Introduction
Current epidemiologic data suggests that the global
prevalence of pulmonary hypertension (PH) is 1%, and this increases
to 10% in individuals >65 years old (1). PH is characterized by elevated
pulmonary vascular resistance, progressive right ventricle (RV)
hypertrophy and, ultimately, RV failure, which is key in the
development of chronic pulmonary heart disease (2). The development of PH is largely due to
hypoxia caused by chronic obstructive pulmonary disease (3); therefore, inhibiting the development of
chronic hypoxia-induced PH (HPH) may effectively prevent the
occurrence of many cardiovascular diseases. Hypoxia may cause
disease by increasing the level of intracellular reactive oxygen
species (ROS) (4). Relatedly, redox
signaling has been suggested to be involved in the regulation of
hypoxic pulmonary vasoconstriction under acute hypoxia, and in cell
proliferation during chronic hypoxia (5,6), the
underlying mechanisms remain unknown. ROS, acting as signaling
molecules, modulate diverse physiological processes including the
regulation of growth factor signaling, the hypoxic response,
inflammation and the immune response in mammalian cells (7,8).
Hypoxia-inducible factor-1 (HIF-1), a master regulator of gene
expression induced by hypoxia, serves an important role in the
origin and development of HPH (9). A
recent study has demonstrated that ROS serve an important role in
the regulation of HIF-1 expression and activity (10).
Although emerging evidence demonstrates the
protective effect of estrogen and its metabolites on pulmonary
arterial hypertension (11,12), it remains unclear whether this occurs
via adjustments of the oxidative stress-hypoxia inducible factor-1α
(OS-HIF-1α) pathway. Previous research by the current authors
indicated that the mean pulmonary artery pressure (mPAP) of
ovariectomized (OVX) rats increased significantly under hypoxic
conditions and that 17β-estradiol (E2) and 2-methoxy estradiol
(2ME) partially reversed HPH development (13). In the current study, an HPH animal
model was established, using OVX rats. Alterations to ROS,
superoxide dismutase (SOD), manganese superoxide dismutase (MnSOD),
copper-zinc superoxide dismutase (Cu/ZnSOD) and HIF-1α were
examined in vivo to determine the effects of E2 and 2ME
treatment on the OS-HIF-1α pathway in this model.
Materials and methods
Animals and experimental design
A total of 32 healthy 6–8 week-old female
Sprague-Dawley rats (weight, 170–190 g) were purchased from the
Animal Center of the Hebei Medical University (Shijiazhuang,
China). In accordance with previous modeling approaches (10), the rats were randomly divided into 4
groups (n=8 per group), as follows: i) Control (group A); ii) OVX +
hypoxia (group B); iii) OVX + hypoxia + E2 (group C); and iv) OVX +
hypoxia + 2ME groups (group D). In groups B, C and D rats were
anesthetized with pentobarbital sodium (40 mg/kg, intraperitoneal;
Shanghai Haling Biotechnology Co., Ltd., Shanghai, China), the
abdominal cavity was subsequently opened and the bilateral ovaries
were removed. In group A, rats were anesthetized with pentobarbital
sodium (40 mg/kg, intraperitoneal; Shanghai Haling Biotechnology
Co., Ltd.), the abdominal cavity was opened and the ovaries were
located but not removed. Following the operation, the rats in
groups A and B received subcutaneous injection of physiological
saline (0.1 ml/day for 8 weeks), group C received a subcutaneous
injection of E2 (20 µg/kg/day for 8 weeks; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) and group D received a subcutaneous
injection of 2ME (240 µg/kg/day for 8 weeks; Sigma-Aldrich; Merck
Millipore). Exposure to hypoxic conditions and E2, 2ME or
physiological saline was initiated simultaneously 1 week after
surgery. To provide hypoxic conditions, rats were maintained in a
normobaric chamber (CJ-DO2245; Changjin Science Co., Ltd.,
Changsha, China) with a controlled O2 concentration of
10.0±0.5%. Soda lime and anhydrous calcium chloride were used to
absorb excess carbon dioxide and water vapor, maintaining the
carbon dioxide concentration at <0.5%. The rats of group A were
housed in normal air. All rats were housed in a
temperature-controlled environment (25°C) with a 12 h light-dark
cycle, fed a standard laboratory diet and provided with water ad
libitum. Bedding was changed once a week. The rats were maintained
under air/hypoxic conditions for 8 weeks in order to establish a
hypoxic PH model. The present study was performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of Hebei Medical University.
Measurement of mPAP and pulmonary
arteriole morphology
Measurement of mPAP was performed as described
previously (14). Briefly, after 8
weeks, the rats were anesthetized by an intraperitoneal injection
of pentobarbital sodium (40 mg/kg; Shanghai Haling Biotechnology
Co., Ltd.). A longitudinal skin incision was made on the right side
of the neck, and blunt layer-by-layer separation of the tissues was
performed until the right external jugular vein was exposed. A
polyethylene catheter was gradually inserted into the pulmonary
artery through an incision in the right external jugular vein, and
the mPAP was recorded using a pressure transducer, which was
interfaced to a BL-420S Bio Lab System (Chengdu TME Technology Co.,
Ltd., Chengdu, China). Following measurement of mPAP, blood samples
(2.0 ml per rat) were drawn from the pulmonary artery of the rats.
These were centrifuged for 10 min at 3,000 × g and the supernatant
was collected and stored at −80°C until use. The rats were then
sacrificed by exsanguination and the lungs were isolated and washed
with physiological saline repeatedly. Three lobes of the right lung
were surgically removed, immediately snap frozen in liquid nitrogen
and stored at −80°C until use. The upper lobe of the left lung was
removed and fixed in a 10% formalin solution overnight, which was
followed by paraffin embedding. Subsequently, lung sections (4-µm)
were prepared and stained with hematoxylin and eosin. Sections were
examined under a light microscope (Eclipse 55i; Nikon Corporation,
Tokyo, Japan) for pulmonary arteriolar morphological analysis.
Assessment of pulmonary arteriolar
ultrastructural changes
To assess the pulmonary arteriolar ultrastructure,
lung tissue was fixed in 4% glutaraldehyde, and postfixed in 1%
OsO4. Ultrathin sections were cut on a microtome, placed
on copper grids, stained with uranyl acetate and lead citrate, and
examined with a transmission electron microscope.
Determination of ROS, SOD, MnSOD, and
Cu/ZnSOD
Serum ROS levels were measured using the Fenton
reaction and Griess color rendering principle according to the ROS
detection kit instructions (cat. no. A018; Jiancheng Bioengineering
Institute, Nanjing, China).
SOD, Cu/ZnSOD, and MnSOD levels in serum were
determined using the xanthine oxidase method according to the
instructions in the SOD activity detection kit (cat. no. A001;
Jiancheng Bioengineering Institute).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to detect the expression of MnSOD
and HIF-1α mRNA, as follows: Total RNA was extracted from lung
tissue using TRIzol (Takara Biotechnology Co., Ltd., Dalian, China)
and 2 µg of each RNA sample was reverse transcribed to cDNA. The
RNA concentration and purity were determined using an ultraviolet
spectrophotometer (756MC; Shanghai Precision & Scientific
Instrument Co., Ltd., Shanghai, China). RT was performed as
follows: 2 µg of total RNA was added into an RT reaction system
tube containing 10 µl avian myeloblastosis virus (AMV) buffer, 5 µl
dNTPs, 2.5 µl oligo(dT) primer, 2.5 µl AMV and 2.5 µl RNase
inhibitor (cat. no. 2313A; all Takara Biotechnology Co., Ltd.).
Diethyl pyrocarbonate (DEPC) solution was added to make up 50 µl
total reaction volume. After mixing and centrifuging at 4°C and 256
× g for 5 sec, the reaction system was subjected to RT. The
conditions for RT were as follows: 42°C for 30 min, 99°C for 5 min
and 5°C for 5 min. The following specific oligonucleotide primers
were used: Forward, 5′-GCCTCAGCAATGTTGTGTCG-3′; and reverse,
5′-TGATTAGAGCAGGCGGCAAT-3′ for MnSOD; and forward,
5′-CTCAGAGGAAGCGAAAAATGG-3′; and reverse,
5′-AATTCTTCACCCTGCAGTAGG-3′ for HIF-1α (Sangon Biotech Co., Ltd.,
Shanghai, China). GAPDH (Forward, 5′-CACCTTTGATGCTGGGGCTG-3′; and
reverse, 5′-TGGTATTCGAGAGAAGGGAGGG-3′; Sangon Biotech Co., Ltd.)
was used as an internal reference to normalize the mRNA expression
levels of MnSOD and HIF-1α. The qPCR reaction system contained 5 µl
10xTaq buffer, 3 µl MgCl2 (25 mmol/l), 0.5 µl dNTP (10
mmol/l), 1 µl forward primer (10 µmol/l), 1 µl reverse primer (10
µmol/l), 2.5 µl cDNA, 0.5 µl Taq DNA polymerase and DEPC solution
to give a total reaction volume of 50 µl (all Takara Biotechnology
Co., Ltd.). The conditions for qPCR were as follows: Initial
denaturation at 94°C for 3 min; 30 cycles of denaturation at 94°C
for 40 sec, annealing at 54°C for 30 sec, and extension at 72°C for
1 min; final extension at 72°C for 10 min. Afterwards, 6 µl of PCR
product was used to perform 1.5% agarose gel electrophoresis. The
electrophoresis image was analyzed using Quantity One Analysis
Software (version 4.6; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Western blotting
Western blot analysis was used to detect the
expression of MnSOD and HIF-1α protein. Total protein was extracted
from lung tissue using radioimmunoprecipitation assay lysis buffer
(BestBio, Shanghai, China), and the protein content was determined
by the improved Lowry method (15).
Equal amounts of protein (60 µg/lane) from each sample were
separated by SDS-PAGE on a 15% polyacrylamide gel, and were
subsequently electrophoretically transferred to a nitrocellulose
membrane. The membranes were blocked with a 5% nonfat dry milk
solution in TBS with 0.1% Tween-20 (TBS-T, pH 8.0) for 2 h at room
temperature and incubated in primary antibody dissolved in the
blocking solution at 4°C overnight. The MnSOD protein was detected
using a rabbit anti-MnSOD monoclonal antibody (1:1,000; ab68155;
Abcam, Cambridge, MA, USA) and HIF-1α was detected using a mouse
anti-HIF-1α monoclonal antibody (1:1,000; NB100-123; Novus
Biologicals LLC, Littleton, CO, USA); a mouse anti-GAPDH monoclonal
antibody (1:1,000; ab9484; Abcam) was used to confirm equal
loading. Following three washes for 5 min in TBS-T, the membranes
were incubated with horseradish peroxidase-conjugated
immunoglobulin g (1:1,000; MAB1799; R&D Systems, Inc.,
Minneapolis, MN, USA) corresponding to the primary antibody in the
blocking buffer for 2.5–3.0 h at room temperature. Following three
washes, the proteins were detected using luminol detection reagent
(Santa Cruz Biotechnology, Inc.) and developed on X-ray film.
Statistical analysis
All quantitative data are expressed as mean ±
standard deviation. Statistical analysis was performed using a
one-way analysis of variance, followed by a least significant
difference test for post hoc multiple comparisons. A P<0.05 was
considered to represent a statistically significant difference.
Analyses were performed using SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
The animal model of PH
During the experiments, rats in the control group
were active, gained weight gradually and their body fur was smooth
and lustrous, whereas in hypoxic groups, the fur of the rats was
less healthy, they took shorter breaths, their food intake was
lower, their bodies were smaller and their activity decreased
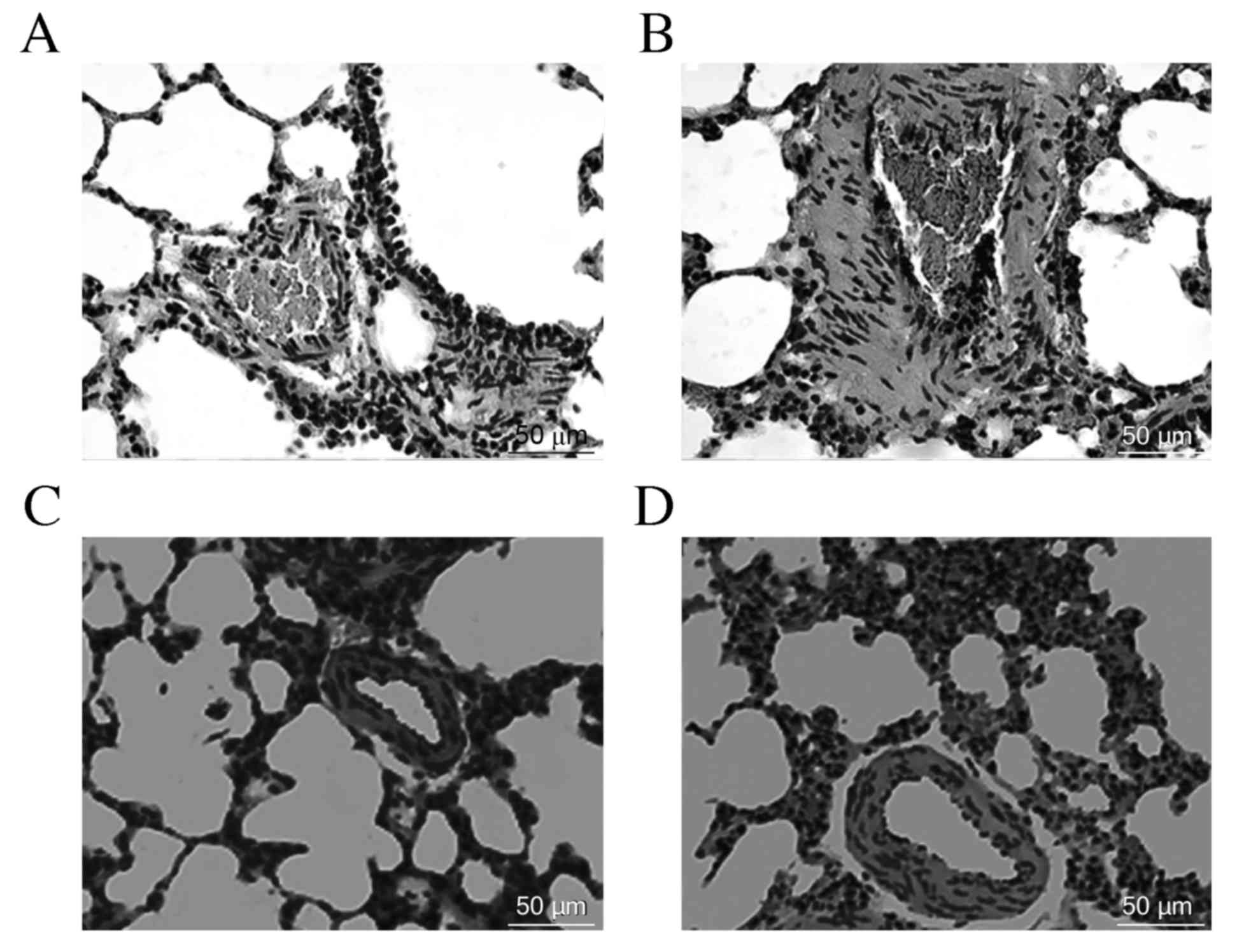
gradually. Following 8 weeks of hypoxia exposure, OVX rats
exhibited pulmonary vascular structural remodeling and PH
characteristics, including a visible pulmonary arterial wall and
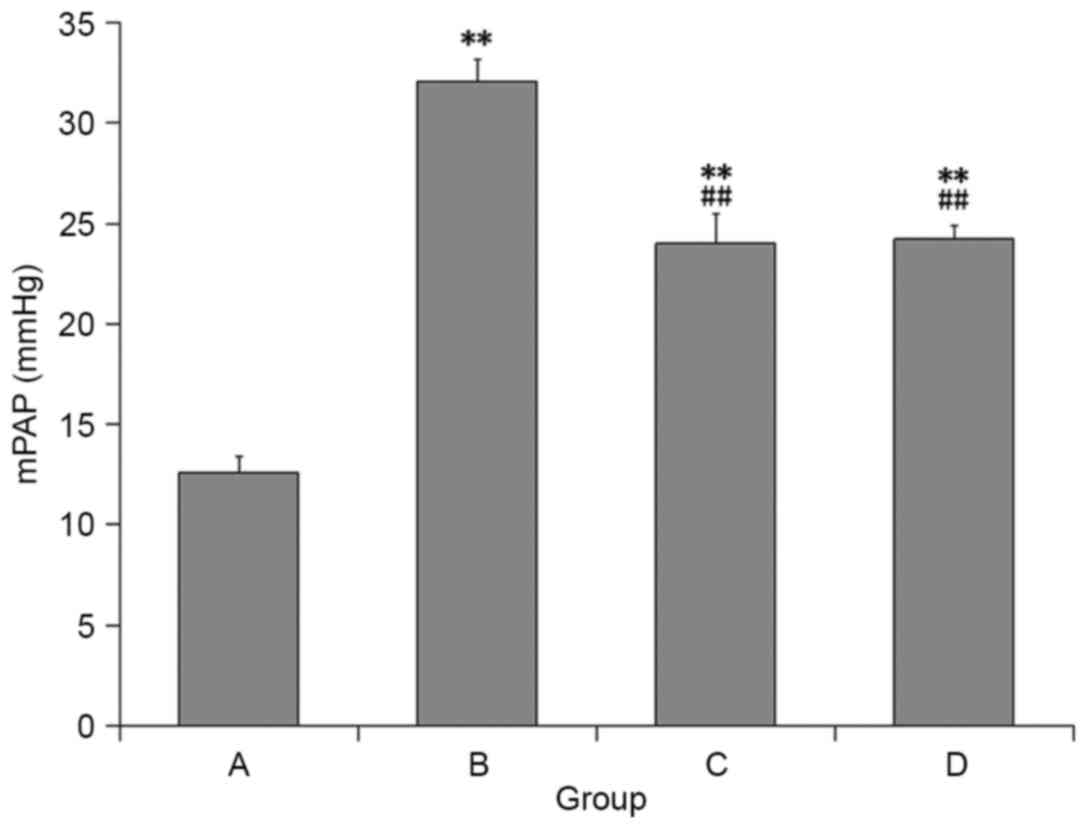
smooth muscle layer thickening, luminal stenosis (Fig. 1) and a significant increase in mPAP
compared with the control rats (Fig.
2). However, the above changes were lessened in the rats
treated with E2 and 2ME as compared with the OVX + hypoxia
rats.
Ultrastructural changes of the
pulmonary arteriole
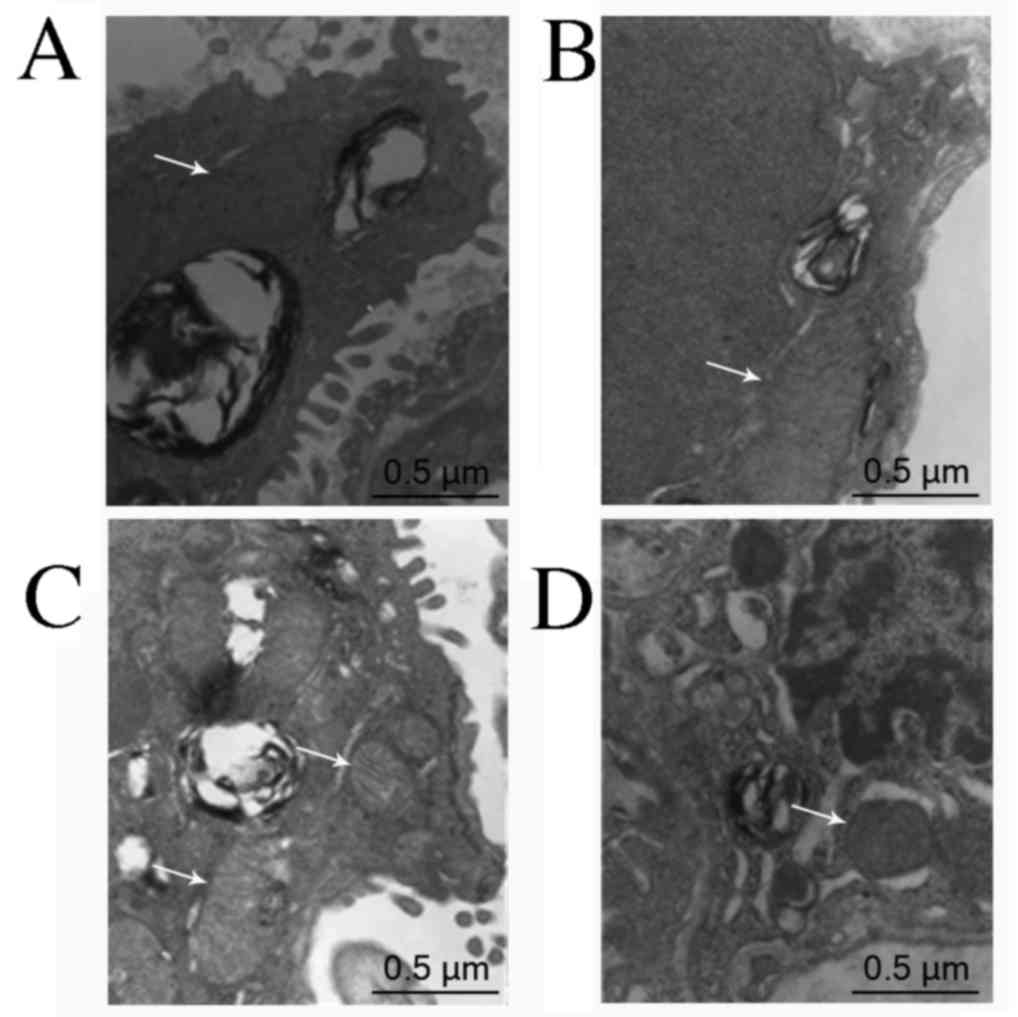
Pulmonary arteriolar ultrastructural changes were
observed via transmission electron microscopy. In the control
group, the mitochondria were distributed evenly and arranged
orderly, and their double membrane structure was clear without
obvious swelling. In the OVX + hypoxia group, the mitochondria were
reduced in number, swollen, and deformed with fractured cristae.
These changes were less apparent the E2 and 2ME intervention groups
than in the OVX + hypoxia group (Fig.
3).
Serum ROS levels
Compared with the control group, the serum ROS level
of the OVX + hypoxia group was significantly higher (P<0.01).
Additionally, compared with the OVX + hypoxia group, the serum ROS
levels in E2 and 2ME intervention groups decreased significantly
(P<0.01), and there was no significant difference between these
two groups (P>0.05) (Fig. 4).
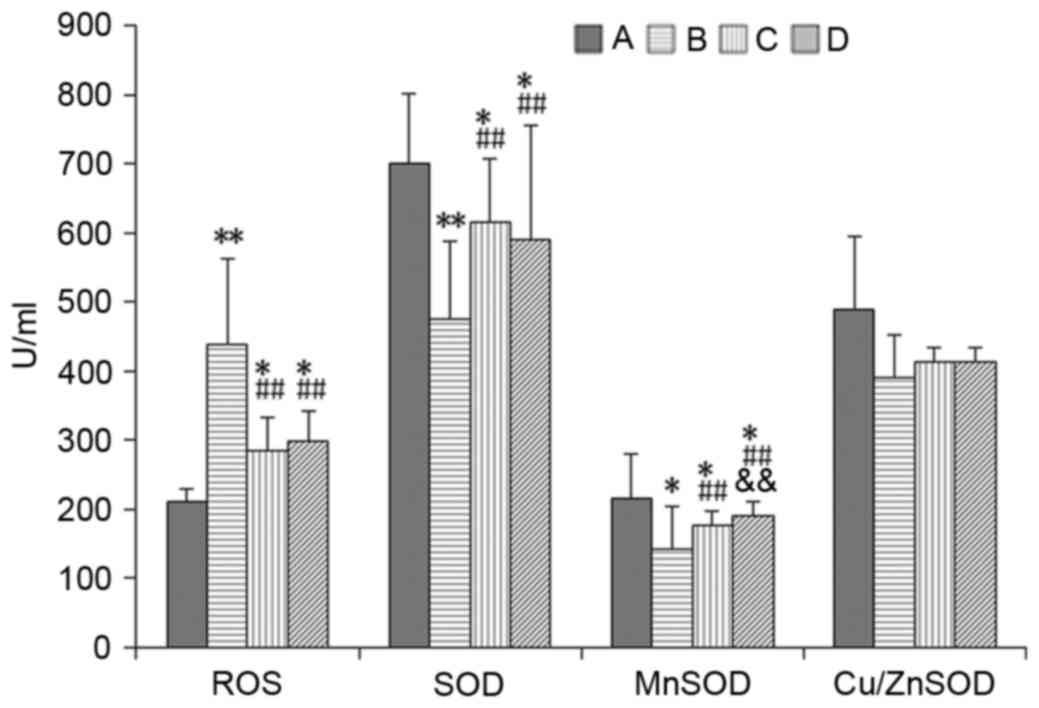 | Figure 4.Comparison of the ROS, SOD, MnSOD,
Cu/ZnSOD levels in serum among the groups. (A) Control group; (B)
OVX + hypoxia group; (C) OVX + hypoxia + E2 group; (D) OVX +
hypoxia + 2ME group. *P<0.05; **P<0.01 vs. A;
##P<0.01 vs. B; &&P<0.01 vs. C.
n=8 rats in each group. ROS, reactive oxygen species; SOD,
superoxide dismutase; MnSOD, manganese superoxide dismutase;
Cu/ZnSOD, copper-zinc superoxide dismutase; OVX, ovariectomized;
E2, estradiol; ME, methoxyestradiol. |
SOD, MnSOD, and Cu/ZnSOD levels
Compared with the control group, SOD and MnSOD
levels in serum were significantly decreased (P<0.05 or
P<0.01). In addition, compared with the OVX + hypoxia group,
levels of these enzymes were significantly higher (P<0.01) in
the E2 and 2ME intervention groups. In the E2 intervention group,
MnSOD was significantly increased compared with the 2ME group
(P<0.01). There was no significant difference in Cu/ZnSOD
activity between the groups (P>0.05) (Fig. 4).
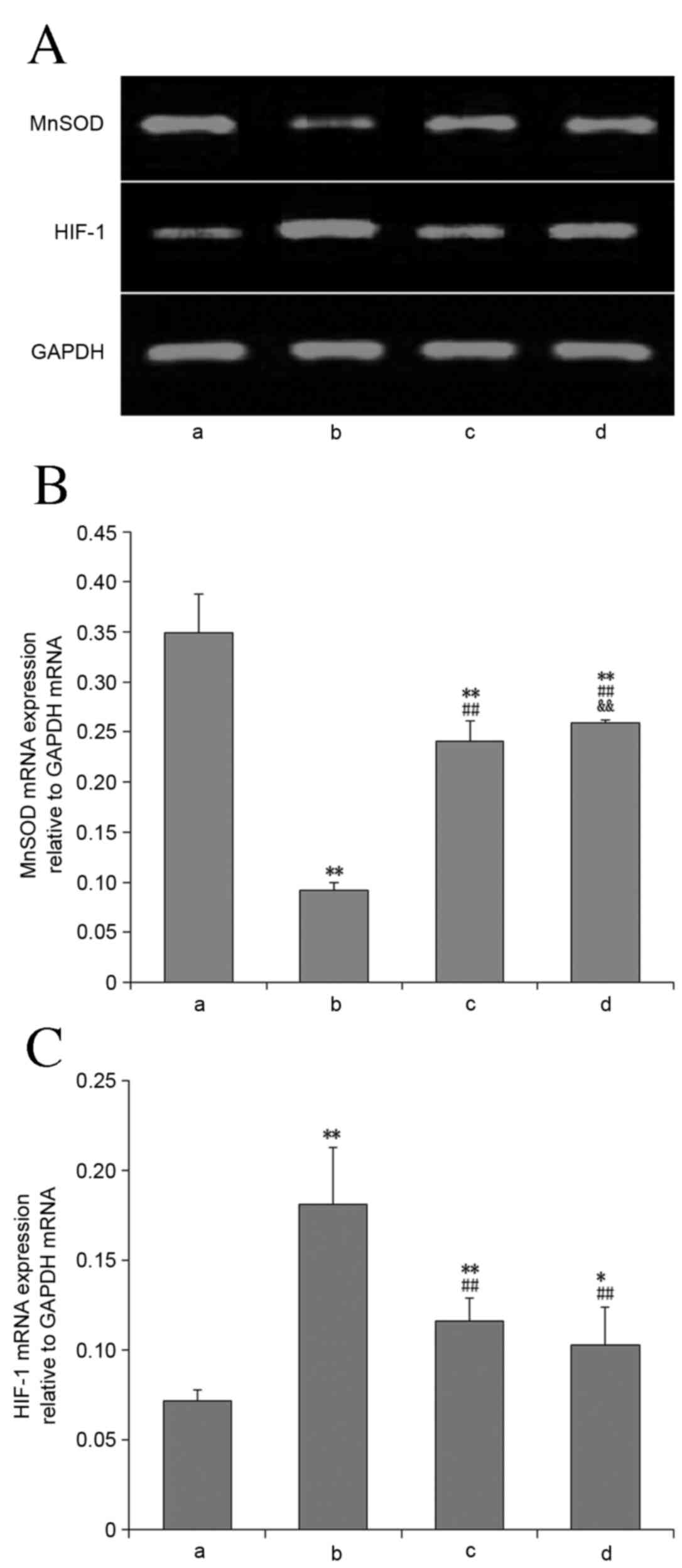
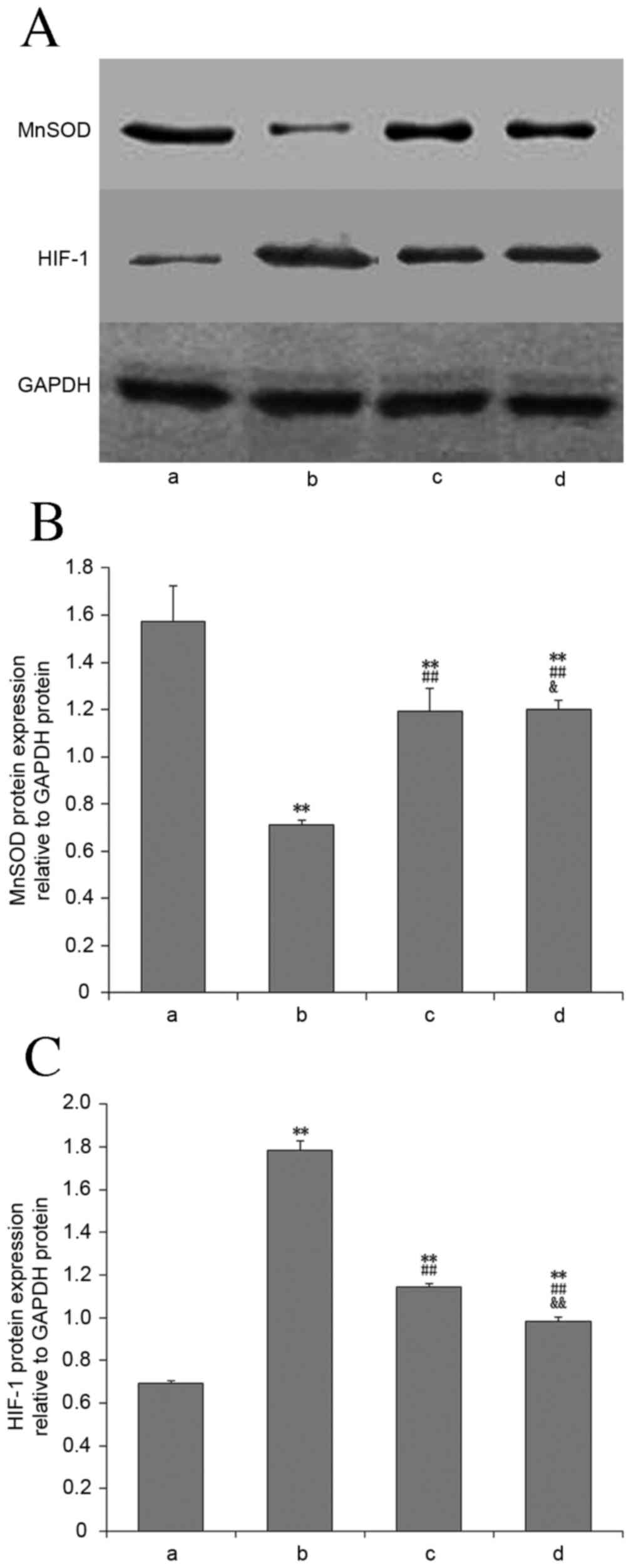
MnSOD mRNA and protein expression
Compared with the control group, MnSOD mRNA
(Fig. 5) and protein expression
(Fig. 6) in lung tissues of the
other groups were significantly decreased (P<0.01). Compared
with the OVX + hypoxia group, the expression of these in the E2 and
2ME intervention groups was significantly increased (P<0.01),
and this increase was greater in the 2ME group.
HIF-1α mRNA and protein
expression
Compared with the control group, HIF-1α mRNA
(Fig. 5) and protein expression
(Fig. 6) in lung tissues was
significantly increased in all experimental groups (P<0.01).
Compared with the OVX + hypoxia group, the HIF-1α mRNA and protein
levels in the E2 and 2ME intervention groups was significantly
reduced (P<0.01). There was no significant difference between
these two groups with regard to mRNA expression level (P>0.05),
but HIF-1α protein expression was significantly lower in the 2ME
group than the E2 group (P<0.01).
Discussion
HPH is characterized by pulmonary vasoconstriction
and pulmonary vascular remodeling, of which the main pathological
change is medial thickening, caused by enhanced proliferation of
pulmonary artery smooth muscle cells (16,17).
However, the potential mechanism of HPH is complicated and remains
poorly defined. HIF-1 is an important transcriptional factor, which
serves an essential role in the hypoxia response and is a
‘molecular switch’, regulating target gene expression and affecting
hypoxia-induced vascular remodeling (18,19).
HIF-1 is a heterodimeric transcription factor composed of a
regulatory α subunit (HIF-1α) and a constitutive β subunit (HIF-1β)
(20). HIF-1α is a functional
subunit that regulates the expression of >100 types of target
genes involved in hypoxic stress and thus serves a crucial role in
the response to hypoxia (21). The
expression and activity of HIF-1α are regulated mainly by cellular
oxygen concentration (21); however,
it is difficult to change a patient's anoxia status. A previous
study demonstrated that factors other than hypoxia may enhance
HIF-1α mRNA expression, as the HIF-1 level did not increase in
direct correlation to oxygen concentration (22). Accumulating evidence suggests that
oxidative stress is involved in the regulation of HIF-1 expression
and activity (23–27).
Oxidative stress (OS) is an imbalance between the
production of ROS, which includes superoxide anion free radicals,
hydrogen peroxide and hydroxyl radicals and the antioxidant
capacity of the body (28). SOD
provides a cellular defense mechanism by scavenging ROS, which
constitutes one of the major defense mechanisms of cells against OS
(29). In pathological conditions,
such as hypoxia, excessive ROS interact with cellular proteins,
lipids and DNA, resulting in oxidative cell and tissue damage,
and/or behave as second messengers, promoting pulmonary vascular
remodeling (30). Mitochondria are a
key site of ROS production, but also represent a target for ROS and
are compromised by severe or prolonged oxidative stress; this
creates a vicious cycle to amplify mitochondrial ROS, which leads
to subsequent mitochondrial dysfunction and oxidant generation
(31). Accumulating evidence
suggests that ROS serve an important role in HIF-1α regulation;
hypoxic exposure may increase ROS, and ROS, behaving as signaling
molecules, activate HIF-1α, inhibit voltage-gated potassium channel
expression and increase cytosolic calcium concentration, thereby
leading to smooth muscle contraction (23). Previous research demonstrated that
augmenting SOD2-increased hydrogen peroxide-mediated redox
signaling inhibited HIF-1α activity and reduced pulmonary artery
smooth muscle cell proliferation (24). Recent studies have reported that
oxidative stress regulates the expression of HIF-1α at both the
protein and mRNA levels (25–27). A
study on arsenic-induced carcinogenesis demonstrated that
arsenic-induced ROS increases HIF-1α transcription via inhibition
of miR-199a expression (25). Sasabe
et al (26) revealed that
intracellular ROS, produced following the knockdown of Mn-SOD,
enhanced HIF-1α expression in oral squamous cell carcinoma cells
through transcriptional, translational and posttranslational
regulation under normoxic and hypoxic conditions. Fijalkowska et
al (27) demonstrated that
decreased expression of mitochondrial MnSOD, which influences
mitochondrial ROS levels and/or NO bioavailability, may be
mechanistically implicated in the enhanced HIF-1α expression in
cultured endothelial cells from patients with idiopathic pulmonary
arterial hypertension. A previous study indicated that oxidative
stress and tissue hypoxia may serve as triggering signals for
HIF-1α activity and expression in irradiated lungs, leading to
radiation-induced inflammation, angiogenesis and fibrosis (32).
In the present study, female rats were used and a
model of HPH was successfully established by treatment with
bilateral OVX and 8 weeks of hypoxia. Rats in group B had
significantly increased mPAP, thickened pulmonary arteriolar walls
and an increased number of smooth muscle cells, in which
mitochondrial swelling, and crista fragmentation and disappearance
were observed. Compared with the control group, serum ROS levels
increased significantly, SOD and MnSOD levels markedly decreased,
lung tissue MnSOD mRNA and protein expression decreased and HIF-1α
mRNA and protein expression were significantly increased in the
model group. These results suggest that oxidative stress may
contribute to the occurrence and development of HPH through the
upregulation of HIF-1α transcription and translation.
Previous studies have focused on the protective
effects of E2 on the pulmonary vasculature, but the mechanisms
behind these are unknown. Miyamoto et al (33) reported that E2 reduced the HIF-1α
mRNA level under hypoxic conditions. Determining whether E2
inhibits the expression of HIF-1α by regulating oxidative stress
will help to further the understanding of the mechanism of action
of E2 in HPH. Wang et al (34) previously revealed that E2 protects
against light-induced retinal damage via its antioxidative effect,
and the etiology of this involves the upregulation of the gene
expression levels of SOD. Liu et al (35) suggested that E2 upregulates SOD2
expression, resulting in reduced ROS generation, which largely
favors cardiovascular function. In the present study,
administration of E2 significantly ameliorated mitochondrial
ultrastructural damage, and alleviated oxidative stress, as
indicated by a significant decrease in the ROS level in serum, a
significant increase in SOD and MnSOD levels in serum and a
significant increase in MnSOD mRNA and protein expression in lung
tissues. E2 therapy also significantly reduced tissue HIF-1α mRNA
and protein expression. The improvement in the aforementioned
parameters was correlated with the observed amelioration of
histological changes of pulmonary arteries. Hence, the protective
effects of E2 on HPH may be mediated by its ability to decrease
HIF-1α mRNA and protein expression through its antioxidant
potential.
2ME, an estrogen derivative, was recently reported
to have antitumor and anti-angiogenesis functions. Recently, 2ME
has been reported to downregulate HIF-1α and inhibit the expression
of its downstream target genes in tumor cells (36). However, the exact mechanism of the
inhibition of HIF-1α by 2ME is remains unclear, which may be due to
a promoting effect on HIF-1α protein degradation (36), an inhibitory effect on the
translation and nuclear translocation of HIF-1α protein (37), or a promoting effect on microtubule
disruption (38). Previous research
indicated that 2ME inhibits ROS generation whilst enhancing SOD
activity, which may be responsible for its anti-angiogenic effect
(39). The present study
demonstrated that 2ME intervention significantly ameliorated
mitochondrial ultrastructural injury, decreased the serum ROS
level, increased serum SOD and MnSOD activities, increased MnSOD
mRNA and protein expression in lung tissues, significantly reduced
tissue HIF-1α mRNA and protein expression and reduced mPAP and
attenuated pulmonary vascular remodeling. These results suggest
that 2ME may also inhibit HPH development by relieving oxidative
stress and thereby downregulating HIF-1α mRNA and protein
expression.
In conclusion, the present report suggests that E2
and 2ME administration attenuates hemodynamic and remodeling
parameters in ovariectomized female HPH rats, and suggests that the
protective effects of E2/2ME may, in part, be mediated by the
OS-HIF-1 pathway; however, the detailed mechanism of this requires
additional study.
Acknowledgements
The present study was supported by the government
Funding Program for Provincial Clinical Medical Talents.
References
|
1
|
Hoeper MM, Humbert M, Souza R, Idrees M,
Kawut SM, Sliwa-Hahnle K, Jing ZC and Gibbs JS: A global view of
pulmonary hypertension. Lancet Respir Med. 4:306–322. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandras SA, Gilkin RJ Jr..Pruett JA and
Raspa S: Pulmonary arterial hypertension: Progress and challenges
in the modern treatment era. Am J Manag Care. 20 Suppl 9:S191–S199.
2014.PubMed/NCBI
|
|
3
|
Weir-McCall JR, Struthers AD, Lipworth BJ
and Houston JG: The role of pulmonary arterial stiffness in COPD.
Respir Med. 109:1381–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waypa GB, Marks JD, Guzy R, Mungai PT,
Schriewer J, Dokic D and Schumacker PT: Hypoxia triggers
subcellular compartmental redox signaling in vascular smooth muscle
cells. Circ Res. 106:526–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schumacker PT: Lung cell hypoxia: Role of
mitochondrial reactive oxygen species signaling in triggering
responses. Proc Am Thorac Soc. 8:477–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sylvester JT, Shimoda LA, Aaronson PI and
Ward JP: Hypoxic pulmonary vasoconstriction. Physiol Rev.
92:367–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al Ghouleh I, Khoo NK, Knaus UG,
Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM,
Kelley EE, Bauer PM, et al: Oxidases and peroxidases in
cardiovascular and lung disease: New concepts in reactive oxygen
species signaling. Free Radic Biol Med. 51:1271–1288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vara D and Pula G: Reactive oxygen
species: Physiological roles in the regulation of vascular cells.
Curr Mol Med. 14:1103–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Zhou Y, Li M and Zhu Y: Expression
of hypoxia-inducible factor-1α, endothelin-1 and adrenomedullin in
newborn rats with hypoxia-induced pulmonary hypertension. Exp Ther
Med. 8:335–339. 2014.PubMed/NCBI
|
|
10
|
Han X, Sun S, Zhao M, Cheng X, Chen G, Lin
S, Guan Y and Yu X: Celastrol stimulates hypoxia-inducible factor-1
activity in tumor cells by initiating the ROS/Akt/p70S6K signaling
pathway and enhancing hypoxia-inducible factor-1α protein
synthesis. PLoS One. 9:e1124702014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frump AL, Goss KN, Vayl A, Albrecht M,
Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB and
Lahm T: Estradiol improves right ventricular function in rats with
severe angioproliferative pulmonary hypertension: Effects of
endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol
Physiol. 308:L873–L890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tofovic SP, Jones T and Petrusevska G:
Dose-dependent therapeutic effects of 2-Methoxyestradiol on
Monocrotaline-Induced pulmonary hypertension and vascular
remodeling. Prilozi. 31:279–295. 2010.PubMed/NCBI
|
|
13
|
Zheng Q, Yuan YD and Zhao J: Effect of
estradiol and its metabolite on hypoxic induced factor-1αand alkane
hydroxylase in experimental rats with ovariectomy and hypoxic
pulmonary hypertension. Chinese Circulation J. 30:884–888.
2015.
|
|
14
|
Yuan M, Duan Z, Sun Y and Yuan Y: Effects
of estrogen on ACE-AngII-AT1 axis in ovariectomy and hypoxic
pulmonary hypertension rats. Zhonghua Yi Xue Za Zhi. 94:1696–1700.
2014.(In Chinese). PubMed/NCBI
|
|
15
|
Shen YX, Xiao K, Liang P, Ma YW and Huang
X: Improvement on the modified Lowry method against interference of
divalent cations in soluble protein measurement. Appl Microbiol
Biotechnol. 97:4167–4178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simonneau G, Gatzoulis MA, Adatia I,
Celermajer D, Denton C, Ghofrani A, Sanchez MA Gomez, Kumar R
Krishna, Landzberg M, Machado RF, et al: Updated clinical
classification of pulmonary hypertension. J Am Coll Cardiol. 62
Suppl 25:D34–D41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Voelkel NF, Gomez-Arroyo J, Abbate A,
Bogaard HJ and Nicolls MR: Pathobiology of pulmonary arterial
hypertension and right ventricular failure. Eur Respir J.
40:1555–1565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prabhakar NR and Semenza GL: Adaptive and
maladaptive cardiorespiratory responses to continuous and
intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2.
Physiol Rev. 92:967–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abud EM, Maylor J, Undem C, Punjabi A,
Zaiman AL, Myers AC, Sylvester JT, Semenza GL and Shimoda LA:
Digoxin inhibits development of hypoxic pulmonary hypertension in
mice. Proc Natl Acad Sci USA. 109:1239–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Semenza GL: Hypoxia-inducible factor 1:
Regulator of mitochondrial metabolism and mediator of ischemic
preconditioning. Biochim Biophys Acta. 1813:1263–1268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bruick RK and McKnight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Archer SL, Marsboom G, Kim GH, Zhang HJ,
Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thébaud B,
Husain AN, et al: Epigenetic attenuation of mitochondrial
superoxide dismutase 2 in pulmonary arterial hypertension: A basis
for excessive cell proliferation and a new therapeutic target.
Circulation. 121:2661–2671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmed LA, Obaid AA, Zaki HF and Agha AM:
Role of oxidative stress, inflammation, nitric oxide and
transforming growth factor-beta in the protective effect of
diosgenin in monocrotaline-induced pulmonary hypertension in rats.
Eur J Pharmacol. 740:379–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He J, Wang M, Jiang Y, Chen Q, Xu S, Xu Q,
Jiang BH and Liu LZ: Chronic arsenic exposure and angiogenesis in
human bronchial epithelial cells via the
ROS/miR-199a-5p/HIF-1α/COX-2 pathway. Environ Health Perspect.
122:255–261. 2014.PubMed/NCBI
|
|
26
|
Sasabe E, Yang Z, Ohno S and Yamamoto T:
Reactive oxygen species produced by the knockdown of
manganese-superoxide dismutase up-regulate hypoxia-inducible
factor-1alpha expression in oral squamous cell carcinoma cells.
Free Radic Biol Med. 48:1321–1329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fijalkowska I, Xu W, Comhair SA, Janocha
AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A,
Erzurum SC and Tuder RM: Hypoxia inducible-factor1alpha regulates
the metabolic shift of pulmonary hypertensive endothelial cells. Am
J Pathol. 176:1130–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duracková Z: Some current insights into
oxidative stress. Physiol Res. 59:459–469. 2010.PubMed/NCBI
|
|
29
|
Li SY, Fu ZJ and Lo AC: Hypoxia-induced
oxidative stress in ischemic retinopathy. Oxid Med Cell Longev.
2012:4267692012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aggarwal S, Gross CM, Sharma S, Fineman JR
and Black SM: Reactive oxygen species in pulmonary vascular
remodeling. Compr Physiol. 3:1011–1034. 2013.PubMed/NCBI
|
|
31
|
Fukai T and Ushio-Fukai M: Superoxide
dismutases: Role in redox signaling, vascular function, and
diseases. Antioxid Redox Signal. 15:1583–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rabbani ZN, Mi J, Zhang Y, Delong M,
Jackson IL, Fleckenstein K, Salahuddin FK, Zhang X, Clary B,
Anscher MS and Vujaskovic Z: Hypoxia inducible factor 1alpha
signaling in fractionated radiation-induced lung injury: Role of
oxidative stress and tissue hypoxia. Radiat Res. 173:165–174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyamoto N, Mandai M, Takagi H, Suzuma I,
Suzuma K, Koyama S, Otani A, Oh H and Honda Y: Contrasting effect
of estrogen on VEGF induction under different oxygen status and its
role in murine ROP. Invest Ophthalmol Vis Sci. 43:2007–2014.
2002.PubMed/NCBI
|
|
34
|
Wang S, Wang B, Feng Y, Mo M, Du F, Li H
and Yu X: 17β-estradiol ameliorates light-induced retinal damage in
Sprague-Dawley rats by reducing oxidative stress. J Mol Neurosci.
55:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Z, Gou Y, Zhang H, Zuo H, Zhang H, Liu
Z and Yao D: Estradiol improves cardiovascular function through
up-regulation of SOD2 on vascular wall. Redox Biol. 3:88–99. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hagen T, D'Amico G, Quintero M,
Palacios-Callender M, Hollis V, Lam F and Moncada S: Inhibition of
mitochondrial respiration by the anticancer agent
2-methoxyestradiol. Biochem Biophys Res Commun. 322:923–929. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma L, Li G, Zhu H, Dong X, Zhao D, Jiang
X, Li J, Qiao H, Ni S and Sun X: 2-Methoxyestradiol synergizes with
sorafenib to suppress hepatocellular carcinoma by simultaneously
dysregulating hypoxia-inducible factor-1 and −2. Cancer Lett.
355:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Verenich S and Gerk PM: Therapeutic
promises of 2-methoxyestradiol and its drug disposition challenges.
Mol Pharm. 7:2030–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Basini G, Santini SE and Grasselli F:
2-Methoxyestradiol inhibits superoxide anion generation while it
enhances superoxide dismutase activity in swine granulosa cells.
Ann N Y Acad Sci. 1091:34–40. 2006. View Article : Google Scholar : PubMed/NCBI
|