Introduction
Muscle atrophy is characterized by an increase in
protein degradation and reduction in protein synthesis. It is
associated with a number of human diseases and catabolic
conditions, including fasting, disuse, aging, cancer, neuromuscular
diseases, stroke, chronic obstructive pulmonary disease, chronic
heart failure, HIV-acquired immunodeficiency syndrome and sepsis
(1–5). Muscle volume shrinking and muscle
weakness induced by muscular dystrophy typically disrupt and
adversely affect the life of patients (3,6–8). Therefore, it is essential to understand
the mechanism by which skeletal muscle atrophy is regulated.
A large amount of protein hydrolysis has previously
been identified to occur during muscle atrophy and the
ubiquitin-proteasome system (UPS) has been demonstrated to be
involved in this process (9–14). In this system, the proteins are first
conjugated into multiple molecules of ubiquitin. The 26S proteasome
then recognizes and degrades the ubiquitinated proteins (15,16).
Multiple enzymes regulate the protein ubiquitination; these include
E1, the ubiquitin-activating enzyme, E2, the ubiquitin conjugating
enzyme and E3 ubiquitin ligases (17,18). E3
ubiquitin ligases serve a major role in the specificity of protein
degradation, because the specific binding between the protein
substrate and E3 occurs prior to the reaction with ubiquitin
(18,19).
It has been demonstrated that the insulin-signaling
pathway is involved in the inhibition of UPS (20). In this pathway, the phosphorylation
of insulin receptor substrate is stimulated when insulin binds its
receptor. Phosphatidylinositol 3-kinase (PI3K), an intracellular
intermediate, is recruited to phosphorylate a serine/threonine
kinase, protein kinase B (AKT) during this process. AKT then
phosphorylates the forkhead box class O transcription factors,
subtype 1 and 3a (FoxO1 and FoxO3a), which prevents the
translocation of these factors into the nucleus from the cytoplasm.
Subsequently, the expression of two muscle-specific E3 ubiquitin
ligases, muscle atrophy F-box (atrogin-1/MAFbx) and muscle ring
finger protein (MuRF-1) is inhibited (21–23).
Accumulating evidence suggests that the
PI3K/AKT-dependent signaling pathway of mammalian target of
rapamycin (mTOR) serves a key role in protein synthesis (24–26).
Activation of mTOR enhances the activity of eukaryotic initiation
factor 4E-binding protein 1 and ribosomal protein S6 kinase and,
ultimately, increases protein synthesis (11,26–28).
One of the most valuable herbs in traditional
Chinese medicine is Panax ginseng. The main bioactive components in
P. ginseng are considered to be ginsenosides, a class of
steroidal glycosides. The ginsenoside ingredients in P.
ginseng vary, depending on the seasons, extraction methods and
cultivating soils (29,30). Ginsenoside Rg1 is one of the main
ingredients in P. ginseng. It has been demonstrated that
ginsenoside Rg1 has an anti-inflammatory effect on human skeletal
muscle during exercise (31). Yu
et al (32) have also
demonstrated that the antioxidant defense system against
exercise-induced oxidative stress is strengthened by oral
supplements of Rg1 in rat skeletal muscle. However, the function of
Rg1 in the resistance to muscle atrophy remains unclear (33–38).
In the present study, Rg1 was observed to increase
the viability of C2C12 muscle cells following starvation by
inhibiting the expression of MuRF-1 and atrogin-1. PI3K dependent
phosphorylation of AKT/FoxO and mTOR was involved in this process,
indicating that Rg1 may resist muscle atrophy by balancing the
protein degradation and protein synthesis pathways.
Materials and methods
Cell culture
C2C12 mouse myoblast cells (American Type Culture
Collection, Manassas, VA, USA) were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal
bovine serum (FBS), 100 µg streptomycin and 100 units penicillin
(all Gibco; Thermo Fisher Scientific, Inc. Waltham, MA, USA). The
cells were maintained in the growing medium at 37°C in a humidified
5% CO2 atmosphere. At confluence, myoblasts were induced
to fuse by changing the medium to medium supplemented with 2% horse
serum (NQBB International Biological Corp., Hong Kong, China). The
cells were maintained in 2% horse serum before the experiments. In
starvation studies, the medium of the differentiated myotubes was
replaced with serum-free medium for 48 h of incubation.
MTT cell activity assay
C2C12 cells were cultured in 96-well plates
(5×104 cells/plate) and were incubated for 24 h in DMEM
containing 0.1% FBS. After differentiation of the C2C12 cell line
to form myotubes, control group cells did not receive any further
treatment in addition to the incubation in medium supplemented with
horse serum medium for 48 h. Following treatment with Rg1
(10−4, 10−3, 10−2 and
10−1 mM; Shanghai Yuanye Biotechnology Co., Ltd.,
Shanghai, China) for 48 h, the starvation model was induced by
incubation of the cells with serum-free basal medium for 48 h.
Myotubes were incubated with MTT solution (Invitrogen; Thermo
Fisher Scientific, Inc.) for 4 h at 37°C. Non-reduced MTT was
removed by aspiration and the formazan crystals were dissolved in
dimethyl sulfoxide (150 µl/well) for 30 min at 37°C. The formazan
was quantified using spectroscopy using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a wavelength of
490 nm.
Western blot analysis
To assess the expression and phosphorylation levels
of AKT, mTOR, FoxO1 and Fox3a, and the expression of atrogin-1 and
MuRF-1, C2C12 cells were cultured in 6-well plates
(1×106 cells/plate) after the differentiation of the
C2C12 cell line to form myotubes. For the specific inhibitor
experiments, following pretreatment with the PI3K inhibitor,
LY294002 (Merck KGaA, Darmstadt, Germany), at 25 µM for 30 min, the
myotubes were cultured in serum-free medium in the absence or
presence of Rg1 (10−4, 10−3, 10−2
and 10−1 mM) for 5, 10, 30, 60 min and 24 h at 37°C in a
humidified 5% CO2 atmosphere. For immunoblotting, cells
were washed with ice-cold phosphate-buffered saline twice.
Subsequently, the cells were immersed in 1 ml precooled RIPA
solution and PMSF (both Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) was added into the RIPA solution, at a
final concentration of 1 mM, for protein extraction. The pyrolysis
liquid was transferred to an EP tube that was precooled on ice for
30 min. Following centrifugation at 13,000 × g for 10 min
(4°C), the quantity of protein in the supernatants was detected
using a bicinchoninic acid protein assay kit (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). The lysates
were collected 60 min later for western blot analysis.
The protein samples were separated by SDS-PAGE
(6–8%) and were transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). The amount of protein used per
lane was 20 µg. Following blocking with 5% non-fat milk for 1 h at
room temperature, the membranes were incubated with primary
antibodies specific for AKT (9272), mTOR (2972), FoxO1 (2880),
FoxO3a (2497), phospho-AKT Ser473 (p-AKT; 4051), phospho-mTOR
Ser2448 (p-mTOR; 2971), phospho-FoxO1 thr24 (p-FoxO1; 9464),
phospho-FoxO3a thr32 (p-FoxO3a; 9464; all Cell Signaling
Technology, Inc., Danvers, MA, USA), atrogin-1 (ab74023), MuRF-1
(ab172479; both Abcam, Shanghai, China) and β-actin (141205;
ZSGB-BIO Technology Co. Ltd. Beijing China) overnight at 4°C. All
primary antibodies were used at a dilution of 1:1,000. Blots
underwent a total of three 8-min washes with Tris-buffered saline
with 0.1% Tween-20 and were then incubated with a secondary,
immunoglobulin G antibody (1:8,000; 14708; Cell Signaling
Technology, Inc.) at room temperature for 1 h. The membranes were
washed as described above and the bands were scanned using the
Tanon 5500 fully automatic digital gel image analysis system (Tanon
Science & Technology Co., Ltd., Shanghai, China).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using analysis of
variance and Tukey's post hoc tests using SigmaPlot version 10.0
software (Systat Software, Inc., San Jose, CA, USA) and P<0.05
was considered to represent a statistically significant
difference.
Results
Ginsenoside Rg1 increases the
viability of mouse C2C12 myoblast cells in the starvation
model
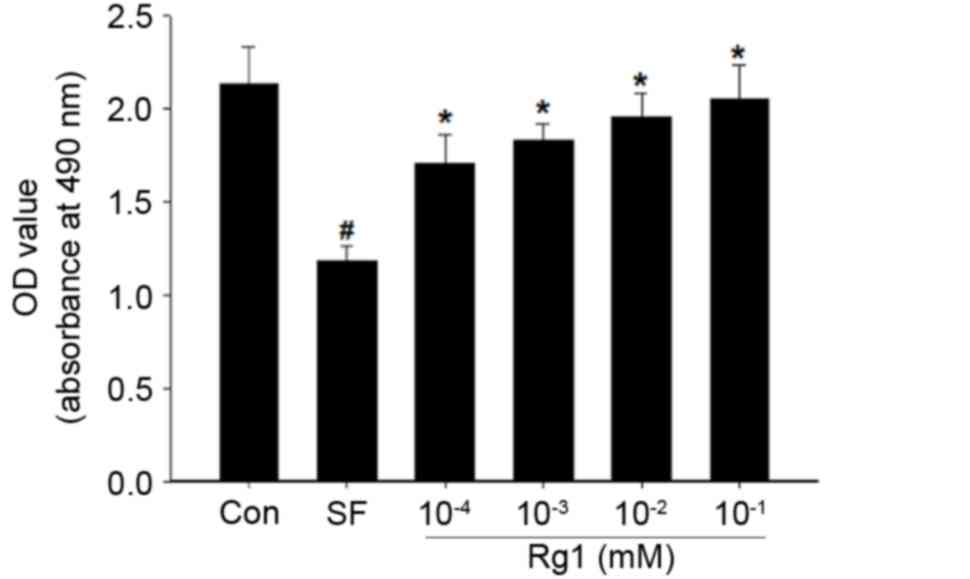
The viability of myoblast C2C12 cells decreases when
they are cultured during starvation (39). The results of the present study are
consistent with this, as a reduction in the viability of the cells
cultured in serum-free medium was observed compared with that in
the control group (Fig. 1). However,
treatment with Rg1 increased the viability of the starved C2C12
cells in a dose-dependent manner. Treatment with 10−1 mM
Rg1 restored the viability of cells to that of the cells in the
normal medium. The results of the present study suggest that
ginsenoside Rg1 promoted the viability of mouse myoblast cells in
the starvation model.
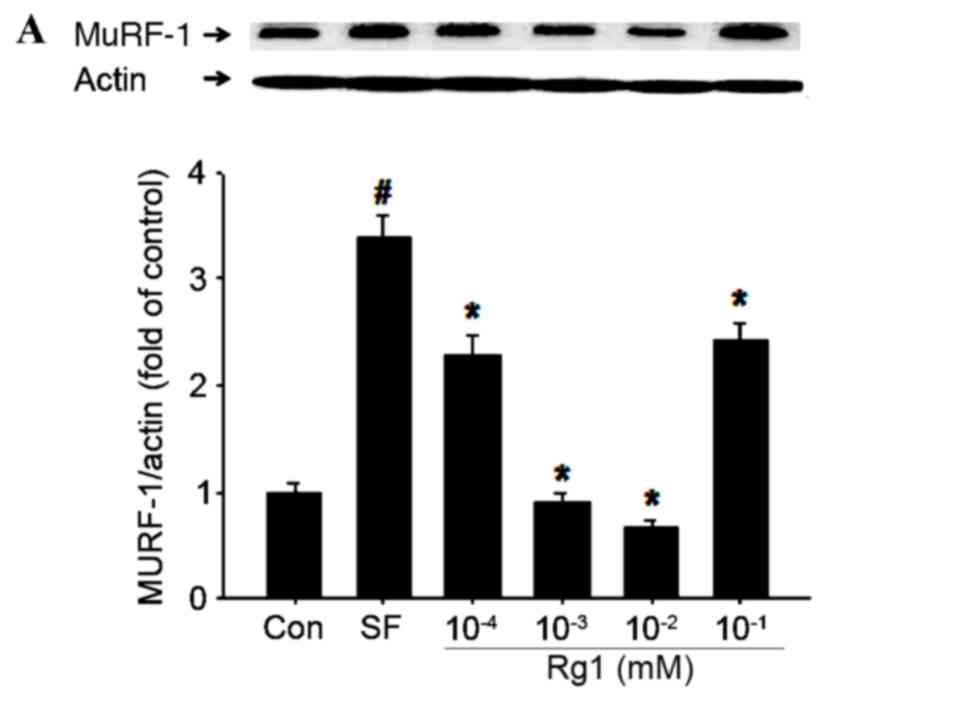
Rg1 inhibits the expression of
atrogin-1 and MuRF-1 in C2C12 cells in the starvation model
In order to assess how Rg1 increased the viability
of C2C12 cells in the starvation model, the present study examined
its effects on the expression of MuRF1 and atrogin-1 (Fig. 2). The results indicated that
treatment with 10−2 mM Rg1 had the largest significant
inhibitory effect on atrogin-1 and MuRF1 expression in C2C12 cells
(P<0.05). However, significantly reduced expression was also
observed in the 10−4 and 10−3 mM Rg1
treatment groups (P<0.05). The expression of atrogin-1 and MuRF1
was less strongly inhibited by treatment with 10−1 mM
Rg1. Therefore, 10−2 mM was considered to be the optimal
dose of Rg1 for inhibiting the expression of atrogin-1 and MuRF1
and increasing the viability of C2C12 cells in the starvation
model.
Rg1 stimulates PI3K-dependent
phosphorylation of AKT and FoxO in C2C12 cells in the starvation
model
The inhibition of atrogin-1 and MuRF1 expression is
primarily caused by the phosphorylation of the transcription
factors, AKT and FoxO (40). The
present study therefore assessed whether Rg1 is able to change the
phosphorylation levels of AKT and FoxO. The cells were treated with
Rg1 at various time points (0, 5, 10, 30, and 60 min and 24 h) for
western blot analysis of targeted protein phosphorylations. Changes
in cellular AKT phosphorylation appeared as early as 5 min and
reached a peak at 60 min after the intervention before decreasing.
PI3K inhibition by LY294002 completely abolished this Rg1-induced
phosphorylation at the indicated time points. The results
demonstrated that the phosphorylation levels of AKT, FoxO1 and
FoxO3a were all increased following the treatment of C2C12 cells in
serum-free medium with 10−2 mM Rg1, whereas the
expression levels of AKT, FoxO1 and FoxO3a were not changed by
treatment with Rg1 (Figs. 3 and
4).
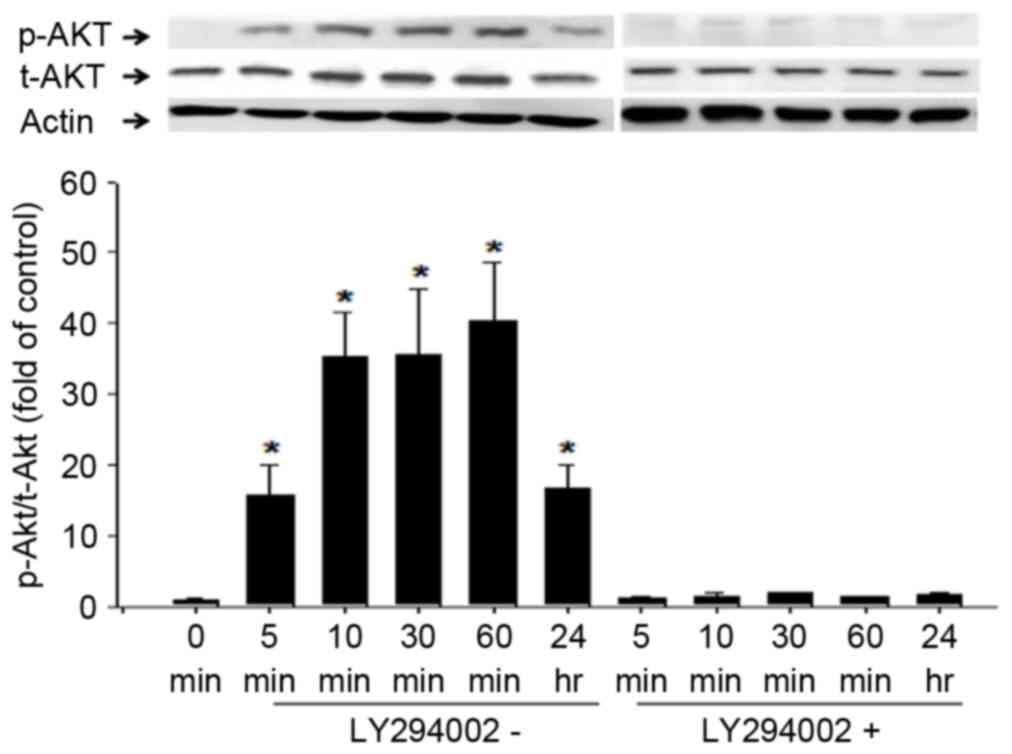 | Figure 3.Rg1-induced phosphorylation of AKT in
C2C12 cells over 24 h. Differentiated C2C12 cells were cultured
with serum-free basal medium for 12 h prior to treatment with
10−4 mM Rg1 in the presence or absence of LY294002, a
PI3K inhibitor. Cells were collected 5, 10, 30 and 60 min and 24 h
later for western blot analysis of AKT phosphorylation at Ser473.
The western blot analysis results are presented using
representative images and the ratio of p-AKT to t-AKT levels at
different time points is indicated using combined quantitative
data. Results are presented as means ± standard deviation, (n=4 for
each group). *P<0.01 vs. 0 min by analysis of variance and Tukey
post hoc tests. AKT, protein kinase B; p-, phosphorylated; t-,
total; Rg1, ginsenoside Rg1; PI3K, phosphatidylinositol 3-kinase;
Ser, Serine; Actin, β-actin. |
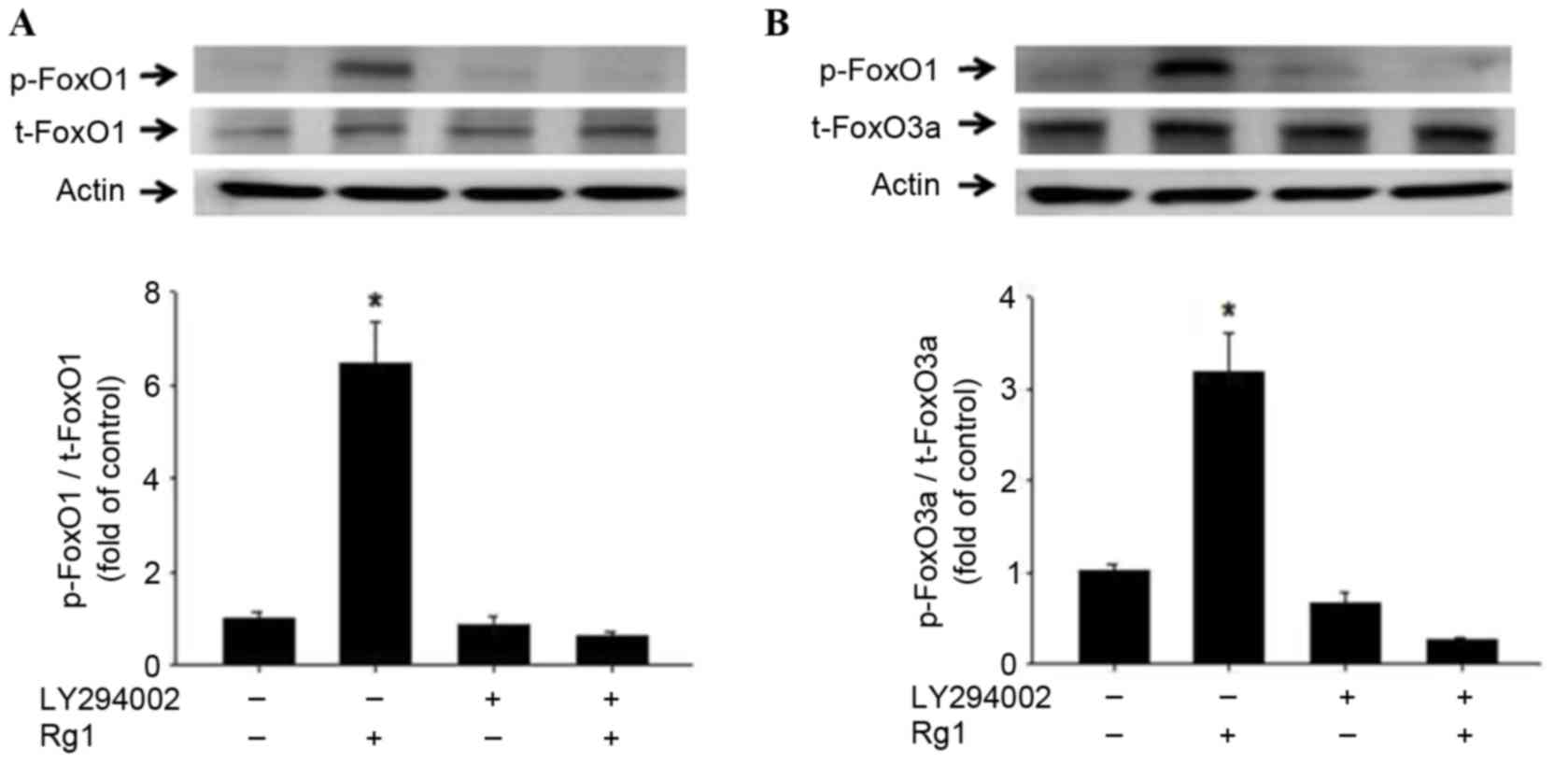 | Figure 4.Phosphorylation of FoxO1 and FoxO3a
in C2C12 cells following Rg1 treatment. Levels of (A) FoxO1 and (B)
FoxO3a phosphorylation in cultured C2C12 cells, incubated in the
presence of 10−2 mM Rg1 and/or the PI3K inhibitor
LY294002. LY294002 was added 30 min prior to the addition of
10−2 mM Rg1. Western blot analysis results are presented
using representative images and the p-FoxO1:t-FoxO1 and
p-FoxO3a:t-FoxO3a ratios are demonstrated using combined
quantitative data. Results are presented as the mean ± standard
deviation of four experiments. *P<0.05 vs. Rg1 group by analysis
of variance and Tukey's post hoc tests. FoxO1, forkhead
transcription factor O, subtype 1; FoxO3a, forkhead transcription
factor O, subtype 3a; Rg1, ginsenoside Rg1; PI3K,
phosphatidylinositol 3-kinase; LY294002, inhibitor of PI3K; p-,
phosphorylated; t-, total; Actin, β-actin. |
The phosphorylation of AKT and FoxO is known to be
regulated by PI3K (4,41). Therefore, the present study
investigated the effects of LY294002, an inhibitor of PI3K. C2C12
cells in serum-free medium were treated with Rg1 and LY294002
individually and together. The Rg1-induced phosphorylation levels
of AKT, FoxO1 and FoxO3a were significantly impaired by treatment
with LY294002 (P<0.05; Figs. 3
and 4). These results indicate that
Rg1 promoted the phosphorylation of AKT, FoxO1 and FoxO3a and was
dependent on PI3K activity.
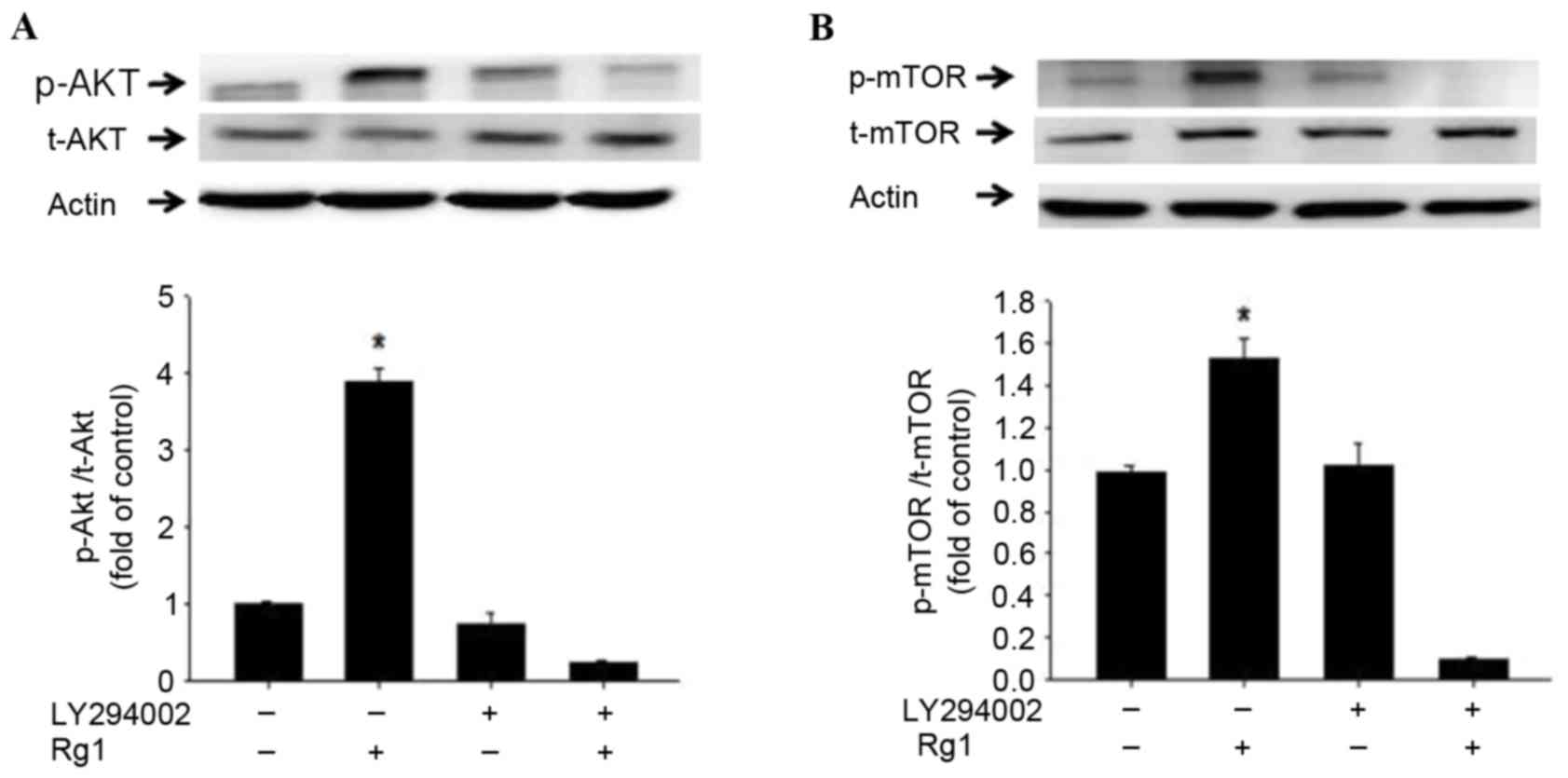
Rg1 activates the PI3K-dependent
phosphorylation of mTOR in C2C12 cells of the starvation model
As a downstream molecule of AKT, mTOR serves a key
role in protein synthesis (42–44). The
results of the present study demonstrated that Rg1 upregulated the
phosphorylation of mTOR in C2C12 cells in the starvation model. The
PI3K inhibitor, LY294002 was observed to inhibit the mTOR
phosphorylation induced by Rg1 (Fig.
5). This indicates that Rg1 promoted the PI3K-dependent
phosphorylation of mTOR in C2C12 cells in the starvation model.
Discussion
Rg1 has been indicated to have an effect on the
function of human diseases via anti-inflammatory and antioxidant
defense systems (45–55). The present study demonstrated that
Rg1 increased the viability of muscle cells within a starvation
model, by inhibiting protein degradation via the AKT/FoxO pathway
and promoting protein synthesis by the mTOR pathway.
The viability of muscle cells is a key factor for
resistance to muscle atrophy. When the rate of protein degradation
exceeds the rate of protein synthesis in adult tissues, muscle
atrophy occurs. The ubiquitin-proteasome pathway is one of most
important protein degradation pathways, which is activated during
muscle atrophy and contributes to the loss of muscle mass. The
ubiquitin proteasome system is controlled by the modulation of
rate-limiting enzyme expression in proteolytic systems, including
atrogin-1/MAFbx and MuRF-1 (21,56–60).
Atrogin-1/MAFbx and MuRF-1 knockout mice have been
demonstrated to be resistant to muscle atrophy induced by
denervation (61). MuRF-1 knockout
mice are also resistant to dexamethasone-induced muscle atrophy
(62), while knockdown of atrogin-1
spares muscle mass in fasting animal models (63). Furthermore, MuRF1 ubiquitinates a
number of structural proteins in the muscle including actin
(64), myosin heavy chains (65,66),
troponin I (67), myosin binding
protein C and myosin light chains 1 and 2 (68). Atrogin-1 promotes degradation of
MyoD, a key muscle transcription factor and eukaryotic translation
initiation factor 3, subunit F, an important activator of protein
synthesis (69,70). Therefore, high expression of
atrogin-1/MAFbx and MuRF-1 may be crucial factors in muscle
atrophy.
The insulin-AKT pathway negatively regulates FoxO
transcription factors, which. were the first to be identified as
critical for the process of atrophy (9). It has been indicated that hypertrophy
may be induced in myotubes by the activation of protein synthesis
through the AKT/mTOR pathway (21).
Cell apoptosis is closely related to muscle protein
degradation in cells, and previous reports have indicated that
apoptosis signaling is essential, and precedes protein degradation,
in wasting skeletal muscle during catabolic conditions (40,71).
These previous reports highlight that an apoptotic signal is
necessary for the activation of skeletal muscle protein
degradation. Activation of muscle protein hydrolysis resulting in
muscle atrophy is a complex process, and it has been demonstrated
that there are various mechanisms involved, including mechanisms
related to cell apoptosis signaling molecules (40). A review article by Argilés et
al (40) also demonstrated that
the inhibition of apoptosis is able to inhibit protein degradation.
In the condition of health, skeletal muscle protein metabolism,
protein synthesis and protein decomposition does not require
caspase-3 activated protein hydrolysis. In fact, an in vitro
experiment demonstrated that using the specific compound,
Ac-DEVD-CHO (a caspase-3 inhibitor), did not inhibit the
degradation of proteins of the basement membrane (71). However, in the case of catabolism,
muscle fibers in excess protein degradation may use the above
inhibitors to block the degradation of protein (71). This conclusion is also supported by
experimental acute induced diabetes (71). In view of the above, under the
condition of catabolism, excessive protein degradation is related
to the activation of apoptotic protease caspase-3 (40). As previously demonstrated, the
inhibition of apoptosis may provide a potential target for a drug
for the treatment of muscular dystrophy (71). This will be the focus of future
research.
In conclusion, the current study observed that Rg1
treatment has an inhibitory effect on Atrogin-1/MAFbx and MuRF1
translation via the activation of Akt, mTOR and FoxO
phosphorylation, which prevents starvation-induced muscle cell
death. The present study therefore provides a theoretical basis for
the use of Rg1 to treat muscle atrophy in a clinical setting.
Acknowledgements
The present study was supported by the National
Natural Science Fund of China (grant no. 51478096) and Jilin
Province Science and Technology Research Projects (grant no.
20140204059YY).
Glossary
Abbreviations
Abbreviations:
|
UPS
|
ubiquitin-proteasome system
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
AKT
|
protein kinase B
|
|
FoxO
|
forkhead box class O
|
|
FoxO1
|
forkhead transcription factor O,
subtype 1
|
|
FoxO3a
|
forkhead transcription factor O,
subtype 3a
|
|
atrogin-1/MAFbx
|
muscle atrophy F-box
|
|
MuRF-1
|
muscle RING-finger protein-1
|
|
mTOR
|
mammalian target of rapamycin
|
|
FBS
|
fetal bovine serum
|
|
DMEM
|
Dulbecco's modified Eagle medium
|
|
Rg1
|
Ginsenoside Rg1
|
References
|
1
|
Kawai N, Hirasaka K, Maeda T, Haruna M,
Shiota C, Ochi A, Abe T, Kohno S, Ohno A, Teshima-Kondo S, et al:
Prevention of skeletal muscle atrophy in vitro using
anti-ubiquitination oligopeptide carried by atelocollagen. Biochim
Biophy Acta. 1853:873–880. 2015. View Article : Google Scholar
|
|
2
|
Langen RC, Gosker HR, Remels AH and Schols
AM: Triggers and mechanisms of skeletal muscle wasting in chronic
obstructive pulmonary disease. Int J Biochem Cell Biol.
45:2245–2256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joassard OR, Durieux AC and Freyssenet DG:
β2-Adrenergic agonists and the treatment of skeletal muscle wasting
disorders. Int J Biochem Cell Biol. 45:2309–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang DT, Yin Y, Yang YJ, Lv PJ, Shi Y, Lu
L and Wei LB: Resveratrol prevents TNF-α-induced muscle atrophy via
regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int
Immunopharmacol. 19:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lecker SH, Jagoe RT, Gilbert A, Gomes M,
Baracos V, Bailey J, Price SR, Mitch WE and Goldberg AL: Multiple
types of skeletal muscle atrophy involve a common program of
changes in gene expression. FASEB J. 18:39–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langen RC, Gosker HR, Remels AH and Schols
AM: Triggers and mechanisms of skeletal muscle wasting in chronic
obstructive pulmonary disease. Int J Biochem Cell Biol.
45:2245–2256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng W, Tu JC, Pouliquin P, Cabrales E,
Shen X, Dulhunty A, Worley PF, Allen PD and Pessah IN: Dynamic
regulation of ryanodine receptor type 1 (RyR1) channel activity by
Homer 1. Cell Calcium. 43:307–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joassard OR, Amirouche A, Gallot YS,
Desgeorges MM, Castells J, Durieux AC, Berthon P and Freyssenet DG:
Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome
pathways in response to formoterol administration in rat skeletal
muscle. Int J Biochem Cell Biol. 45:2444–2455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sandri M: Protein breakdown in muscle
wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int J
Biochem Cell Biol. 45:2121–2129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawai N, Hirasaka K, Maeda T, Haruna M,
Shiota C, Ochi A, Abe T, Kohno S, Ohno A, Teshima-Kondo S, et al:
Prevention of skeletal muscle atrophy in vitro using
anti-ubiquitination oligopeptide carried by atelocollagen. Biochim
Biophys Acta. 1853:873–880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kazi AA, Hong-Brown L, Lang SM and Lang
CH: Deptor knockdown enhances mTOR activity and protein synthesis
in myocytes and ameliorates disuse muscle atrophy. Mol Med.
17:925–936. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng B, Ohkawa S, Li HY, Roberts-Wilson
TK and Price SR: FOXO3a mediates signaling crosstalk that
coordinates ubiquitin and atrogin-1/MAFbx expression during
glucocorticoid-induced skeletal muscle atrophy. FASEB J.
24:2660–2669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You JS, Lincoln HC, Kim CR, Frey JW,
Goodman CA, Zhong XP and Hornberger TA: The role of diacylglycerol
kinase ζ and phosphatidic acid in the mechanical activation of
mammalian target of rapamycin (mTOR) signaling and skeletal muscle
hypertrophy. J Biol Chem. 289:1551–1563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moylan JS, Smith JD, Chambers MA,
McLoughlin TJ and Reid MB: TNF induction of atrogin-1/MAFbx mRNA
depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J
Physiol Cell Physiol. 295:C986–C993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tisdale MJ: The ubiquitin-proteasome
pathway as a therapeutic target for muscle wasting. J Support
Oncol. 3:209–217. 2005.PubMed/NCBI
|
|
16
|
Argiles JA, López-Soriano FJ and Busquets
S: Apoptosis signalling is essential and precedes protein
degradation in wasting skeletal muscle during catabolic conditions.
Int J Biochem Cell Biol. 40:1674–1678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wray CJ, Mammen JM, Hershko DD and
Hasselgren PO: Sepsis upregulates the gene expression of multiple
ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol.
35:698–705. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong JF, Yan X, Zhu MJ and Du M:
AMP-activated protein kinase enhances the expression of
muscle-specific ubiquitin ligases despite its activation of
IGF-1/Akt signaling in C2C12 myotubes. J Cell Biochem. 108:458–468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Argilés JM, López-Soriano FJ and Busquets
S: Apoptosis signalling is essential and precedes protein
degradation in wasting skeletal muscle during catabolic conditions.
Int J Biochem Cell Biol. 40:1674–1678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stitt TN, Drujan D, Clarke BA, Panaro F,
Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD and Glass DJ: The
IGF-1/PI3K/Akt pathway prevents expression of muscle
atrophy-induced ubiquitin ligases by inhibiting FOXO transcription
factors. Mol Cell. 14:395–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandri M, Sandri C, Gilbert A, Skurk C,
Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH and Goldberg
AL: Foxo transcription factors induce the atrophy-related ubiquitin
ligase atrogin-1 and cause skeletal muscle atrophy. Cell.
117:399–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baviera AM, Zanon NM, Navegantes LC and
Kettelhut IC: Involvement of cAMP/Epac/PI3K-dependent pathway in
the antiproteolytic effect of epinephrine on rat skeletal muscle.
Mol Cell Endocrinol. 315:104–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharp ZD and Bartke A: Evidence for
down-regulation of phosphoinositide 3-kinase/Akt/mammalian target
of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory
signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med
Sci. 60:293–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goodman CA, Mayhew DL and Hornberger TA:
Recent progress toward understanding the molecular mechanisms that
regulate skeletal muscle mass. Cell Signal. 23:1896–1906. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimura K, Cheng XW, Inoue A, Hu LN, Koike
T and Kuzuya M: β-Hydroxy-β-methylbutyrate facilitates
PI3K/Akt-dependent mammalian target of rapamycin and FoxO1/3a
phosphorylations and alleviates tumor necrosis factor α/interferon
γ-induced MuRF-1 expression in C2C12 cells. Nutr Res. 34:368–374.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia L, Li YF, Wu GF, Song ZY, Lu HZ, Song
CC, Zhang QL, Zhu JY, Yang GS and Shi XE: MiRNA-199a-3p regulates
C2C12 myoblast differentiation through IGF-1/AKT/ mTOR signal
pathway. Int J Mol Sci. 15:296–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Risson V, Mazelin L, Roceri M, Sanchez H,
Moncollin V, Corneloup C, Richard-Bulteau H, Vignaud A, Baas D,
Defour A, et al: Muscle inactivation of mTOR causes metabolic and
dystrophin defects leading to severe myopathy. J Cell Biol.
187:859–874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sievenpiper JL, Arnason JT, Leiter LA and
Vuksan V: Variable effects of American ginseng: A batch of American
ginseng (Panax quinquefolius L.) with a depressed ginsenoside
profile does not affect postprandial glycemia. Eur J Clin Nutr.
57:243–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Um JY, Chung HS, Kim MS, Na HJ, Kwon HJ,
Kim JJ, Lee KM, Lee SJ, Lim JP, Do KR, et al: Molecular
authentication of Panax ginseng species by RAPD analysis and
PCR-RFLP. Biol Pharm Bull. 24:872–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hou CW, Lee SD, Kao CL, Cheng IS, Lin YN,
Chuang SJ, Chen CY, Ivy JL, Huang CY and Kuo CH: Improved
inflammatory balance of human skeletal muscle during exercise after
supplementations of the ginseng-based steroid Rg1. PLoS One.
10:e01163872015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu SH, Huang HY, Korivi M, Hsu MF, Huang
CY, Hou CW, Chen CY, Kao CL, Lee RP, Lee SD and Kuo CH: Oral Rg1
supplementation strengthens antioxidant defense system against
exercise-induced oxidative stress in rat skeletal muscles. J Int
Soc Sport Nutr. 9:232012. View Article : Google Scholar
|
|
33
|
Yang XD, Yang YY, Ouyang DS and Yang GP: A
review of biotransformation and pharmacology of ginsenoside
compound K. Fitoterapia. 100:208–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu QF, Deng ZY, Ye JM, He AL and Li SS:
Ginsenoside Rg1 protects chronic cyclosporin a nephropathy from
tubular cell apoptosis by inhibiting endoplasmic reticulum stress
in rats. Transplant Proc. 47:pp. 566–569. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim WK, Song SY, Oh WK, Kaewsuwan S, Tran
TL, Kim WS and Sung JH: Wound-healing effect of ginsenoside Rd from
leaves of Panax ginseng via cyclic AMP-dependent protein kinase
pathway. Eur J Pharmacol. 702:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi B, Liu L, Zhang H, Zhou GX, Wang S,
Duan XZ, Bai XY, Wang SM and Zhao DQ: Anti-fatigue effects of
proteins isolated from Panax quinquefolium. J Ethnopharmacol.
153:430–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhuang CL, Mao XY, Liu S, Chen WZ, Huang
DD, Zhang CJ, Chen BC, Shen X and Yu Z: Ginsenoside Rb1 improves
postoperative fatigue syndrome by reducing skeletal muscle
oxidative stress through activation of the PI3K/Akt/Nrf2 pathway in
aged rats. Eur J Pharmacol. 740:480–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei HJ, Yang HH, Chen CH, Lin WW, Chen SC,
Lai PH, Chang Y and Sung HW: Gelatin microspheres encapsulated with
a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial
injection in a rat model with infarcted myocardium. J Control
Release. 120:27–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato S, Ogura Y and Kumar A: TWEAK/Fn14
signaling axis mediates skeletal muscle atrophy and metabolic
dysfunction. Front Immunol. 5:182014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Argilés JM, López-Soriano FJ and Busquets
S: Apoptosis signalling is essential and precedes protein
degradation in wasting skeletal muscle during catabolic conditions.
Int J Biochem Cell Biol. 40:1674–1678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thomas MP, Mills J and Engelbrecht AM:
Phosphatidylinositol-3-kinase (PI3K) activity decreases in C2C12
myotubes during acute simulated ischemia at a cost to their
survival. Life Sci. 91:44–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giresi PG, Stevenson EJ, Theilhaber J,
Koncarevic A, Parkington J, Fielding RA and Kandarian SC:
Identification of a molecular signature of sarcopenia. Physiol
Genomics. 21:253–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kandarian SC and Jackman RW: Intracellular
signaling during skeletal muscle atrophy. Muscle Nerve. 33:155–165.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schulze PC, Fang J, Kassik KA, Gannon J,
Cupesi M, MacGillivray C, Lee RT and Rosenthal N: Transgenic
overexpression of locally acting insulin-like growth factor-1
inhibits ubiquitin-mediated muscle atrophy in chronic
left-ventricular dysfunction. Circ Res. 97:418–426. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim WK, Song SY, Oh WK, Kaewsuwan S, Tran
TL, Kim WS and Sung JH: Wound-healing effect of ginsenoside Rd from
leaves of Panax ginseng via cyclic AMP-dependent protein kinase
pathway. Eur J Pharmacol. 702:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X: Cardiovascular protection by
ginsenosides and their nitric oxide releasing action. Clin Exp
Pharmacol Physiol. 23:728–732. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gillis CN: Panax ginseng pharmacology: A
nitric oxide link? Biochem Pharmacol. 54:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nag SA, Qin JJ, Wang W, Wang MH, Wang H
and Zhang R: Ginsenosides as anticancer agents: In vitro and in
vivo activities, structure-activity relationships and molecular
mechanisms of action. Front Pharmacol. 3:252012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie JT, Mehendale S and Yuan CS: Ginseng
and diabetes. Am J Chin Med. 33:397–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ,
Kim MJ, Kim HS, Ha J, Kim MS and Kwon DY: Anti-obesity effects of
ginsenoside Rh2 are associated with the activation of AMPK
signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun.
364:1002–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cho WC, Chung WS, Lee SK, Leung AW, Cheng
CH and Yue KK: Ginsenoside Re of Panax ginseng possesses
significant antioxidant and antihyperlipidemic efficacies in
streptozotocin-induced diabetic rats. Eur J Pharmacol. 550:173–179.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shang W, Yang Y, Zhou L, Jiang B, Jin H
and Chen M: Ginsenoside Rb1 stimulates glucose uptake through
insulin-like signaling pathway in 3T3-L1 adipocytes. J Endocrinol.
198:561–569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Song Z, Moser C, Wu H, Faber V, Kharazmi A
and Høiby N: Cytokine modulating effect of ginseng treatment in a
mouse model of Pseudomonas aeruginosa lung infection. J Cyst
Fibros. 2:112–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee E, Ko E, Lee J, Rho S, Ko S, Shin MK,
Min BI, Hong MC, Kim SY and Bae H: Ginsenoside Rg1 enhances CD4(+)
T-cell activities and modulates Th1/Th2 differentiation. Int
Immunopharmacol. 4:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Foletta VC, White LJ, Larsen AE, Léger B
and Russell AP: The role and regulation of MAFbx/atrogin-1 and
MuRF1 in skeletal muscle atrophy. Pflugers Arch. 461:325–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jagoe RT and Goldberg AL: What do we
really know about the ubiquitin-proteasome pathway in muscle
atrophy? Curr Opin Clin Nutr Metab Care. 4:183–190. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wray CJ, Mammen JM, Hershko DD and
Hasselgren PO: Sepsis upregulates the gene expression of multiple
ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol.
35:698–705. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hershko A: The ubiquitin system. Springer
US; 1998
|
|
60
|
Krawiec BJ, Nystrom GJ, Frost RA,
Jefferson LS and Lang CH: AMP-activated protein kinase agonists
increase mRNA content of the muscle-specific ubiquitin ligases
MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab.
292:E1555–E1567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Baehr LM, Furlow JD and Bodine SC: Muscle
sparing in muscle RING finger 1 null mice: Response to synthetic
glucocorticoids. J Physiol. 589:4759–4776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cong H, Sun L, Liu C and Tien P:
Inhibition of atrogin-1/MAFbx expression by adenovirus-delivered
small hairpin RNAs attenuates muscle atrophy in fasting mice. Hum
Gene Ther. 22:313–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Polge C, Heng AE, Jarzaguet M, Ventadour
S, Claustre A, Combaret L, Béchet D, Matondo M, Uttenweiler-Joseph
S, Monsarrat B, et al: Muscle actin is polyubiquitinylated in vitro
and in vivo and targeted for breakdown by the E3 ligase MuRF1.
FASEB J. 25:3790–3802. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Clarke BA, Drujan D, Willis MS, Murphy LO,
Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E
and Glass DJ: The E3 Ligase MuRF1 degrades myosin heavy chain
protein in dexamethasone-treated skeletal muscle. Cell Metab.
6:376–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fielitz J, Kim MS, Shelton JM, Latif S,
Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R and Olson EN:
Myosin accumulation and striated muscle myopathy result from the
loss of muscle RING finger 1 and 3. J Clin Invest. 117:2486–2495.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kedar V, McDonough H, Arya R, Li HH,
Rockman HA and Patterson C: Muscle-specific RING finger 1 is a bona
fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl
Acad Sci USA. 101:pp. 18135–18140. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cohen S, Brault JJ, Gygi SP, Glass DJ,
Valenzuela DM, Gartner C, Latres E and Goldberg AL: During muscle
atrophy, thick, but not thin, filaments components are degraded by
MuRF1-dependent ubiquitylation. J Cell Biol. 185:1083–1095. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Csibi A, Cornille K, Leibovitch MP, Poupon
A, Tintignac LA, Sanchez AM and Leibovitch SA: The translation
regulatory subunit eIF3f controls the kinase-dependent mTOR
signaling required for muscle differentiation and hypertrophy in
mouse. PLoS One. 5:e89942010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tintignac LA, Lagirand J, Batonnet S,
Sirri V, Leibovitch MP and Leibovitch SA: Degradation of MyoD
mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem.
280:2847–2856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Du J, Wang X, Miereles C, Bailey JL,
Debigare R, Zheng B, Price SR and Mitch WE: Activation of caspase-3
is an initial step triggering accelerated muscle proteolysis in
catabolic conditions. J Clin Invest. 113:115–123. 2004. View Article : Google Scholar : PubMed/NCBI
|