Introduction
Hepatitis B virus (HBV) is responsible for ~2
billion cases of liver infection worldwide, including ~40% of
chronic carriers at the risk of developing fulminant hepatitis,
cirrhosis and hepatocellular carcinoma (1,2). In
total, >50% of the worldwide population lives in areas where HBV
infection is highly endemic and >75% of this occurs in Asia,
Africa and the Middle East, including the Kingdom of Saudi Arabia
(1,3,4).
Unfortunately, despite their high efficacies, all currently
approved drugs against chronic hepatitis B have their own
limitations. While long-term treatment with nucleot(s)ide analogues
lead to the emergence of drug-resistance, chemotherapy with
interferon-α is associated with a high incidence of adverse effects
(3,4). Despite the global success of HBV
vaccination programs, vaccine-escape mutants of the virus present
another bottleneck in the preventive measures (1,2). In
addition, the marketed anti-HBV agents are too expensive for the
majority of developing countries. Therefore, there is an urgent
need to search for novel anti-HBV agents with greater efficacy and
safety.
Currently, there is an ongoing effort to identify
anti-HBV products from a variety of plants and natural sources.
Notably, it has been estimated that ~80% of Chinese patients with
chronic hepatitis B (CHB) rely on traditional herbal remedies.
Compared with the treatment of conventional drugs, such as
interferons or lamivudine, a meta-analysis of clinical trials
suggested that herbal preparations from Phyllanthus urinaria
and Scutellaria baicalensis alone may have equivalent or
better effects than lamivudine in the treatment of CHB (5). Additionally, many phytocompounds of
different chemical classes have been reported to have promising
anti-HBV activities (6–11).
Out of >1,000 species of medicinal plants
documented in Saudi Arabia, at least 35 plants are used in Saudi
folk medicine for the treatment of liver disorders (12,13).
However, this wealth of herbal medicine has not been subjected to
sustained scientific evaluations of their anti-HBV potentials to
date. Therefore, the primary aim of the present study was to
investigate the in vitro anti-HBV activities of 60 candidate
plants and to perform a qualitative phytochemical analysis in order
to identify the major secondary metabolites.
Materials and methods
Selection criteria
Candidate plants in the present study were selected
on the basis of following one or more criteria: i) Claimed
efficacies in treating various liver diseases in folk or
traditional medicine; ii) reported in vitro or in
vivo hepatoprotective potentials; iii) published antiviral
activities against genetically-close human viruses, such as human
immunodeficiency virus (HIV) and herpes simplex virus (HSV); and
iv) taxonomically related to plants known for their antiviral
activities.
Plant materials
A total of 60 medicinal plants were collected from
different regions of the Kingdom of Saudi Arabia (n=57) as well as
Sudan (n=3) (Table I). Plants were
identified by an experienced plant taxonomist at the College of
Pharmacy, King Saud University, (Riyadh, Saudi Arabia) and voucher
specimens (Table I) were deposited
at the college herbarium.
 | Table I.List of medicinal plants (n=60)
screened in the present study. |
Table I.
List of medicinal plants (n=60)
screened in the present study.
| No. | Plant name | Family | Part used | Voucher no. | Collection
location |
|---|
| 1 | Abutilon
figarianum |
Malvaceae | L | 16,082 | Riyadh, KSA |
| 2 | Acacia
hamulosa |
Fabaceae | L + S | 16,221 | South, KSA |
| 3 | Acacia
asak |
Fabaceae | L | 16,387 | South, KSA |
| 4 | Acacia
ehrenbergiana |
Fabaceae | S | 16,385 | South, KSA |
| 5 | Acacia
laeta |
Fabaceae | S | 16,390 | South, KSA |
| 6 | Acacia
oerfota |
Fabaceae | S | 16,389 | South, KSA |
| 7 | Acacia
salicina |
Fabaceae | L | 15,007 | South, KSA |
| 8 | Acacia
tortilis |
Fabaceae | S | 14,977 | South, KSA |
| 9 | Achyranthes
aspera |
Amaranthaceae | Aerial parts (S, L,
Fr) | 16,011 | Gabeel, KSA |
| 10 | Albizia
procera |
Fabaceae | L | 16,182 | Taif, KSA |
| 11 | Alternanthera
pungens |
Amaranthaceae | Aerial parts (S, L,
Fr, Fl) | 16,391 | South, KSA |
| 12 | Amaranthus
alba |
Amaranthacea | Aerial parts (S, L,
Fr, Fl) | 16,189 | Riyadh, KSA |
| 13 | Anagallis
arvensis, var. caerulea |
Primulaceae | Aerial parts (S, L,
Fr, Fl) | 16,296 | South, KSA |
|
|
| 14 | Argemone
ochroleuca |
Papaveraceae | Aerial parts (S, L,
Fr) | 16,185 | Taif, KSA |
| 15 | Atriplex
suberecta |
Chenopodiaceae | Aerial parts (S, L,
Fr) | 16,195 | Taif, KSA |
| 16 | Aerva
Javanica |
Amaranthaceae | Aerial parts (S, L,
Fl) | 16,196 | Taif, KSA |
| 17 | Bacopa
monieri |
Scrophulariaceae | L + S | 16,300 | South, KSA |
| 18 | Balanites
aegyptiaca |
Zygophyllaceae | B |
560 | Khartoum,
Sudan |
| 19 | Boerhavia
diffusa |
Nyctaginaceae | L | 16,184 | Taif, KSA |
| 20 | Bougainvillea
spectabilis |
Nyctaginaceae | L | 16,177 | Taif, KSA |
| 21 | Capparis
decidua |
Capparaceae | S | 15,841 | Tabouk, KSA |
| 22 | Cassytha
filiformis |
Lauraceae | S | 15,716 | Taif, KSA |
| 23 | Chenopodium
ambrosioides |
Chenopodiaceae | Aerial parts (S, L,
Fr) | 16,181 | Taif, KSA |
| 24 | Chenopodiumg
laucum |
Chenopodiaceae | L + S | 16,197 | Taif, KSA |
| 25 | Citrus
maxima |
Rutaceae | L | 16,173 | Riyadh, KSA |
| 26 | Cleome
droserifolia |
Crassulaceae | Aerial parts (S, L,
Fl) | 15,830 | Taif, KSA |
| 27 | Clerodendrum
inerme |
Verbenaceae | L + S | 12,788 | Riyadh, KSA |
| 28 | Coccinia
grandis |
Cucurbitaceae | L + S | 16,275 | South, KSA |
| 29 | Combretum
molle |
Combretaceae | B | 15,496 | South, KSA |
| 30 | Corallocarpus
epigeus |
Cucurbitaceae | L | 16,393 | South, KSA |
| 31 | Daturai
noxia |
Solanaceae | L | 15,604 | Riyadh, KSA |
| 32 | Delonix
elata |
Fabaceae | L | 16,035 | South, KSA |
| 33 | Delonix
regia |
Fabaceae | L | 16,183 | Taif, KSA |
| 34 | Dodonea
angustifolia |
Sapindaceae | L | 15,787 | South, KSA |
| 35 | Eruca
sativa |
Brassicaceae | L + S | 16,318 | South, KSA |
| 36 | Euphorbia
tirucalli |
Euphorbiaceae | S | 16,172 | Riyadh, KSA |
| 37 | Euphorbia
hirta |
Euphorbiaceae | Aerial parts (S, L,
Fr) | 16,084 | Omdurman,
Sudan |
| 38 | Ficus
benghalensis |
Moraceae | L + B | 16,080 | Riyadh, KSA |
| 39 | Ficus
palmata |
Moraceae | L | 15,448 | Tanoma, KSA |
| 40 | Flaveria
trineriva |
Asreraceae | Arial parts (S, L,
Fr) | 16,198 | Taif, KSA |
| 41 | Fumaria
parviflora |
Fumariaceae | L + S | 16,301 | South, KSA |
| 42 | Guiera
senegalensis |
Combretaceae | L |
798 | Kordofan,
Sudan |
| 43 | Haplophyllum
tuberculum |
Rutaceae | Aerial parts (S, L,
Fl) | 16,324 | South, KSA |
| 44 | Indigofera
caerulea |
Fabaceae | Aerial parts (S, L,
Fl) | 16,392 | South, KSA |
| 45 | Ipomoea cairica
(L.) sweet |
Convolvulaceae | Aerial parts (S, L,
Fr) | 16,075 | Riyadh, KSA |
| 46 | Indigofera
tinctoria |
Fabaceae | Aerial parts (S, L,
Fr) | 16,390 | South, KSA |
| 47 | Jatropha
curcas |
Euphorbiaceae | Seeds | 15,189 | Riyadh, KSA |
| 48 | Juniperus
phonicea |
Cupressaceae | S + L | 16,179 | Taif, KSA, |
| 49 | Juniperus
procera |
Cupressaceae | S + L | 16,194 | Taif KSA, |
| 50 | Marrubium
vulgare |
Labiatae | Aerial parts (S, L,
Fr) | 16,043 | Hadah, KSA |
| 51 | Momordica
balsamina |
Cucurbitaceae | L | 16,395 | South, KSA |
| 52 | Pergularia
tomentosa |
Asclepiadaceae | Aerial parts (S, L,
Fr) | 16,075 | Riyadh, KSA |
| 53 | Psidiumg
uajava |
Myrtaceae | L | 16,085 | South, KSA |
| 54 | Pulicaria
crispa |
Asteraceae | Aerial parts (S, L,
Fr) | 16,083 | Riyadh, KSA |
| 55 | Ricinus
communis |
Euphorbiaceae | L | 14,005 | Riyadh, KSA |
| 56 | Rumex
dentatus |
Polygonaceae | Aerial parts (S, L,
Fr) | 16,186 | Taif, KSA |
| 57 | Senna
obtusifolia |
Fabaceae | Fr | 160,322 | South, KSA |
| 58 | Senna
occidentalis |
Fabaceae | Fr | 155,009 | South, KSA |
| 59 | Senna
alexandrina |
Fabaceae | L | 16,245 | South, KSA |
| 60 | Solanum
surrattense |
Solanaceae | L | 16,386 | South, KSA |
Preparation of the plants
extracts
Dried plant materials were ground to a coarse powder
using a mortar and pestle, extracted with 80% ethanol for 3 days
followed by filtering with Whatman Grade 1 paper and were
concentrated using a rotary evaporator (Buchi Labortechnik AG,
Flawil, Switzerland) under reduced pressure at 4°C. Plants extracts
showing anti-HBV activity were further extracted sequentially with
different organic solvents of increasing polarity: Hexane, ethyl
acetate, dichloromethane, methanol (all from Merck, Darmstadt,
Germany), including the aqueous phase. Briefly, 100 g of each plant
powder was soaked in a suitable volume of hexane with intermittent
shaking for 24 h, and filtered using Whatman Grade 1 paper. Each of
the residues were further extracted twice with fresh solvent, and
the filtrates were pooled together. The residue was air-dried
followed by sequential extractions with dichloromethane, ethyl
acetate, methanol and double-distilled water similar to the
procedure performed for hexane. Finally, solvents were removed
under reduced pressure at 4°C using a rotary evaporator (Buchi
Labortechnik AG). Following complete drying, the yield percentage
of each extract was calculated (Table
II). For biological screening, each extract was dissolved in
dimethyl sulfoxide (DMSO; Sigma-Aldrich, Merck KGaA), and the
stocks (100 mg/ml) were stored at −20°C until subsequent use.
 | Table II.Determination of cytotoxicity
concentration (CC50) and anti-hepatitis B virus activity
(inhibition of HBsAg secretion via IC50) and the
corresponding TI of the plant extracts. |
Table II.
Determination of cytotoxicity
concentration (CC50) and anti-hepatitis B virus activity
(inhibition of HBsAg secretion via IC50) and the
corresponding TI of the plant extracts.
| Plant name | Extraction
solvent | Yield (%) | CC50
(µg/ml) | IC50
(µg/ml) | TI |
|---|
| Abutilon
figarianum | EtOH | 8.71 | 366.67 | 106.46 | 3.44 |
|
| Hex | 1.10 | 1700.00 | NA | ND |
|
| DCM | 0.64 | 1375.02 | 99.76 | 13.78 |
|
| EtAc | 0.48 | 332.30 | NA | ND |
|
| MeOH | 8.02 | 284.70 | NA | ND |
|
| Aqua | 2.21 | NC | NA | ND |
| Acacia
oerfota | EtOH | 11.13 | 1375.00 | 101.46 | 13.55 |
|
| Hex | 2.64 | 1150.10 | NA | ND |
|
| DCM | 0.19 | 960.00 | NA | ND |
|
| EtAc | 0.79 | NC | NA | ND |
|
| MeOH | 5.54 | 383.30 | 118.90 | 3.22 |
|
| Aqua | 9.60 | 422.20 | 106.84 | 3.95 |
| Capparis
decidua | EtOH | 10.31 | 366.67 | 76.85 | 4.77 |
|
| Hex | 0.27 | 383.30 | NA | ND |
|
| DCM | 0.51 | 400.00 | NA | ND |
|
| EtAc | 0.18 | 833.10 | NA | ND |
|
| MeOH | 3.86 | 1667.67 | NA | ND |
|
| Aqua | 6.86 | 520.00 | 66.82 | 7.78 |
| Coccinia
grandis | EtOH | 8.71 | 219.44 | 31.57 | 6.95 |
|
| Hex | 1.33 | 800.00 | NA | ND |
|
| DCM | 0.86 | 480.01 | 57.14 | 8.40 |
|
| EtAc | 0.32 | NC | NA | ND |
|
| MeOH | 7.28 | 800 | NA | ND |
|
| Aqua | 5.61 | 557.14 | 87.21 | 6.38 |
| Corallocarpus
epigeus | EtOH | 7.35 | 1275.00 | 71.90 | 17.73 |
|
| Hex | 0.86 | 150.00 | NA | ND |
|
| DCM | 0.50 | 112.90 | NA | ND |
|
| EtAc | 0.31 | 153.37 | NA | ND |
|
| MeOH | 0.72 | 2500.00 | NA | ND |
|
| Aqua | 2.61 | 1094.00 | 70.91 | 15.42 |
| Fumaria
parviflora | EtOH | 9.35 | NC | 79.84 | ND |
|
| Hex | 0.88 | 425.00 | 35.44 | 11.99 |
|
| DCM | 0.62 | 188.10 | NA | ND |
|
| EtAc | 1.02 | 221.98 | NA | ND |
|
| MeOH | 8.10 | 1950.00 | 79.19 | 24.62 |
|
| Aqua | 2.61 | 766.67 | NA | ND |
| Guiera
senegalensis | EtOH | 9.76 | 1566.00 | 73.21 | 21.39 |
|
| Hex | 0.52 | 3330.00 | NA | ND |
|
| DCM | 0.74 | 200.00 | 10.65 | 18.77 |
|
| EtAc | 0.62 | 450.10 | NA | ND |
|
| MeOH | 7.32 | 1000.06 | NA | ND |
|
| Aqua | 2.08 | 370.69 | 76.67 | 4.83 |
| Indigofera
caerulea | EtOH | 8.61 | 1455.00 | 84.62 | 17.19 |
|
| Hex | 0.62 | 230.43 | NA | ND |
|
| DCM | 0.66 | 208.00 | NA | ND |
|
| EtAc | 0.42 | 642.80 | NA | ND |
|
| MeOH | 2.47 | 1566 | 73.21 | 21.39 |
|
| Aqua | 4.06 | 1250.00 | 125.10 | 9.99 |
| Pulicaria
crispa | EtOH | 12.17 | 686.71 | 23.10 | 29.72 |
|
| Hex | 1.24 | 160.10 | NA | ND |
| Pulicaria
crispa | DCM | 1.64 | 79.31 | NA | ND |
|
| EtAc | 0.48 | 603.00 | 14.45 | 41.04 |
|
| MeOH | 7.80 | 258.33 | 141.72 | 1.82 |
|
| Aqua | 4.41 | 733.30 | NA | ND |
Cell culture and drug
The HBV reporter cell line (HepG2.2.15) was a
generous gift from Dr Shahid Jameel (International Center for
Genetic Engineering & Biotechnology, New Delhi, India).
HepG2.2.15 cells were grown in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 1X penicillin-streptomycin, and 1X sodium pyruvate
streptomycin (HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
at 37°C in a humidified chamber (5% CO2). Lamivudine
(Sigma-Aldrich; Merck KGaA), the approved anti-HBV nucleoside
analog-based drug was used as a standard.
Cytotoxicity assessment of the plants
extracts
Cytotoxicity of extracts was tested on HepG2.2.15
cells using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) cell proliferation assay kit (Tervigen, Gaithersburg,
MD, USA) to determine the extract concentrations (doses) that did
not affect cell viability, and were used in subsequent assays. MTT
assay is based on the metabolic reduction of soluble MTT by
mitochondrial enzyme activity of viable cells, into an insoluble
colored formazan product which can be measured optically (14). Cells were seeded (0.5×105
cells/100 µl/well) in flat-bottom 96-well tissue culture plates
(Corning Inc., Corning, NY, USA). Following 24 h of incubation, the
cells were treated (in triplicate) with various concentrations of
test samples (0, 6.25, 12.5, 25, 50 and 100 µg/ml) prepared in
culture media, and incubated at 37°C for 48 h. The final
concentration of DMSO never exceeded 0.1% in any of the assays and
therefore, had no signs of toxicity. Blank (only media) and
untreated/negative (0.1% DMSO in media) controls were also
included. Cells were treated with MTT reagent (10 µl/well) and
further incubated at 37°C for 3–4 h. Upon appearance of a purple
color, the detergent solution (100 µl) from the kit was added to
each well and further incubated at 37°C for 1 h. Optical density
(OD) was recorded at 570 nm using a microplate reader (ELx800;
BioTek Instruments, Inc., Winooski, VT, USA). Non-linear regression
analysis was performed using Excel software (Microsoft Corp.,
Redmond, WA, USA) to determine the concentration that resulted in a
50% cytotoxicity concentration (CC50) using the
following equation:
Survivalfraction=OD[s]–OD[b]OD[c]–OD[b]
Where OD(s), OD(b) and OD(c) are the absorbances of
the sample, blank and negative control, respectively.
Microscopy
At 24 and 48 h post-treatment, cells were visually
monitored for morphological changes, such as lesions of the cell
membrane and the compactness of cytoplasmic components under an
inverted microscope (Bio Optical, Milan, Italy) at a magnification
of ×200.
Dose-dependent analysis of HBsAg
expression in treated cells
HepG2.2.15 cells were seeded in 96-well plates
(0.5×105/well) and incubated at 37°C. The following day,
the culture media was replaced with fresh media (150 µl, each in
triplicate) containing various doses (0, 6.25, 12.5, 25 and 50
µg/ml) of the test samples and controls, and incubated at 37°C for
48 h. The culture supernatants of each sample were collected and
stored at −20°C. The secreted HBsAg in the culture supernatants was
analyzed using an ELISA kit (cat. no. 72348; Monolisa HBsAg ULTRA;
Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the
manufacturer's instructions. OD was recorded using an ELx800
microplate reader and analyzed according to the manufacturer's
instructions. Non-linear regression analysis was performed using
Excel software to determine the half maximal (50%) inhibitory
concentration (IC50) of HBsAg secretion.
Time-course analysis of HBsAg
inhibition
Based on the dose-dependent inhibition results,
time-course (day 1, 3 and 5) analyses were performed to further
investigate the antiviral potential of the most active extracts.
The HBsAg expression study was performed by treating cells with the
safest single-dose (50 µg/ml), as determined by the IC50
values.
Time-course analysis of HBeAg
inhibition
Extracts exhibiting the most promising inhibitory
effects on HBsAg secretions were further subjected to time-course
(day 1, 3 and 5) analyses of HBeAg expression at a dose of 50
µg/ml. ELISA was performed on the culture media using an
HBeAg/anti-HBe ELISA kit (cat. no. KAPG4BNE3; DIAsource
ImmunoAssays; SA, Louvain-la-Neuve, Belgium) according to the
manufacturer's instructions. OD was recorded using an ELx800
microplate reader, and analyzed following the DIASource manual.
Phytochemical constituent
screening
Plants exhibiting the most promising anti-HBV
activities were subjected to qualitative phytochemical screening
for major secondary metabolites, including alkaloids, flavonoids,
anthraquinones, tannins and saponins, following standard procedures
(15–17) with minor modifications. Briefly, for
alkaloids, the Mayer's test was performed. A total of 0.5 gm of the
extract was dissolved in 2% hydrochloric acid (Sigma-Aldrich; Merck
KGaA), and filtered. Mayer's reagent was freshly prepared by
dissolving 0.68 g of mercuric chloride (Sigma-Aldrich; Merck KGaA)
and 2.5 g of potassium iodide (Sigma-Aldrich; Merck KGaA) and made
to 50 ml with distilled water. A few drops of the reagent were
added to the 3 ml of extract solution in a test tube where
formation of a yellowish precipitate indicated the presence of
alkaloids. For flavonoids, the sodium hydroxide test was performed.
A total of 5 ml of the extract solution was treated with few drops
of 20% sodium hydroxide (Sigma-Aldrich; Merck KGaA) in a test tube.
Formation of an intense yellow color, which becomes colorless on
addition of diluted hydrochloric acid, indicated the presence of
flavonoids. For tannins, the ferric chloride test was performed. A
total of 0.25 gm of the extract was dissolved in 10 ml of distilled
water in a test tube and few drops of 5% ferric chloride
(Sigma-Aldrich; Merck KGaA) was added. Appearance of brownish green
or blueish-black color indicated the presence of tannins. For
saponins, the frothing test was performed. A total of 0.5 gm of the
extract was dissolved in 10 ml of distilled water in a test tube
and shaken vigorously. Formation of a thick persistent froth that
persisted for at least 15 min indicated the presence of saponins.
And lastly, for anthraquinone the Borntrager's test was performed.
A total 0.5 gm of the extract residue was boiled with 5 ml of
dilute hydrochloric acid and filtered while hot. The filtrate was
combined with 5 ml of chloroform and shaken. The chloroform layer
was transferred into a test tube and 2 ml of dilute ammonia
solution (Sigma-Aldrich; Merck KGaA) was added. The appearance of a
rose-pink to cherry-red color indicated the presence of
anthraquinones.
Results
Effects of plants extracts on cell
viability
CC50 values of different plants extracts
were calculated (Table II), which
allowed for the determination of the single optimal dose (50 µg/ml)
with no sign of cytotoxicity. This observation was confirmed by
microscopic observation of the cell morphology/growth at 24 and 48
h post-treatment with different concentrations of each extract
(data not shown). Therefore, extracts at 50 µg/ml were used in the
subsequent antiviral assays.
Dose-dependent inhibition of HBsAg by
different extracts
Firstly, the total ethanolic-extracts of 60
medicinal plants were screened for anti-HBV activity by measuring
the expression levels of viral HBsAg at 48 h. Of these, 9 plants
showed inhibition of HBsAg production in a dose-dependent manner.
These were Abutilon figarianum, Acacia oerfota,
Capparis decidua, Coccinea grandis, Corallocarpus
epigeus, Fumaria parviflora, Guiera senegalensis,
Indigofera caerulea and Pulicaria crispa with
IC50 values of 106.46, 101.46, 76.85, 31.57, 71.90,
79.84, 73.21, 84.62 and 23.10 µg/ml, respectively (Table II). Based on these results, 5
sequential extracts (hexane, dichloromethane, ethyl acetate,
methanol and aqueous) of each of the 9 selected plants were
prepared, and tested for cytotoxicity. The sequential extracts were
further evaluated for dose-dependent HBsAg inhibition. The
extraction yield percentage, IC50, CC50 and
their corresponding TI values were calculated (Table II). Of these, 24 different extracts
(from the 9 selected plants) that showed marked HBsAg inhibition
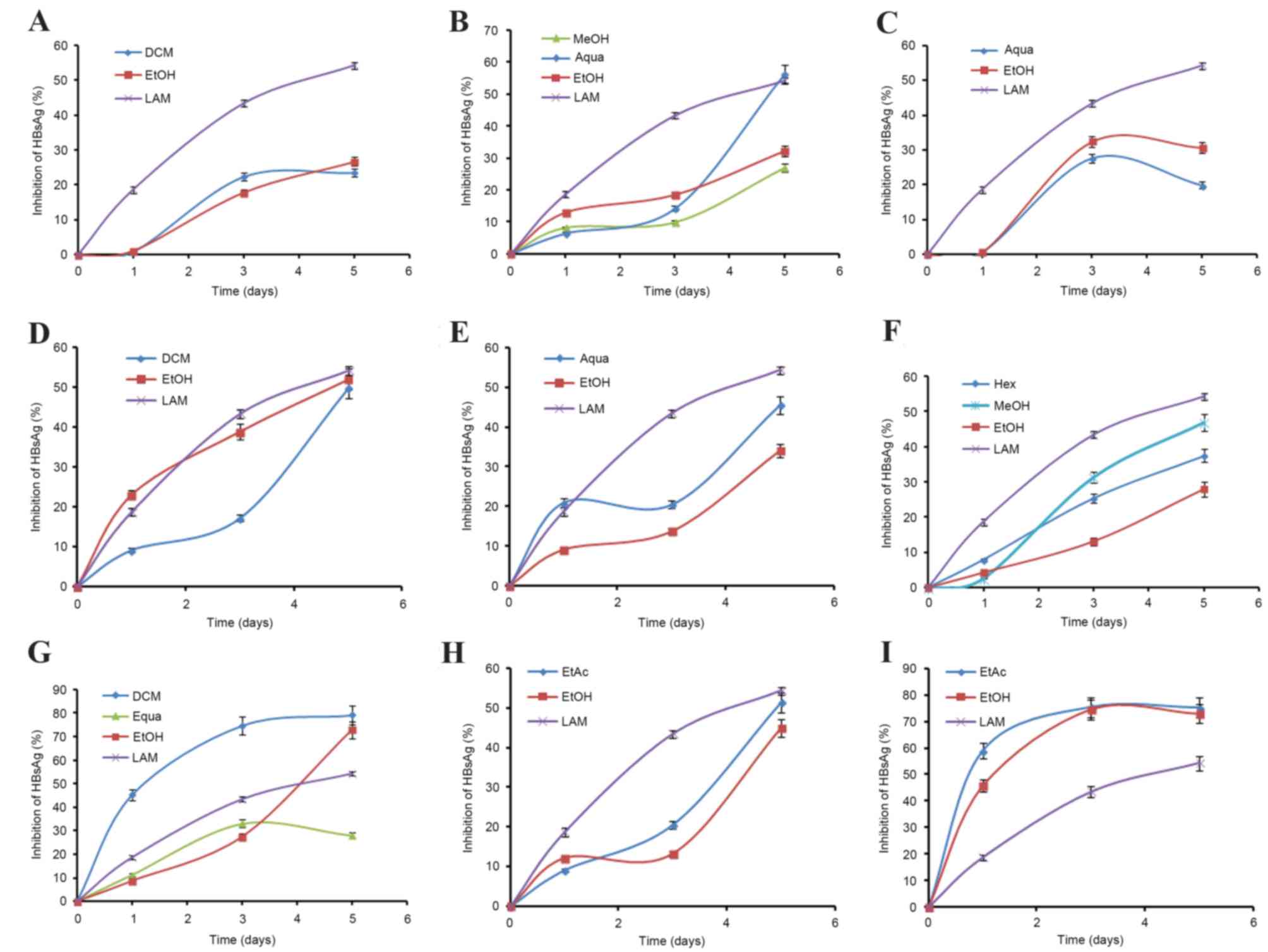
were evaluated in a time-course study.
Time-course inhibition of HBsAg by
selected extracts
The selected extracts (of 9 plants) that showed
marked HBsAg inhibition were evaluated in a time-course study,
using 50 µg/ml doses for 5 days (Fig.
1). While prolonged treatment beyond day 5 did not show any
notable inhibitory effect, further continuation of the culture
resulted in cell overgrowth and death (data not shown). The optimal
antiviral activities, in order, on day 5 post-treatment were: G.
senegalensis (dichloromethane extract; IC50=10.65),
P. crispa (ethyl acetate extract; IC50=14.45),
C. gardis (total ethanol extract; IC50=31.57),
F. parviflora (hexane extract; IC50=35.44), C.
decidua (aqueous extract; IC50=66.82), C.
epigeus (total ethanol extract; IC50=71.9), I.
caerulea (methanol extract; IC50=73.21), A.
figarianum (dichloromethane extract; IC50=99.76) and
A. oerfota (total ethanol extract; IC50=101.46)
(Table II).
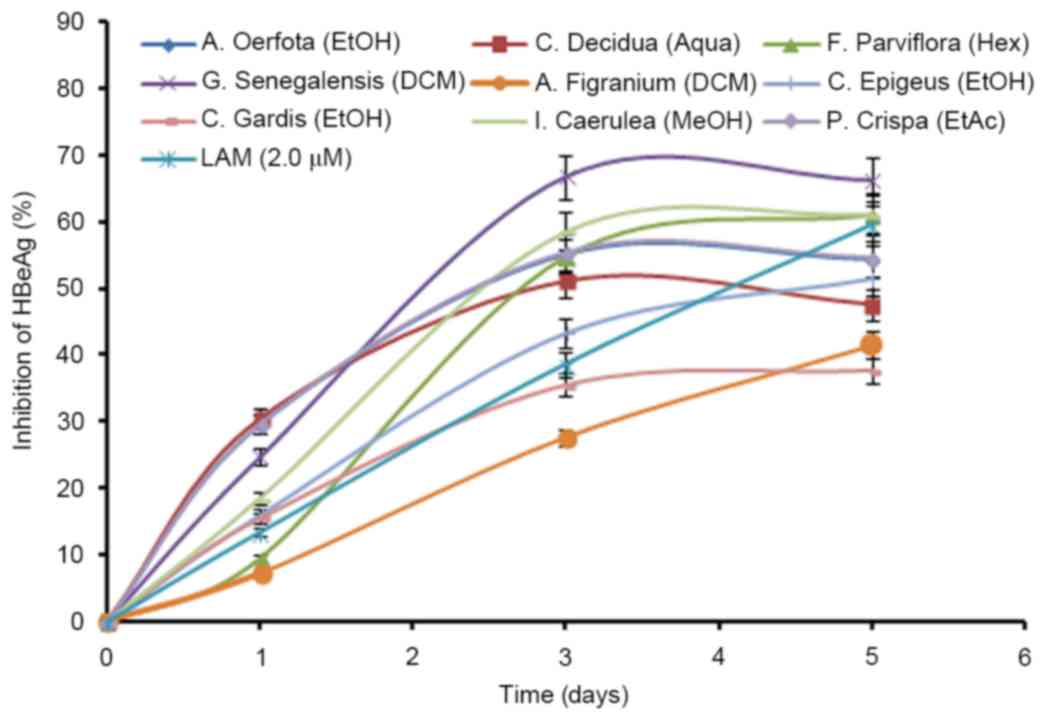
Time-course downregulation of HBV
replication by the active extracts
The HBeAg is a processed product of the ‘pre-Core’
gene that is co-translated with the ‘Core’ gene by bicistronic
subgenomic-RNA. Therefore, in a natural infection, seropositivity
of HBeAg is a hallmark of active viral DNA replication. Notably,
this is analogous to the HIV ‘p24’ antigen where ELISA is a valid
tool to monitor retroviral RNA replication. Therefore, the most
promising active extracts that greatly suppressed HBsAg synthesis
(Fig. 1), were analyzed for
time-course effect on HBeAg production in the culture supernatants.
While HBeAg secretion was inhibited maximally at day 3, there were
no further improvements in antiviral activities at day 5 (Fig. 2). Limited by cell overgrowth and
death, and unaffected virus replication (inhibition of HBeAg), the
study was terminated at day 5. Furthermore, the antiviral
activities on downregulating virus replication, as measured by
HBeAg production, were: G. senegalensis (dichloromethane
extract; 66%); I. caerulea (methanol extract; 58.60%); P.
crispa (ethyl acetate extract; 55.30%); F. parviflora
(hexane-extract; 54.68%); C. decidua (aqueous extract;
51.52%); C. epigeus (total ethanol extract; 43.31%); C.
grandis (total ethanol extract; 35.51%); A. oerfota
(total ethanol extract; 35.28%); and A. figarianum (dichloro
methane extract; 27.56%) compared to the untreated control
(Fig. 2). It is noteworthy that,
with the exception of C. grandis and A. figarianum,
all plant extracts (50 µg/ml) showed greater antiviral activity
than lamivudine (2 µM).
Phytochemical screening
Plants that exhibited anti-HBV activity showed the
presence of alkaloids, flavonoids, tannins, saponins and
anthraquinones (Table III), which
have been previously reported for their antiviral activities
(7,8).
 | Table III.Qualitative phytochemical screening
of plants with anti-hepatitis B virus activities. |
Table III.
Qualitative phytochemical screening
of plants with anti-hepatitis B virus activities.
|
| Phytochemicals |
|---|
|
|
|
|---|
| Active plant
extracts | Alkaloids | Flavonoids | Tannins | Saponins | Anthraquinones |
|---|
| Abutilon
figarianum | + | − | − | − | − |
| Acacia
oerfota | + | + | + | − | − |
| Capparis
deciduas | + | + | + | + | − |
| Coccinea
grandis | + | + | + | + | − |
| Corallocarpus
epigeus | + | + | + | − | − |
| Fumaria
parviflora | + | + | + | + | − |
| Guiera
senegalensis | + | + | + | + | − |
| Indigofera
caerulea | + | + | + | + | − |
| Pulicaria
crispa | + | + | + | − | − |
Discussion
Development of anti-HBV therapies has been impeded
until recently by the lack of suitable in vitro and in
vivo experimental models that were able mimic natural chronic
hepatitis B (18–21). Several lines of hepatoma cells stably
transfected with HBV genome have been developed as an in
vitro model to screen and identify potential antiviral
therapeutic agents. Of these, in the present study, the widely used
Hep G2.2.15 cells were used to evaluate the anti-HBV potential of
candidate plants by measuring the expression of HBsAg (serological
marker of viral infection) and HBeAg (serological marker of active
DNA replication), respectively (22,23). A
total of 60 medicinal plants were investigated for the first time,
based on information on either their use in traditional medicine
for curing liver diseases or experimental evidence of
hepatoprotective or anti-retroviral potentials (12,13,24–37). The
preliminary cell viability assay of the plants' total ethanolic
extracts in the present study showed no cytotoxicity at
concentrations up to 50 µg/ml. Further initial screening
(dose-dependent HBsAg inhibition) identified 9 plants with notable
anti-HBV activity that were therefore selected for sequential
extractions (organic and aqueous phase) and subsequent
screening.
The highest level of anti-HBV activity was observed
in the dichloromethane extract of G. senegalensis, known as
the ‘cure all’ medicine in African traditional medicine due to its
wide applications (24). It is used
to treat venereal, stomach, respiratory, dermatological and
microbial diseases (25), including
malaria (26). In agreement with its
reported anti-HSV potential (27),
G. senegalensis is likely to have exhibited anti-HBV
activity because HSV and HBV are biologically and genetically
similar. In addition to this, it has previously been reported that
administration of G. senegalensis extract to Wistar rats for
6 months did not cause any significant hematological, biochemical
or histological toxicity (28),
confirming its in vivo safety. Assuming there was no synergy
among the phytochemical constituents present in the
dichloromethane-extract, it can be implied that the active
compound(s) could be more potent than the lamivudine used as
standard reference drugs.
F. parviflora has traditionally been used in
Saudi folk medicine for the treatment of jaundice and hepatobiliary
disorders (29). At day 5
post-treatment, hexane and methanol extracts of F.
parviflora showed the best anti-HBV activities by ~37.45 and
46.86%, respectively. Besides its use in Sudanese traditional
medicine to treat fever and jaundice, an aqueous-extract of C.
decidua has been demonstrated to show hepatoprotective activity
in rats (30). Notably, in line with
its reported antiviral activity against HIV reverse-transcriptase
(31), the aqueous-extract of C.
decidua exhibited anti-HBV potential in the present study.
Acacia spp. constitute a large variety of medicinal
plants worldwide, and of these, A. catechu has been
previously reported for its anti-HIV activity (23). The present authors have previously
demonstrated that A. mellifera ethyl acetate, n-butanol and
aqueous extracts also have hepatoprotective and anti-HBV effects
(32). In the present study,
anti-HBV evaluation of A. oerfota extracts at a
non-cytotoxic dose showed its association with the methanolic and
aqueous extracts. Furthermore, the highest anti-HBV activity of
C. grandis and C. epigeus was associated with the
crude ethanolic-extract, indicating the possibility of synergy
among the antiviral phytochemical constituents of the extract.
Synergistic activity of antiviral components of plant extracts that
act by different mechanisms has been reported previously (33). Traditionally, crude extracts of C.
grandis are used to treat coughing, bronchitis, skin diseases,
tongue sores and liver disorders (34). In previous studies, in vivo
antioxidant and hepatoprotective efficacies of C. grandis
(crude ethanolic-extract) and C. epigeus (ethanolic and
aqueous extracts) have been demonstrated (35–37).
A variety of active phytochemicals (alkaloids,
flavonoids, lignans, tannins, terpenoids, saponins and
anthraquinones) of diverse geographic origins have already been
reported to be effective against HBV in vitro and/or in
vivo (7,8,38,39). Of
these, promising anti-HBV phytoproducts such as picroliv
(Picrorhiza kurroa), andrographolide (Andrographis
paniculata), artemisinin (Artemisia annua) and silymarin
have been reported for a long time (40). Notably, the most potent anti-HBV
phytochemicals include isolated niranthin and hinokinin (lignans).
From Phyllanthus spp. (41–43),
helioxanthin from the Chinese Taiwania cryptomerioides
(44), wogonin, another flavonoid
from Scutellaria radix (45),
the polyphenolic extract from Geranium carolinianum L.
(46), protostane triterpenes from
Alisma orientalis (47),
dihydrochelerythrine alkaloids from Corydalis saxicola
(48), Saikosaponin C from
Bupleurum species (49),
extracts from Rheum palmatum L. (50), and LPRP from Liriope
platyphylla (51). Furthermore,
the qualitative phytochemical analyses of the selected plant
extracts in the present study that showed promising anti-HBV
potential also revealed the presence of alkaloids, flavonoids,
triterpenoids and tannin, which may have contributed to the
antiviral activities observed. However, a detailed phytochemical
investigation of these extracts is essential to elucidate the
active principle(s) responsible for the anti-HBV potential.
In conclusion, antiviral screening in the present
study discovered that extracts of G. senegalensis, F.
parviflora and P. crispa had the most promising anti-HBV
potential, followed by those of A. figarianum, A.
oerfota, C. decidua, C. grandis, C.
epigeus and I. caerulea with notable activities. From
the results of the present study, it is possible to demonstrate the
importance of the application of ethnobotanical information in the
search and selection of traditionally used plants, which may
provide new opportunities for the treatment of chronic hepatitis B.
However, a detailed phytochemical study of these extracts is
required in order to elucidate the active principle(s) responsible
for their novel anti-HBV potential.
Acknowledgements
The project was supported by the National Plan for
Science, Technology and Innovation (MARIFAAH) funded by King
Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia
(grant no. MED11-1585-02).
References
|
1
|
Teo CG and Locarnini SA: Potential threat
of drug-resistant and vaccine-escape HBV mutants to public health.
Antivir Ther. 15:445–449. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torresi J: Hepatitis B antiviral
resistance and vaccine escape: Two sides of the same coin. Antivir
Ther. 13:337–340. 2008.PubMed/NCBI
|
|
3
|
Lok AS, Zoulim F, Locarnini S,
Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M,
Kuiken C and Hepatitis B; Virus Drug Resistance Working Group, :
Antiviral drug-resistant HBV: Standardization of nomenclature and
assays and recommendations for management. Hepatology. 46:254–265.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Locarnini S: Primary resistance, multidrug
resistance, and cross-resistance pathways in HBV as a consequence
of treatment failure. Hepatol Int. 2:147–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y and Zhu J: Anti-HBV effect of
individual traditional chinese herbal medicine in vitro and in
vivo: An analytic review. J Viral Hep. 20:445–452. 2013. View Article : Google Scholar
|
|
6
|
Qiu LP and Chen KP: Anti-HBV agents
derived from botanical origin. Fitoterapia. 84:140–157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parvez MK, Arbab AH, Al-Dosari MS and
Al-Rehaily AJ: Antiviral natural products against chronic hepatitis
B: Recent developments. Curr Pharm Des. 22:286–293. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu YH: Naturally derived anti-hepatitis B
virus agents and their mechanism of action. World J Gastroenterol.
22:188–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HC, Chou CK, Lee SD, Wang JC and Yeh
SF: Active compounds from Saussurea lappa Clarks that suppress
hepatitis B virus surface antigen gene expression in human hepatoma
cells. Antiviral Res. 27:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chou SC, Huang TJ, Lin EH, Huang CH and
Chou CH: Anti-hepatitis B virus constituents from Solanum
erianthum. Nat Prod Commun. 78:153–156. 2012.
|
|
11
|
Li J, Huang H, Zhou W, Feng M and Zhou P:
Anti-hepatitis B virus activities of Geranium carolinianum L.
extracts and identification of the active components. Biol Pharma
Bull. 31:743–747. 2008. View Article : Google Scholar
|
|
12
|
Rahman MA, Mossa JS, Al-Said MS and
Al-Yahya MA: Medicinal plant diversity in the flora of Saudi Arabia
1: A report on seven plant families. Fitoterapia. 75:149–161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Asmari AK, Al-Elaiwi AM, Athar MT,
Tariq M, Al Eid A and Al-Asmary SM: A review of hepatoprotective
plants used in saudi traditional medicine. Evid Based Complement
Alternat Med. 2014.8908422014.PubMed/NCBI
|
|
14
|
van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: The MTT assay. Methods Mol Biol.
731:237–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satyajit D, Sarkar ZL and Gray AI: Natural
Products Isolation. Humana Press; Totowa, NJ, USA: 2nd. 2006
|
|
16
|
Kar A: Pharmacognosy and
Pharmacobiotechnology. New Age International; New Delhi, India:
2nd. 2007
|
|
17
|
Tiwari P, Mandeep BK, Kaur KG and Kaur H:
Phytochemical screening and extraction: A review. Interl Pharma
Sci. 1:98–1062. 2011.
|
|
18
|
Hantz O and Zoulim F: Duck hepatitis B
virus primary hepatocyte culture model. Methods Mol Med.
96:189–197. 2004.PubMed/NCBI
|
|
19
|
Engler OB, Dai WJ, Sette A, Hunziker IP,
Reichen J, Pichler WJ and Cerny A: Peptide vaccines against
hepatitis b virus: From animal model to human studies. Mol Immunol.
38:457–465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thung SN, Gerber MA, Purcell RH, London
WT, Michalik KB and Popper H: Animal model of human disease:
Chimpanzee carriers of hepatitis B virus. Chimpanzee hepatitis B
carriers. Am J Pathol. 105:328–332. 1981.PubMed/NCBI
|
|
21
|
Aljofan M, Netter HJ, Aljarbou AN, Hadda
TB, Orhan IE, Sener B and Mungall BA: Anti-hepatitis B activity of
isoquinoline alkaloids of plant origin. Arch Virol. 159:1119–1128.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonino F, Hoyer B, Nelson J, Engle R,
Verme G and Gerin J: Hepatitis B virus DNA in the sera of HBsAg
carriers: A marker of active hepatitis B virus replication in the
liver. Hepatology. 1:386–391. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arbab AH, Parvez MK, Al-Dosari MS,
Al-Rehaily AJ, Al-Sohaibani M, Zaroug EE, AlSaid MS and Rafatullah
S: Hepatoprotective and antiviral efficacy of Acacia mellifera
leaves extracts against hepatitis B virus. Biomed Res Int.
2015:9291312015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akuodor GC, Essien AD, David-Oku E,
Chilaka KC, Akpan JL, Ezeokpo B and Ezeonwumelu JO:
Gastroprotective effect of the aqueous leaf extract of Guiera
senegalensis in albino rats. Asian Pacific J Trop Med. 6:771–775.
2013. View Article : Google Scholar
|
|
25
|
Silva O, Serrano R and Gomes ET: Botanical
characterization of Guiera senegalensis leaves. Microsc Microanal.
14:398–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abubakar MS, Sule MI, Pateh UU, Abdurahman
EM, Haruna AK and Jahun BM: In vitro snake venom detoxifying action
of the leaf extract of Guiera senegalensis. J Ethnopharmacol.
69:253–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silva O, Barbosa S, Diniz A, Valdeira ML
and Gomes E: Plant extracts antiviral activity against herpes
simplex virus type 1 and African swine fever virus. Interl J
Pharmacog. 35:12–16. 1997. View Article : Google Scholar
|
|
28
|
Diouf A, Cisse A, Gueye SS, Mendes V, Siby
T, Diop RM Diouf and Bassene E: Toxocological study of Guiera
senegalensis Lam (Combretaceae). Dakar Med. 45:89–94. 2000.(In
French). PubMed/NCBI
|
|
29
|
Najeeb-ur-Rehman Bashir S, Al-Rehaily AJ
and Gilani AH: Mechanisms underlying the antidiarrheal,
antispasmodic and bronchodilator activities of Fumaria parviflora
and involvement of tissue and species specificity. J
Ethnopharmacol. 144:128–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ali H, König GM, Khalid SA, Wright AD and
Kaminsky R: Evaluation of selected Sudanese medicinal plants for
their in vitro activity against hemoflagellates, selected bacteria,
HIV-RT and tyrosine kinase inhibitory, and for cytotoxicity. J
Ethnopharmacol. 83:219–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ali SA, Gameel AA, Mohamed AH and Hassan
T: Hepatoprotective activity of Capparis decidua aqueous and
methanolic stems extracts against carbon tetrachloride induced
liver histological damage in rats. J Pharmacol Toxicol. 6:62–68.
2011. View Article : Google Scholar
|
|
32
|
Nutan M, Modi C, Dezzutti CS, Kulshreshtha
S, Rawat AK, Srivastava SK, Malhotra S, Verma A, Ranga U and Gupta
SK: Extracts from Acacia catechu suppress HIV-1 replication by
inhibiting the activities of the viral protease and tat. Virology
J. 10:3092013. View Article : Google Scholar
|
|
33
|
Dhivya R, Enzo AP, Tiong CY, Lim SL Diana,
Chu LT, Francois M and Lara G: Evidence of synergistic activity of
medicinal plant extracts against neuraminidase inhibitor resistant
strains of influenza viruses. Adv Microbiol. 4:1260–1277. 2014.
View Article : Google Scholar
|
|
34
|
Niazi J, Singh P, Bansal Y and Goel RK:
Anti-inflammatory, analgesic and antipyretic activity of aqueous
extract of fresh leaves of Coccinia indica. Inflammopharmacology.
17:239–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gopalakrishnan V, Rao KN, Devi M, Padmaha
N, Lakshmi PM, Srividya T and Vadivukarasi G: Antihepatotoxic
acticity of Coccinia indica. Anc Sci Life. 21:12–15.
2001.PubMed/NCBI
|
|
36
|
Rangu MV, Suresh B, Sandeep VN, Narendra N
and Ramesh M: Hepatoprotective activity of leaves of Corallocarpus
epigaeus in carbon tetrachloride induced rats. Interl J Biol Pharma
Res. 3:567–570. 2012.
|
|
37
|
Madrigal-Santillán E, Madrigal-Bujaidar E,
Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J,
Bautista M, Morales-González Á, García-Luna y González-Rubio M,
Aguilar-Faisal JL and Morales-González JA: Review of natural
products with hepatoprotective effects. World J Gastroenterol.
20:14787–14804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wohlfarth C and Efferth T: Natural
products as promising drug candidates for the treatment of
hepatitis B and C. Acta Pharmacol Sin. 30:25–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, McIntosh H and Lin H: Chinese
medicinal herbs for chronic hepatitis B: A systematic review.
Liver. 21:280–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mehrotra R, Rawat S, Kulshreshtha DK,
Patnaik GK and Dhawan BN: In vitro studies on the effect of certain
natural products against hepatitis B virus. Indian J Med Res.
92:133–138. 1990.PubMed/NCBI
|
|
41
|
Huang RL, Huang YL, Ou JC, Chen CC, Hsu FL
and Chang C: Screening of 25 compounds isolated from Phyllanthus
species for anti-human hepatitis B virus in vitro. Phytother Res.
17:449–453. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ott M, Thyagarajan SP and Gupta S:
Phyllanthus amarus suppresses hepatitis B virus by interrupting
interactions between HBV enhancer I and cellular transcription
factors. European J Clin Invest. 27:908–915. 1997. View Article : Google Scholar
|
|
43
|
Mehortra R, Rawat S, Kulshreshtha DK,
Goyal P, Patnaik GK and Dhawan BN: In vitro effect of Phyllanthus
amarus on hepatitis B virus. Indian J Med Res. 93:71–73.
1991.PubMed/NCBI
|
|
44
|
Cheng YC, Ying CX, Leung CH and Li Y: New
targets and inhibitors of HBV replication to combat drug
resistance. J Clin Virol. 34 Suppl 1:S147–S150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo Q, Zhao L, You Q, Yang Y, Gu H, Song
G, Lu N and Xin J: Anti-hepatitis B virus activity of wogonin in
vitro and in vivo. Antiviral Res. 74:16–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li J, Huang H, Feng M, Zhou W, Shi X and
Zhou P: In vitro and in vivo anti-hepatitis B virus activities of a
plant extract from Geranium carolinianum L. Antiviral Res.
79:114–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang Y, Zhang XM, Zhang FX, Liu N, Zhao
F, Zhou J and Chen JJ: A new triterpene and anti-hepatitis B virus
active compounds from Alisma orientalis. Planta Med. 72:951–954.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu YR, Ma YB, Zhao YX, Yao SY, Zhou J,
Zhou Y and Chen JJ: Two new quaternary alkaloids and anti-hepatitis
b virus active constituents from Corydalis saxicola. Planta Medica.
73:787–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chiang LC, Ng LT, Liu LT, Shieh DE and Lin
CC: Cytotoxicity and anti-hepatitis B virus activities of
saikosaponins from Bupleurum species. Planta Med. 69:705–709. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Z, Li LJ, Sun Y and Li J:
Identification of natural compounds with anti-hepatitis B virus
activity from Rheum palmatum L. ethanol extract. Chemotherapy.
53:320–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang TJ, Tsai YC, Chiang SY, Wang GJ, Kuo
YC, Chang YC, Wu YY and Wu YC: Anti-viral effect of a compound
isolated from Liriope platyphylla against hepatitis B virus in
vitro. Virus Res. 192:16–24. 2014. View Article : Google Scholar : PubMed/NCBI
|