Introduction
Spinal cord injury (SCI) is a common type of motor
system trauma which may cause varying degrees of four limbs or
paraplegia with loss of labor ability, potentially resulting in a
heavier burden on society and families in 2010 (1). Epidemiological data indicated that in
the USA, traffic accidents and high-altitude falls were the
predominant causes of SCI, accounting for ~67.5%. The majority of
cases were in young men with an average age of 41.0 years, the
annual average morbidity is ~40 per million people, and ~12,000
people per year and increasing (2).
At present in the field of sports injury, recovery after SCI
remains difficult. However, scholars worldwide continue to
investigate new treatment strategies for SCI.
At present the SCI mechanism is divided into primary
and secondary SCI (3). The former
consists of instantaneous mechanical damage that is reversible.
Secondary damage gradually forms in the weeks following primary
damage, and is accompanied by a series of cell metabolism and gene
expression changes, including excitatory amino acid release, free
radical damage, decrease in ion inflow, inflammation and cell
apoptosis, resulting in more harm than primary damage (4,5).
Numerous studies have shown that post changes of SCI gene
expression play an important role in the pathological process
(4,5).
Transforming growth factor (TGF)-β is a
multifunctional cytokine, and its function in mammals involves
damage repair and the formation of scar tissue (6). The biological function of TGF-β is
mediated via the conduction of types I (RI) and II (RII)
transmembrane receptors (7). TGF-β
is present in almost all cells; therefore, it can influence the
physiological function of the majority of tissues correspondingly
(8). In vitro, TGF-β is a
potent agent for astrocyte chemotaxis, which can result in the
hypertrophy of astrocytes and upregulation of the synthesis of
fibronectin and collagen IV (8).
Tocotrienols are isomers of vitamin E, located
primarily in plants, with antioxidative (9), anti-tumor (10) and neuroprotective functions (11). Tocotrienols inhibit proliferation of
breast cancer cells, show G1 phase retardation and can promote cell
apoptosis (12,13). However, to the best of our knowledge,
the potential protective effect and underlying molecular mechanism
of tocotrienol on SCI has not been investigated. In addition,
investigated the hypothesis that the effect of tocotrienol on SCI
is mediated via the suppression of TGF-β.
Materials and methods
Animals and surgery
A total of 45 female Sprague Dawley rats weighing
250–270 g were obtained from the Animal Resource Center of Xinjiang
Medical University (Urumqi, China). Animal experiments were
performed in accordance to the institutional guidelines provided by
the Committee on Animal Research at Xinjiang Medical
University.
Drugs and chemicals
Tocotrienol [D-(−Tocotrienol; purity, >98%)] was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany),
shown in Fig. 1. Malondialdehyde
(MDA), superoxide dismutase (SOD), catalase (CAT), activity of
glutathione peroxidase (GSH-PX), NF-κB p65 unit, tumor necrosis
factor (TNF)-α, IL-1β and IL-6 ELISA kits were purchased from
R&D Systems, Inc. (catalog nos. A003-1, A001-3, A007-2, A005,
H202, H052, H002 and H007; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). A bicinchoninic assay kit was bought
from Beyotime Institute of Biotechnology (Nantong, China).
Inducible nitric oxide synthase (iNOS) commercial kit was bought
from Imgenex (catalog no. A014-1; Nanjing Jiancheng Bioengineering
Institute).
Experimental groups and
procedures
Sprague Dawley rats were randomly divided into four
groups (n=10 per group): i) Sham group, normal rats received
physiological saline 0.1 ml/100 g (i.p.); ii) SCI group (SCI)
(14), SCI model rats received
physiological saline 0.1 ml/100 g (i.p.); iii) methylprednisolone
sodium succinate (MPSS) group (positive control group), SCI model
rats were treated with 100 mg/kg MPSS (i.p.); and iv) tocotrienol
group, which experienced SCI and received 120 mg/kg/day tocotrienol
once a day for eight consecutive weeks (15).
Evaluation of Basso Beattie Bresnahan
(BBB) locomotor rating scale
The evaluation of locomotor function after treatment
with tocotrienol on SCI was performed using the BBB locomotor
rating scale of 0 (complete paralysis) to 21 (normal locomotion)
(16).
Evaluation of the volume of spinal
cord contusions after SCI
The volume of spinal cord contusions after SCI was
evaluated by calculating as wet weight after treatment with
doctrinal on SCI. Spinal cord tissue samples were dried at 80°C for
48 h and calculated as dry weight. The volume of spinal cord
contusions was calculated by the following formula: Water content
of spinal cord (%)=(Wet weight-dry weight)/wet weight × 100%.
Evaluation of serum oxidative stress
and inflammation
After the experimental administration of tocotrienol
on SCI rats, serum was collected for analysis. Levels of MDA, SOD,
CAT, GSH-PX, NF-κB p65 unit, TNF-α, IL-1β and IL-6 were analyzed
using respective commercial immunoassay kits according to the
manufacturers protocols (Wuhan Elabscience Biotechnology, Co.,
Ltd., Wuhan, China).
Western blot analysis
Spinal cord tissue samples (50 mg) were homogenized
in an ice-cold lysis buffer (Beyotime Institute of Biotechnology).
The mixed liquor was centrifuged at 12,000 × g for 10 min at 4°C.
The supernatant was analyzed protein quantification using a
bicinchoninic acid assay kit (catalog no. P0013B; Beyotime
Institute of Biotechnology). Total proteins (10 µg) were separated
by electrophoresis using 10–12% SDS-polyacrylamide gels and
transferred onto nitrocellulose membranes (Merck Millipore).
Membranes were washed with Tris buffer saline Tween-20 (TBST) three
times and blocked with 5% skim milk powder inTBST for 1 h at 37°C.
Subsequently, they were incubated using anti-iNOS (catalog no.
sc-8310; 1:2,000), anti-TGF-β (catalog no. sc-130348; 1:2,000),
anti-collagen type IV (catalog no. sc-9301; 1:3,000),
anti-fibronectin (catalog no. sc-69681; 1:4,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) anti-β-actin (catalog no.
D110007; 1:2,000; Sangon Biotech, Shanghai, China) primary
antibodies at 4°C overnight. Then, membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse
secondary antibody (1:5,000; Santa Cruz Biotechnology, Inc.) at
37°C for 1 h and visualized using enhanced chemiluminescence agents
(ECL Plus; Pierce Protein Biology; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The optical densities of immunopositive bands
were determined using Gene Tools analysis software (GeneTools, LLC,
Philomath, OR, USA).
Determination of iNOS activity
Following experimentation, spinal cord tissues were
collected from different groups. iNOS activity was determined using
ELISA commercial kits according to the manufacturer instructions
(catalog no. A014-1; Nanjing Jiancheng Bioengineering
Institute).
Evaluation of plasma NO
production
Following experimentation, the plasma supernatant
was collected and underwent 12,000 × g centrifugation for 20 min at
4°C. Nitrite concentration was determined using Griess reagent (1%
sulfanilamide and 0.1% naphthylethylenediamide in 5% phosphoric
acid (Sangon Biotech) at a wavelength of 450 nm by a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and analyzed using one-way analysis of variance followed by
Dunnett's test. All statistical analyses were conducted using
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of tocotrienol on functional
recovery after SCI
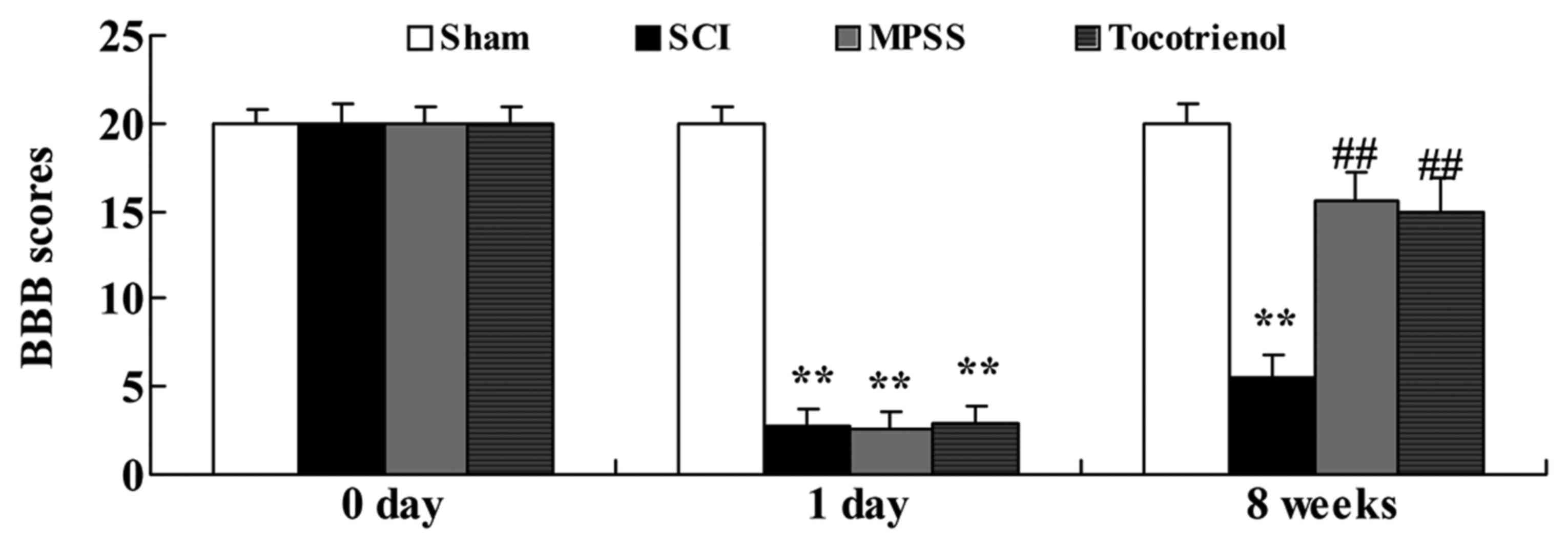
To assess the effects of tocotrienol on functional
recovery after SCI, functional recovery was assessed using BBB
behavioral testing one day before SCI, one day post-SCI, and for
eight weeks post-SCI. Reduced BBB scores were observed in SCI group
at one day post-SCI or eight weeks post-SCI, compared to that of
control group (P<0.05, Fig. 2).
At one day post-SCI, BBB scores were not significantly different in
the MPSS or tocotrienol groups compared with the SCI group
(Fig. 2). However, at eight weeks
post-SCI, BBB scores were significantly higher in MPSS and
tocotrienol groups compared with the SCI group (P<0.05, Fig. 2). There was no significant difference
between MPSS and tocotrienol groups at any time point (P>0.05,
Fig. 2).
Effect of tocotrienol on the volume of
gray matter contusions in the injured spinal cords
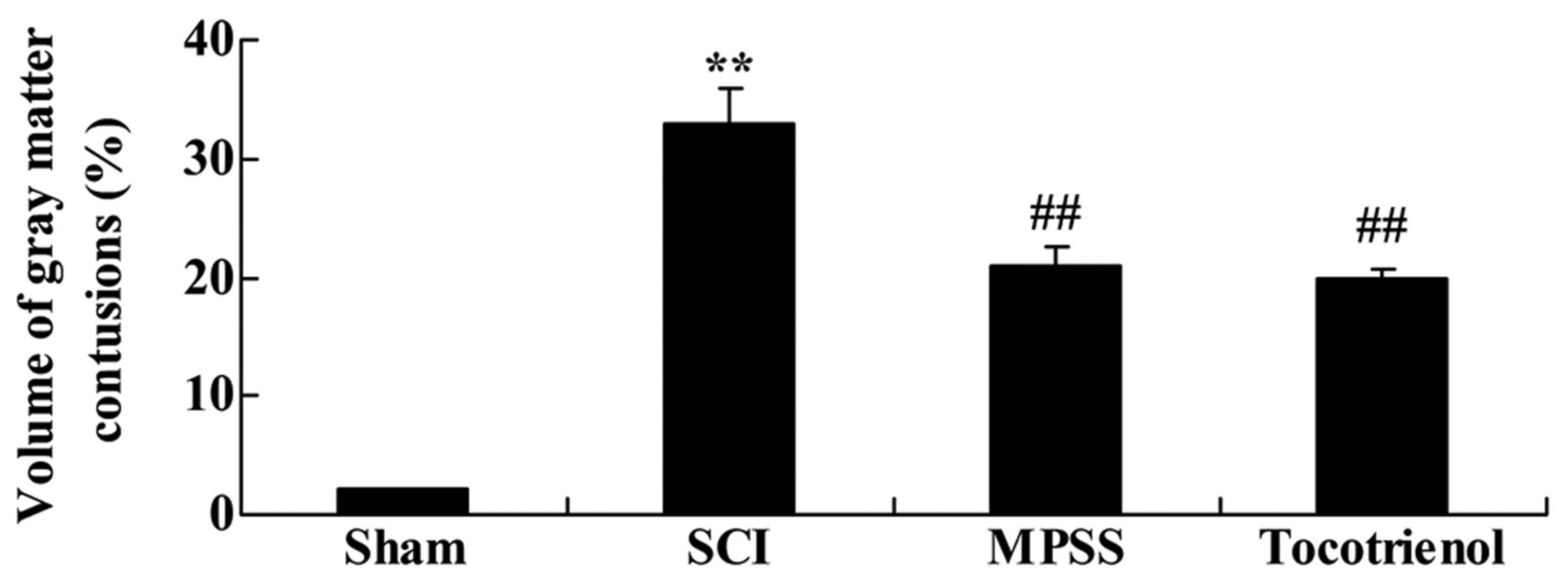
Next, the effects of tocotrienol were investigated
on the volume of gray matter contusions in the injured spinal
cords. Contused volume was significantly increased in the SCI
group, compared with the control group (P<0.05, Fig. 3). Furthermore, contused volume was
significantly reduced in the tocotrienol and MPSS groups compared
with the SCI group (P<0.05, Fig.
3). However, there was no significant difference between the
MPSS and tocotrienol groups (P>0.05, Fig. 3).
Effect of tocotrienol on oxidative
stress
To elucidate the effects of tocotrienol on oxidative
stress in SCI rats, the serum levels of MDA, SOD, CAT and GSH-PX
were evaluated. Initially, serum MDA levels were elevated and serum
SOD, CAT and GSH-PX levels were suppressed in the SCI group
compared with the control group (P<0.05, Fig. 4). Furthermore, these SCI-induced
changes in serum MDA, SOD, CAT and GSH-PX levels were significantly
reversed by the MPSS and tocotrienol treatments, compared with the
SCI group (P<0.05, Fig. 4).
However, no significant inter-group difference was observed between
the MPSS and tocotrienol groups for anti-oxidative effects in SCI
model rats (P>0.05, Fig. 4).
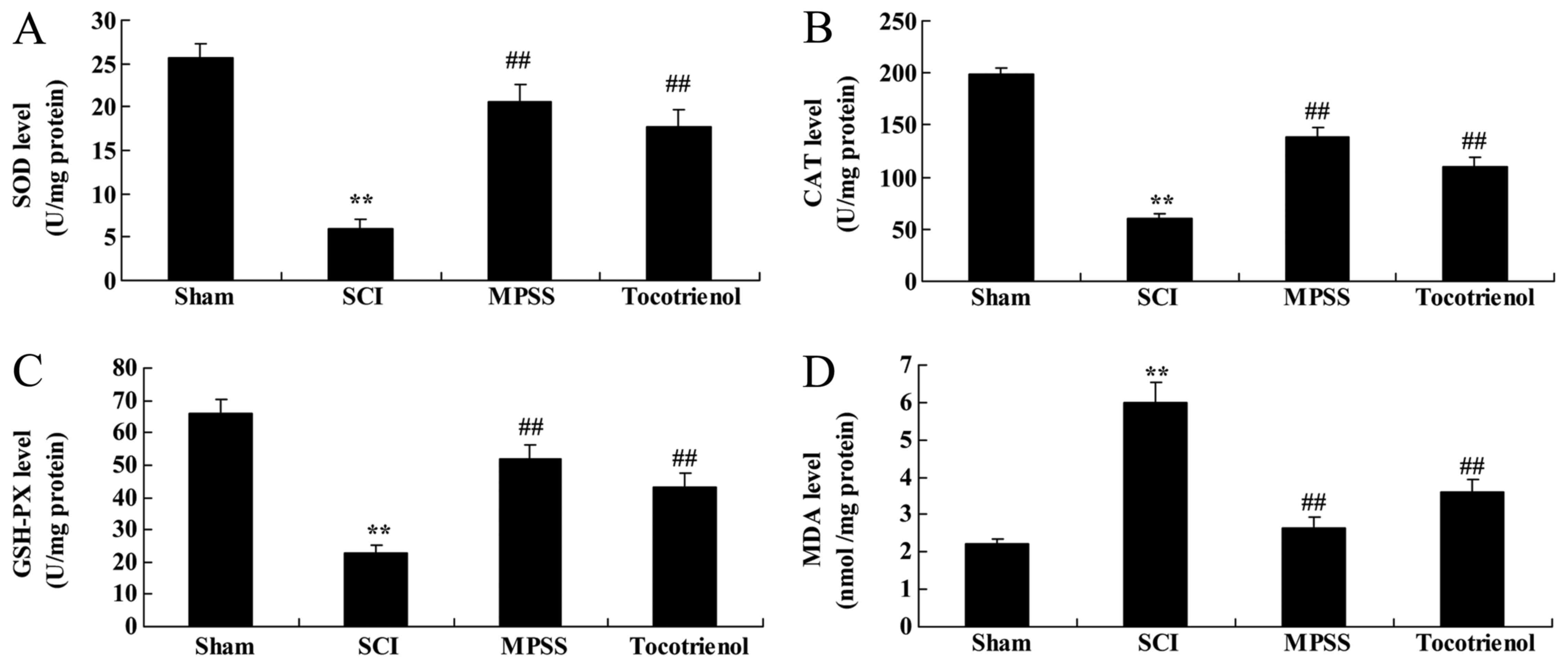 | Figure 4.Effects of tocotrienol on oxidative
stress. The effects of tocotrienol on (A) SOD, (B) CAT, (C) GSH-PX
and (D) MDA levels in SCI rats. **P<0.05 vs. sham group;
##P<0.05 vs. SCI group. Sham, Sham group; SCI, spinal
cord injury group; MPSS, methylprednisolone-treated; Tocotrienol,
tocotrienol (120 mg/kg) -treated; SOD, superoxide dismutase; CAT,
catalase; GSH-PX, glutathione peroxidase; MDA, malondialdehyde. |
Effect of tocotrienol on
inflammation
To elucidate the effects of tocotrienols on
inflammation of SCI rat, the serum levels of NF-κB p65 unit, TNF-α,
IL-1β and IL-6 were evaluated. SCI induced inflammation reactions
in rats, compared to the control group, as indicated by
significantly elevated levels of the detected proteins (P<0.05,
Fig. 5). Notably, the serum NF-κB
p65 unit, TNF-α, IL-1β and IL-6 levels were significantly reduced
by treatment with MPSS and tocotrienol, compared to the SCI group
(P<0.05, Fig. 5). However, no
significant changes in the serum levels of NF-κB p65 unit, TNF-α,
IL-1β and IL-6 were detected between the MPSS and tocotrienol
groups (P>0.05, Fig. 5A-D).
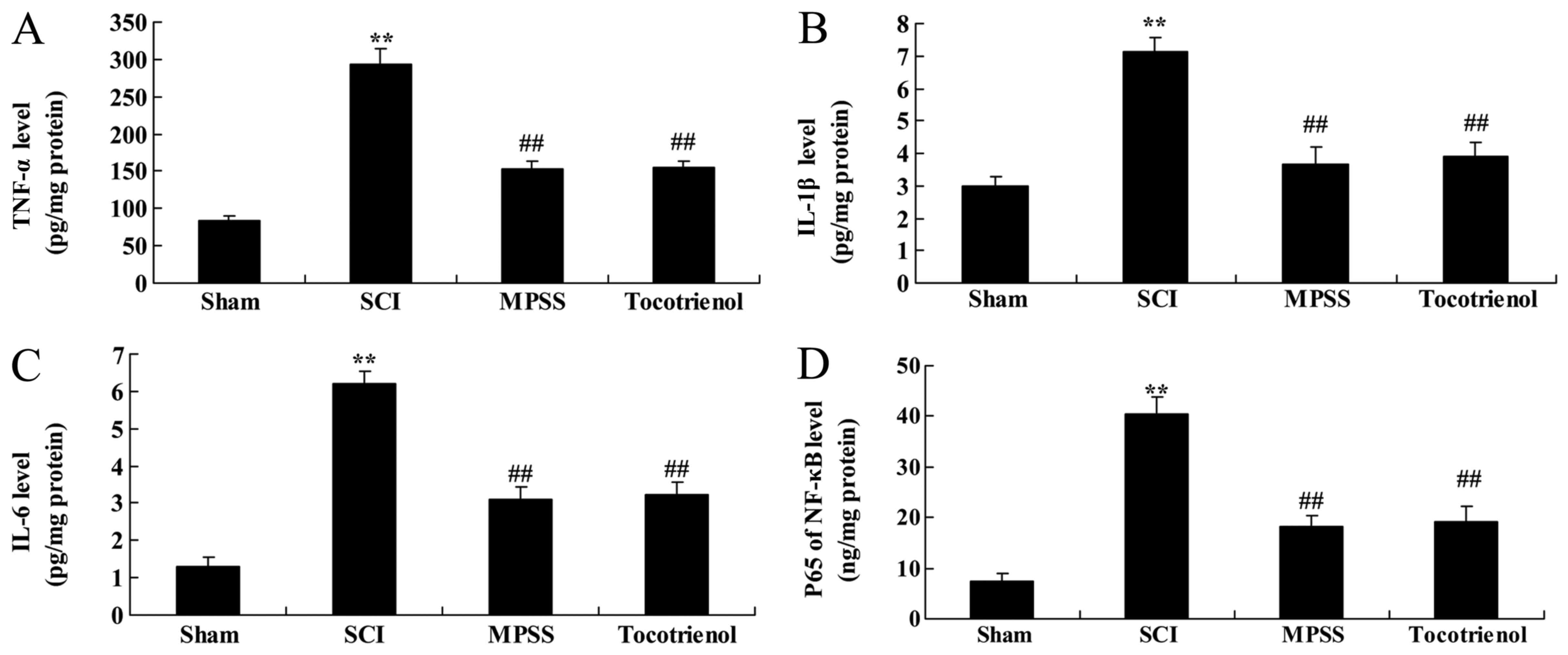 | Figure 5.Effects of tocotrienol on
inflammation. The effects of tocotrienol on (A) TNF-α, (B) IL-1β,
(C) IL-6 and (D) NF-κB p65 unitin SCI rats. **P<0.05 vs. sham
group; ##P<0.05 vs. SCI group. Sham, Sham group; SCI,
spinal cord injury group; MPSS, methylprednisolone-treated;
Tocotrienol, tocotrienol (120 mg/kg) -treated; TNF-α, tumor
necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6;
NF-κB p65 unit, nuclear factor-κB p65 unit. |
Effect of tocotrienol on iNOS
expression, iNOS activity and plasma NO production
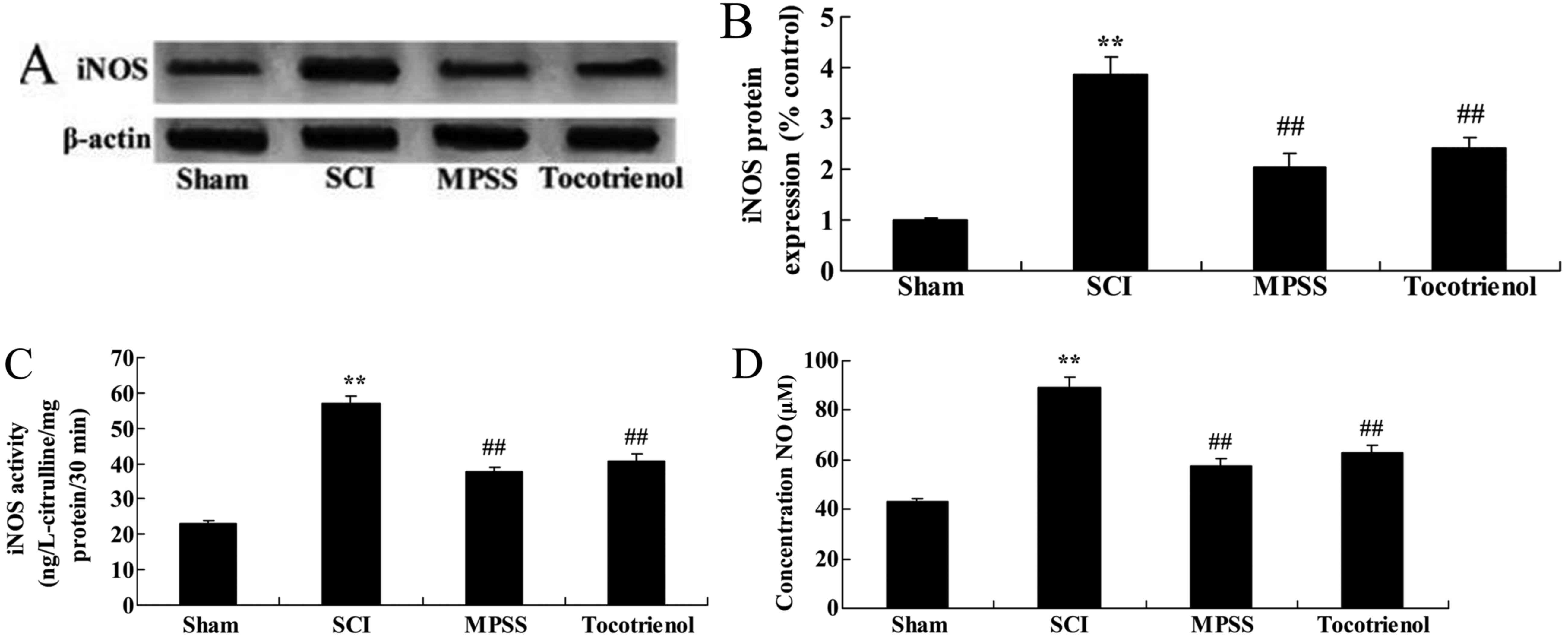
To explain the effects of tocotrienol on iNOS
protein expression in SCI rats, iNOS protein expression, iNOS
activity and plasma NO production were evaluated. SCI promoted iNOS
protein expression in rats compared with the control group
(P<0.05, Fig. 6A and B).
Treatment with MPSS and tocotrienol reduced iNOS protein expression
compared to the SCI group (P<0.05, Fig. 6A and B). SCI activated iNOS activity
and increased plasma NO production in SCI rats compared with the
control group (P<0.05, Fig. 6C and
D). However, these changes were significantly reversed by
treatment with MPSS and tocotrienol compared to the SCI group
(P<0.05, Fig. 6C and D).
Furthermore, no significant differences were detected in iNOS
protein expression, iNOS activity and increased plasma NO
production between the MPSS and tocotrienol groups (P>0.05,
Fig. 6A-D).
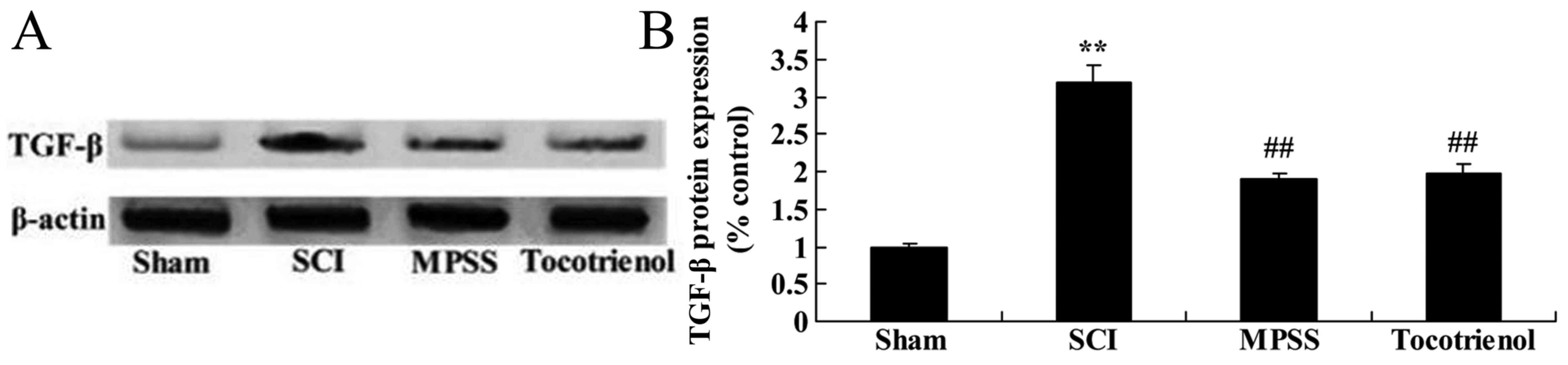
Effect of tocotrienol on the
expression of TGF-β
Western blot analysis was used to further elucidate
the effects of tocotrienol on the expression of TGF-β protein in
SCI rats. Western blot analysis demonstrated that the expression of
TGF-β protein was significantly elevated in the SCI group compared
with the sham group (P<0.05, Fig. 7A
and B). By contrast, treatment with MPSS and tocotrienol
significantly inhibited TGF-β protein expression, compared with the
SCI group (P<0.05, Fig. 7A and
B). However, there was no significant difference between the
MPSS and tocotrienol groups (P>0.05, Fig. 7A and B).
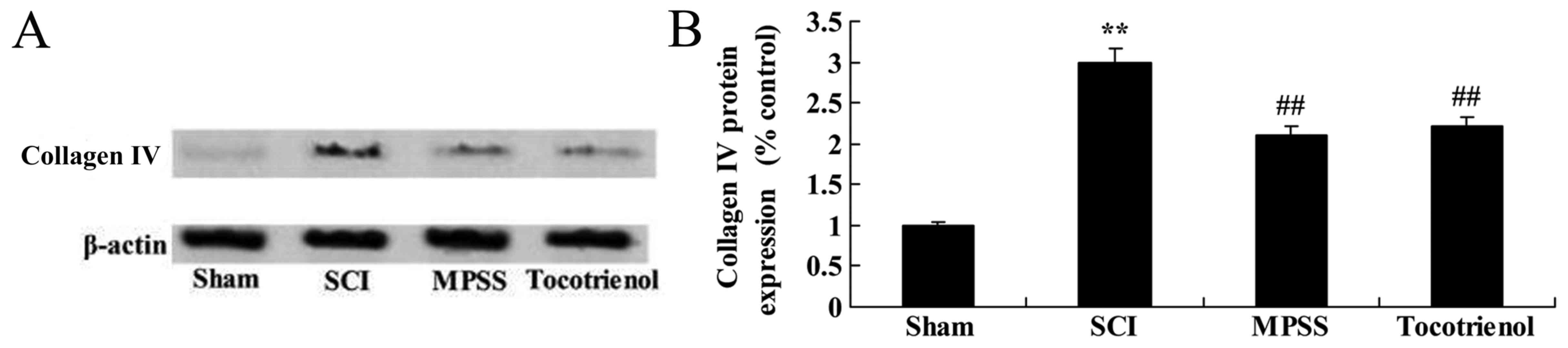
Effect of tocotrienol on the
expression of collagen type IV
Western blot analysis was used to evaluate the
effects of tocotrienol on the protein expression levels of collagen
type IV in SCI rats. The expression of collagen type IV protein was
significantly increased in SCI model group, compared to the sham
group (P<0.05, Fig. 8A and B).
However, MPSS and tocotrienol significantly reduced the collagen
type IV protein expression compared with the SCI group (P<0.05,
Fig. 8A and B). However, there was
no significant difference in collagen type IV protein expression
between the MPSS and tocotrienol groups (P>0.05, Fig. 8A and B).
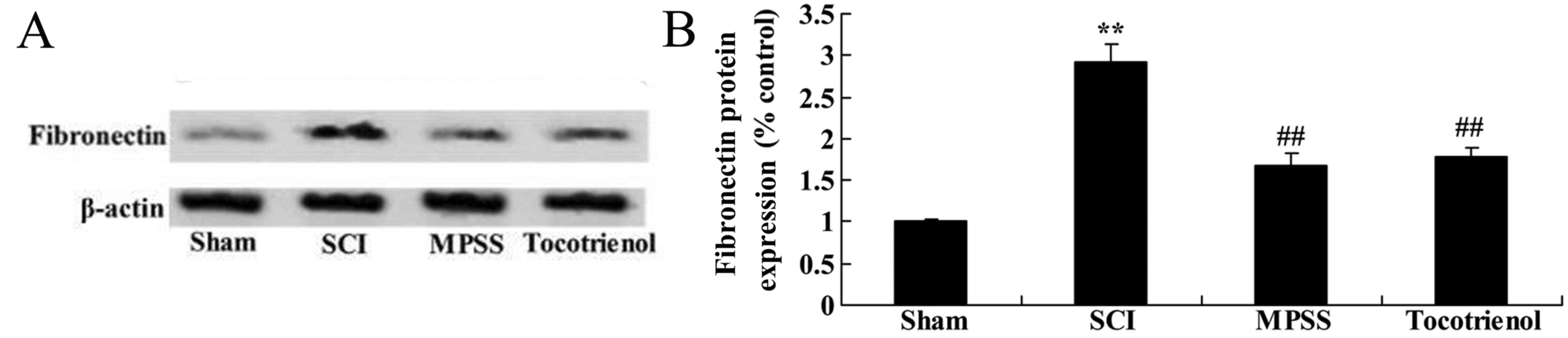
Effect of tocotrienol on the
expression of fibronectin
Western blot analysis was used to further evaluate
the effects of tocotrienol on the expression of fibronectin protein
in SCI rats. As shown in Fig. 9A and
B, there was a significant increase in fibronectin protein
expression in the SCI model rats compared with the sham group. In
contrast to the SCI model rats, MPSS and tocotrienol treatments
significantly suppressed the protein expression of fibronectin
(P<0.05, Fig. 9A and B). However,
in the expression no significant changes in fibronectin protein
expression were detected between the MPSS and tocotrienol groups
were observed (P>0.05, Fig. 9A and
B).
Discussion
As a result of the development of modern
transportation and industrial and mining processes, SCI has become
a commonly reported condition in clinical practice (14). The two damage mechanisms of primary
and secondary SCI may combine to seriously affect human health and
quality of life; potentially causing disabling injuries (14). Primary SCI occurs instantly after
injury of spinal cord tissue due to exposure to external forces,
and is an irreversible form of damage (17). On the basis of primary injury in the
spinal cord tissue, the progressive, self-destructive cascade
amplification destruction process caused by a variety of factors is
known as secondary SCI; the degree of damage is substantially more
than in primary SCI (17–19). The present study further showed that
BBB scores and contused volume in rats post-SCI were improved and
suppressed by treatment with tocotrienol, respectively. Frank et
al (18) reported that
tocotrienol has potential as a neuroprotective dietary factor.
Mitochondrial dysfunction is an important factor
that causes the deaths of a large number of nerve cells following
SCI; this occurrence is directly associated with the accumulation
of Ca2+ in the cell after the damage (20). Post-SCI oxidative stress damages the
ion steady state inside and outside the mitochondrial membrane,
Ca2+ accumulates inside the mitochondria, causing
mitochondrial damage and aerobic energy metabolic disorders and
inhibiting the synthesis of ATP (4).
Following SCI, excessive activity of free radicals may be applied
to the postsynaptic neuron, activating nearby astrocytes and
microglia, causing ionic imbalance at the neuronal cell membrane
(21). In addition,
Ca2+-ATPase of the plasma and endoplasmic reticulum on
the retina is highly sensitive to oxidative damage. Following SCI,
the lipid peroxidation process can inhibit the activity of
Ca2+-ATPase and interfere with the movement of
Ca2+ out of the cell membrane, causing intracellular
Ca2+ overload and ionic imbalance (4). The present results suggest that the
neuroprotective effect of tocotrienol reduced the serum MDA level
and increased the serum SOD, CAT and GSH-PX levels in SCI rats
(21). Siddiqui et al showed
that protective effects of tocotrienol may protect against
nephropathy in experimental type-2 diabetic rats by suppression of
oxidative stress (21). Nizar et
al (22) found that the
antioxidative effect of tocotrienol protects osteoblasts through
reduction of oxidative stress.
Inflammation serves a crucial function in the onset
of SCI, inflammatory reaction of local damage tissue can increase
the degree of secondary SCI (23).
Following local damage, the secondary inflammatory environment may
cause the injury of spinal cord cell necrosis and apoptosis, and
irreversible pathological changes, thus aggravating SCI (24). Neutrophils may gather to the site of
injury and release materials such as elastase; aggravating tissue
edema, necrosis and promoting apoptosis of neurons and
oligodendrocytes, and leading to local glial scar (24). The present findings support the
protective effects of tocotrienol, which inhibited the serum levels
of NF-κB p65 unit, TNF-α, IL-1β and IL-6 in SCI rats. González
et al (25) indicated that
tocotrienols exhibit anti-inflammatory properties and protect from
arginine chronic-like pancreatitis. Yam et al (26) suggested that tocotrienols suppress
proinflammatory markers in RAW264.7 macrophages.
Numerous studies have shown that central nerve
damage caused by ischemia and trauma can lead to substantial and
persistent expression of neuronal iNOS, and synthesis of excessive
quantities of NO, which has direct neurotoxic effects (27). NO is a type of small molecular free
radical with a wide range of biological activity, including
vasodilation, nerve information transfer and cytotoxic effects
(27). NO and iNOS are crucially
involved in central nervous system injury. Excessive NO can induce
cell apoptosis, and apoptosis is believed to be important factor of
SCI secondary change (28). The
present results showed that the protective effects of tocotrienol
reduced iNOS protein expression, iNOS activity and plasma NO
production in SCI rats. Qureshi et al reported that
tocotrienol inhibits lipopolysaccharide-induced proinflammatory
cytokines through suppression of iNOS gene expression in
macrophages of female mice (29).
The positive expression of TGF-β is visualized as
red granular staining in the cytoplasm of articular cartilage in
rats (30). The expression level of
TGF-β in rats with cartilage articular is is relatively low except
that the expression on the surface of the articular cartilage is
significantly higher than that of the middle and deep layer of
cartilage (31). It has been
previously reported that TGF-β1, -β2 and -β3 are strongly expressed
on the surface layer, transitional layer and the low-maturity cell
layer of cartilage cells during the process of chondrogenesis
(30). Experiments have confirmed
that TGF-β is expressed in the early stage of cartilage formation,
in cartilage callus mesenchymal cells, immature chondrocytes and
mature chondrocytes (32).
Furthermore, previous studies show that the extracellular
expression of TGF-β1 initially appeared in the hematoma in injured
area after the spinal cord injury, and the expression is
subsequently enhanced in the cytoplasm and nucleus of astrocytes,
intramedullary and extramedullary capillary endothelial cells and
motor neurons (33,34). The present results indicate that
treatment with tocotrienols significantly inhibited TGF-β protein
expression in SCI rats. Siddiqui et al (21) reported that tocotrienol mitigates the
damage of lipid-induced nephropathy through suppression of TGF-β
and collagen type IV.
Fibronectin is a multifunctional glycoprotein found
in the extracellular matrix and plasma (35). Widely existing in animal tissues and
tissue fluid, fibronectin is a V-shaped macromolecular glycoprotein
with a molecular weight of ~450 kDa and a variety of biological
activities (36). Numerous
international studies have shown that the fibronectin molecule is
highly conserved in the evolution process, and various animal body
fluids share similar structure, properties and biological function,
therefore, fibronectin from different organisms may be
interchangeable (33,34,37).
Fibronectin has many different subunits which are the expression
products of the same gene but different in the RNA splicing after
transcription and thus different mRNA are produced (38). Fibronectin is widely distributed in
hematoma, the surface of numerous types of cells and extracellular
matrix (36). Fibronectin is
crucially involved in cell adhesion, migration, differentiation and
functions. In recent years, fibronectin has been found to play an
important role in the occurrence and development of arthritis and
cartilage degeneration (39). In the
present study, tocotrienol treatment significantly reduced the
collagen type IV and fibronectin protein expression levels of SCI
rats. González et al reported that tocotrienol prevents
against lipid-induced nephropathy through suppression of TGF-β and
collagen type IV (25).
SCI can directly cause necrosis and apoptosis of
vascular endothelial cells, basement membrane components and
structural damage of the microvascular wall, which are contributed
to the disruption of the blood-spinal cord barrier and lead to the
leakage of vascular material, and further aggravate the
inflammatory reaction, edema and other secondary injury (40). As the basement membrane component,
collagen IV and laminin begin degradation following spinal cord
injury; however, the secretion of collagen IV and laminin are
enhanced accordingly as the mechanism of vascular regeneration is
activated rapidly by pathophysiological requirements (41). Previous results have shown that the
early formation of collagen IV and laminin after spinal cord injury
contribute to the restriction of inflammatory reaction and the
promotion of neovascularization (40,41).
Furthermore, pretreatment with tocotrienol treatment significantly
reduced the protein expression of fibronectin in SCI rats, in the
present study. González et al (25) reported that oral tocotrienols improve
quantitative measures of chronic pancreatic damage through
fibronectin.
In conclusion, tocotrienol attenuated the functional
recovery and the volume of gray matter contusions of SCI rats.
Prevention of oxidative damage, inflammation and iNOS may be the
key mechanisms underlying the effect of tocotrienol on SCI through
collagen type IV and fibronectin, thereby promoting functional
recovery in SCI rats, potentially in part via the TGF-β signaling
pathway.
References
|
1
|
Rosado IR, Lavor MS, Alves EG, Fukushima
FB, Oliveira KM, Silva CM, Caldeira FM, Costa PM and Melo EG:
Effects of methylprednisolone, dantrolene, and their combination on
experimental spinal cord injury. Int J Clin Exp Pathol.
7:4617–4626. 2014.PubMed/NCBI
|
|
2
|
Song Q, Xu R, Zhang Q, Ma M and Zhao X:
Therapeutic effect of transplanting bone mesenchymal stem cells on
the hind limbs' motor function of rats with acute spinal cord
injury. Int J Clin Exp Med. 7:262–267. 2014.PubMed/NCBI
|
|
3
|
Liu C, Huang Z, Jiang H and Shi F: The
sirtuin 3 expression profile is associated with pathological and
clinical outcomes in colon cancer patients. Biomed Res Int.
2014:8712632014.PubMed/NCBI
|
|
4
|
Guo J, Li Y, He Z, Zhang B, Li Y, Hu J,
Han M, Xu Y, Li Y, Gu J, et al: Targeting endothelin receptors A
and B attenuates the inflammatory response and improves locomotor
function following spinal cord injury in mice. Int J Mol Med.
34:74–82. 2014.PubMed/NCBI
|
|
5
|
Grasso G, Meli F, Graziano F, Stagno V,
Imbrucè P, Florena AM, Maugeri R and Iacopino DG: Chronic
inflammation causing spinal cord compression in human
immunodeficiency virus infection. Med Sci Monit. 14:CS134–CS137.
2008.PubMed/NCBI
|
|
6
|
Ji H, Tang H, Lin H, Mao J, Gao L, Liu J
and Wu T: Rho/Rock cross-talks with transforming growth
factor-β/Smad pathway participates in lung fibroblast-myofibroblast
differentiation. Biomed Rep. 2:787–792. 2014.PubMed/NCBI
|
|
7
|
Yu Z, Yu P, Chen H and Geller HM: Targeted
inhibition of KCa3.1 attenuates TGF-β-induced reactive astrogliosis
through the Smad2/3 signaling pathway. J Neurochem. 130:41–49.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banh A, Deschamps PA, Gauldie J, Overbeek
PA, Sivak JG and West-Mays JA: Lens-specific expression of TGF-beta
induces anterior subcapsular cataract formation in the absence of
Smad3. Invest Ophthalmol Vis Sci. 47:3450–3460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loganathan R, Selvaduray KR, Nesaretnam K
and Radhakrishnan AK: Tocotrienols promote apoptosis in human
breast cancer cells by inducing poly(ADP-ribose) polymerase
cleavage and inhibiting nuclear factor kappa-B activity. Cell
Prolif. 46:203–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das S, Mukherjee S, Lekli I, Gurusamy N,
Bardhan J, Raychoudhury U, Chakravarty R, Banerji S, Knowlton AA
and Das DK: Tocotrienols confer resistance to ischemia in
hypercholesterolemic hearts: Insight with genomics. Mol Cell
Biochem. 360:35–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zarogoulidis P, Cheva A, Zarampouka K,
Huang H, Li C, Huang Y, Katsikogiannis N and Zarogoulidis K:
Tocopherols and tocotrienols as anticancer treatment for lung
cancer: Future nutrition. J Thorac Dis. 5:349–352. 2013.PubMed/NCBI
|
|
12
|
Tanito M, Itoh N, Yoshida Y, Hayakawa M,
Ohira A and Niki E: Distribution of tocopherols and tocotrienols to
rat ocular tissues after topical ophthalmic administration. Lipids.
39:469–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sylvester PW, Akl MR, Malaviya A, Parajuli
P, Ananthula S, Tiwari RV and Ayoub NM: Potential role of
tocotrienols in the treatment and prevention of breast cancer.
Biofactors. 40:49–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Chen C, Ma S, Wang Y, Zhang X and
Su X: Inhibition of monocyte chemoattractant peptide-1 decreases
secondary spinal cord injury. Mol Med Rep. 11:4262–4266.
2015.PubMed/NCBI
|
|
15
|
Wong WY, Poudyal H, Ward LC and Brown L:
Tocotrienols reverse cardiovascular, metabolic and liver changes in
high carbohydrate, high fat diet-fed rats. Nutrients. 4:1527–1541.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, et al: MASCIS evaluation of open field locomotor scores:
Effects of experience and teamwork on reliability. Multicenter
Animal Spinal Cord Injury Study. J Neurotrauma. 13:343–359. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Genovese T, Esposito E, Mazzon E, Muià C,
Di Paola R, Meli R, Bramanti P and Cuzzocrea S: Evidence for the
role of mitogen-activated protein kinase signaling pathways in the
development of spinal cord injury. J Pharmacol Exp Ther.
325:100–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frank J, Chin XW, Schrader C, Eckert GP
and Rimbach G: Do tocotrienols have potential as neuroprotective
dietary factors? Ageing Res Rev. 11:163–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahsan H, Ahad A, Iqbal J and Siddiqui WA:
Pharmacological potential of tocotrienols: A review. Nutr Metab
(Lond). 11:522014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bao F, Chen Y, Schneider KA and Weaver LC:
An integrin inhibiting molecule decreases oxidative damage and
improves neurological function after spinal cord injury. Exp
Neurol. 214:160–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siddiqui S, Ahsan H, Khan MR and Siddiqui
WA: Protective effects of tocotrienols against lipid-induced
nephropathy in experimental type-2 diabetic rats by modulation in
TGF-β expression. Toxicol Appl Pharmacol. 273:314–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nizar AM, Nazrun AS, Norazlina M, Norliza
M and Ima Nirwana S: Low dose of tocotrienols protects osteoblasts
against oxidative stress. Clin Ter. 162:533–538. 2011.PubMed/NCBI
|
|
23
|
Sribnick EA, Wingrave JM, Matzelle DD,
Wilford GG, Ray SK and Banik NL: Estrogen attenuated markers of
inflammation and decreased lesion volume in acute spinal cord
injury in rats. J Neurosci Res. 82:283–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamai H, Sawamura S, Takeda K, Orii R and
Hanaoka K: Anti-allodynic and anti-hyperalgesic effects of
nociceptin receptor antagonist, JTC-801, in rats after spinal nerve
injury and inflammation. Eur J Pharmacol. 510:223–228. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
González AM, Garcia T, Samper E, Rickmann
M, Vaquero EC and Molero X: Assessment of the protective effects of
oral tocotrienols in arginine chronic-like pancreatitis. Am J
Physiol Gastrointest Liver Physiol. 301:G846–G855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yam ML, Hafid SR Abdul, Cheng HM and
Nesaretnam K: Tocotrienols suppress proinflammatory markers and
cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids.
44:787–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen G, Zhang Z, Wang S and Lv D: Combined
treatment with FK506 and nerve growth factor for spinal cord injury
in rats. Exp Ther Med. 6:868–872. 2013.PubMed/NCBI
|
|
28
|
Liu C, Wu W, Zhang B, Xiang J and Zou J:
Temporospatial expression and cellular localization of glutamine
synthetase following traumatic spinal cord injury in adult rats.
Mol Med Rep. 7:1431–1436. 2013.PubMed/NCBI
|
|
29
|
Qureshi AA, Reis JC, Papasian CJ, Morrison
DC and Qureshi N: Tocotrienols inhibit lipopolysaccharide-induced
pro-inflammatory cytokines in macrophages of female mice. Lipids
Health Dis. 9:1432010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Chen W, Liu W, Wu J, Shao Y and
Zhang X: The role of thrombospondin-1 and transforming growth
factor-beta after spinal cord injury in the rat. J Clin Neurosci.
16:818–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park SM, Jung JS, Jang MS, Kang KS and
Kang SK: Transforming growth factor-beta1 regulates the fate of
cultured spinal cord-derived neural progenitor cells. Cell Prolif.
41:248–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Andrew JG, Hoyland J, Andrew SM, Freemont
AJ and Marsh D: Demonstration of TGF-beta 1 mRNA by in situ
hybridization in normal human fracture healing. Calcif Tissue Int.
52:74–78. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gensel JC and Zhang B: Macrophage
activation and its role in repair and pathology after spinal cord
injury. Brain Res. 1619:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kandasamy M, Lehner B, Kraus S, Sander PR,
Marschallinger J, Rivera FJ, Trümbach D, Ueberham U, Reitsamer HA,
Strauss O, et al: TGF-beta signalling in the adult neurogenic niche
promotes stem cell quiescence as well as generation of new neurons.
J Cell Mol Med. 18:1444–1459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bachman H, Nicosia J, Dysart M and Barker
TH: Utilizing fibronectin integrin-binding specificity to control
cellular responses. Adv Wound Care (New Rochelle). 4:501–511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu Y, Soderblom C, Trojanowsky M, Lee DH
and Lee JK: Fibronectin matrix assembly after spinal cord injury. J
Neurotrauma. 32:1158–1167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia M and Zhu Y: Fibronectin enhances
spinal cord astrocyte proliferation by elevating P2Y1 receptor
expression. J Neurosci Res. 92:1078–1090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
King VR, Hewazy D, Alovskaya A, Phillips
JB, Brown RA and Priestley JV: The neuroprotective effects of
fibronectin mats and fibronectin peptides following spinal cord
injury in the rat. Neuroscience. 168:523–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia M: Fibronectin blocks the recovery of
spinal cord injury by increasing P2Y1 receptor level. Int J Dev
Neurosci. 47:20–21. 2015. View Article : Google Scholar
|
|
40
|
Koopmans G, Hasse B and Sinis N: Chapter
19: The role of collagen in peripheral nerve repair. Int Rev
Neurobiol. 87:363–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liesi P and Kauppila T: Induction of type
IV collagen and other basement-membrane-associated proteins after
spinal cord injury of the adult rat may participate in formation of
the glial scar. Exp Neurol. 173:31–45. 2002. View Article : Google Scholar : PubMed/NCBI
|