient was initiated with a stress dose of hydrocortisone,
followed by a small dose of oral levothyroxine under the state of
high stress. Glucocorticoid is replaced prior to levothyroxine to
avoid aggravating pituitary crisis. In contrast to previous
patients, the patient of the present study was also given positive
inotropic agents, digoxin and dopamine, which improved hemodynamics
and increased cardiac pump function. Her blood pressure did not
return to normal until vasopressor blood pressure support and
hormone replacement therapy were provided for 20 days. It is
speculated that a longstanding adverse influence on cardiac
function may be the primary cause of refractory hypotension. The
patient was discharged whilst receiving a physiological dosage of
hydrocortisone and levothyroxine. At 3-month follow-up, cardiac
function was partly reversed with preserved LVEF, normal cardiac
size, small pericardial effusion and abnormal LV diastolic
filling.
The definite reason why the patient's
hormone-induced cardiac dysfunction was not completely resolved
remains elusive. One explanation that long-standing hormone
deficiencies drive the irreversible change of myocardial damage
appears to not be dependable (11,12). Bao
and Fisher (11) reported on a
35-year-old woman with long-standing hypopituitarism for 15 years
and heart failure for at least 10 years with severely compromised
cardiac function. At 9 months after discharge, echocardiography
showed completely normalized cardiac function without growth
hormone replacement (11).
Similarly, Kissell et al (12) reported on a 40-year-old patient with
cardiogenic shock due to non-ischemic cardiomyopathy induced by
severe anterior hypopituitarism initially treated with
levothyroxine and hydocortisone replacement therapy, who had 20
years of history of undiagnosed Sheehan syndrome. Eighteen months
later, echocardiography revealed that LVEF was partially reversed
(12). One hypothesis is that GH
deficiency is involved in pathogenic mechanisms of cardiomyopathy,
although there is a possibility of complete recovery of cardiac
function without growth hormone replacement (10,11). In
the present case, the irreversibility of cardiac damage may have
been associated with the short duration of hormone replacement
therapy, while there is a possibility that cardiomyopathy may be
completely reversed in the future.
The importance of a rapid diagnosis of Sheehan
syndrome, particularly intercurrent hypopituitary crisis, should be
emphasized, as otherwise, treatment is delayed and patients remain
in a critical condition. Kaufmann et al (13) reported on a 52-year-old woman who
developed hypopituitary crisis with severe hypotension and coma
secondary to unrecognized chronic anterior hypophysitis.
Unfortunately, she succumbed to refractory cardiac arrest without
prompt hormone replacement treatment. In the present case, the
important information that the patient had a history of Sheehan
syndrome was available, which provided a hint for selecting an
appropriate treatment.
In conclusion, a prompt diagnosis and specific
treatment of Sheehan syndrome is vital. The present case
illustrated that refractory hypotension is a rare and serious
complication of longstanding hypopituitarism induced by Sheehan
syndrome with pituitary crisis, of which volume depletion and low
cardiac output are common pathological mechanisms. The case also
exemplified that glucocorticoid, thyroid hormone and growth hormone
deficiencies may contribute to cardiomyopathy by varying degrees,
and cardiac function may be restored with hormone replacement
therapy.
References
|
1
|
Shivaprasad C: Sheehan syndrome's
syndrome: Newer advances. Indian J Endocrinol Metab. 15:(Suppl 3).
S203–S207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vesely DL, Mastrandrea P, Samson C,
Argyelan G and Charvit S: Post-herpes encephalitic anterior
pituitary insufficiency with hypothermia and hypotension. Am J Med
Sci. 320:273–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vargas-Uricoechea H and Sierra-Torres CH:
Thyroid hormones and the heart. Horm Mol Biol Clin Invest.
18:15–26. 2014.
|
|
4
|
Galli E, Pingitore A and Lervasi G: The
role ofthyroid hormone in the pathophysiology of heart failure:
Clinical evidence. Heart Fail Rev. 15:155–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt-Ott UM and Ascheim DD: Thyroid
hormone and heart failure. Curr Heart Fail Rep. 3:114–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cleghorn RA: Cardiovascular failure in
experimental adrenal insufficiency: A history revival. Perspect
Biol Med. 27:135–155. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Narayanan N: Effects of adrenalectomy and
in vivo administration of dexamethasone on ATP-dependent calcium
accumulation by sarcoplasmic reticulum from rat heart. J Mol Cell
Cardiol. 15:7–15. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castellano G, Affuso F, Conza PD and Fazio
S: The GH/IGF-1 axis and heart failure. Curr Cardiol Rev.
5:203–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lombardi G, Di Somma C, Grasso LF,
Savanelli MC, Colao A and Pivonello R: The cardiovascular system in
growth hormone excess and growth hormone deficiency. J Endocrinol
Invest. 35:1021–1029. 2012.PubMed/NCBI
|
|
10
|
Laway BA, Alai MS, Gojwari T, Ganie MA and
Zargar AH: Sheehan syndrome with reversible dilated cardiomyopathy.
Ann Saudi Med. 30:321–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao SS and Fisher SJ: Repairing a ‘broken
heart’ with hormone replacement therapy: Case report of cardiogenic
shock due to undiagnosed pituitary insufficiency. Endocr Pract.
18:e26–e31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kissell N, Mudd JO, Gelow JM, Chong LE and
Yuen KC: Cardiogenic shock due to non-ischemic cardiomyopathy
induced by severe anterior hypopituitarism. Endocr Pract. Nov
4–2014.(Epub ahead of print). PubMed/NCBI
|
|
13
|
Kaufmann P, Lax SF, Radner H, Eber B,
Leuger A and Smolle KH: Severe hypotension and coma secondary to
unrecognized chronic anterior hypophysitis. Intensive Care Med.
21:847–849. 1995. View Article : Google Scholar : PubMed/NCBI
|