Introduction
Warfarin therapy effectively reduces ischemic stroke
and mortality amongst patients with various types of
thromboembolism, such as deep venous thrombosis, as well as heart
valve prosthesis, atrial fibrillation and stroke (1).
It is the most frequently used oral anti-coagulant.
Bleeding is an important adverse drug response (ADR) of
anti-coagulants due to its narrow therapeutic index and the wide
variability in drug responses among individuals.
To date, the warfarin dose response has been
associated with race, environmental, clinical status, and
particularly genetic factors (2).
Warfarin exerts its anti-coagulant effect by antagonizing vitamin K
epoxide reductase complex (VKORC1), thereby reducing the activation
of vitamin K-dependent clotting factors II, VII, IX and X. A series
of genetic variations related with the pharmacodynamics and
pharmacokinetics of warfarin have been reported, such as VKORC1,
cytochrome P450 (CYP) 2C9 and CYP4F2 (3,4).
Only few reports on warfarin-induced side effects
associated with CYP3A4 mutations are currently available, and the
present study reported on warfarin-induced major bleeding in a
patient with acute limb ischemia (ALI) associated with a CYP3A4
loss-of-function mutation, which may promote the development of
warfarin research.
Case report
The present study was approved by the institutional
review board (CWO) of Ren Ji Hospital, School of Medicine, Shanghai
Jiao Tong University. Patients provided written informed consent. A
60-year-old man was admitted to the vascular surgery department due
to ALI in the left lower extremity in May 2015. On reviewing his
medical history, it was revealed that the patient's blood pressure,
blood glucose, cholesterol, triglyceride, homocysteine, erythrocyte
sedimentation rate, anti-thrombin-III as well as protein C and S
were within normal ranges. His life style was healthy and he had
never smoked. He had neither atrial fibrillation nor a ventricular
aneurysm. According to the angiography results, his left profunda
femoris was completely occluded, and his distal superficial femoral
artery (SFA) to popliteal artery (PA) was filled with thrombus. A
4F/30 cm Uni-Fuse™ Thrombolytic catheter (AngioDynamics,
Queensbury, NY, USA) was inserted and the patient was
intra-arterially injected 1 million units urokinase by exact pump
over the next 24 h. One day later, his SFA-PA-peroneal artery track
was visualized and the thrombus was basically cleared. The occluded
proximal anterior tibial artery (ATA) and strait distal superficial
femoral artery were then re-canalized by implanting a XIENCE-PRIME
3.5×38 mm (Abbott Pharmaceutical Co. Ltd., Lake Bluff, IL, USA) and
an Everflex 7×120 mm (Covidien/Medtronic, Dublin, Ireland). With
the ATA and peroneal artery serving as below-the-knee (BTK) outflow
tracks, his ischemic symptoms totally disappeared and the left
ankle-brachial index was recovered from 0.43 to 1.14. The patient
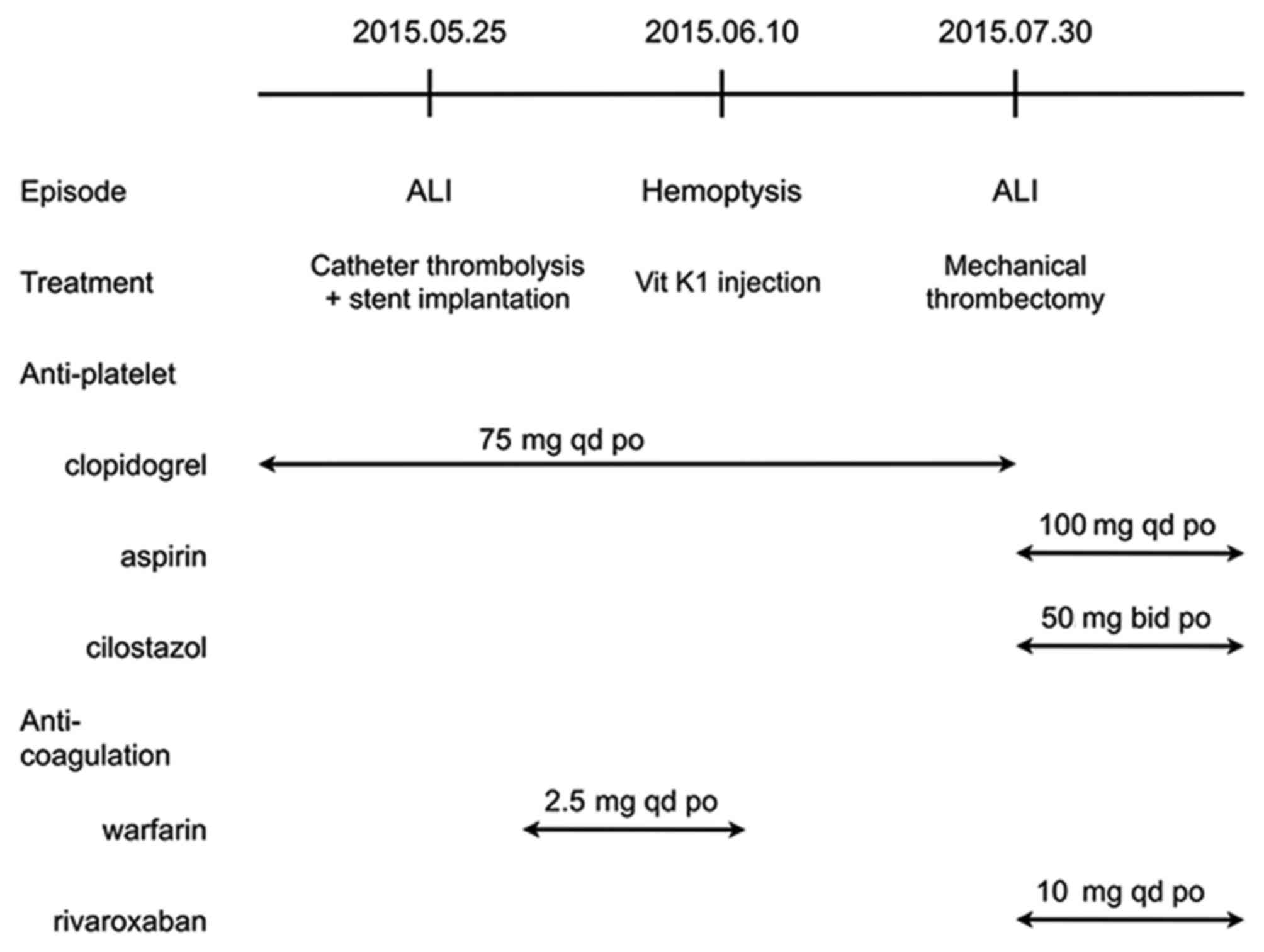
was discharged after 6 days and was prescribed warfarin (2.5 mg per
day) and clopidogrel (75 mg per day).
Unexpectedly, at 9 days after discharge, the patient
presented to the emergency room with a sudden onset of hemoptysis.
Laboratory analysis revealed that the international normalized
ratio (INR) of the prothrombin time was 5.02 R. After intravenous
infusion of 40 mg vitamin K1, his INR was decreased to 1.06R
(reference value: 0.8–1.5). The patient's prescription was
therefore modified by eliminating warfarin and retaining
clopidogrel when he was discharged 24 h later. The patient's
clinical characteristics on second admission are listed in Table I.
 | Table I.Patient characteristics at the
time-point of second admission (sudden onset of hemoptysis after
warfarin + clopidogrel treatment for 9 days). |
Table I.
Patient characteristics at the
time-point of second admission (sudden onset of hemoptysis after
warfarin + clopidogrel treatment for 9 days).
| Variable | Result | Reference value |
|---|
| Ethnicity | Chinese-Han |
|
| Sex | Male |
|
| Height | 175 cm |
|
| Weight | 64 kg |
|
| Fever | No |
|
| Smoking | No |
|
| Alcohol
consumption | No |
|
| Diabetes
mellitus | No |
|
| Hypertension | No |
|
| Hepatic disease | No |
|
| Chronic kidney
disease | No |
|
| Protein C/S
deficiency | No |
|
| Age (years) | 60 |
|
| Alanine
aminotransferase (IU/l) | 19.5 | 13–69 |
| Aspartate
aminotransferase (U/l) | 26.0 | 15–46 |
| Blood urea nitrogen
(µmol/l) | 2.80 | 2.5–7.1 |
| Creatinine
(µmol/l) | 51.0 | 53–115 |
| White blood cells
(×109/l) | 8.99 | 3.97–9.15 |
| Platelets
(×109/l) | 176 | 85–303 |
Fifty days later, the patient presented with another
left ALI with typical 6P symptoms. The angiogram revealed that a
thrombus had formed in the upper part of the left SFA, which was
more proximal to BTK arteries than the previous time. Mechanical
thrombectomy using an Angioget system (Boston Scientific Corp.,
Marlborough, MA, USA) and traditional balloon angioplasty to
re-establish the SFA-PA-ATA/peroneal artery tracks. The patient was
prescribed aspirin (100 mg per day) and cilostazol (100 mg per day)
to inhibit platelet aggregation, as well as rivaroxaban (10 mg per
day) to neutralize coagulation factor X. To date, the patient has
remained in remission. The treatment of the patient is illustrated
in a flow diagram in Fig. 1.
The genetic information was also analyzed. DNA
extraction was performed using a QIAamp DNA Blood Mini kit (Syngen,
Inc., Sacramento, CA, USA) according to the manufacturer's
protocol. The concentration of isolated DNA was measured using a
NanoDrop spectrophotometer (NanoDrop; Thermo Fisher Scientific,
Inc., Pittsburgh, PA, USA) according to the manufacturer's
protocol. The associated genetic variation in the pharmacogenomics
knowledge base (https://www.pharmgkb.org/) was obtained by
whole-genome sequencing (Illumina Hiseq2500; Illumina Inc. San
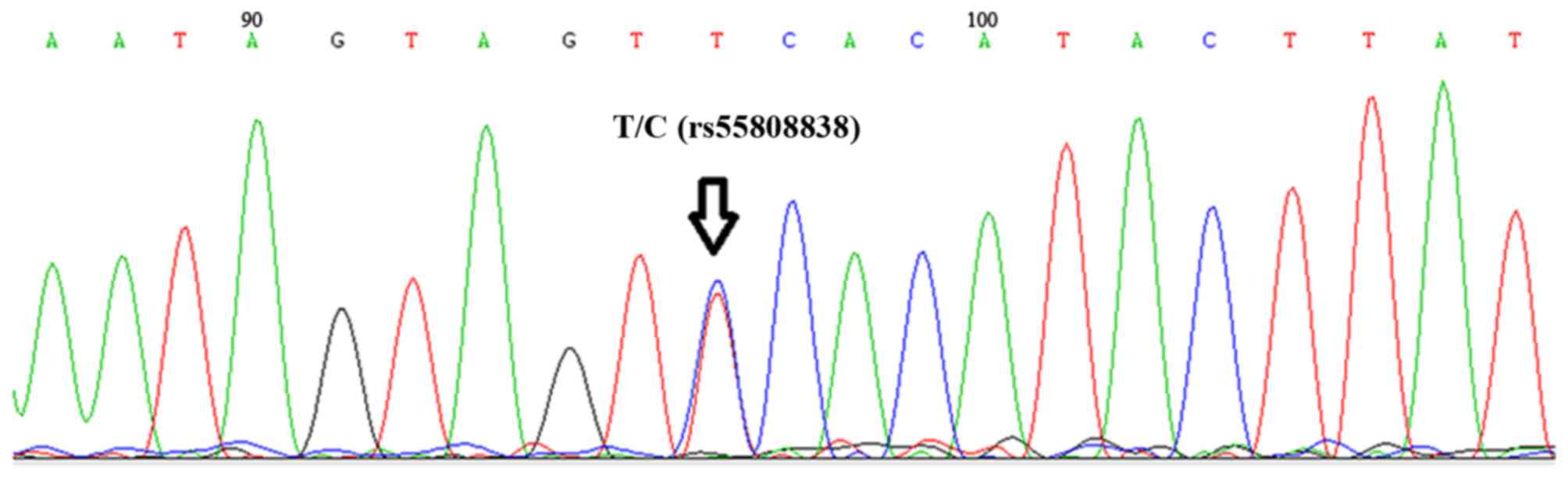
Diego, CA, USA; sequencing depth, ×100). Rare genetic variations
[minor allele frequency (MAF) <1%] were analyzed in this
patient. There was no rare mutation in CYP2C9 or VKORC1, but a
splicing mutation in CYP3A4 (rs55808838; MAF=0.04%) was identified
by the Sanger method (Fig. 2).
Discussion
As is known, the dose-response association of
warfarin is influenced by several factors, including ethnicity,
environmental factors, drug interactions, clinical status and
genetic factors. With regard to bleeding complications, as many
aspects as possible were considered for the present case:
i) Clinical
characteristics. It has been reported that abnormal kidney/liver
function and several complications, such as fever, hypertension and
diabetes mellitus affect the effective dose of warfarin (5–7).
Recently, the white blood cell count was also found to affect
inter-patient variations in the response to warfarin (8).
ii) Drug interactions. A
multitude of drugs have been associated with the effective dose of
warfarin, such as amiodarone, fluconazole and antibiotics,
according to the warfarin product monograph. The patient of the
present study was only prescribed clopidogrel (75 mg/day) besides
warfarin. To the best of our knowledge, there is no evidence that
clopidogrel affects the dose of warfarin and it was not a major
factor associated with the bleeding.
iii) Vitamin K
supplementation. Another common concern regarding the use of
warfarin is the interaction with food rich in vitamin K (9). It has been reported that vitamin K
intake was responsible for ~3% of dosage variations in Chinese
patients (10), while certain
studies found a negative correlation with vitamin K intake
(11). The available evidence does
not support the modification of dietary vitamin K intake when
starting therapy with warfarin. The patient of the present study
was on a regular Chinese diet and it was not the major factor
associated with the bleeding.
iv) Genetic factors.
Given that the clinicopathological findings were associated with
genetic factors, written informed consent was obtained from the
patient to perform genetic testing. Five genetic variations
associated with a required increase in the warfarin dose, namely
CYP4F2, γ glutamyl carboxylase, protease, serine 53 and NAD(P)H
quinone dehydrogenase 1 were identified, besides the common
mutation in VKORC1 (rs7294) in Chinese individuals (Table II) (12–17).
 | Table II.Genetic variations. |
Table II.
Genetic variations.
| Gene | Single nucleotide
polymorphism | Evidence level | Genotype | Effect on dose |
|---|
| CYP2C9 | rs1799853 | 1A | CC |
|
| CYP2C9 | rs1057910 | 1A | AA |
|
| VKORC1 | rs9923231 | 1A | AA | Decrease |
| VKORC1 | rs7294 | 1B | CC |
|
| VKORC1 | rs9934438 | 1B | GG |
|
| CYP4F2 | rs2108622 | 1B | CT | Increase |
| CYP2C9 | rs7900194 | 2A | GG |
|
| CYP2C9 | rs4917639 | 2A | AA |
|
| CYP2C9 | rs56165452 | 2A | TT |
|
| CYP2C9 | rs28371686 | 2A | CC |
|
| VKORC1 | rs2359612 | 2A | AA |
|
| VKORC1 | rs8050894 | 2A | CC |
|
| VKORC1 | rs17708472 | 2A | GG |
|
| VKORC1 | rs2884737 | 2A | AA |
|
| VKORC1 | rs61742245 | 2A | CC |
|
| CALU | rs339097 | 2B | AA |
|
| CALU | rs12777823 | 2B | GG |
|
| CALU | rs7196161 | 2B | GG |
|
| NR1I3 | rs2501873 | 3 | CC |
|
| EPHX1 | rs1877724 | 3 | CC |
|
| GGCX | rs2592551 | 3 | GA | Increase |
| GGCX | rs699664 | 3 | CT | Increase |
|
| rs12714145 | 3 | CC |
|
| CYP2C9 | rs9332096 | 3 | CC |
|
| CYP2C9 | rs7089580 | 3 | AA |
|
| CYP2C9 | rs9332131 | 3 | AA |
|
| CYP2C9 | rs10509680 | 3 | GG |
|
| CYP2C9 | rs28371685 | 3 | CC |
|
| CYP2C9 | rs1057910 | 3 | AA |
|
| STX4 | rs10871454 | 3 | CC |
|
| PRSS53 | rs11150606 | 4 | CC | Increase |
| VKORC1 | rs7200749 | 4 | GG |
|
| PRSS53, VKORC1 | rs17886199 | 4 | AA |
|
| VKORC1 | rs9934438 | 4 | GG |
|
| VKORC1 | rs17880887 | 4 | GG |
|
| VKORC1 | rs61162043 | 4 | AA |
|
| NQO1 | rs10517 | 4 | AA |
|
| NQO1 | rs1800566 | 4 | GA | Increase |
|
| rs2189784 | 4 | GG |
|
| THBD | rs1042580 | 4 | TT |
|
| HNF4A | rs3212198 | 4 |
|
|
| VKORC1 | rs104894542 | 4 | AA |
|
|
| rs104894541 |
| TT |
|
| VKORC1 | rs104894540 | 4 | AA |
|
| VKORC1 | rs104894539 | 4 | CC |
|
The patient's warfarin dose was adjusted using five
built-in pharmacogenetics-based warfarin dosing algorithms for the
Chinese population and one by the International Warfarin
Pharmacogenetics Consortium algorithm, as the equations based on
Caucasian populations may not be suitable for predicting the dose
of warfarin in Chinese patients (Table
III).
 | Table III.Six published pharmacogenetics-based
warfarin dosing algorithms. |
Table III.
Six published pharmacogenetics-based
warfarin dosing algorithms.
| Author/(Ref.),
year | Ethnicity |
Pharmacogenetics-based warfarin
algorithm | Calculated dose
(mg/day) |
|---|
| IWPC; Klein et
al (12), 2009 | Mixed | √Weekly dose =
5.6044–0.2614 (age in decades) +0.0087 (height in cm) +0.0128
(weight in kg) −0.8677 (VKORC1 A/G) −1.6974 (VKORC1 A/A)-0.9357
(CYP2C9*1/*3) −0.1092 (Asian)a | 3 |
| Miao et al
(13), 2007 | Chinese | Daily dose =
6.22–0.011 (age in years) +0.017 (weight in kg) −0.775
(CYP*3)-3.397 (VKORC1 × 1) −4.803 (VKORC1 × 2)a | 2.02 |
| Huang et al
(14), 2009 | Chinese | Daily dose = exp
[0.727–0.007 (age in years)+0.384 (BSA in square meters)+0.403
(VKORC1 6484TC) −0.482 (CYP2C9*1/*3)-1.583 (CYP2C9*3/*3)] | 2.67 |
| Zhong et al
(15), 2012 | Chinese | √Daily dose =
1.68143–0.0029 (age, years) +0.30784 (BSA, m2) −0.2633
(VKORC1 g.3588G.A) −0.19114 (CYP2C9*3) +0.14735(CYP4F2
c.1297G>A) −0.1797 (amiodarone) −0.4138 (fluconazole) -(0.1888)
diltiazem | 2.79 |
| Tan et al
(16), 2012 | Chinese | √Daily dose =
2.140–0.370 (VKORC1-1639 G>A)-0.332 (CYP2C9*3)+0.324 (BSA,
m2)-0.004 (age, years) −0.231 (no. of INR-increasing
drugs) +0.105 (smoking habit) −0.135 (pre-operative stroke history)
−0.108 (hypertension) | 2.99 |
| Chen et al
(17), 2014 | Chinese | Daily dose =
0.135+1.7816 (rs7294) −1.2146 (rs1057910) +1.2886 (BSA,
m2) −0.0196 (age, years) +0.7086 (target INR) +0.1596
(rs2108622) +0.3736 (diabetes mellitus) −0.5816 (amiodarone)
−0.2526 (digoxin) | 2.84 |
Even if no other genetic variations were included in
the dosing algorithms, not all of the dosing algorithms listed may
be suitable for predicting the dose of warfarin in the present
Chinese patient. In addition, the patient had a similar response to
warfarin treatment ~10 years previously.
Compared to the normal Chinese population, the
patient was unusually sensitive to warfarin. It was suggested that
he had a rare genetic mutation leading to the high sensitivity to
warfarin, as no other decrease-dose factors were found in this
patient.
To the best of our knowledge, the present study was
the first to report on warfarin-induced life threatening bleeding
associated with a CYP3A4 loss-of-function mutation. S-warfarin is
metabolized via CYP3A4 and the splicing mutation of CYP3A4 may
therefore affect the effective dose of warfarin.
Only few studies have previously assessed CYP3A4
polymorphisms (18,19), and to the best of our knowledge, the
rs55808838 polymorphism has not been previously reported. These
previous studies on pharmacogenetic polymorphisms reported that
other CYP3A4 polymorphisms may be associated with drug-induced
thrombosis. CYP3A4*1G is the most frequent mutant allele of CYP3A4
in Asians and may be associated with a lower rate of clopidogrel
resistance, which was, however, not the case in the patient of the
present study.
The major limitation of the present study was that
the effect of this rare mutation on the effect of warfarin in a
single case was hard to verify. Therefore, as many aspects of this
case as possible were reviewed, such as the patient's
clinicopathological characteristics, drug interactions and vitamin
K supplementation. All known genetic variations were considered and
included into the pharmacogenetics-based warfarin dosing
algorithms. However, all not all factors reviewed explain for the
sensitivity to the warfarin in this patient. However, it is likely
that this rare genetic mutation of CYP3A4 was associated with the
recurrent warfarin-induced bleeding.
The present case report illustrated the importance
of considering genetic mutations for assessing unusual
warfarin-induced bleeding and presented the methods used for this.
Although this was a rare case, clinicians should be alert to the
bleeding risk associated with such rare genetic mutations.
Acknowledgements
The present study was supported by the China
National Natural Science Foundation (nos. 81500372, 81322025,
81171623, 81371875 and 81421001) and the Shanghai Pujiang Program
(no. 15PJ1405000).
References
|
1
|
Lam MP and Cheung BM: The pharmacogenetics
of the response to warfarin in Chinese. Br J Clin Pharmacol.
73:340–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi H and Echizen H:
Pharmacogenetics of warfarin elimination and its clinical
implications. Clin Pharmacokinet. 40:587–603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vecsler M, Loebstein R, Almog S, Kurnik D,
Goldman B, Halkin H and Gak E: Combined genetic profiles of
components and regulators of the vitamin K-dependent
gamma-carboxylation system affect individual sensitivity to
warfarin. Thromb Haemost. 95:205–211. 2006.PubMed/NCBI
|
|
4
|
Li S, Zou Y, Wang X, Huang X, Sun Y, Wang
Y, Dong L and Jiang H: Warfarin dosage response related
pharmacogenetics in Chinese population. PLoS One. 10:e01164632015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gage BF, Yan Y, Milligan PE, Waterman AD,
Culverhouse R, Rich MW and Radford MJ: Clinical classification
schemes for predicting hemorrhage: Results from the National
Registry of Atrial Fibrillation (NRAF). Am Heart J. 151:713–719.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pisters R, Lane DA, Nieuwlaat R, de Vos
CB, Crijns HJ and Lip GY: A novel user-friendly score (HAS-BLED) to
assess 1-year risk of major bleeding in patients with atrial
fibrillation: The euro heart survey. Chest. 138:1093–1100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Self TH, Oliphant CS, Reaves AB,
Richardson AM and Sands CW: Fever as a risk factor for increased
response to vitamin K antagonists: A review of the evidence and
potential mechanisms. Thromb Res. 135:5–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harada T, Ariyoshi N, Shimura H, Sato Y,
Yokoyama I, Takahashi K, Yamagata S, Imamaki M, Kobayashi Y, Ishii
I, et al: Application of Akaike information criterion to evaluate
warfarin dosing algorithm. Thromb Res. 126:183–190. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holmes MV, Hunt BJ and Shearer MJ: The
role of dietary vitamin K in the management of oral vitamin K
antagonists. Blood Rev. 26:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You JH, Wong RS, Waye MM, Mu Y, Lim CK,
Choi KC and Cheng G: Warfarin dosing algorithm using clinical,
demographic and pharmacogenetic data from Chinese patients. J
Thromb Thrombolysis. 31:113–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kakkar AK, Mueller I, Bassand JP,
Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GY,
Mantovani LG, et al: Risk profiles and antithrombotic treatment of
patients newly diagnosed with atrial fibrillation at risk of
stroke: Perspectives from the international, observational,
prospective GARFIELD registry. PLoS One. 8:e634792013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
International Warfarin Pharmacogenetics
Consortium, ; Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE,
Lee MT, Limdi NA, Page D, Roden DM, et al: Estimation of the
warfarin dose with clinical and pharmacogenetic data. N Engl J Med.
360:753–764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miao L, Yang J, Huang C and Shen Z:
Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to
the anticoagulant response to warfarin: Proposal for a new dosing
regimen in Chinese patients. Eur J Clin Pharmacol. 63:1135–1141.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang SW, Chen HS, Wang XQ, Huang L, Xu
DL, Hu XJ, Huang ZH, He Y, Chen KM, Xiang DK, et al: Validation of
VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance
dose: A prospective study in Chinese patients. Pharmacogenet
Genomics. 19:226–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong SL, Yu XY, Liu Y, Xu D, Mai LP, Tan
HH, Lin QX, Yang M and Lin SG: Integrating interacting drugs and
genetic variations to improve the predictability of warfarin
maintenance dose in Chinese patients. Pharmacogenet Genomics.
22:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan SL, Li Z, Song GB, Liu LM, Zhang W,
Peng J, Zhang T, Jia FF, Zhou G, Zhou HH and Zhou XM: Development
and comparison of a new personalized warfarin stable dose
prediction algorithm in Chinese patients undergoing heart valve
replacement. Pharmazie. 67:930–937. 2012.PubMed/NCBI
|
|
17
|
Chen J, Shao L, Gong L, Luo F, Wang J, Shi
Y, Tan Y, Chen Q, Zhang Y, Hui R and Wang Y: A
pharmacogenetics-based warfarin maintenance dosing algorithm from
Northern Chinese patients. PLoS One. 9:e1052502014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blanco F, Muriel C, Labrador J,
Gonzalez-Porras JR, Gonzalez-Sarmiento R and Lozano FS: Influence
of UGT2B7, CYP3A4, and OPRM1 gene polymorphisms on transdermal
buprenorphine pain control in patients with critical lower limb
ischemia awaiting revascularization. Pain Pract. 16:842–849. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu R, Zhou ZY, Chen YB, Li JL, Yu WB,
Chen XM, Zhao M, Zhao YQ, Cai YF, Jin J and Huang M: Associations
of CYP3A4, NR1I2, CYP2C19 and P2RY12 polymorphisms with clopidogrel
resistance in Chinese patients with ischemic stroke. Acta Pharmacol
Sin. 37:882–888. 2016. View Article : Google Scholar : PubMed/NCBI
|