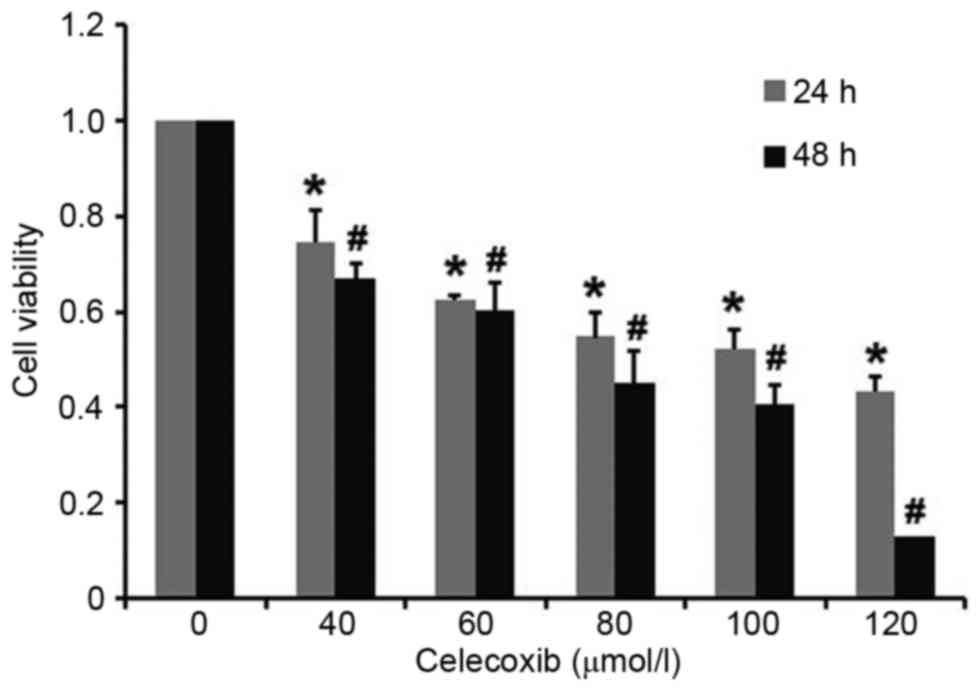
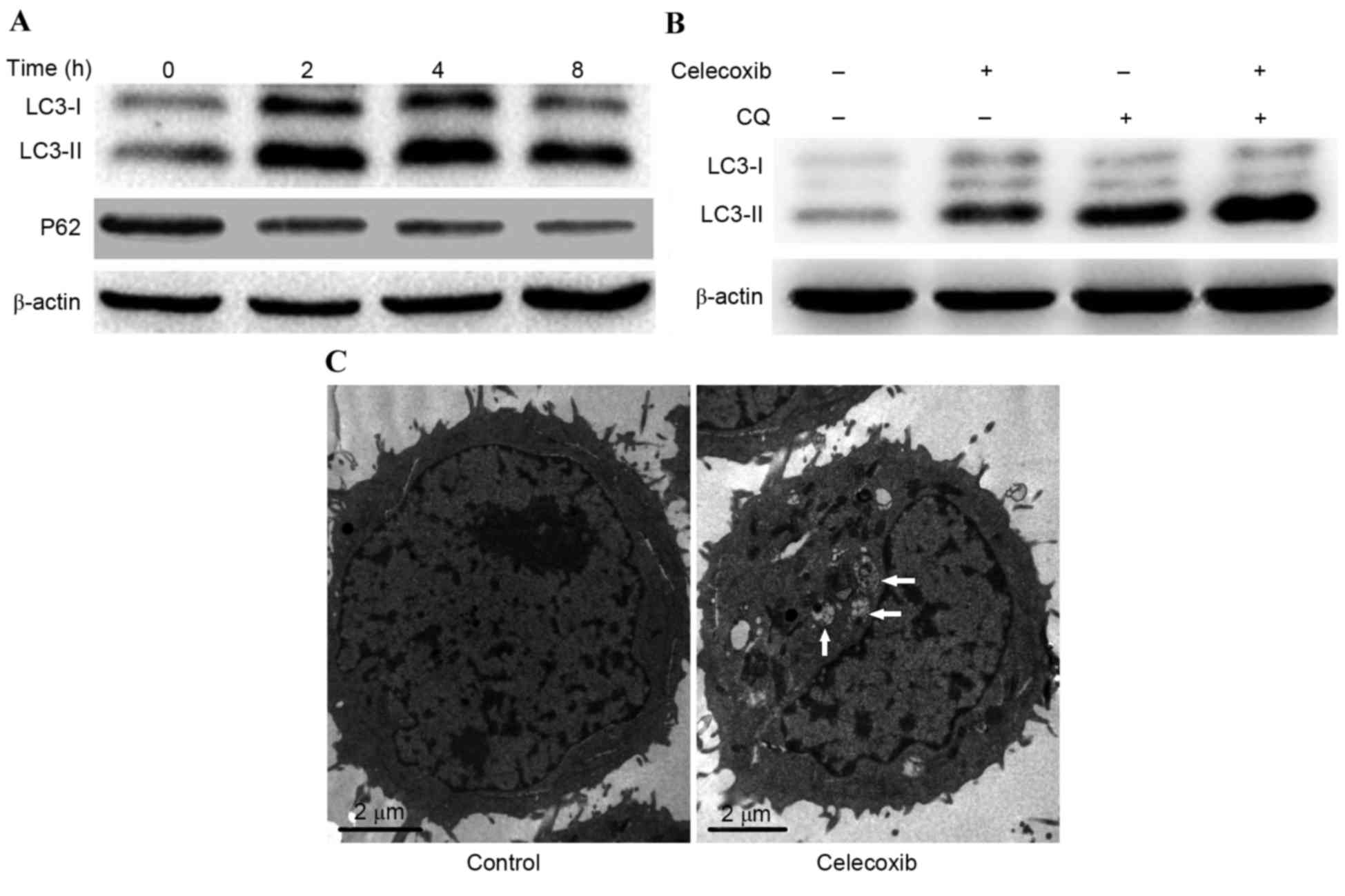
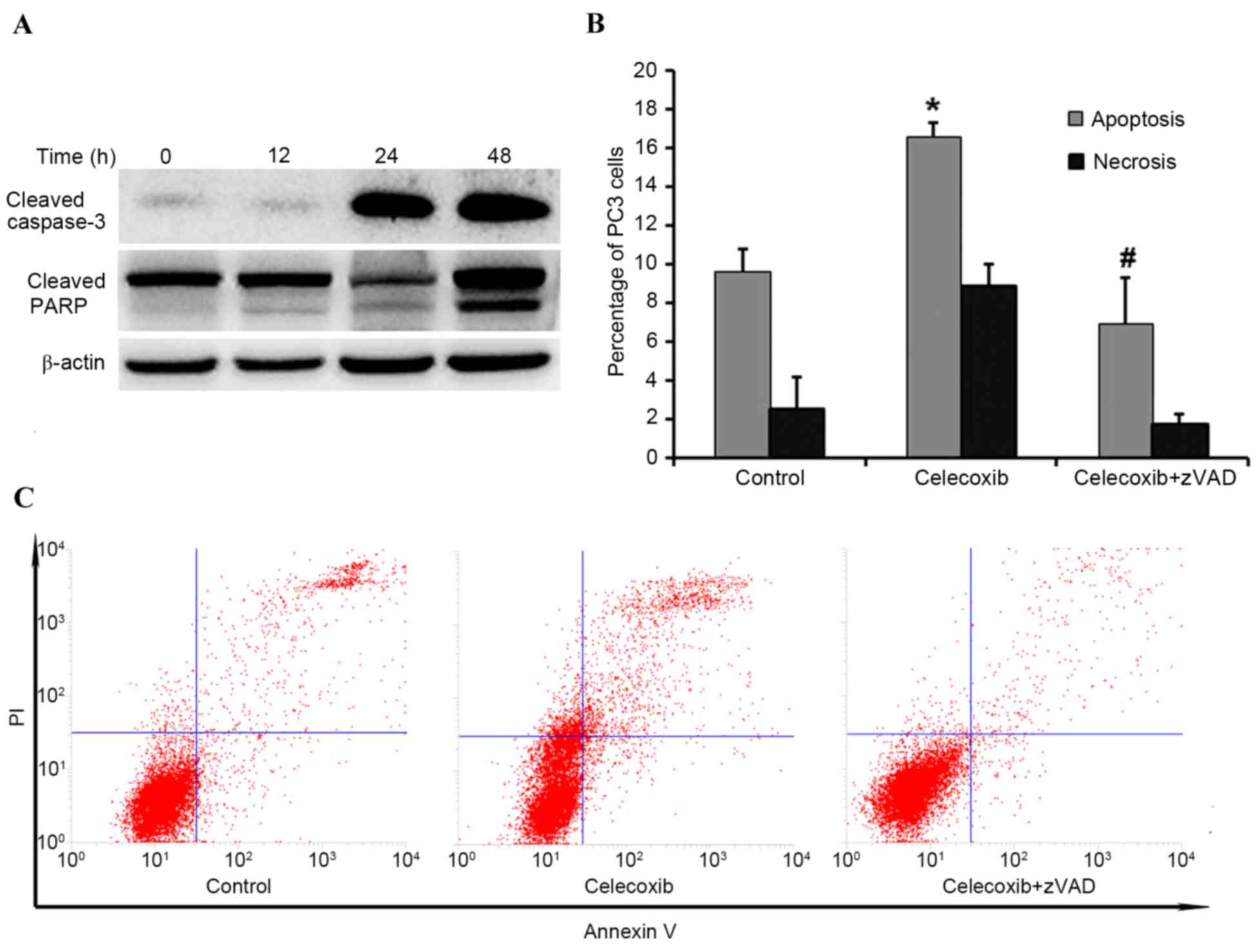
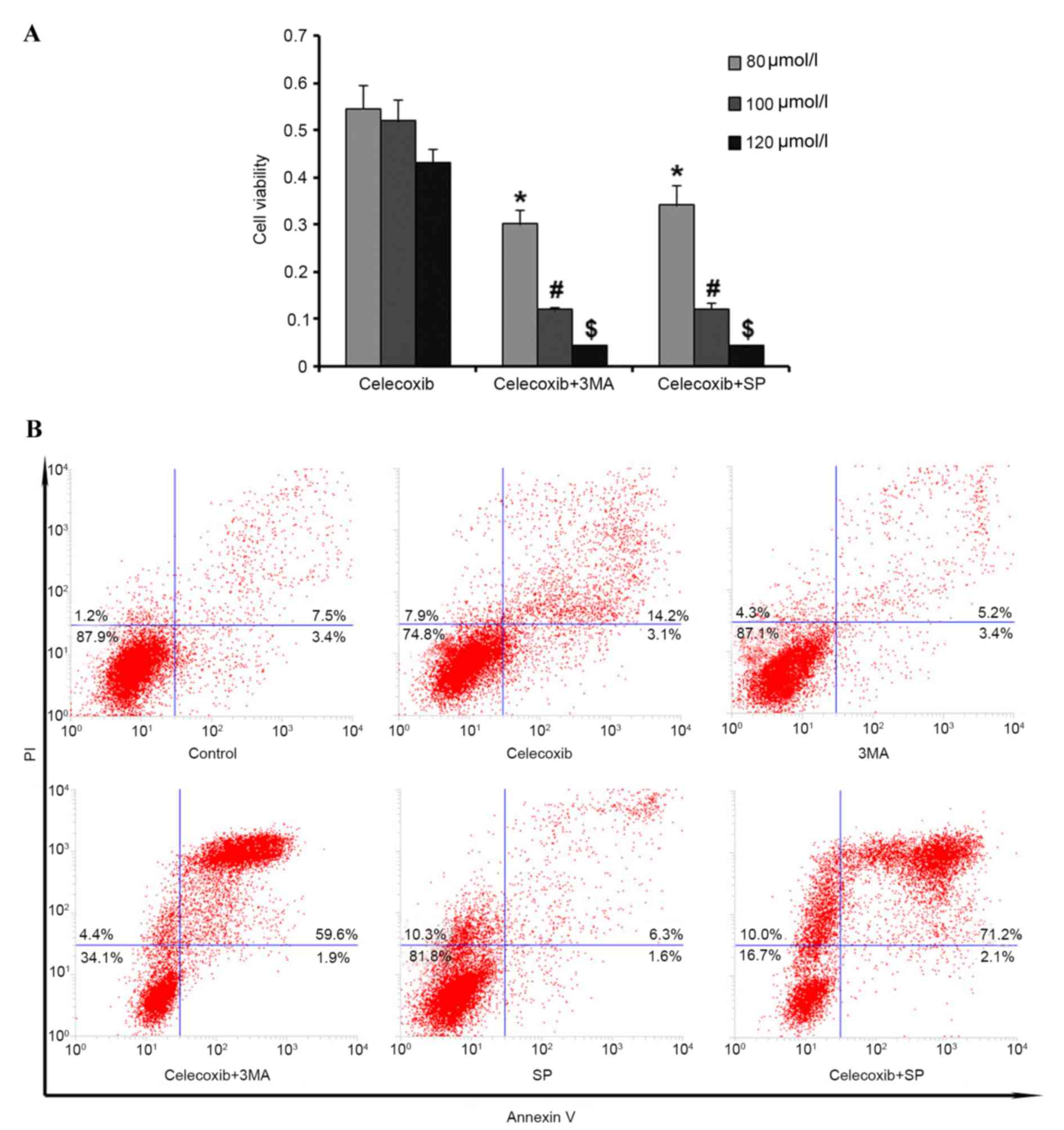
|
1
|
Center MM, Jemal A, Lortet-Tieulent J,
Ward E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taketo MM: Cyclooxygenase-2 inhibitors in
tumorigenesis (part I). J Natl Cancer Inst. 90:1529–1536. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel MI, Subbaramaiah K, Du B, Chang M,
Yang P, Newman RA, Cordon-Cardo C, Thaler HT and Dannenberg AJ:
Celecoxib inhibits prostate cancer growth: Evidence of a
cyclooxygenase-2-independent mechanism. Clin Cancer Res.
11:1999–2007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S and Sinicrope FA:
Celecoxib-induced apoptosis is enhanced by ABT-737 and by
inhibition of autophagy in human colorectal cancer cells.
Autophagy. 6:256–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li DD, Wang LL, Deng R, Tang J, Shen Y,
Guo JF, Wang Y, Xia LP, Feng GK, Liu QQ, et al: The pivotal role of
c-Jun NH2-terminal kinase-mediated Beclin 1 expression during
anticancer agents-induced autophagy in cancer cells. Oncogene.
28:886–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith FM, Reynolds JV, Kay EW, Crotty P,
Murphy JO, Hollywood D, Gaffney EF, Stephens RB and Kennedy MJ:
COX-2 overexpression in pretreatment biopsies predicts response of
rectal cancers to neoadjuvant radiochemotherapy. Int J Radiat Oncol
Biol Phys. 64:466–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishikawa H, Ohno T, Kato S, Wakatsuki M,
Iwakawa M, Ohta T, Imai T, Mitsuhashi N, Noda SE, Nakano T and
Tsujii H: Cyclooxygenase-2 impairs treatment effects of
radiotherapy for cervical cancer by inhibition of radiation-induced
apoptosis. Int J Radiat Oncol Biol Phys. 66:1347–1355. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao JT, Roth MD, Fishbein MC, Aberle DR,
Zhang ZF, Rao JY, Tashkin DP, Goodglick L, Holmes EC, Cameron RB,
et al: Lung cancer chemoprevention with celecoxib in former
smokers. Cancer Prev Res (Phila). 4:984–993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perroud HA, Rico MJ, Alasino CM, Queralt
F, Mainetti LE, Pezzotto SM, Rozados VR and Scharovsky OG: Safety
and therapeutic effect of metronomic chemotherapy with
cyclophosphamide and celecoxib in advanced breast cancer patients.
Future Oncol. 9:451–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basler JW and Piazza GA: Nonsteroidal
anti-inflammatory drugs and cyclooxygenase-2 selective inhibitors
for prostate cancer chemoprevention. J Urol. 171:S59–S63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Shen P, Zhang XC, Zhao MD, Zhang
XG and Yang L: Efficacy and safety profile of celecoxib for
treating advanced cancers: A meta-analysis of 11 randomized
clinical trials. Clin Ther. 36:1253–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hussain T, Gupta S and Mukhtar H:
Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett.
191:125–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gallego G Aparicio, Díaz Prado S, Jiménez
Fonseca P, García Campelo R, Cassinello Espinosa J and Antón
Aparicio LM: Cyclooxygenase-2 (COX-2): A molecular target in
prostate cancer. Clin Transl Oncol. 9:694–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khor LY, Bae K, Pollack A, Hammond ME,
Grignon DJ, Venkatesan VM, Rosenthal SA, Ritter MA, Sandler HM,
Hanks GE, et al: COX-2 expression predicts prostate-cancer outcome:
Analysis of data from the RTOG 92-02 trial. Lancet Oncol.
8:912–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ziparo E, Petrungaro S, Marini ES, Starace
D, Conti S, Facchiano A, Filippini A and Giampietri C: Autophagy in
prostate cancer and androgen suppression therapy. Int J Mol Sci.
14:12090–12106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin S: P53, Autophagy and tumor
suppression. Autophagy. 1:171–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozpolat B and Benbrook DM: Targeting
autophagy in cancer management-strategies and developments. Cancer
Manag Res. 7:291–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Jiang K, Jiang H and Wei P:
MiR-155 mediates drug resistance in osteosarcoma cells via inducing
autophagy. Exp Ther Med. 8:527–532. 2014.PubMed/NCBI
|
|
23
|
Aita VM, Liang XH, Murty VV, Pincus DL, Yu
W, Cayanis E, Kalachikov S, Gilliam TC and Levine B: Cloning and
genomic organization of beclin 1, a candidate tumor suppressor gene
on chromosome 17q21. Genomics. 59:59–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kung HJ: Targeting tyrosine kinases and
autophagy in prostate cancer. Horm Cancer. 2:38–46. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Merseburger AS, Hammerer P, Rozet F,
Roumeguère T, Caffo O, da Silva FC and Alcaraz A: Androgen
deprivation therapy in castrate-resistant prostate cancer: How
important is GnRH agonist backbone therapy? World J Urol.
33:1079–1085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen HG, Yang JC, Kung HJ, Shi XB, Tilki
D, Lara PN Jr..White RW DeVere, Gao AC and Evans CP: Targeting
autophagy overcomes enzalutamide resistance in castration-resistant
prostate cancer cells and improves therapeutic response in a
xenograft model. Oncogene. 33:4521–4530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bennett HL, Stockley J, Fleming JT, Mandal
R, O'Prey J, Ryan KM, Robson CN and Leung HY: Does
androgen-ablation therapy (AAT) associated autophagy have a
pro-survival effect in LNCaP human prostate cancer cells? BJU Int.
111:672–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chhipa RR, Wu Y and Ip C: AMPK-mediated
autophagy is a survival mechanism in androgen-dependent prostate
cancer cells subjected to androgen deprivation and hypoxia. Cell
Signal. 23:1466–1472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chhipa RR, Wu Y, Mohler JL and Ip C:
Survival advantage of AMPK activation to androgen-independent
prostate cancer cells during energy stress. Cell Signal.
22:1554–1561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Verheij M, Bose R, Lin XH, Yao B, Jarvis
WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, et al:
Requirement for ceramide-initiated SAPK/JNK signalling in
stress-induced apoptosis. Nature. 380:75–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng R, Li W, Guan Z, Zhou JM, Wang Y, Mei
YP, Li MT, Feng GK, Huang W, Liu ZC, et al: Acetylcholinesterase
expression mediated by c-Jun-NH2-terminal kinase pathway during
anticancer drug-induced apoptosis. Oncogene. 25:7070–7077. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurinna SM, Tsao CC, Nica AF, Jiffar T and
Ruvolo PP: Ceramide promotes apoptosis in lung cancer-derived A549
cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer
Res. 64:7852–7856. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei Y, Pattingre S, Sinha S, Bassik M and
Levine B: JNK1-mediated phosphorylation of Bcl-2 regulates
starvation-induced autophagy. Mol Cell. 30:678–688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu GY, Jiang XX, Zhu X, He WY, Kuang YL,
Ren K, Lin Y and Gou X: ROS activates JNK-mediated autophagy to
counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta
Pharmacol Sin. 36:1473–1479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J and Yao S: JNK-Bcl-2/Bcl-xL-Bax/Bak
pathway mediates the crosstalk between matrine-induced autophagy
and apoptosis via interplay with beclin 1. Int J Mol Sci.
16:25744–25758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
García Rodríguez LA and González-Pérez A:
Inverse association between nonsteroidal anti-inflammatory drugs
and prostate cancer. Cancer Epidemiol Biomarkers Prev. 13:649–653.
2004.PubMed/NCBI
|