Introduction
Chronic heart failure (CHF) has currently become a
global epidemic with high prevalence, incidence and mortality, and
is one of the deadliest diseases (1). It is diagnosed as a state of chronic
inflammation with elevated T-cell activation and inflammatory
cytokine production in the circulatory system (2). A recent study revealed strong evidence
that T lymphocytes are important in the pathogenesis of cardiac
disease (3). In addition,
inflammatory cytokines have been investigated as targets of heart
failure therapy (4).
The development and progression of CHF itself are
closely associated with the imbalance between pro- and
anti-inflammatory mediators, and patients with CHF are
characterized by an increased expression of inflammatory mediators
in circulating leukocytes. For example, using blood samples,
T-lymphocytes of patients with CHF demonstrated increased
expression of interferon (IFN) and interleukin (IL)-10 in addition
to increased surface activation markers, including CD69 and CD25
(5). Hence, IFN-γ and IL-10 have
been considered to be candidate cytokines involved in the
pathomechanism leading to CHF (6).
Another surface marker, the forkhead/winged helix transcription
factor (Foxp3), which is expressed by regulatory T cells (Treg),
has an anti-inflammatory role and maintains tolerance to
self-components by contact-dependent suppression or by releasing
anti-inflammatory cytokines, including IL-10 (7). Within the blood, IL-10 itself has
potent deactivating properties in macrophages and T-cells and thus
acts as a downregulator of cell-mediated immune responses, which
have already been described as being important in CHF (8).
Circulating inflammatory markers, including IL-6,
may be useful in establishing a diagnosis and in gauging a
prognosis in patients with heart failure (HF) (4). IL-6 is a multifunctional cytokine that
mediates both immune and inflammatory responses. It has been shown
that increased plasma IL-6 levels may contribute to disease
progression, and may be associated with enhanced mortality in
patients with CHF (9). Within the
myocardium, IL-6-type cytokines convey their signals predominantly
through the signal transducer and activator of the transcription 3
pathway via a glycoprotein 130 (gp130) receptor subunit. Moreover,
the circulating levels of the common receptor subunit, soluble
gp130 (sgp130), may potentially indicate the activity of the whole
IL-6 family, including myocardial activation. It is the common
receptor of IL-6, which is elevated in patients with CHF (10).
Pericardial fluid may be used as a biomarker for
inferring pathological alteration to the heart (11), and its accumulation can be attributed
to an underlying systemic or local inflammatory process (12). Therefore, it is important to identify
the association between the progression of CHF and the inflammatory
response in the pericardial fluid in order to find a future
treatment strategy and prognosis of HF. In order to further
evaluate the role of pericardial fluid in CHF patients, the
pericardial fluid expression of several cytokines and also surface
expression of activity markers in T-cells from CHF patients and
controls was examined.
Materials and methods
Patients
The present study population consisted of 74
patients with various degrees of CHF who were undergoing cardiac
surgery in Nanjing First Hospital (Nanjing, China). Diagnosis of
the etiology of HF was based on the clinical history, symptoms,
physical examination, echocardiography, chest X-rays,
electrocardiography and cardiac catheterization, conforming to
available guidelines regarding CHF (13,14).
Echocardiographic evaluation was performed according to the
guidelines of the American Society of Echocardiography (15) before cardiac surgery using a
commercially available probe and system to assess cardiac function
and measure the left ventricular ejection fraction. Coronary
angiography was performed in all patients before surgery to exclude
patients with coronary artery disease. The following laboratory
tests were also performed within the first 24 h after admission:
Complete blood count, tests of liver function, measurements of
blood urea nitrogen and serum creatinine, body mass index (BMI) and
serum electrolytes.
Patients meeting the criteria for the present study
were prospectively assigned to the CHF or NHF group. Moreover, the
functional severity of HF was assessed prior to the surgery using
the New York Heart Association (NYHA) classification (16,17), and
the patients were classified by an independent investigator as: i)
Symptomatic, which had a history or presence of clinical symptoms
and sign of CHF including, orthopnea, paroxysmal nocturnal dyspnea,
pulmonary congestion on chest radiograph or a significant
exertional dyspnea or fatigue (NYHA II, n=26; NYHA III, n=21; NYHA
IV, n=3) for the CHF group or ii) asymptomatic (NYHA I, n=24) for
the NHF group. Patients were excluded if they had a history of
recent myocardial infarction or unstable angina in the last 12
months. Other exclusion criteria included acute coronary syndrome,
end-stage renal failure, shock, cancer and severe infection.
Moreover, the study conformed to the principles outlined in the
Declaration of Helsinki, the Ethics Committee of Nanjing Medical
University Affiliated Nanjing First Hospital approved the trial,
and each patient provided written informed consent.
Data collection
Sociodemographics (i.e., gender, age, education,
marital status, living status and employment status) and
information pertaining to medical history (i.e. CHF etiology and
co-morbidities, including dyslipidemia and chronic kidney disease)
were collected. Information about medication use, history of
hypertension, diabetes mellitus, coronary heart disease, chronic
obstructive pulmonary disease and stroke were also obtained. The
baseline characteristics of the patient demographic variables in
the present study, HF classification and echocardiographic
parameters of patients are shown in Table I.
 | Table I.Baseline characteristics in non-heart
failure patients and in patients with chronic heart failure. |
Table I.
Baseline characteristics in non-heart
failure patients and in patients with chronic heart failure.
|
Characteristics | NHF (n=24) | CHF (n=50) |
|---|
| Age (years) | 57.79±9.81 | 57.30±10.92 |
| Gender
(male/female) | 11/13 | 21/29 |
| Body mass index
(kg/m2) | 22.98±3.64 | 24.01±3.57 |
| New York Heart
Association |
|
|
| I | 24 | – |
| II | – | 26 |
|
III | – | 21 |
| IV | – | 3 |
| Diabetes mellitus,
n (%) | 1 (4) | 2 (4) |
| COPD, n (%) | – | 1 (2) |
| Stroke, n (%) | – | 2 (4) |
| Echocardiographic
data |
|
|
| LVEF
(%) | 64.21±1.98 | 69.04±17.21 |
|
Creatinine (mmol/l) | 62.19±18.76 | 69.04±17.21 |
Measurement of biomarkers
Pericardial fluid samples were obtained during
surgery under general anesthesia. Undiluted samples of the
pericardial fluid were obtained immediately after the creation of a
small incision in the pericardium and before heparinization.
Samples contaminated with blood were discharged. Pericardial fluid
was collected for the measurement of total protein. Arterial blood
samples were withdrawn from the cannulated brachial artery at the
same time for the measurement of plasma N-terminal propeptide of
B-type natriuretic peptide (NT-proBNP). In addition, all the
samples were collected into sterile tubes and immediately placed on
ice. These were then immediately centrifuged at 3,000 × g for 15
min at 4°C and rapidly frozen and stored at −80°C until further
analysis.
Histological analysis
Cells obtained from the pericardial fluid samples
were fixed in 4% paraformaldehyde (PFA) smear section, which was
stained with hematoxylin and eosin (H&E) to assess the cell
proportions within each group for histopathology. Stained sections
were performed according to a previously described protocol with
minor revisions (18), and were then
observed under a BX41TF microscope (Olympus Corporation, Tokyo,
Japan). True color digital images were then captured.
Immunohistochemical staining
The expression levels of Foxp3 and CD25 receptor
protein were examined immunohistochemically in pericardial fluid
biopsy samples obtained from the patients with CHF and NHF.
Staining of CD25 (1:100; ab61195, Abcam, Cambridge, MA, USA) and
Foxp3 (1:100; sc-28705, Santa Cruz Biotechnology Inc., Santa Cruz,
CA, USA) immunohistochemistry was performed on formalin-fixed
sections, followed by incubation with PV-6001 (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd., Beijing, China) according to
the manufacturer's instructions. Each stained histological section
was examined under an Olympus BX53F microscope (Olympus
Corporation) connected to a computerized image-analysis system
(Image-Pro v6.0; Media Cybernetics Inc., Silver Spring, MD,
USA).
Immunofluorescence staining
Immunofluorescence staining for IL-6 (1:100; ab6672;
Abcam, Cambridge, MA, USA), IL-10 (1:100; 250713; Abbiotec, San
Diego, CA, USA) and IFN-γ (1:100; ab175878; Abcam) was performed in
a smear section. Prior to immunostaining, cells were washed with
phosphate-buffered saline, fixed with 4% PFA for 10 min and
permeabilized for 10 min using 0.05% Triton-100. Cells were then
incubated for 20 min (37°C) with 5% bovine serum albumin blocking
solution in order to block non-specific binding. Cells were then
incubated at 4°C overnight with primary antibodies and 1 h with the
appropriate secondary antibody conjugated to Alexa-488 (1787900) or
Alexa 594 (1827987; both Invitrogen; Thermo Fisher Scientific,
Inc., Carlsbad, CA, USA). DAPI was used to counterstain the nuclei.
Samples were then covered with mounting media (Invitrogen; Thermo
Fisher Scientific, Inc.), overlaid with coverslips and examined
under a fluorescence microscope (Axio Scope A1; Carl Zeiss AG,
Oberkochen, Germany) following the method described in previous
studies (19,20).
ELISA
The concentration of NT-proBNP and Sgp130 levels
were evaluated using the highly sensitive ELISA. NT-proBNP was
measured by the ELISA kit for human NT-proBNP (Cloud-Clone Corp,
Houston, TX, USA). Sgp130 was measured using a human sgp130
Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA)
all following the manufacturer's instructions. The assays were
quantified in an ultra-microplate reader at a wavelength of 450 nm
(ELX808; BioTek Instruments, Inc., Winooski, VT, USA). The results
are expressed as pg/ml.
Statistical analysis
Comparisons of CHF patients vs. control subjects
were performed using Student's t-test where distributions of data
were normal and homogeneity of variance. For skewed variables the
Mann-Whitney U test was used for the analysis. For the ranked data,
Pearson's χ2 or Fisher's exact tests were used for the
comparison between two groups. Continuous values are expressed as
the mean ± standard deviation. Data were analyzed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was used to
indicate a statistically significant difference.
Results
Clinical characteristics in CHF
patients
In total, 74 patients were enrolled in the present
study. The baseline clinical characteristics of the study subjects
are detailed in Table I, which
summarizes the demographic data, medical history, NYHA function
classification and BMI in the CHF and NHF groups. There were no
significant differences in age, gender and BMI between the two
groups. Moreover, according to the NYHA classification, the
severity of HF was class I in 24, class II in 26, class III in 21
and class IV in three patients.
Concentration of plasma NT-proBNP in
patients with CHF
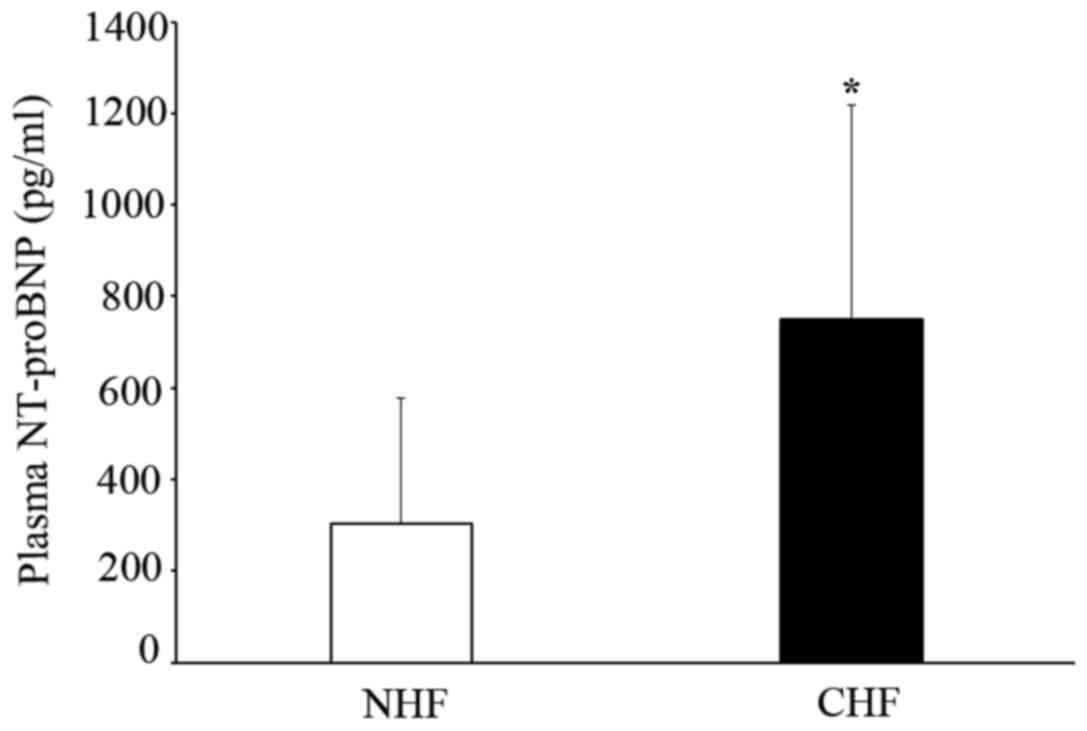
Plasma NT-pro BNP was observed in CHF and NHF
patients. The level of plasma NT-proBNP significantly increased in
the CHF group compared to the NHF group (749.60±468.06 vs.
303.45±275.00 pg/ml; P<0.05) (Fig.
1).
Pericardial fluid expression in
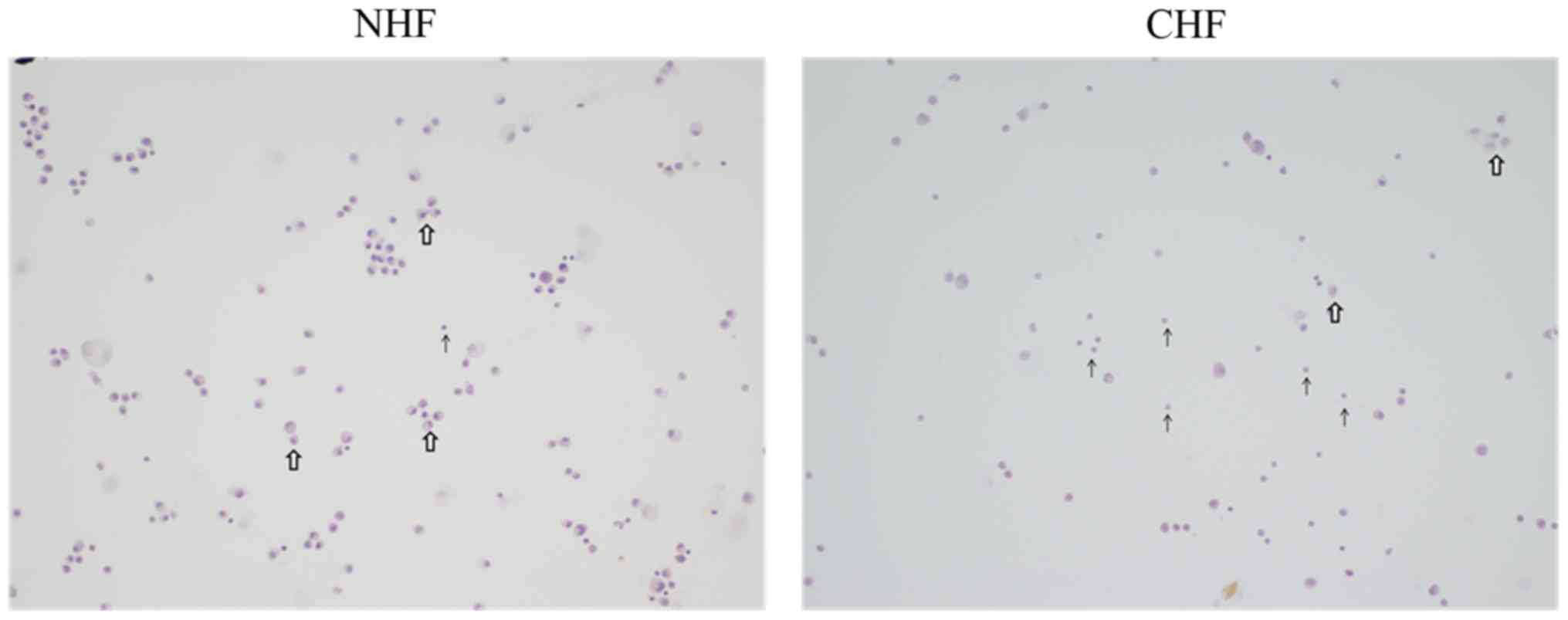
cellular proportion of patients with CHF
The pericardial fluid of CHF patients exhibited
marked infiltration of inflammatory cells, whereas the pericardial
fluid of the NHF patients had less of such infiltrates. Moreover,
CHF patients showed an increased number of circulating lymphocytes
within its pericardial fluid compared to NHF patients as evidenced
by H&E staining (Fig. 2).
Pericardial fluid expression on
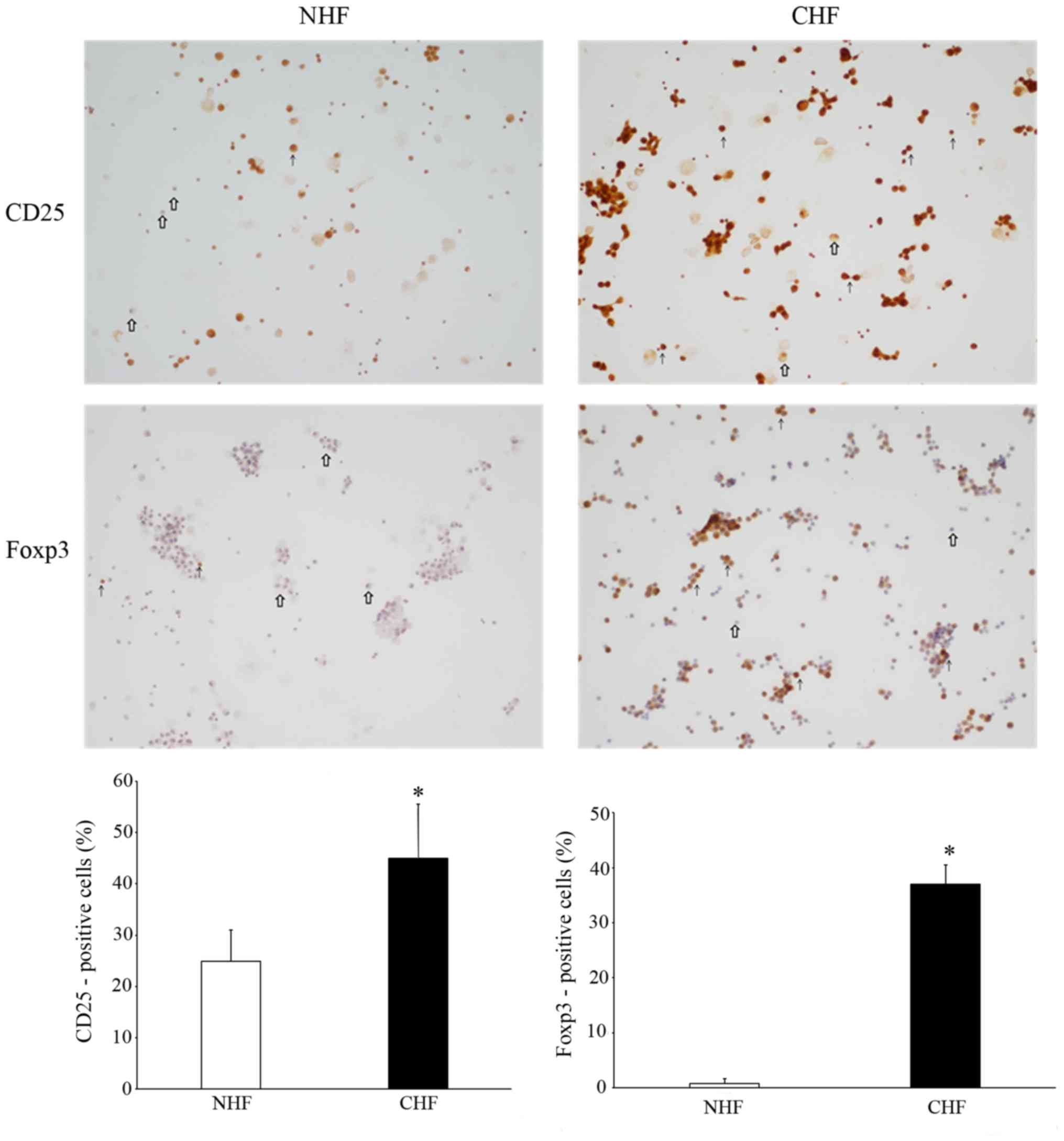
surface markers CD25 and Foxp3
In order to further elucidate the role of lymphocyte
cells in the systemic inflammatory response during CHF, the surface
expression of the T cell activation markers, CD25 and Foxp3, on
pericardial fluid were detected using immunohistochemistry. The
T-cell activation marker CD 25 was observed to be significantly
increased in CHF patients compared to the controls (P<0.05;
Fig. 3). Foxp3 staining by
immunohistochemistry also revealed that there was an enhanced
expression of cells present in the pericardial fluid of CHF
patients compared with NHF patients. The quantitative analysis of
the percentage of positive cells (n=100 cells each) was also
measured by immunostaining. The proportion of CD25-positive cells
was significantly higher in the CHF group compared to the NHF group
(45.00±10.58 vs. 24.92±6.13%; P<0.05); while Foxp3-positive
cells were also significantly higher in the CHF group compared to
the NHF group (36.97±3.50% vs. 0.76±0.90%; P<0.05) (Fig. 3), suggesting T cell activation within
the pericardial fluid of the CHF patients.
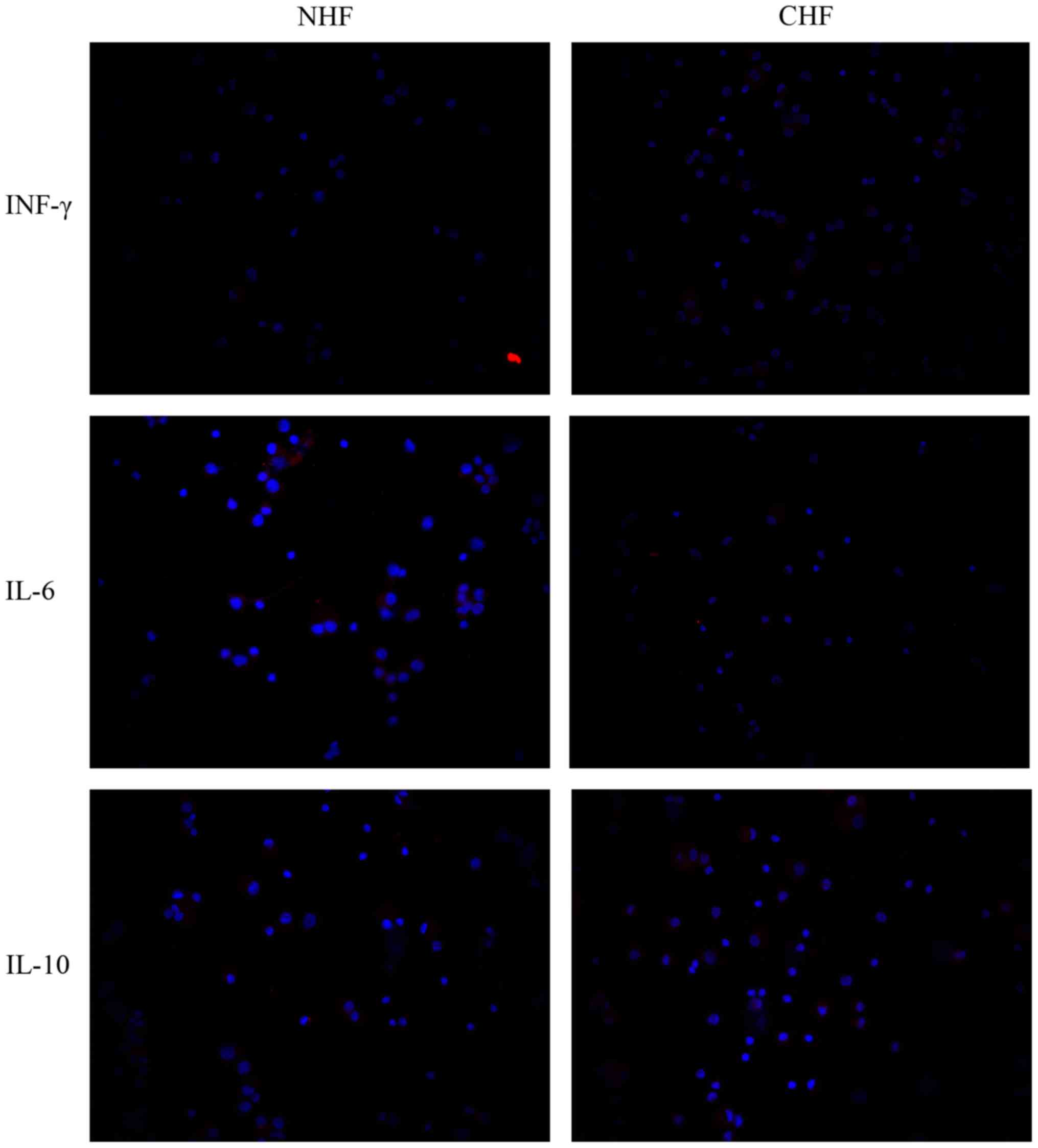
Pericardial fluid expression of the
pro- and anti-inflammatory cytokines IFN-γ, IL-6 and IL-10
Given the inflammatory reactions in the hearts of
CHF patients, it was of interest to examine whether pro- and
anti-inflammatory cytokines were detectable in the pericardial
fluid of such patients. Therefore, the present study investigated
the pericardial fluid expression of IFN-γ, IL-6 and IL-10. The
pro-inflammatory cytokine expression of the pericardial fluid IFN-γ
was markedly increased in the CHF compared to the NHF patients.
Moreover, pericardial fluid expression of IL-6 was also markedly
increased in the CHF compared with the NHF patients. On the other
hand, pericardial fluid expression of the anti-inflammatory
cytokine IL-10 was also showing a markedly increase in cells of CHF
compared to NHF patients (Fig.
4).
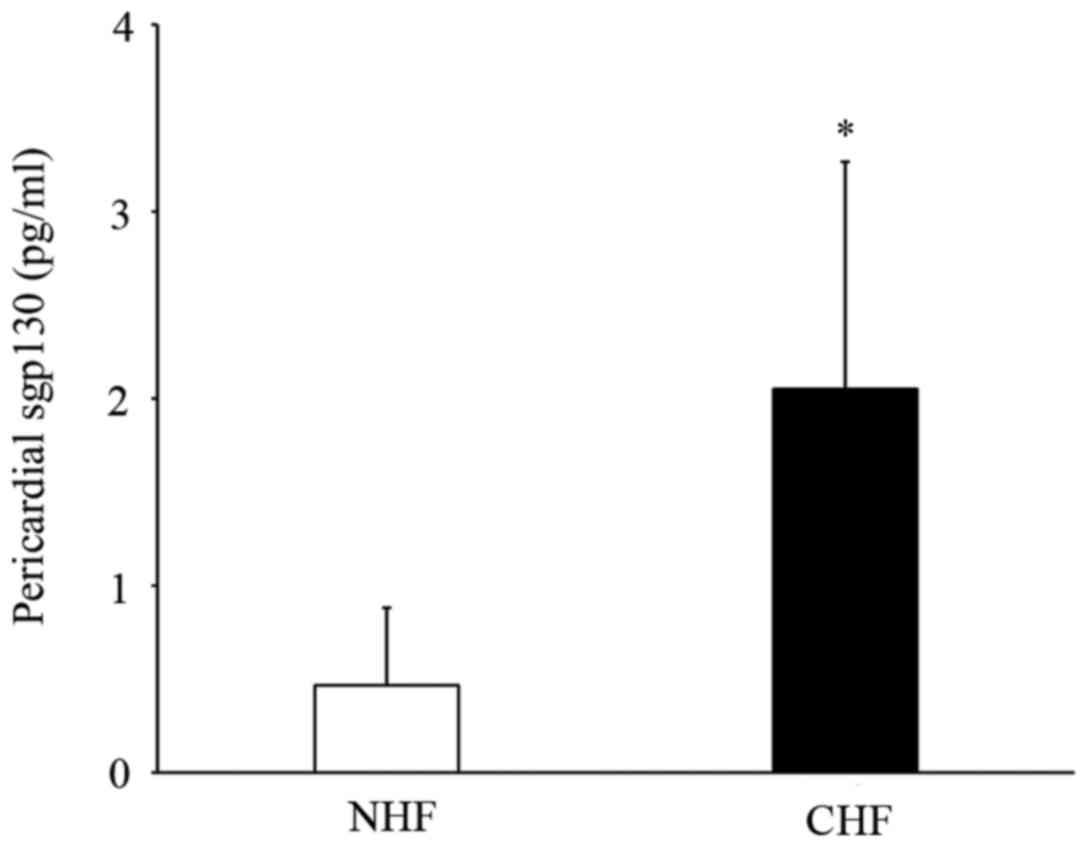
Circulating levels of sgp130 in the
pericardial fluid of patients with CHF
Finally, the pericardial fluid expression of the
IL-6 receptor sgp130 levels in patients with CHF was examined in
the present study. Patients with CHF had significantly increased
levels of pericardial sgp130 fluid compared with NHF patients
(2.05±1.21 vs. 0.46±0.40 pg/ml; P<0.05) (Fig. 5).
Discussion
CHF is an increasingly prevalent disease, which
claims thousands of lives every year (21). Previous studies have identified some
prognostic markers indicating adverse outcomes in patients with CHF
(22–24). However, researchers have still not
reached a consensus on the incidence, characteristics and
importance of pericardial fluid in patients with CHF. To the best
of our knowledge, the present study investigated for the first time
the expression of pericardial fluid T cells and the related
inflammatory cytokines in CHF patients.
NT-proBNP has recently become a subject of interest
amongst medical researchers due to its possible role in monitoring
HF and distinguishing acute coronary syndromes (25). It has recently been reported that
plasma, fresh and frozen urine levels of NT-proBNP were
significantly higher in CHF patients (26). A previous study suggests that the
severity of CHF could be determined on the basis of plasma
NT-proBNP levels, whereas the NT-proBNP assay may be more sensitive
than BNP under certain circumstances (27). Therefore, in the present study the
NT-proBNP levels were evaluated. These were used to confirm the
diagnosis of CHF in the population of the present study, regardless
of history, etiology and symptoms of CHF itself. In agreement with
previous results (26–28), the plasma levels of NT-proBNP in the
present study were significantly increased in patients with CHF,
suggesting that NT-proBNP is a useful marker for detecting CHF.
HF occurs due to the inability of the heart to
respond to the circulatory demand; main causes include ischemic and
valvular injuries, while toxic, metabolic or genetic (congenital)
origins are less common. Alterations in the heart may lead to major
changes in the myocardial structure and function. The pathogenesis
of HF itself is determined by numerous factors, including
inflammation, neuroendocrine activation, oxidative stress, severe
angiogenesis, apoptosis pathway changes and vascular remodeling
(29). Inflammation may stimulate
cardiac remodeling and fibrosis, and thus participates in the
progression and pathogenesis of HF. Gruson et al (30) reported that circulating levels of
cytokines are enhanced in the failing myocardium, and increased
production of pro-inflammatory cytokines may challenge the
surrounding tissue through propagation of the inflammatory response
and direct effects on the cardiac myocyte structure and its
function. Pro-inflammatory cytokines such as the tumor necrosis
factor-α and IL family (IL-6, IL-1 and IL-18) appear to cause
cardiomyocyte apoptosis and necrosis as well as cell hypertrophy,
leading to CHF (30). In the present
study, which investigated pericardial fluid samples, an aim was to
detect biomarkers of inflammation associated with CHF that may
provide additional options, rather than NT-proBNP alone, for
diagnosing CHF. There was a notable limitation within our study.
Although the concentration of plasma NT-proBNP increased in the CHF
group, the standard deviation of these data are quite large, which
may have been caused by a deficiency of the samples. Furthermore,
although we have attempted to decrease the influence of other
clinical factors, there are still some factors that we did not
recognize that may affect the results.
Activation of the immune system and inflammation in
HF patients are considered to be important in the progression of HF
(22,31–33). In
particular, variations in different types of leukocytes, including
lymphocyte, monocyte, eosinophil and mast cells, have been
identified in a high-risk subset of HF patients (34). It has been hypothesized that the
typical hallmarks for the involvement of immune mechanisms in CHF
pathogenesis are the infiltration of cardiac tissue by leukocytes
(6). We hypothesized that T cell
infiltration will also appear within the pericardial fluid of CHF
patients, the present results demonstrated that CHF patients had an
increasing number of circulating T lymphocytes within its
pericardial fluid compared to NHF patients, which indicates
increased T cell infiltration within the pericardial fluid of CHF
patients.
T cells in CHF patients are evidently activated, as
evidenced by enhanced gene expression of chemokines and
inflammatory cytokines, in addition to the surface expression of
activated markers (35). CD25 T
lymphocytes represent the most well characterized subset of
regulatory T cells on their surface, and recently, nuclear
transcription factor Foxp3 has been demonstrated to a specific
marker for and to regulate the development and function of
CD4+ CD25+ Tregs (36). A previous publication stated that
circulating Treg cells were reduced and their function was altered
in CHF patients, regardless of etiology, indicating that the
defects in Treg cells are responsible for the aberrant chronic
immune activation in CHF patients (2). In the present study, the result
demonstrated that T-cells in the pericardial fluid of CHF patients
expressing CD25 and Foxp3 activation markers were significantly
increased compared to the NHF patients. This observation was
consistent with other reports (37,38) that
showed that T-cells from CHF patients had an enhanced surface
expression of the activation marker CD25. This study demonstrated
that circulating T-cells are markedly activated in CHF as assessed
by both enhanced mRNA levels of several inflammatory cytokines as
well as by an increased surface expression of activation markers
(37). Moreover, the results of the
present study suggested that CD25 is not only expressed within T
lymphocytes but also within other cells, including monocytes.
However, the present study focused on T lymphocytes that expressed
the surface marker CD25. Other studies also reported that among the
leukocyte subsets, monocytes have been shown to contribute to the
systemic inflammation in HF patients (38,39), and
through this study it was suggested that T cells may be an
important cellular source for inflammatory cytokines in CHF.
However, the expression and functional role of CD25 within other
cell types requires further study.
Besides monocyte function being modulated in CHF
enhanced expression of pro-inflammatory cytokines and activation
markers of T-cells has also been reported. However, in the present
study, which based on the pericardial fluid sample, the results
showed that pro-inflammatory cytokine expression of the pericardial
fluid IFN-γ was significantly increased in CHF compared to the NHF
patients. There is clinical evidence that T cellular IFN-γ gene
expression is increased in patients with CHF (6). Cappuzzello et al (40) have described for the first time, that
there was an increase of IL-9, and a decrease of IL-5, IL-7 and
IFN-γ plasma levels in patients with CHF. Moreover, Fukunaga et
al (41) demonstrated that in
CHF patients more CD4+ T-lymphocytes produce IFN, and
that the number of CD4+ IFN producing T-lymphocytes
increases with disease activity. IFN-γ may protect against the
development of dilated cardiomyopathy, HF and the incidence of
mortality by reducing mast cell degranulation in the pericardium
and preventing the adhesive, fibrous form of pericarditis (42).
During the last few years, an interest in the
involvement of inflammatory factors in HF has emerged. The role of
inflammation in the pathogenesis of HF has been strengthened with
the implication of several cytokines, including IL-6, in the
disease process in some experimental studies and the demonstration
of elevated inflammatory markers in patients with milder degrees of
HF, including those with asymptomatic left ventricular dysfunction
as well as in patients at risk to develop HF (32). In the present study, the results
demonstrated that pericardial fluid expression of IL-6 was also
significantly increased in CHF patients. Recently, a study by
Hilfiker-Kleiner et al (43)
has raised the warning that continuous gp130-mediated signal
transducer and activator of transcription 3 (STAT3) activation
promotes inflammation, left ventricular rupture and adverse
remodeling in myocardial infarction. Moreover, these authors
hypothesized that the elevation of IL-6 levels caused continuous
activation of the gp130/STAT3 pathway, leading to HF. In addition,
the serum IL-6 level is increased in patients with HF. Furthermore,
Kinugawa et al (44) reported
that cardiac expression of IL-6 mRNA is also increased in the
myocardium in patients with advanced HF. IL-6 may contribute to the
progression of myocardial damage and dysfunction in the CHF
syndrome (23). For several reasons,
it is possible that the enhanced IL-6 activity in the failing
myocardium may contribute to the increased gp130 and IL-6 levels in
patients with CHF.
In patients with HF, a decrease in IL-10 plasma
concentration has been reported and it is positively correlated
with a decrease in the left ventricular ejection fraction (45). In the present study, the results
revealed that the anti-inflammatory cytokine IL-10 was increased in
T-cells from CHF patients within its pericardial fluid compared to
NHF patients. This result is supported by a previous study
performed by Amir et al (46), which reported that the IL-10
circulating levels are higher in HF patients compared with
healthycontrols. Moreover, Yndestad et al (39) reported a rise in inflammatory
mediators that appears not to be accompanied by a corresponding
increase in anti-inflammatory cytokines, including IL-10 in HF
patients, and resulting in an inflammatory effect. Moreover in the
present study, pericardial fluid expression of both pro- and
anti-inflammatory mediators demonstrated cellular infiltrations of
abundant T-cells within the pericardial fluid of CHF patients. This
result suggests that the possible interaction in the complex of
inflammatory and anti-inflammatory network may require further
study.
It has previously been demonstrated that elevated
sgp130 levels are associated with cardiovascular damage, and
mortality resulting from worsening of HF (47). In the present study, the results
showed that patients with CHF had significantly increased levels of
pericardial fluid sgp130 compared with NHF patients, indicating
that sgp130 may be involved in the progression of CHF. It has also
been reported (48,49) that elevated serum levels of IL-6
cytokines and gp130 proteins are strong prognostic markers for
morbidity. Askevold et al (22) demonstrated that serum sgp130 was
associated with fatal outcomes in patients with chronic systolic HF
of ischemic causes, but did not appear to predict vascular events,
and sgp130 did not enhance risk prediction when troponin T was
accounted for and mortality in patients with HF or after myocardial
infarction. gp130 is the common signal-transducing receptor subunit
of the IL-6 family, which may be involved in the progression of HF.
Sgp130 itself reflecting inflammatory processes, which has been
reported is elevated in CHF patients (10). Moreover, the gp130 receptor system is
important in the transition of left ventricular hypertrophy to
overt HF (49).
It has been reported that increased levels of
pro-inflammatory cytokines, in response to myocardial damage, are
important prognostic factors correlating with increased mortality
rates in HF patients (29). In
addition, another study has indicated that the assessment of
markers associated with the different pathways of HF pathogenesis
may facilitate diagnosis and the prediction of mortality risk in
patients with HF based on five years of observation (50). From this study it may be concluded
that cytokines are increased in the pericardial fluid of the CHF,
which may induce alteration in the structural matrix of the
myocardium, and cause myocyte apoptosis leading to the inflammatory
response, which may reflect T cell activation. Therefore, more data
on the risk of stratification and therapy using presented
biomarkers are required, and may warrant further studies and with
larger populations.
In conclusion, to the best of our knowledge the
present study reported for the first time that there is
infiltration of inflammatory cells and enhanced expression of
inflammatory cytokines within the pericardial fluid of patients
with CHF, which reflects T cell activation. Moreover, it confirmed
that the progression of CHF is associated with systemic
inflammation. Furthermore, this evidence could represent a possible
novel target that may lead to an improved understanding and future
therapeutics and prevention to improve the clinical outcome in
patients with CHF.
Acknowledgements
The authors thank all the participants and people
who worked in the present study. Dr Xin Chen is a fellow at the
Collaborative Innovation Center for Cardiovascular Disease
Translational Medicine. The present study was supported by grants
from the National Science Foundation of China (grant no. 81370259)
and the Clinical Scientific Grant of Jiangsu Province (grant no.
BE2015621).
References
|
1
|
Zhu ZF, Li JJ, Liu J, Tang TT, Ding YJ,
Liao YH, Cheng X and Wang X: Circulating Th17 cells are not
elevated in patients with chronic heart failure. Scand Cardiovasc
J. 46:295–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang TT, Zhu ZF, Wang J, Zhang WC, Tu X,
Xiao H, Du XL, Xia JH, Dong NG, Su W, et al: Impaired thymic export
and apoptosis contribute to regulatory T-cell defects in patients
with chronic heart failure. PloS One. 6:e242722011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lichtman AH: The heart of the matter:
Protection of the myocardium from T cells. J Autoimmun. 45:90–96.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haugen E, Gan LM, Isic A, Skommevik T and
Fu M: Increased interleukin-6 but not tumour necrosis factor-alpha
predicts mortality in the population of elderly heart failure
patients. Exp Clin Cardiol. 13:19–24. 2008.PubMed/NCBI
|
|
5
|
Fritzenwanger M, Jung C, Franz M, Foerster
M and Figulla HR: Immunomodulatory effects of cardiotrophin-1 on in
vitro cytokine production of monocytes & CD4 + T-lymphocytes.
Indian J Med Res. 136:471–476. 2012.PubMed/NCBI
|
|
6
|
Reifenberg K, Lehr HA, Torzewski M, Steige
G, Wiese E, Küpper I, Becker C, Ott S, Nusser P, Yamamura K, et al:
Interferon-gamma induces chronic active myocarditis and
cardiomyopathy in transgenic mice. Am J Pathol. 171:463–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ,
Tang TT, Yao R, Chen Y and Liao YH: The Th17/Treg imbalance in
patients with acute coronary syndrome. Clin immunol. 127:89–97.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stumpf C, Lehner C, Yilmaz A, Daniel WG
and Garlichs CD: Decrease of serum levels of the anti-inflammatory
cytokine interleukin-10 in patients with advanced chronic heart
failure. Clin Sci. 105:45–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pudil R, Tichý M, Andrýs C, Rehácek V,
Bláha V, Vojácek J and Palicka V: Plasma interleukin-6 level is
associated with NT-proBNP level and predicts short and long term
mortality in patients with acute heart failure. Acta Medica (Hradec
Kralove). 53:225–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hofmann U and Frantz S: How can we cure a
heart ‘in flame’? A translational view on inflammation in heart
failure. Basic Res Cardiol. 108:3562013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiang F, Guo X, Chen W, Wang J, Zhou T,
Huang F, Cao C and Chen X: Proteomics analysis of human pericardial
fluid. Proteomics. 13:2692–2695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frohlich GM, Keller P, Schmid F, Wolfrum
M, Osranek M, Falk C, Noll G, Enseleit F, Reinthaler M, Meier P, et
al: Haemodynamically irrelevant pericardial effusion is associated
with increased mortality in patients with chronic heart failure.
Eur Heart J. 34:1414–1423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hunt SA, Abraham WT, Chin MH, Feldman AM,
Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K,
et al: 2009 focused update incorporated into the ACC/AHA 2005
guidelines for the diagnosis and management of heart failure in
adults: A report of the American college of cardiology
foundation/American heart association task force on practice
guidelines: Developed in collaboration with the International
society for heart and lung transplantation. Circulation.
119:e391–e479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eren M: Diagnosis and treatment of acute
and chronic heart failure: What has changed in the new European
society of cardiology guideline 2008. Turk Kardiyol Dern Ars.
37:295–300. 2009.PubMed/NCBI
|
|
15
|
Cheitlin MD, Armstrong WF, Aurigemma GP,
Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD,
Kimball TR, et al: ACC/AHA/ASE 2003 guideline update for the
clinical application of echocardiography: Summary article: A report
of the American college of cardiology/American heart association
task force on practice guidelines (ACC/AHA/ASE committee to update
the 1997 guidelines for the clinical application of
echocardiography). Circulation. 108:1146–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hurst JW: The value of using the entire
New York heart association's classification of heart and vascular
disease. Clin Cardiol. 29:415–417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hunt SA, Abraham WT, Chin MH, Feldman AM,
Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K,
et al: ACC/AHA 2005 guideline update for the diagnosis and
management of chronic heart failure in the adult: A report of the
American college of cardiology/American heart association task
force on practice guidelines (writing committee to update the 2001
guidelines for the evaluation and management of heart failure):
Developed in collaboration with the American college of chest
physicians and the international society for heart and lung
transplantation: Endorsed by the heart rhythm society. Circulation.
112:e154–e235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Chen W, Zhu Y, Wang X, Jiang DS,
Huang F, Wang L, Xiang F, Qin W, Wang Q, et al: Caspase recruitment
domain 6 protects against cardiac hypertrophy in response to
pressure overload. Hypertension. 64:94–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao C, Zhu Y, Chen W, Li L, Qi Y, Wang X,
Zhao Y, Wan X and Chen X: IKKe knockout prevents high fat diet
induced arterial atherosclerosis and NF-kB signaling in mice. PloS
One. 8:e649302013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao C, Li L, Chen W, Zhu Y, Qi Y, Wang X,
Wan X and Chen X: Deficiency of IKKe inhibits inflammation and
induces cardiac protection in high-fat diet-induced obesity in
mice. Int J Mol Med. 34:244–252. 2014.PubMed/NCBI
|
|
21
|
Ziaeian B and Fonarow GC: Epidemiology and
aetiology of heart failure. Nat Rev Cardiol. 13:368–378. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Askevold ET, Nymo S, Ueland T, Gravning J,
Wergeland R, Kjekshus J, Yndestad A, Cleland JG, McMurray JJ,
Aukrust P and Gullestad L: Soluble glycoprotein 130 predicts fatal
outcomes in chronic heart failure: Analysis from the Controlled
Rosuvastatin multinational trial in heart failure (CORONA). Circ
Heart Fail. 6:91–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanda T and Takahashi T: Interleukin-6 and
cardiovascular diseases. Jpn Heart J. 45:183–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda K, Tsutamoto T, Wada A, Mabuchi N,
Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T and
Kinoshita M: High levels of plasma brain natriuretic peptide and
interleukin-6 after optimized treatment for heart failure are
independent risk factors for morbidity and mortality in patients
with congestive heart failure. J Am Coll Cardiol. 36:1587–1593.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maries L and Manitiu I: Diagnostic and
prognostic values of B-type natriuretic peptides (BNP) and
N-terminal fragment brain natriuretic peptides (NT-pro-BNP).
Cardiovasc J Afr. 24:286–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toufan M, Namdar H, Abbasnezhad M,
Habibzadeh A, Esmaeili H, Yaraghi S and Samani Z: Diagnostic values
of plasma, fresh and frozen urine NT-proBNP in heart failure
patients. J Cardiovasc Thorac Res. 6:111–115. 2014.PubMed/NCBI
|
|
27
|
Chang HR, Hsieh JC, Hsu BG, Wang LY, Chen
MY and Wang JH: Inverse association of N-terminal pro-B-type
natriuretic peptide with metabolic syndrome in patients with
congestive heart failure. PloS One. 8:e790962013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taylor CJ, Roalfe AK, Iles R and Hobbs FD:
The potential role of NT-proBNP in screening for and predicting
prognosis in heart failure: A survival analysis. BMJ Open.
4:e0046752014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szczurek W and Szyguła-Jurkiewicz BS:
Oxidative stress and inflammatory markers - the future of heart
failure diagmostics? Kardiochir Torakochirurgia Pol. 2015:145–149.
2015.
|
|
30
|
Gruson D, Ahn SA and Rousseau MF:
Biomarkers of inflammation and cardiac remodeling: The quest of
relevant companions for the risk stratification of heart failure
patients is still ongoing. Biochem Med (Zagreb). 21:254–263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoare GS, Birks EJ, Bowles C, Marczin N
and Yacoub MH: In vitro endothelial cell activation and
inflammatory responses in end-stage heart failure. J Appl Physiol
(1985). 101:1466–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ruiz-Ruiz FJ, Ruiz-Laiglesia FJ,
Lasierra-Diaz P, Legarre Samperiz P, Morales-Rull JL,
Sánchez-Marteles M, Amores M and Perez-Calvo JI: Lack of clinical
usefulness of interleukin-6 in long-term follow-up of acutely
decompensated heart failure. Singapore Med J. 48:532–536.
2007.PubMed/NCBI
|
|
33
|
Rumalla VK, Calvano SE, Spotnitz AJ,
Krause TJ, Hilkert RJ, Lin E and Lowry SF: Alterations in
immunocyte tumor necrosis factor receptor and apoptosis in patients
with congestive heart failure. Ann Surg. 236:254–260. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okamoto N, Noma T, Ishihara Y, Miyauchi Y,
Takabatake W, Oomizu S, Yamaoka G, Ishizawa M, Namba T, Murakami K,
et al: Prognostic value of circulating regulatory T cells for
worsening heart failure in heart failure patients with reduced
ejection fraction. Int Heart J. 55:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang TT, Ding YJ, Liao YH, Yu X, Xiao H,
Xie JJ, Yuan J, Zhou ZH, Liao MY, Yao R, et al: Defective
circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients
with chronic heart failure. Cell Physiol Biochem. 25:451–458. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ,
Agreda P, Satpute SR, Crow MT, King LS and Rabb H:
Foxp3+ regulatory T cells participate in repair of
ischemic acute kidney injury. Kidney Int. 76:717–729. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yndestad A: Enhanced expression of
inflammatory cytokines and activation markers in T-cells from
patients with chronic heart failure. Cardiovasc Res. 60:141–146.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fritzenwanger M, Jung C, Franz M, Foerster
M and Figulla HR: Immunomodulatory effects of cardiotrophin-1 on in
vitro cytokine production of monocytes & CD4 + T-lymphocytes.
Indian J Med Res. 136:471–476. 2012.PubMed/NCBI
|
|
39
|
Yndestad A, Damås JK, Oie E, Ueland T,
Gullestad L and Aukrust P: Systemic inflammation in heart
failure-the whys and wherefores. Heart Fail Rev. 11:83–92. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cappuzzello C, Di Vito L, Melchionna R,
Melillo G, Silvestri L, Cesareo E, Crea F, Liuzzo G, Facchiano A,
Capogrossi MC and Napolitano M: Increase of plasma IL-9 and
decrease of plasma IL-5, IL-7, and IFN-γ in patients with chronic
heart failure. J Transl Med. 9:282011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fukunaga T, Soejima H, Irie A, Sugamura K,
Oe Y, Tanaka T, Kojima S, Sakamoto T, Yoshimura M, Nishimura Y and
Ogawa H: Expression of interferon-gamma and interleukin-4
production in CD4+ T cells in patients with chronic
heart failure. Heart Vessels. 22:178–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fairweather D, Frisancho-Kiss S, Yusung
SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB and Rose NR:
Interferon-gamma protects against chronic viral myocarditis by
reducing mast cell degranulation, fibrosis and the profibrotic
cytokines transforming growth factor-beta 1, interleukin-1 beta,
and interleukin-4 in the Heart. Am J Pathol. 165:1883–1894. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hilfiker-Kleiner D, Shukla P, Klein G,
Schaefer A, Stapel B, Hoch M, Müller W, Scherr M, Theilmeier G,
Ernst M, et al: Continuous glycoprotein-130-mediated signal
transducer and activator of transcription-3 activation promotes
inflammation, left ventricular rupture, and adverse outcome in
subacute myocardial infarction. Circulation. 122:145–155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kinugawa T, Kato M, Yamamoto K, Hisatome I
and Nohara R: Proinflammatory cytokine activation is linked to
apoptotic mediator, soluble Fas level in patients with chronic
heart failure. Int Heart J. 53:182–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Júnior ML Jr, Lopes RD, Seelaender MC and
Lopes AC: Anti-inflammatory effect of physical training in heart
failure role of TNF-alpha and IL-10. Arq Bras Cardiol. 93:643–651.
2009.PubMed/NCBI
|
|
46
|
Amir O, Rogowski O, David M, Lahat N,
Wolff R and Lewis BS: Circulating interleukin-10: Association with
higher mortality in systolic heart failure patients with elevated
tumor necrosis factor-alpha. Isr Med Assoc J. 12:158–162.
2010.PubMed/NCBI
|
|
47
|
Ritschel VN, Seljeflot I, Arnesen H,
Halvorsen S, Weiss T, Eritsland J and Andersen GØ: IL-6 signalling
in patients with acute ST-elevation myocardial infarction. Results
Immunol. 4:8–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pudil R, Tichý M, Andrýs C, Rehácek V,
Bláha V, Vojácek J and Palicka V: Plasma interleukin-6 level is
associated with NT-proBNP level and predicts short- and long-term
mortality in patients with acute heart failure. Acta Medica (Hradec
Kralove). 53:225–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fischer P and Hilfiker-Kleiner D: Role of
gp130-mediated signalling pathways in the heart and its impact on
potential therapeutic aspects. Br J Pharmacol. 153:(Suppl 1).
S414–S427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Richter B, Koller L, Hohensinner PJ, Zorn
G, Brekalo M, Berger R, Mörtl D, Maurer G, Pacher R, Huber K, et
al: A multi-biomarker risk score improves prediction of long-term
mortality in patients with advanced heart failure. Int J Cardiol.
168:1251–1257. 2013. View Article : Google Scholar : PubMed/NCBI
|