Introduction
Echis coloratus is a venomous viper species
native to several Middle Eastern countries including Saudi Arabia
and Egypt (1). Its venom induces
functional alteration of many systems and organs which may lead to
death. Viper venoms contain an abundance of proteins that disrupt
the coagulation cascade, the normal hemostatic system and tissue
repair (2). Some of the enzyme
proteins include serine proteinases,
Zn+2-metalloproteinases L-amino acid oxidase and group
II phospholipases A2 (3). Such
enzymes interfer in several physiological processes, induce a
variety of pharmacologic effects and cause breakdown of
intracellular organelles leading to necrosis and organs dysfunction
(4–6). Human envenomation by Echis
coloratus is manifested by local swelling and necrosis, pain,
respiratory failure, arrhythmia, hypotension and circulatory
collapse leading to loss of renal function and hepatocellular
damage (5–9).
Limited concentrations of reactive oxygen species
(ROS) including superoxide anions (SOA), hydrogen peroxide
(H2O2), lipid peroxides (LPO) and hydroxyl
radicals are generated during normal cellular oxidative metabolism.
This occurs as a result of the activity of the complexes of the
mitochondrial respiratory chain and other enzymes and pathways
(10). Although these activities
consume most of the oxygen utilised by cells, about 2% undergoes
reduction and results in ROS production. Normal baseline ROS levels
are essential regulators of many cellular functions. They act as
messengers for the activation of specific transcription factors and
mediators of signaling transduction pathways in cell growth,
proliferation and apoptosis (11).
However, increased cellular ROS generation causes oxidative stress
(OS) which results in damage of cellular organelles, structural
changes of macromolecules including lipids, proteins and DNA and
alteration in gene expression of apoptosis related genes resulting
in cytotoxicity and cell death (11–14). To
counteract OS cells synthesise antioxidant enzymes which neutralize
ROS. These include superoxide dismutase (SOD) which transforms SOA
to H2O2 which along with LPO get converted to
water by glutathione peroxidase (GPx) and catalase (CAT). GPx acts
to transfer the energy of peroxides to reduced glutathione (GSH)
thus forming oxidized glutathione (GSSG) which is then reduced back
to GSH by glutathione reductase (GR) (15).
Besides causing many human pathologies (16), OS seems to be a major causative
factor of venom-induced toxicity and has been associated with renal
failure, hepatic impairment and acute pancreatitis in viper and
other envenomed experimental animals and humans (6,17–20). To
this end, ROS generation has been demonstrated during scorpion
envenomation (21). Echis
pyramidum venom has also been shown to cause the formation of
highly reactive LPO and OS in several mouse organs (22), and to significantly lower hepatic CAT
and SOD activities in rats (23).
Similarly, Echis ocellatus envenomed mice exhibited lowered
serum GPx, SOD and CAT activities (24). In another study, whereas hepatic and
renal H2O2, LPO and carbonyl proteins levels
were significantly increased, CAT and SOD activities underwent
pronounced decreases in Naja Haje envenomed mice (25).
The use of large amounts of ascorbate (Asc) was
shown to provide protection against oxidative damage both in
vivo (26) and in vitro
using cultured human fibroblasts (27). The vitamin was shown to combat
arsenic-induced OS in mouse liver (28), and provided protection against both
metal ion-dependent oxidation of low density lipoproteins and
lipids (29), and as a hepato and
cardioprotective agent after carbon tetrachloride treatment
(30). The use of mega Asc doses
showed that it acted as a reducing agent, an oxidizing agent, an
anti-histamine, anti-toxins and anti-infective agent (31). Treatment of snake envenomation using
Asc was started by Klenner by administering 4 g of the vitamin
intravenously (32). However, there
is a distinct lack of reports related to the effect of Asc on
venom-induced oxidative injury. Only one recent study (33), reported that administration of Asc
(50 mg/kg body weight) to Bitis arietans envenomed rats
improved the elevated serum AST, ALT, creatinine and BUN levels,
reduced liver peroxidation levels and increased GPx, SOD and CAT
activities.
Due to the paucity of data regarding the protective
role of Asc against viper envenomation, the current comprehensive
study was conducted to investigate the effect of Asc in combating
OS induced by Echis coloratus envenomation of human tissue.
The activities of several antioxidant enzymes including GPx, GR,
glutathione S-transferase (GST), CAT and SOD, as well as GSH levels
and the corresponding oxidant generation rates including
H2O2, LPO, SOA and GSSG were assayed in
venom-free cultures and in cultures incubated with a sub-lethal
dose of crude Echis coloratus venom (EcV). In addition, the
gene expression levels of the investigated antioxidant enzymes were
studied in EcV-treated cultures in the presence of increasing Asc
concentrations and incubation periods.
Materials and methods
Echis coloratus crude venom was purchased
from Latoxan, (Rosans, France). Fibroblast culture reagents
including Eagle's Minimum Essential Medium (MEM), Hanks Buffered
Salt Solution (HBSS), fetal calf serum, trypsin, and tissue culture
flasks were obtained from Flow Laboratories, Inc. (McLean, VA,
USA). Analytical grade chemicals and biochemical were purchased
from Sigma Chemical Co., Poole, Dorset, UK.
Preparation of human skin fibroblast
cultures
Primary human fibroblast cultures were established
from ten epidermal forearm skin biopsies (~15 mg in weight) taken
from healthy adult donors (average age, 25.9±1.73 years).
Acquisition of the biopsies was approved by the Ethics Committee,
College of Medicine and King Khalid University Hospital, King Saud
University (CMIRB-KKUH-KSU). Fibroblasts were cultivated in MEM (20
ml) containing 10% fetal calf serum and harvested by
trypsinisation. The composition as well as procedures related the
preparation of culture, trypsinisation and harvesting media and
cells are as detailed by us elsewhere (27). Cells were cultured in 75
cm2 flasks in a Gelaire BSB 4A Laminar Flow cabinet
(Sydney, Australia) in an atmosphere containing 18% O2.
Confluent passage 5 fibroblasts at an early stage of their
proliferative lifespan were used for investigation.
Preparation of EcV and/or Asc treated
media and experimental design
The only source of Asc in normal growth MEM is fetal
calf serum which gives it a 60–100 µM concentration of the vitamin
depending on the batch of serum used. Hence, a serum-free medium
will be devoid of Asc. In the present study four groups of
triplicate 75 cm2 flasks of ten passage 5 confluent
fibroblast cultures were set up for investigation. Group I were
control cultures grown to confluence in normal routine MEM. Group
II consisted of EcV-incubated cultures where normal MEM was removed
and replaced with serum-free MEM containing an aliquot of crude EcV
(dissolved in HBSS, pH 7.4) to give a final venom concentration
equivalent to 0.5 µg/ml, and cells further incubated in this medium
for 4 h at 37°C. Group III were Asc-incubated cultures where normal
MEM was replaced with serum-free MEM containing 400 µM Asc and
cells further incubated for 12 h at 37°C. Finally, group IV
consisted of confluent fibroblast cultures incubated with
serum-free MEM containing 0.5 µg/ml EcV for 4 h then supplemented
with Asc (400 µM) and cells further incubated for 12 h at 37°C. The
use of the above concentrations and incubation periods of EcV and
Asc were based on data obtained and presented later in the result
section. Post-incubation cell cultures of all groups were harvested
by trypsinisation, resuspended in harvesting medium, thoroughly
washed and centrifuged at 2,000 × g for 5 min. The pellets were
kept on ice and immediately sonicated for 20 sec in 0.1 M phosphate
buffer (pH 7.0, 0.5 ml) using a Fisher Sonic Dismembrator Model 150
(Thermo Fisher Scientific, Waltham, MA, USA) at 50% of the power
output equivalent to 1,000 Hz frequency. Appropriate sonicate
aliquots were then used for the assay of various parameters.
Determination of the viability of EcV
and Asc incubated cells
A modified MTT
[3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide]
assay based on that documented by Mosmann (34) was used to establish EcV and Asc doses
and incubation periods at which fibroblasts maintain normal
metabolic activity and proliferation. Triplicate passage 5
fibroblasts were grown in 96-well microplates with 8×104
cells/ml initial concentration using routine MEM. At confluence,
the medium was removed and replaced with serum-free MEM (100 µl)
containing increasing amounts of EcV equivalent to 0.10, 0.25,
0.50, 1.00, 1.50, 2.50 and 4.00 µg/ml and cells incubated for
either 4, 12 or 24 h at 37°C. The EcV-containing medium was removed
and replaced with buffered saline (pH 7.2) containing sterilized
MTT (2.4 mM, 400 µl). After a 2-h incubation, the MTT solution was
removed and formazan crystals (formed as a result of the cleavage
of MTT by succinate dehydrogenase of viable cells) were solubilized
using acidified isopropanol (300 µl/well). Finally, absorbance of
all samples was measured at 570 nm using an EIA plate reader (model
2550; Bio-Rad Laboratories, Inc., Hercules, CA, USA) against a
background absorbance at 690 nm. The above experiment was repeated
by incubating confluent fibroblast cultures in serum-free media
containing increasing Asc concentrations equivalent to 200, 300,
400 and 500 µM for 4, 12 and 24 h. The viability of either EcV or
Asc-incubated cells was then expressed as mean ± SD percentages at
each venom or vitamin concentration against venom-free controls or
controls cultured in normal MEM containing ~100 µM Asc, both of
which were considered to have absorbance values representative of
100% viability.
Oxidative status of cultures with
respect to EcV concentration and incubation time
In this experiment routine MEM of confluent passage
5 cultures (n=10) was replaced with serum-free media containing
0.10, 0.25, 0.50 and 1.00 µg EcV/ml and cells further incubated
with these media for 4, 12 and 24 h. Fibroblasts were then
harvested, pelleted and sonicated as described earlier and protein
carbonyl content (PCC) were assayed as described later. PCC was
chosen to serve as a biomarker of the oxidative status of cultures
at increasing EcV concentrations and incubation periods.
Antioxidant/oxidant status of
EcV-treated cells with respect to Asc concentration and incubation
time
A pilot study was run to determine the Asc
concentration and incubation period required to produce maximal
change in marker antioxidant enzymatic activity and oxidant
generation in viable EcV-treated cell cultures. For this purpose
triplicates of the ten passage 5 cultures were grown to confluence
in normal MEM which was then replaced with serum-free media
containing 0.5 µg EcV/ml and cells were incubated in these media
for 4 h. Asc was then added to give final concentrations equal to
200, 300, 400 and 500 µM and cells further incubated for 4, 12 and
24 h at 37°C. Fibroblasts were then harvested, pelleted and
sonicated as described earlier, and SOD activity and the
corresponding SOA generation rates were assayed in appropriate
aliquots of the sonicates according to methodologies presented
later. SOD was chosen for this pilot study since it has cytosolic,
mitochondrial and other compartmental isoforms, thus allowing for
variations in intracellular Asc transport the rate of which could
be affected by its concentration and incubation time. Results were
compared to those obtained for the control cultures grown in normal
venom-free MEM containing an approximate 100 µM Asc concentration
contributed by fetal calf serum.
Biochemical assays
GPx, CAT, SOD and GR specific activities as well as
the generation rates of H2O2, SOA and LPO and
GSH and GSSG levels were spectrophotometrically assayed using
appropriate volumes of fibroblasts sonicates according to the
respective methodologies previously detailed and documented by us
(35,36).
GST activity was measured according to Habig et
al (37). The assay measures
total GST activity and is based on the conjugation of
1-chloro-2,4-dinitrobenzene (CDNB) with GSH. Fibroblast sonicates
(50 µl) were added to potassium phosphate buffer (2 ml, pH 6.5)
containing 0.1% Triton X-100, CDNB (1.0 mM) and 5.0 mM GSH and
incubated at 25°C in a cuvette. The increase in absorbance at 340
nm (the rate of which is directly proportional to GST activity) was
monitored for 3 min in a recording thermostated spectrophotometer
(Model UV-2401 PC; Shimadzu, Dubai, United Arab Emirates).
Total protein content of fibroblast sonicates (20
µl) was assayed according to Bradford (38).
PCC was assayed using dinitrophenylhydrazine (DNPH)
according to Reznick and Packer (39) with minor modifications. Fibroblast
sonicates (100 µl) were incubated with 10 mM DNPH (0.5 ml)
dissolved in 2 M HCl and blanks using 2 M HCl only (1 ml) were run
in parallel. Samples were left standing in the dark for 1 h
accompanied by frequently mixing. Protein hydrazone derivatives
were then precipitated with 20% TCA (0.5 ml) by centrifugation
(12,000 × g for 5 min at 4°C) and pellets were washed three times
using ethanol: ethylacetate (1:1, 1 ml). The final pellets were
then dissolved in guanidine (6 M, 1 ml), centrifuged at 12,000 × g
for 15 min and PCC (n mol/mg tissue) measured
spectrophotometrically at 360 nm using an absorption coefficient of
22×103 M−1 cm−1.
Gene expression profiling of hsGPx,
hsGR, hsGST, hsCAT and hsSOD using real-time quantitative PCR
(RT-qPCR)
Freshly collected pellets were stored in
RNAlater® RNA stabilization solution at −80°C and
homogenized using a Tissue Lyser LT (both from Qiagen, Hilden,
Germany) in 1.0 ml TRIzol® Reagent (Invitrogen, Paisley,
UK) and total RNA was extracted according to standard procedures.
Genomic DNA was then eliminated and cDNA synthesized from RNA (1
µg) in a final reaction volume (20 µl) using the QuantiTect Reverse
Transcriptase kit (Qiagen). RT-qPCR was subsequently performed as
described by us earlier (40) using
a QuantiTect SYBR-Green PCR kit (Qiagen) with the following gene
primer assays for each antioxidant gene: GPx (QT00203392), GR
(QT00038325), GST (QT00063357), CAT (QT000796764) and SOD
(QT01664327) in a final reaction volume (25 µl) containing the
diluted cDNA sample (5 µl), 2X SYBR-Green PCR Master mix (12.5 µl),
each forward and reverse primer (10 µM stock, 2.5 µl) and
RNAase-free water (2.5 µl). The amplification program and PCR
amplicon specificity were performed and assessed as previously
reported (40). Each fibroblast
tissue sample was represented by biological replicas and three
technical replicas, with the inclusion of a no-template control.
Raw data were analysed using the Rotor-Gene cycler software 2.3 to
calculate the threshold cycle using the second derivative maximum.
The fold-change in each gene was determined after normalization to
the expression levels of 18 S as a house-keeping gene.
Statistical analysis
Analysis of variance followed by post hoc Tukey HSD
test were performed to evaluate statistical differences between
mean ± SD values of all parameters assayed in control venom-free
cultures against those incubated with different concentrations of
EcV, Asc and EcV plus Asc for different periods. This was done
using the SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to be statistically significant.
Results
Effect of increasing concentrations
and incubation periods of EcV and Asc on viability of the cultured
cells
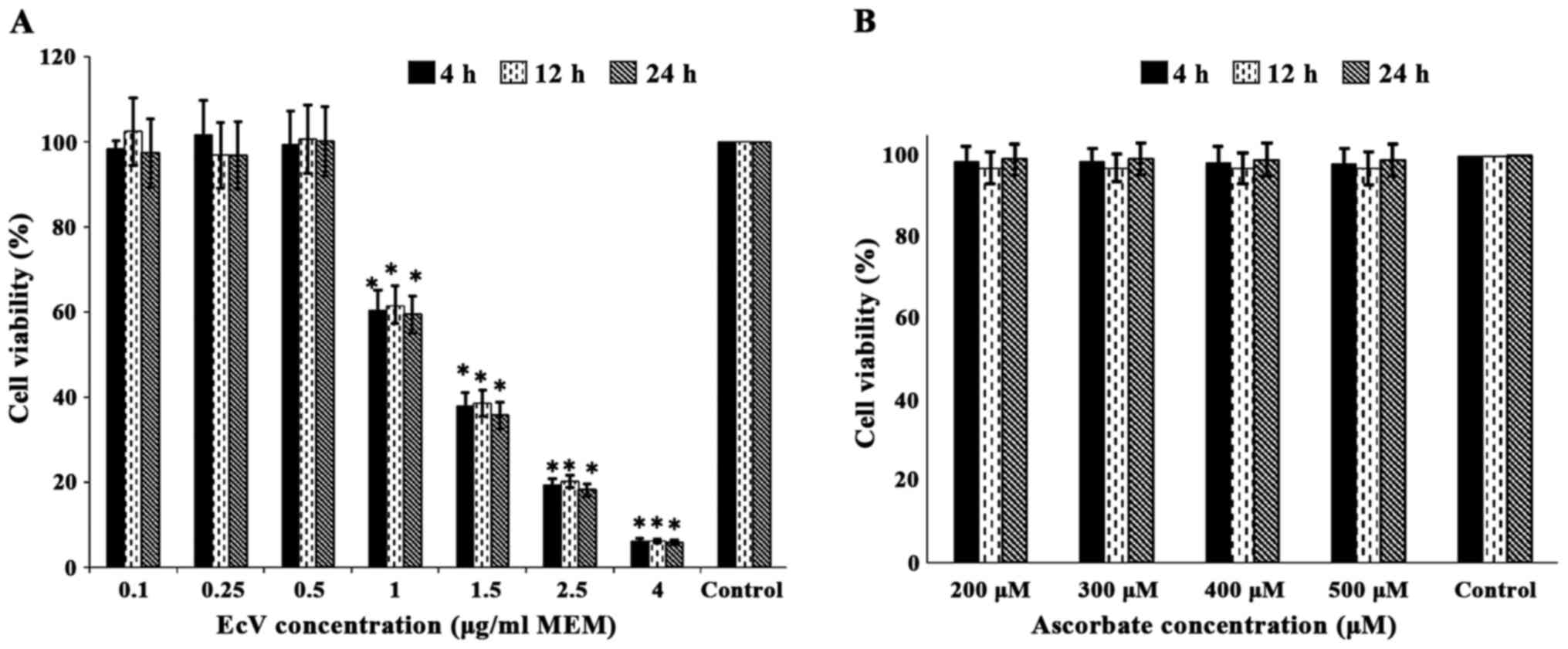
Results presented in Fig.
1A indicated that fibroblast cultures grown in normal MEM and
incubated with increasing EcV concentrations of 0.10, 0.25 and 0.50
µg/ml MEM for 4, 12 and 24 h did not cause significant loss of cell
viability compared to venom-free controls. As an example percentage
cell viabilities equaled 98.3±4.12, 101.6±4.34 and 99.4±4.16% in
cultures incubated with 0.10, 0.25 and 0.50 µg/ml for 4 h,
respectively, against 100% assigned to venom-free controls.
Moreover, very similar values were obtained for cultures incubated
with the same venom concentrations for 12 and 24 h. Other Fig. 1A data however, revealed that
incubation of cultures with 1.0, 1.5, 2.5 and 4.0 µg EcV/ml MEM for
4, 12 and 24 h resulted in very significant and progressive losses
of cell viability proportional to venom concentration and very
similar in magnitude regardless of the incubation period. As an
example percentage cell viabilities equaled 60.3±2.41, 37.8±1.48,
19.3±0.77 and 6.14±0.23% in cultures incubated with 1.0, 1.5, 2.5
and 4.0 µg EcV/ml, respectively, against 100% assigned for
venom-free controls (P<0.001 for all comparisons). In light of
Fig. 1A results, cultures were
incubated with 0.5 µg EcV/ml MEM for 4 h prior to investigation of
the oxidative status of cells.
In contrast, Fig. 1B
data show that incubation of fibroblast cultures with increasing
Asc concentrations equivalent to 200, 300, 400 and 500 µM (chosen
to approximately represent double, triple, quadruple and quintuple
human plasma levels), did not result in any significant loss of
cell viability when compared to control cultures cultivated in
routine MEM approximately containing 100 µM Asc. In addition, cell
viabilities were very similar in magnitude regardless of whether
the incubation was performed for 4, 12 or 24 h.
Effect of increasing EcV
concentrations and incubation time on PCC of fibroblast
cultures
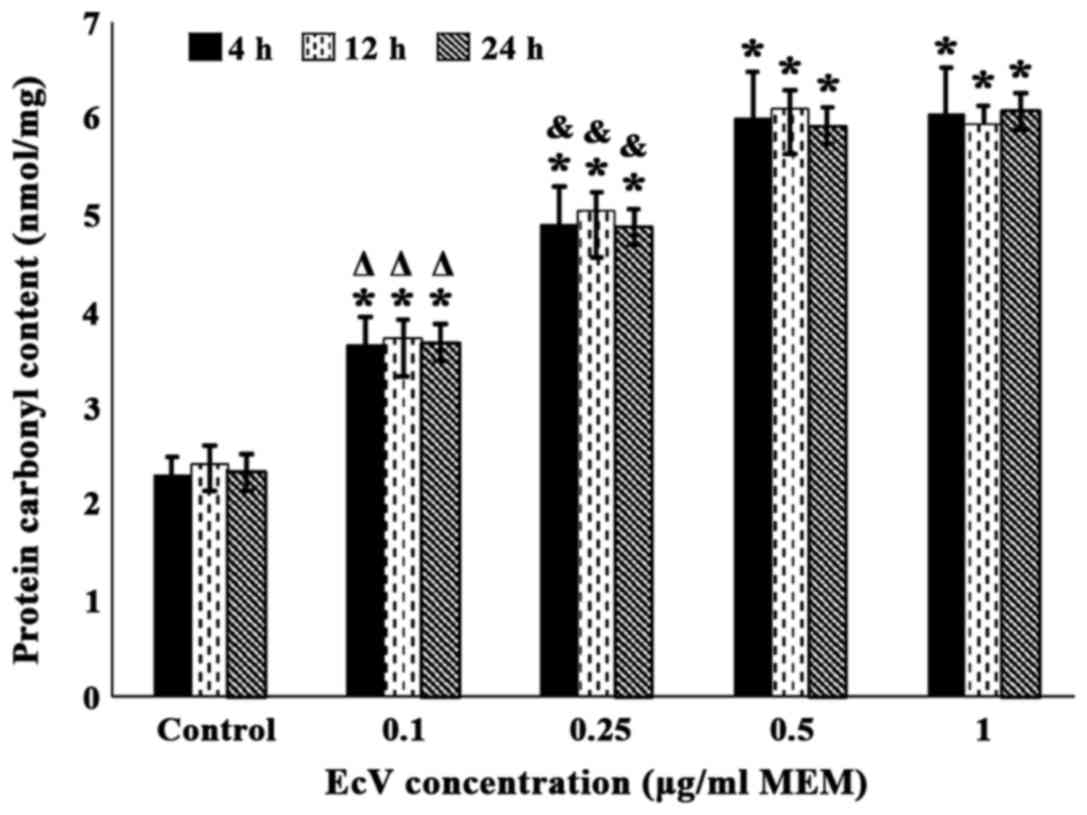
As illustrated in Fig.
2, incubation of venom-free control cultures with increasing
EcV concentrations (0.10–1.00 µg/ml MEM) for 4, 12 and 24 h
resulted in very significant progressive increases in PCC that were
dose-dependent. After incubation for 4 h, values equaled 3.65±0.29,
4.91±0.39, 6.01±0.48 and 6.05±0.48 nmol/mg tissue at 0.10, 0.25,
0.50 and 1.00 µg EcV/ml MEM, respectively, against 2.31±0.18
nmol/mg tissue recorded for venom-free controls (P<0.001 for all
comparisons). As also evident from Fig.
2, such PCC values were very similar in magnitude regardless of
whether the incubation period with EcV was performed for 4, 12 or
24 h. Furthermore, PCC values in cultures incubated with 0.50 and
1.00 µg EcV/ml MEM reached maximal levels and were very similar in
value regardless of the incubation period.
In light of the above results and those related to
the effect of increasing EcV concentrations and incubation time on
cell viability (presented in Fig.
1A), cultures were incubated with 0.50 µg EcV/ml MEM for 4 h
prior to investigation of the antioxidant/oxidant status of cells.
Under such conditions envenomed cells are metabolically viable and
proliferate normally but are being subjected to OS.
Effect of increasing Asc
concentrations and incubation time of EcV-treated cultures on SOD
and SOA as markers of OS
As evident from Fig.
3A data, incubation of EcV-treated cultures with increasing Asc
concentrations for 4 h resulted in very significant progressive SOD
activity increases that were dose dependent (11.2±1.01, 20.2±1.71
and 23.5±1.97 µmol/min/mg protein at 300, 400 and 500 µM Asc,
respectively, against 8.51±0.74 µmol/min/mg protein recorded in
EcV-treated cultures incubated with 200 µM Asc; P<0.001 for all
comparisons). However, such increased values were very
significantly lower compared to that obtained in venom-free
controls (26.8±2.28 µmol/min/mg protein; P<0.001 for 200–400 µM
Asc and P<0.05 for 500 µM Asc). In addition, Fig. 3A data revealed that very significant
higher enzyme activity increases were obtained when EcV-treated
cultures were incubated with the same Asc concentrations for 12 h
(14.8±1.26, 27.2±2.32 and 26.1±2.31 µmol/min/mg protein at 300, 400
and 500 µM Asc, respectively, against 11.9±0.99 µmol/min/mg protein
recorded in cultures incubated with 200 µM Asc; P<0.001 for all
comparisons). Furthermore, although SOD activities in EcV-treated
cultures incubated with 200 and 300 µM Asc were still significantly
lower than that obtained for venom-free controls (11.9±0.99 and
14.8±1.26, respectively, against 26.9±2.36 µmol/min/mg protein;
P<0.001 for both comparisons), the enzyme activities in
venom-treated cultures incubated with 400 and 500 µM Asc were of
very similar magnitude and not statistically different when
compared to that of venom-free controls (27.2±2.32 and 26.1±2.31,
respectively. against 26.9±2.36 µmol/min/mg protein). Fig. 3A data also indicated a very similar
pattern and magnitude of SOD activity increases when EcV-treated
cultures were incubated with the same Asc concentrations for 24 h
compared to those incubated for 12 h (15.5±1.36, 26.7±2.22 and
25.1±2.13 µmol/min/mg protein at 300, 400 and 500 µM Asc,
respectively, against 12.3±1.02 µmol/min/mg protein obtained for
venom-treated cultures incubated with 200 µM Asc; P<0.001 for
all comparisons). Moreover, the above enzyme activities at 400 and
500 µM Asc were of very similar magnitude with that obtained in
venom-free cultures (27.5±2.39 µmol/min/mg protein).
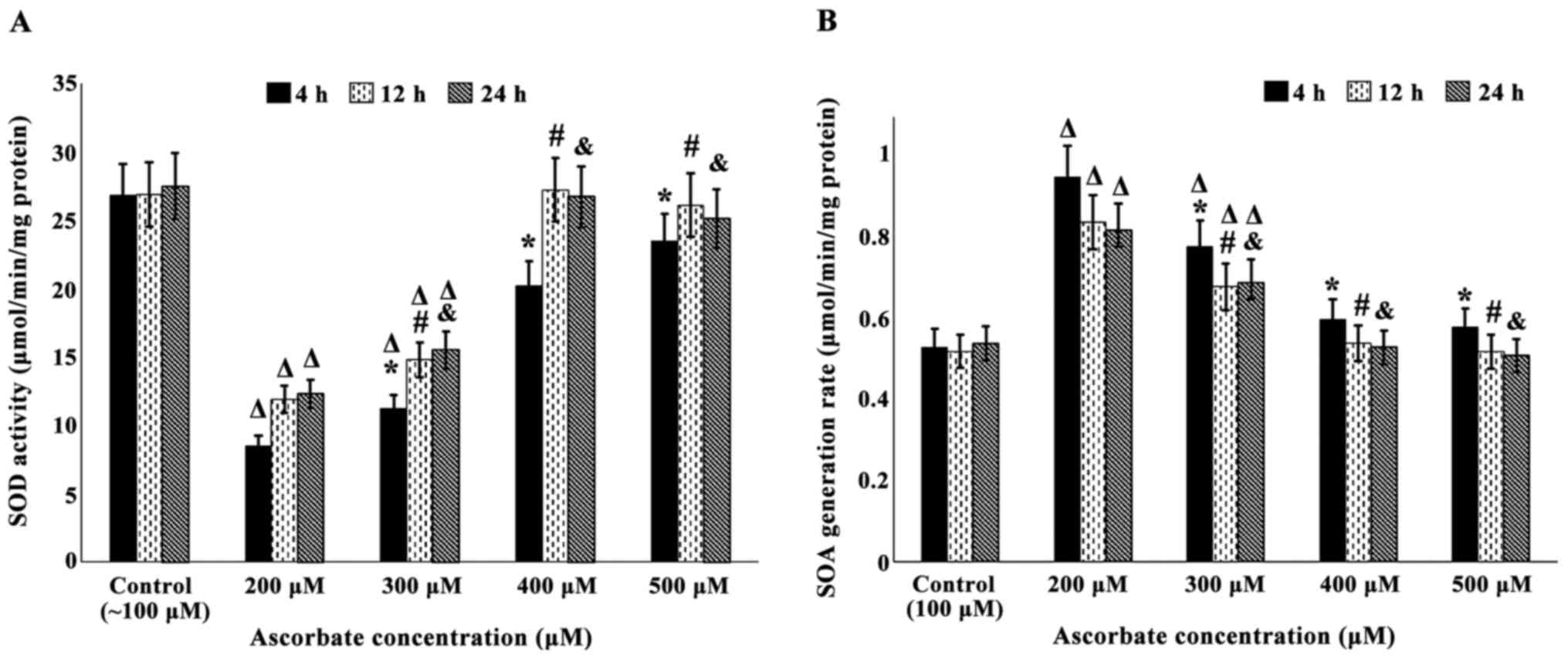 | Figure 3.(A) Effect of incubation of EcV
treated fibroblast cultures (0.5 µg/ml MEM for 4 h) with ascorbate
(200–500 µM) for 4, 12 and 24 h on SOD activity. Confluent passage
5 cultures were used. Control cultures (venom-free) were grown in
routine MEM. Values shown are means ± SD of triplicate
determinations of 10 cultures. *,#,&P<0.001 when
comparing SOD activities in EcV-treated cultures supplemented with
300, 400 and 500 µM ascorbate for 4 h (*), 12 h (#) and 24 h
(&), against those supplemented with 200 µM ascorbate.
ΔP<0.001 when SOD activities in EcV-treated cultures
supplemented with 200 and 300 µM ascorbate were compared to either
those supplemented with 400 and 500 µM ascorbate, or with
venom-free controls at each incubation period. (B) Effect of
incubation of EcV-treated cultures (0.5 µg/ml MEM for 4 h) with
ascorbate (200–500 µM) for 4, 12 and 24 h on SOA generation rates.
Confluent passage 5 cultures were used. Controls (venom-free) were
grown in routine MEM. Values shown are means ± SD of triplicate
determinations of 10 cultures. *,#,&P<0.001 when
comparing SOA generation rates in EcV-treated cultures supplemented
with 300, 400 and 500 µM ascorbate for 4 h (*), 12 h (#) and 24 h
(&), against those supplemented with 200 µM ascorbate.
ΔP<0.001 when SOA generation rates in EcV-treated
cultures supplemented with 200 and 300 µM ascorbate were compared
to either those supplemented with 400 and 500 µM ascorbate, or with
venom-free controls at each incubation period. EcV, Echis
coloratus venom; MEM, Eagles Minimum Essential Medium; SOD,
superoxide dismutase; SOA, superoxide anions. |
Concurrently, Fig. 3B
demonstrates that incubation of EcV-treated cultures with
increasing Asc concentrations for 4 h resulted in very significant
dose-dependent gradual reductions in SOA generation. Rates equaled
0.78±0.064, 0.6±0.049 and 0.58±0.047 µmol/min/mg protein at 300,
400 and 500 µM Asc, respectively, against 0.95±0.079 µmol/min/mg
protein recorded in EcV-treated cultures incubated with 200 µM Asc
(P<0.001 for all comparisons). However, such lower rates were
still significantly higher than that recorded for SOA generation in
venom-free controls (0.53±0.045 µmol/min/mg protein; P<0.001 for
200 and 300 µM Asc, P<0.01 for 400 µM Asc and P<0.05 for 500
µM Asc). Data also showed that lower magnitude SOA rate reductions
were obtained when EcV-treated cultures were incubated with the
same Asc concentrations for 12 h (0.68±0.057, 0.54±0.044 and
0.52±0.042 µmol/min/mg protein at 300, 400 and 500 µM Asc
respectively against 0.84±0.067 µmol/min/mg protein recorded in
cultures incubated with 200 µM Asc; P<0.001 for all
comparisons). Although the rates in envenomed cultures incubated
with 200 and 300 µM Asc were still very significantly higher than
that obtained for venom-free controls (0.84±0.067 and 0.68±0.057
µmol/min/mg protein, respectively, against 0.52±0.041 µmol/min/mg
protein; P<0.001 for both comparisons), those in cultures
incubated with 400 and 500 µM Asc were of very similar magnitude
and not significantly different from the rate in venom-free
controls (0.54±0.044 and 0.52±0.042 µmol/min/mg protein,
respectively, against 0.52±0.041 µmol/min/mg protein). Additionally
Fig. 3B data indicate a very similar
pattern as well as magnitude of SOA generation rate reductions when
EcV-treated cultures were incubated with the same Asc
concentrations for 24 h rather than 12 h (0.69±0.056, 0.53±0.042
and 0.51±0.041 µmol/min/mg protein at 300, 400 and 500 µM Asc,
respectively, against 0.82±0.065 µmol/min/mg protein recorded in
cultures incubated with 200 µM Asc; P<0.001 for all
comparisons). Moreover, the above-mentioned rates at 400 and 500 µM
Asc were of very similar magnitude to that obtained in venom-free
controls (0.54±0.043 µmol/min/mg protein).
Thus, Fig. 3 results
indicate that a 400 or 500 µM Asc concentration and an incubation
period of either 12 or 24 h were required to achieve maximal
restoration of SOD activities and SOA generation rates in envenomed
cultures to values similar to those recorded in venom-free
controls. Hence, in all subsequent experiments investigating the
effect of Asc on the activities and levels of antioxidants and
pro-oxidants, EcV-treated cultures were incubated with 400 µM Asc
for 12 h.
Effect of incubation of cultures with
EcV, Asc and EcV plus Asc on antioxidant enzyme activities
Table I data clearly
indicate that incubation of control fibroblast cultures with EcV
(0.5 µg/ml MEM for 4 h) resulted in highly significant reductions
in the activities of all investigated antioxidant enzymes. All
enzymes underwent activity percentage reductions of similar
magnitude when compared to activities recorded in venom-free
control cultures (P<0.001 for all comparisons). Percentage
reductions equaled 47.3±4.18, 44.6±4.09, 49.1±4.34, 44.2±4.18 and
52.3±4.46% of control activities for GPx, CAT, SOD, GR and GST,
respectively. In contrast, incubation of control cultures in
serum-free MEM supplemented with 400 µM for 12 h did not cause any
significant changes in the activities of any of the studied
enzymes. Also, Table I data show
that incubation of the EcV-treated cultures with serum-free MEM
containing 400 µM Asc for 12 h resulted in the restoration of GPx,
CAT, SOD and GR activities to values very similar and not
significantly different from those recorded for venom-free
controls. However, GST activity was partially restored to levels
significantly lower compared to those documented for venom-free
controls (84.9±6.12 nmol/min/mg protein against 92.8±7.88
nmol/min/mg protein; P<0.05).
 | Table I.Effect of incubation of fibroblast
cultures with EcV, Asc and EcV plus Asc on antioxidant enzyme
activities. |
Table I.
Effect of incubation of fibroblast
cultures with EcV, Asc and EcV plus Asc on antioxidant enzyme
activities.
|
| Enzymatic
parameters |
|---|
|
|
|
|---|
| Sample
groups/incubation time (n=10) | GPx | CAT | SOD | GR | GST |
|---|
| Group I |
|
|
|
|
|
| Control
cultures | 1.88±0.15 | 3.75±0.31 | 25.2±2.21 | 2.70±0.25 | 92.8±7.88 |
| Group II |
|
|
|
|
|
| Cultures + EcV (4
h) |
1.01±0.10a |
2.08±0.19a |
12.8±1.14a |
1.48±0.13a |
44.6±3.81a |
| Group III |
|
|
|
|
|
| Cultures + Asc (12
h) | 1.97±0.17 | 3.80±0.30 | 23.5±2.12 | 2.68±0.24 | 95.0±7.94 |
| Group IV |
|
|
|
|
|
| Cultures + EcV (4
h) + Asc (12 h) | 1.95±0.16 | 3.62±0.29 | 23.7±2.22 | 2.76±0.27 |
84.9±6.21b |
Effect of incubation of cultures with EcV, Asc and
EcV plus Asc on oxidant generation. Table II data demonstrate that SOA,
H2O2 and LPO generation rates in
EcV-incubated cultures (0.5 µg/ml for 4 h) underwent very
significant increases compared to those obtained for venom-free
controls (0.92±0.09 against 0.58±0.06 µmol/min/mg protein for SOA,
2.59±0.25 against 1.69±0.17 pmol/min/mg protein for
H2O2 and 51.2±4.56 against 35.1±3.34
pmol/min/mg protein for LPO; P<0.001 for all comparisons). Such
increases amounted to 37.4±3.69, 52.2±5.44 and 44.7±4.56% of
control levels for SOA, H2O2 and LPO,
respectively. In contrast, incubation of venom-free control
cultures with serum-free MEM containing 400 µM Asc for 12 have did
not significantly alter the generation rates of any of the studied
oxidants. Table II data also show
that incubation of the EcV-treated cultures with serum-free MEM
containing 400 µM Asc for 12 h, caused very significant decline in
all oxidant generation rates to values very similar and not
statistically different from those recorded for venom-free cultures
(0.53±0.06 µmol/min/mg protein for SOA and 1.64±0.15 and 34.7±3.29
pmol/min/mg protein for H2O2 and LPO,
respectively).
 | Table II.Effect of incubation of fibroblast
cultures with EcV, Asc and EcV plus Asc on oxidant generation
rates. |
Table II.
Effect of incubation of fibroblast
cultures with EcV, Asc and EcV plus Asc on oxidant generation
rates.
|
| Oxidant generation
rates |
|---|
|
|
|
|---|
| Samples
groups/incubation time (n=10) | SOA |
H2O2 | LPO |
|---|
| Group I |
|
|
|
| Control
cultures | 0.58±0.06 | 1.69±0.17 | 35.1±3.34 |
| Group II |
|
|
|
| Cultures + EcV (4
h) |
0.92±0.09a |
2.59±0.25a |
51.2±4.56a |
| Group III |
|
|
|
| Control + Asc (12
h) | 0.56±0.06 | 1.67±0.15 | 35.8±.26 |
| Group IV |
|
|
|
| Cultures + EcV (4
h) + Asc (12 h) | 0.53±0.06 | 1.64±0.15 | 34.7±3.29 |
Effect of incubation of cultures with
EcV, Asc and EcV plus Asc on GSH and GSSG levels
Results presented in Table III indicated that cellular GSH
levels in EcV-treated cultures significantly declined by
34.4±0.29%, and those of GSSG significantly increased by 40.1±0.33%
of levels recorded in venom-free control cultures. Levels equaled
32.1±2.24 and 1.11±0.092 nmol/mg protein against 48.5±3.95 and
0.79±0.068 nmol/mg protein for GSH and GSSG, respectively
(P<0.001 for both comparisons). Consequently the GSH/GSSG ratio
was significantly decreased in the EcV-treated cultures compared to
controls (28.9±2.15 against 60.7±4.97). However, these levels were
restored upon incubation of the envenomed cultures with serum-free
MEM containing 400 µM Asc for 12 h reaching very similar values to
those recorded in venom-free controls (46.9±3.67 against 48.5±3.95
nmol/mg protein for GSH and 0.76±0.052 against 0.79±0.068 nmol/mg
protein for GSSG). This resulted in restoration of the GSH/GSSG
ratio to a value similar to that obtained for venom-free controls
(59.2±4.90 against 60.7±4.97).
 | Table III.Effect of incubation of fibroblast
cultures with EcV, Asc and EcV plus Asc on GSH and GSSG levels. |
Table III.
Effect of incubation of fibroblast
cultures with EcV, Asc and EcV plus Asc on GSH and GSSG levels.
| Samples
groups/incubation time (n=12) | GSH | GSSG | GSH/GSSG |
|---|
| Group I |
|
|
|
| Control
cultures | 48.5±3.95 | 0.79±0.068 | 60.7±4.97 |
| Group II |
|
|
|
| Cultures + EcV (4
h) |
32.1±2.24a |
1.11±0.092a |
28.1±2.15a |
| Group III |
|
|
|
| Control + Asc (12
h) | 46.7±3.74 | 0.82±0.071 | 57.3±4.57 |
| Group IV |
|
|
|
| Cultures + EcV (4
h) + Asc (12 h) | 46.9±3.67 | 0.76±0.052 | 59.2±4.90 |
Effect of incubation of cultures with
EcV, Asc and EcV plus Asc on relative gene expression of
antioxidant enzymes
As evident from Fig.
4, fibroblast hsGPx, hsGR, hsGST,
hsCAT and hsSOD gene expression levels were very
significantly downregulated by 52.2±4.18, 45.9±3.62, 59.8±4.84,
52.4±4.35 and 53.0±4.38% of control levels, respectively
(P<0.001 upon comparison of the fold-change in the gene
expression levels of all enzymes in EcV-treated cells relative to
venom-free controls). However, gene expression levels of all
enzymes except GST were restored to values similar and not
significantly different from those of control cultures when the
venom-treated cultures were incubated with Asc (400 µM for 12 h).
For GST, the fold-changes in envenomed cultures were moderately but
significantly lower than those recorded for controls (0.99±0.086
against 1.10±0.090; P<0.01).
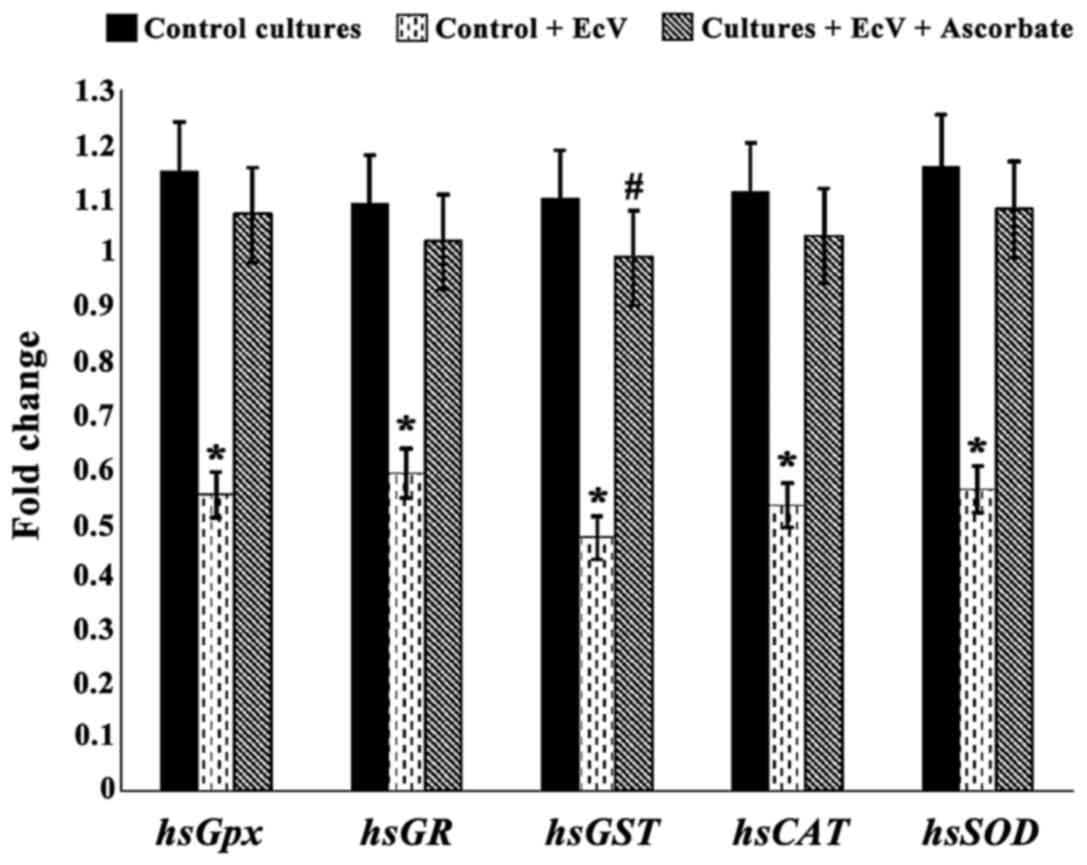 | Figure 4.Relative gene expression of
hsGPx, hsGR, hsGST, hsCAT and
hsSOD in control, EcV-treated and EcV-treated plus ascorbate
fibroblasts. Confluent passage 5 cultures were used. Control
(venom-free) cultures were grown in routine MEM. Concentrations of
EcV and ascorbate are indicated in the text. Fold-change values are
means ± SD for triplicates of the 10 cultures. *P<0.001 when
comparing fold-change in the gene expression levels of each enzyme
in EcV-treated fibroblasts relative to venom-free controls.
#P<0.01 upon fold-change comparison in the gene
expression level of GST in EcV plus ascorbate-treated fibroblasts
relative to controls. EcV, Echis coloratus crude venom;
hsGpx, hsGR, hsGST, hsCAT and
hsSOD: Homo sapiens glutathione peroxidase,
glutathione reductase, glutathione S-transferase, catalase and
superoxide dismutase respectively; MEM, Eagles Minimum Essential
Medium. |
Discussion
Human fibroblast cultures have been previously
extensively used by us for the study of metabolic changes related
to different pathologic conditions including incubation of cells
with snake venom proteins (41–43). The
in vitro maintained human tissue model system provided in
the present study is an appropriate experimental tool for the
investigation of the effect of different concentrations and
incubation periods of EcV, Asc and EcV plus Asc on the
antioxidant/oxidant status of envenomed fibroblasts. To this end,
it was essential to choose an EcV concentration and incubation
period that would minimize kinetic errors without affecting the
proliferative and metabolic viability of the cells. Thus, any
observed changes in the oxidative status of cells can be attributed
to the activity of the venom. Fig.
1A data suggest that incubating cultures with EcV
concentrations up to 0.5 µg/ml MEM for 4, 12 and 24 h maintained
control cellular viability. However, the use of 1.0, 1.5, 2.5 and
4.0 µg/ml for the same incubation periods caused progressive loss
of viability in a dose-dependent fashion regardless of the
incubation time. Results also showed that incubation of cultures
with EcV concentrations at 0.10, 0.25, 0.50 and 1.00 µg/ml MEM
caused significant progressive increases in the PCC of cells which
peaked at 0.50 and 1.00 µg of the venom (Fig. 2). Furthermore, the magnitude of such
increases were very similar regardless of whether the incubation
was performed for 4, 12 or 24 h. In light of the above results, it
was decided that in all subsequent experiments, cell cultures will
be incubated with EcV (0.50 µg/ml MEM for 4 h) prior to harvesting
for investigation. This ensured that although cells at such venom
concentrations were metabolically and proliferatively viable, they
were being subjected to OS.
It was also important to choose an Asc concentration
and incubation time that would not affect the proliferative and
metabolic viability of cells. To this end, Fig. 1B data show that incubation of
cultures with Asc (200–500 µM) for 4, 12 and 24 h did not cause any
significant changes in cell viability regardless of the incubation
period. However, incubation of EcV-treated oxidatively-stressed
cultures with the same Asc increasing concentrations and incubation
times caused progressive statistically very significant increases
in SOD activity that was chosen as a marker antioxidant (Fig. 3A). Such increases were dose-dependent
and reached very similar peak values in envenomed cultures
incubated with 400 and 500 µM Asc regardless of whether the
incubation was performed for 12 or 24 h. Furthermore, incubation of
the EcV-treated cultures with the vitamin at the same
concentrations and incubation periods caused progressive decreases
in the levels of SOA chosen as a marker oxidant (Fig. 3B). Such decreases were also shown to
reach similar lowest levels when the oxidatively stressed cells
were incubated with 400 and 500 µM Asc for 12 and 24 h. Hence, in
all subsequent experiments that investigated the activities and
levels of a variety of antioxidants and oxidants, EcV-treated
cultures were incubated with 400 µM Asc for 12 h thus minimizing
kinetic errors.
Throughout the present study passage 5 cultures were
used since we previously showed that fibroblasts beyond passages 10
and 15 enter an early phase of senescence causing many metabolic
changes including lowered rates of growth and replication, protein
synthesis and changes in the activities of many key and antioxidant
enzymes as well as alterations in cellular morphology (27,36,44,45).
Other optimal culture conditions were also provided to ensure
maximal rates of fibroblast growth, multiplication and metabolism.
These included the use of sufficient MEM volumes, addition of Hepes
buffer to both culture and trypsinisation media and streptomycin
and penicillin to prevent contamination.
As illustrated in Table
I, incubation of cultures with EcV resulted in highly
significant decreases of similar magnitude in the activities of
GPx, GR, GST, SOD and CAT compared to those documented for control
cultures. These findings are in broad agreement with previous
studies which reported that Echis pyramidum, Echis
ocellatus and Naja Haje envenomation of rats and mice
caused significant decreases of hepatic and renal GPx, CAT and SOD
activities (22–25). In the present study all enzyme
activities were expressed in terms of cellular protein, however the
ratios of protein/DNA for the 10 cultures were similar in value
regardless of EcV incubation (mean = 14.6±1.35 µg protein/µg DNA).
Furthermore, incubation of cultures with 0.5 µg/ml EcV for 4 h did
not significantly change the protein yield (671±41.1 µg/75
cm2 flask of cells) indicating no proteolytic activity
of the venom at the above concentration and incubation time.
Although proteolytic activity has been reported for some venoms,
none was detected by us for Echis coloratus purified
fractions (41). The absence of
proteolytic activity, however, could have been a result of protease
inhibitors contributed by the fetal calf serum component of MEM.
Furthermore, the possibility of cell membrane rupture that could
have resulted from the venoms phospholipase A2 activity is ruled
out since no antioxidant enzyme activity was detected in EcV or MEM
prior to or post-incubation of cells with the venom. In contrast,
incubation of cultures with EcV (>8 µg/ml) resulted in rounding
and lysis of cells. Our previous study (41) also showed that incubation of
fibroblast sonicates with EcV (0.5 µg/ml MEM for 3 h) did not cause
any significant changes in the activities of key cytosolic and
mitochondrial enzymes. This finding coupled with the fact all
presently investigated antioxidant enzyme activities underwent
reductions of similar magnitude (44–52% of control activities),
suggest that EcV executes its effect at the cellular level rather
than directly at the protein enzyme molecules. Several of our
previous studies reported similar findings where TCA cycle enzyme
activities reduced by 50–60% (44),
and phosphofructokinase and citrate synthase activities by 60–62%
(41) of control values upon
incubation of fibroblast cultures with Walterinnesia
aegyptia and EcVs, respectively. Furthermore, these effects
were venom dose-dependent and exhibited saturation kinetics. In the
present study Figs. 1A and 2 data show that loss of cell viability and
the increased levels of PCC were also proportional to EcV
concentrations and reached maximal values at 0.50–1.00 µg EcV/ml
MEM. These findings further indicate that venom proteins execute
their effect at the cellular level possibly via cellular or
mitochondrial receptors, the mechanism of which needs
elucidation.
Concurrent with the above antioxidant enzyme
activity reductions, EcV-treated cultures exhibited very
significant increases in the generation rates of
H2O2, LPO and SOA (Table II) which were similar in magnitude
and ranged from 37–52% of control levels. In addition, although GSH
levels were significantly decreased, GSSG levels underwent
significant increases leading to a drastically lowered GSH/GSSG
ratio equivalent to about 51% of the value recorded for control
cultures (Table III). GSH is an
important antioxidant for the maintenance of hemeostasis and redox
balance as well as the prevention of lipid peroxidation (46). The significant decrease presently
noted in GR activity of EcV-treated cells (Table I), could have resulted in the lowered
GSH levels. Alternatively, the GSH decline could have been a result
of GSH reacting directly with excessively generated
H2O2 leading to increased GSSG formation.
These findings indicated that the venom-treated cells were
subjected to OS which is in broad agreement with results reported
by other workers (21–25). However, such studies only
investigated a few parameters of the antioxidant/oxidant status of
envenomed animals and were not related to EcV. In comparison, the
present study examined the effect of EcV on a comprehensive list of
antioxidants and their corresponding oxidants using human tissue.
Furthermore, results showed that the antioxidant capacity decreases
and those of the corresponding oxidant generation increases were of
similar magnitude. Other results unique to the present study
demonstrated that the expression levels of all investigated
antioxidant genes in EcV-treated cultures underwent significant
downregulations of similar magnitude ranging from 46–60% of the
levels recorded in control cultures (Fig. 4). Such downregulation was also in a
similar range of the corresponding reductions seen in antioxidant
enzyme activities. These results further suggest that EcV executes
its effect at the cellular and compartmental levels. The lowered
gene expression levels in the EcV-treated cells must have caused
the subsequent reduction in antioxidant enzyme activities, and were
probably a result of DNA damage incurred by the increased ROS
generation leading to downregulation of transcription and
translation processes. To this end, SOA have been reported to
activate key cellular hallmark events including DNA damage and
mitochondrial alterations (14) thus
trigering apoptosis. Moreover, SOD loss has been shown to induce
phosphorylation of a DNA damage marker (γ-H2AX), and upregulation
of p21, a target gene of p53 in fibroblasts (47). The demonstrated increased
H2O2 generation could have also interacted
with SOA thus producing the more reactive hydroxyl radicals
(48) known to react with purines
and pyrimidines causing DNA damage and lowered antioxidant gene
expression.
The antioxidant property of vitamin C stems from its
reducing and electron donating ability. It donates two electrons
from a double bond between the second and third carbon atoms of its
molecule. The donated electrons are received by compounds with
unpaired electrons like ROS, which thus get non-enzymatically
neutralized (49). When vitamin C
loses one electron it becomes oxidized to Asc which is relatively
stable and fairly unreactive making it a potent free radical
scavenger (50). Asc is not
synthesized by human cells including fibroblasts (50), and is taken up by
NA+-dependent protein transporters hSVCT1 and hSVCT2
which are products of separate genes (51). Although Asc plasma concentration is
60–100 µM, its intracellular levels are several orders of magnitude
higher indicating that it is normally concentrated and accumulated
in cellular compartments by the transporter proteins (52). Results of the present study (Tables I–III) demonstrated very significant
restoration of the activities and levels of all the investigated
antioxidants and oxidants to values very similar to those recorded
in control cultures when the EcV-treated cultures were incubated
with 400 µM Asc for 12 h, suggesting that Asc ameliorates the
venom-induced OS. Similar findings are scarce and have been
reported in only one study where administration of Asc (50 mg/kg
body weight) to Bitis arietans envenomed rats increased GPx,
SOD and CAT activities, and reduced liver peroxidation levels
(32,33). Unique to the present study is that
the noted downregulation of the investigated antioxidant gene
expression levels in the envenomed cultures were restored to
fold-change levels similar to those recorded for venom-free
controls when the former were incubated with 400 µM Asc for 12 h
(Fig. 4). The percentage
upregulation of the antioxidant gene expression levels incurred by
Asc approximately equaled 93, 73, 110, 94 and 93% of control levels
for GPx, GR, GST, CAT and SOD, respectively, and correlated well
with the recorded corresponding increases in the enzyme activities
which equaled 91, 75, 86, 87 and 90% respectively.
In light of the present study findings, it is
concluded that incubation of EcV-treated cultures with high Asc
concentrations (400 or 500 µM) acted to scavenge ROS thus
preventing OS and helped to aleviate DNA damage and the
downregulation of antioxidant gene expression levels. Asc could
have also acted to aleviate ROS-related DNA damage possibly causing
downregulation of the expression levels of genes responsible for
the synthesis of the hsSVCT1 and hsSVCT2 transporter proteins.
Acknowledgements
The present study was financially supported by King
Saud University, Vice Deanship of Research Chairs.
References
|
1
|
Mallow D, Ludwig D and Nilson G: True
Vipers: Natural History and Toxinology of Old World Vipers. Kreiger
Publication Company; Malabar, FL: 2003
|
|
2
|
Serrano SM, Shannon JD, Wang D, Camargo AC
and Fox JW: A multifaceted analysis of viperid snake venoms by
two-dimensional gel electrophoresis: an approach to understanding
venom proteomics. Proteomics. 5:501–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mackessy SP: Handbook of Venoms and Toxins
of Reptiles. CRC press; Boca Raton, FL: 2009, View Article : Google Scholar
|
|
4
|
Kini RM: Excitement ahead: structure,
function and mechanism of snake venom phospholipase A2 enzymes.
Toxicon. 42:827–840. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Jammaz I: Physiological effects of LD50
of Echis coloratus crude venom on rat at different time intervals.
J King Saud Univ Sci. 15:121–129. 2003.
|
|
6
|
Al-Asmari AK, Manthiri RM Abbas, Osman
NMA, Al-Otaibi AF and Al-Asmari SA: Beneficial role of quercetin on
Echis coloratus snake venom induced hepato-renal toxicity in rats.
J Biol Sci. 16:112–119. 2016. View Article : Google Scholar
|
|
7
|
Annobil SH: Complications of Echis
colorata snake bites in the Asir region of Saudi Arabia. Ann Trop
Paediatr. 13:39–44. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boviatsis EJ, Kouyialis AT, Papatheodorou
G, Gavra M, Korfias S and Sakas DE: Multiple hemorrhagic brain
infarcts after viper envenomation. Am J Trop Med Hyg. 68:253–257.
2003.PubMed/NCBI
|
|
9
|
Fernandez S, Hodgson W, Chaisakul J,
Kornhauser R, Konstantakopoulos N, Smith AI and Kuruppu S: In vitro
toxic effects of puff adder (Bitis arietans) venom, and their
neutralization by antivenom. Toxins (Basel). 6:1586–1597. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sena LA and Chandel NS: Physiological
roles of mitochondrial reactive oxygen species. Mol Cell.
48:158–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pickering AM, Vojtovich L, Tower J and A
Davies KJ: Oxidative stress adaptation with acute, chronic, and
repeated stress. Free Radic Biol Med. 55:109–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai Z and Yan LJ: Protein oxidative
modifications: beneficial roles in disease and health. J Biochem
Pharmacol Res. 1:15–26. 2013.PubMed/NCBI
|
|
14
|
Aboul-Soud MA, Al-Othman AM, El-Desoky GE,
Al-Othman ZA, Yusuf K, Ahmad J and Al-Khedhairy AA:
Hepatoprotective effects of vitamin E/selenium against
malathion-induced injuries on the antioxidant status and
apoptosis-related gene expression in rats. J Toxicol Sci.
36:285–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants, and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al Asmari AK, Khan HA, Manthiri RA, Al
Yahya KM and Al Otaibi KE: Effects of Echis pyramidum snake venom
on hepatic and renal antioxidant enzymes and lipid peroxidation in
rats. J Biochem Mol Toxicol. 28:407–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamasaki SC, Villarroel JS, Barone JM,
Zambotti-Villela L and Silveira PF: Aminopeptidase activities,
oxidative stress and renal function in Crotalus durissus terrificus
envenomation in mice. Toxicon. 52:445–454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valenta J, Stach Z and Svítek M: Acute
pancreatitis after viperid snake cerastes cerastes envenoming: a
case report. Prague Med Rep. 111:69–75. 2010.PubMed/NCBI
|
|
20
|
Sagheb MM, Sharifian M, Moini M and Salehi
O: Acute renal failure and acute necrotizing pancreatitis after
Echis carinatus sochureki bite, report of a rare complication from
southern Iran. Prague Med Rep. 112:67–71. 2011.PubMed/NCBI
|
|
21
|
Dousset E, Carrega L, Steinberg JG,
Clot-Faybesse O, Jouirou B, Sauze N, Devaux C, Autier Y, Jammes Y,
Martin-Eauclaire MF and Guieu R: Evidence that free radical
generation occurs during scorpion envenomation. Comp Biochem
Physiol C Toxicol Pharmacol. 140:221–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al Asmari A, Al Moutaery K, Manthari RA
and Khan HA: Time-course of lipid peroxidation in different organs
of mice treated with Echis pyramidum snake venom. J Biochem Mol
Toxicol. 20:93–95. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asmari AK, Khan HA, Banah FA, Buraidi AA
and Manthiri RA: Serum biomarkers for acute hepatotoxicity of Echis
pyramidum snake venom in rats. Int J Clin Exp Med. 8:1376–1380.
2015.PubMed/NCBI
|
|
24
|
Onyeama HP, Ebong PE and Eteng MU:
Evaluation of the effects of Calliandra portoricensis extracts on
oxidative stress enzymes in Wistar rats challenged with venom of
Echis oscellatus. J Appl Pharm Sci. 2:199–202. 2012.
|
|
25
|
Tohamy AA, Mohamed AF, Moneim AE Abdul and
Diab MSM: Biological effects of Naja haje crude venom on hepatic
and renal tissues of mice. J King Saud Univ Sci. 26:205–212. 2014.
View Article : Google Scholar
|
|
26
|
Halliwell B: Vitamin C: antioxidant or
pro-oxidant in vivo? Free Radic Res. 25:439–454. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghneim HK and Al-Sheikh YA: The effect of
aging and increasing ascorbate concentrations on respiratory chain
activity in cultured human fibroblasts. Cell Biochem Funct.
28:283–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banerjee P, Bhattacharyya SS,
Bhattacharjee N, Pathak S, Boujedaini N, Belon P and Khuda-Bukhsh
AR: Ascorbic acid combats arsenic-induced oxidative stress in mice
liver. Ecotoxicol Environ Saf. 72:639–649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Retsky KL and Frei B: Vitamin C prevents
metal ion-dependent initiation and propagation of lipid
peroxidation in human low-density lipoprotein. Biochim Biophys
Acta. 1257:279–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen K, Suh J, Carr AC, Morrow JD, Zeind J
and Frei B: Vitamin C suppresses oxidative lipid damage in vivo,
even in the presence of iron overload. Am J Physiol Endocrinol
Metab. 279:E1406–E1412. 2000.PubMed/NCBI
|
|
31
|
ElShama SS, EL-Meghawry A, El-Kenawy AE
and Osman HE: Vitamin C daily supplements and its ameliorative
effects. Vitamin C..Guiné R: Nova Science Publishers, Inc.;
Hauppauge, NY: pp. 47–64. 2013
|
|
32
|
Klenner FR: Observations on the dose of
administration of ascorbic acid when employed beyond the range of a
vitamin in human pathology. J Appl Nutr. 23:61–68. 1971.
|
|
33
|
Khan W, Osman NA, Alahmari AM, Amaan A and
Al-Asmari A: Vitamin C protects against viper venom induced
hepatotoxicity and oxidative damage in rat liver. MOJ Toxicol.
2:000262016. View Article : Google Scholar
|
|
34
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghneim HK and Alshebly MM: Biochemical
markers of oxidative stress in Saudi women with recurrent
miscarriage. J Korean Med Sci. 31:98–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al-Sheikh YA and Ghneim HK: ‘The effect of
micronutrients on superoxide dismutase in senescent fibroblasts’.
Cell Biochem Funct. 29:384–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases. The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974.PubMed/NCBI
|
|
38
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reznick AZ and Packer L: Oxidative damage
to proteins: spectrophotometric method for carbonyl assay. Methods
Enzymol. 233:357–363. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghneim HK, Al-Sheikh YA, Alshebly MM and
Aboul-Soud MA: Superoxide dismutase activity and gene expression
levels in Saudi women with recurrent miscarriage. Mol Med Rep.
13:2606–2612. 2016.PubMed/NCBI
|
|
41
|
Al-Saleh SS, Ghneim HK, Haddad HY and Khan
SU: Separation and purification of Echis coloratus venom and some
biological and biochemical effects of the proteins. Cell Biochem
Funct. 20:153–162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Al-Saleh S, Ghneim H and Khan S: The
effect of crude and purified Cerastes vipera venom protein
fractions on respiratory chain function in cultured human
fibroblasts. Cell Physiol Biochem. 13:315–320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ghneim HK, Al-Sheikh YA and Aboul-Soud MA:
The effect of Walterinnesia aegyptia venom proteins on TCA cycle
activity and mitochondrial NAD(+)-redox state in cultured human
fibroblasts. Biomed Res Int. 2015:7381472015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghneim HK and Al-Sheikh YA: Effect of
selenium supplementation on glutathione peroxidase and catalase
activities in senescent cultured human fibroblasts. Ann Nutr Metab.
59:127–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghneim HK: Enzymatic variations related to
glucose and glycogen catabolism in serially subcultured human
fibroblasts. Cell Physiol Biochem. 4:44–56. 1994. View Article : Google Scholar
|
|
46
|
Couto N, Malys N, Gaskell SJ and Barber J:
Partition and turnover of glutathione reductase from Saccharomyces
cerevisiaea proteomic approach. J Proteome Res. 12:2885–2894. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lei XG, Zhu JH, McClung JP, Aregullin M
and Roneker CA: Mice deficient in Cu, Zn-superoxide dismutase are
resistant to acetaminophen toxicity. Biochem J. 399:455–461. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kehrer JP: The Haber-Weiss reaction and
mechanisms of toxicity. Toxicology. 149:43–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon
O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK and Levine M: Vitamin
C as an antioxidant: evaluation of its role in disease prevention.
J Am Coll Nutr. 22:18–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Welch RW, Bergsten P, Butler JD and Levine
M: Ascorbic acid accumulation and transport in human fibroblasts.
Biochem J. 294:505–510. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Savini I, Rossi A, Pierro C, Avigliano L
and Catani MV: SVCT1 and SVCT2: key proteins for vitamin C uptake.
Amino Acids. 34:347–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Linster CL and Van Schaftingen E: Vitamin
C. Biosynthesis, recycling and degradation in mammals. FEBS J.
274:1–22. 2007. View Article : Google Scholar : PubMed/NCBI
|