Introduction
The two most common knee joint disorders involving
cartilage damage are knee osteoarthritis (OA) and meniscus injury
(MI). OA, which is an age-related disease with increasing incidence
due to increase in the aging population (1), is characterized by loss and damage of
articular cartilage and other structures surrounding the joint:
ligaments, synovial membrane, subchondral bone and menisci
(2). OA pathogenesis is poorly
known, whereas some factors that contribute to the development and
progression of OA have been identified encompassing inflammation
and altered biomechanical conditions (3).
The menisci are fibro-cartilage-like tissue mainly
consisting of collagen and water, with cells interposed (4). They are important for stability, shock
absorption, and transmission of load and play a pivotal role in the
the normal function and long-term health of the knee joint. As the
menisci become worn with age they are more prone to lesions and
tears caused by degenerative disease, trauma, or a conjunction of
both, leading to significant musculoskeletal morbidity (4). Similar to OA, MI is a degenerative
disease, the incidence of which correlates with age. MI is
considered vital to the development of knee OA and its abnormal
condition bears greater risk for the subsequent radiographic OA
development (5). The interaction
between chondrocytes and the extracellular matrix (ECM) exerts an
important role in the pathophysiology of OA (6). Abnormal chondrocyte/ECM interaction
leads to the imbalance of destructive cytokines, particularly
matrix metalloproteinase-13 (MMP-13), over regulatory factors,
resulting in enzymatic degradation of cartilage matrix without
adequate inhibition. The balance between matrix synthesis and
metalloproteinase inhibition is also crucial for meniscus repair
(7). The level of growth factors and
MMPs in the synovial fluid may reflect the severity and progression
of OA and MI as the synovial fluid has physical contact directly
with structures of the joint and functions to reduce friction
during movement (8). Collagenase 2
is involved in the metabolism of subchondral bone (collagen type I)
and cartilage (collagen type II). It is a candidate biomarker in
the pathological tissue remodeling associated with OA and MI
(9,10). Vascular endothelial growth factor
(VEGF) serves as a specific angiogenic factor secreted from
endothelial cells, synoviocytes and chondrocytes (11). There is a positive association
between synovial fluid VEGF and severity of OA. In this study, we
compared the levels of inflammatory cytokines, MMPs, collagenase,
and VEGF of the synovial fluid in cases with OA and MI and to
examine the association between the levels of these synovial
proteins and the severity of OA and MI.
Patients and methods
The study was a case-control study (level III
evidence). During the prospective cross-sectional study, patients
with OA and MI provided informed consent and were then recruited by
the Shanghai Jiao Tong University Affiliated Sixth People's
Hospital. The present study obtained the approval from the Ethics
Committee of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital.
The inclusion criteria were as follows: patients
with symptomatic primary knee OA diagnosed based on the American
College of Rheumatology criteria or patients with MI diagnosed
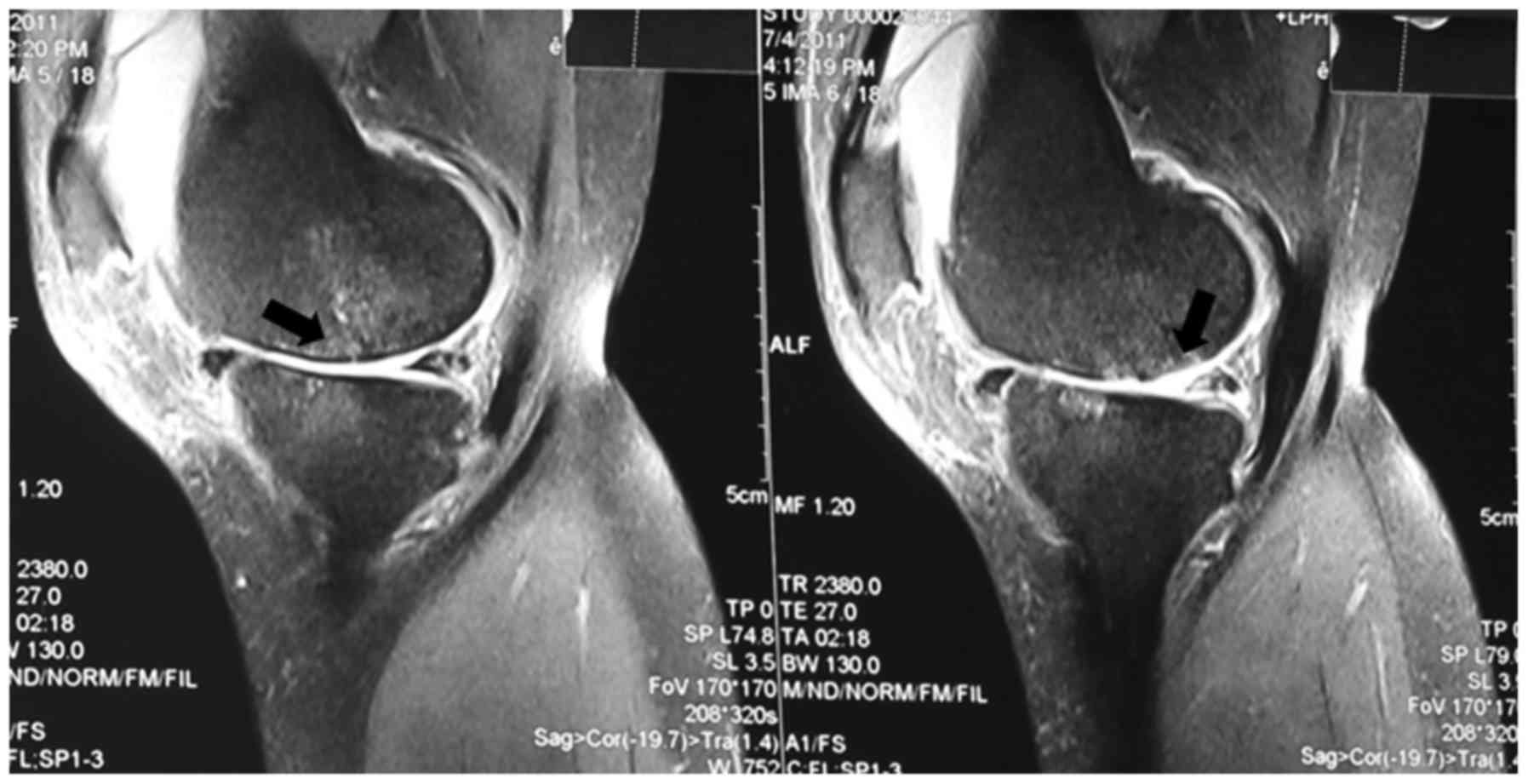
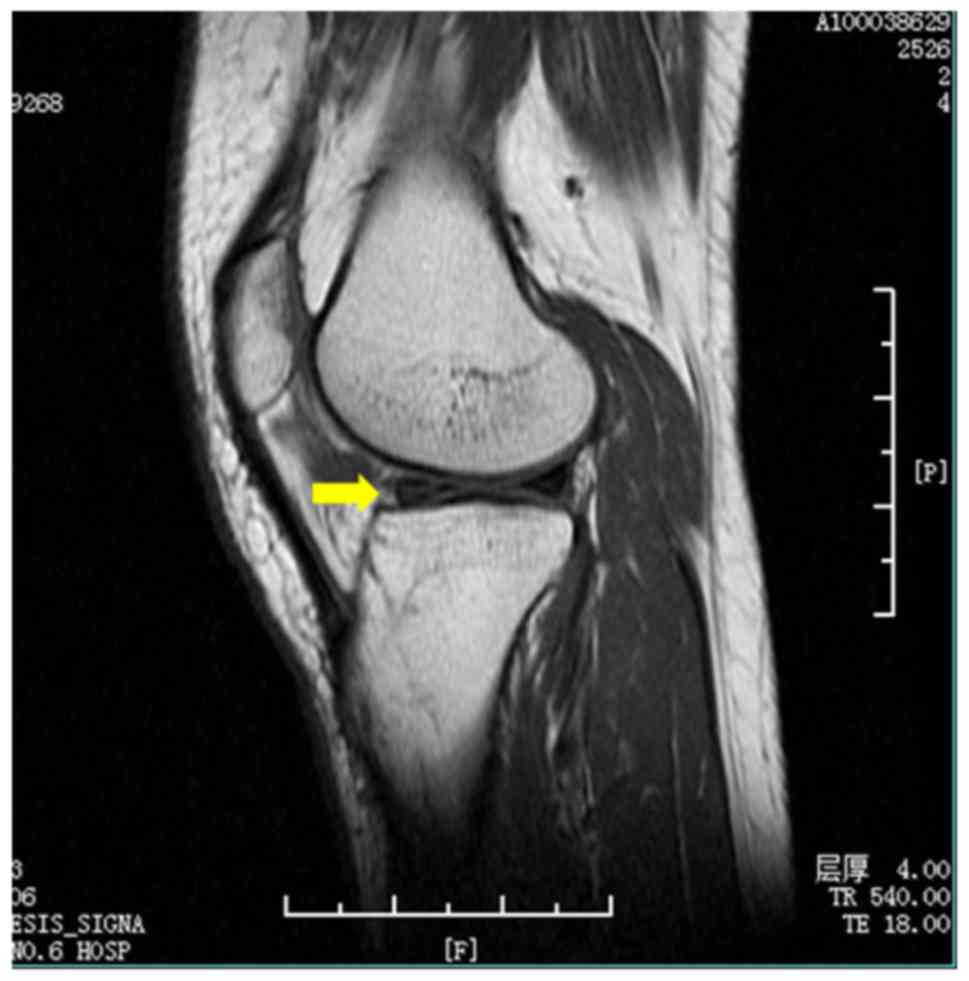
based on magnetic resonance imaging (MRI) findings of an abnormally
shaped meniscus and a strong signal intensity at the meniscus on
MRI images (12). Patients with OA
and MI were excluded. All the patients received consensus diagnosis
from three senior orthopedic surgeons. Radiological grading
(Kellgren-Lawrence score, K-L score) (13) and Stoller grade, assessed by MRI,
were also confirmed by at least two of the three senior orthopedic
surgeons. The exclusion criteria were as follows: patients with OA
or MI who had received immunosuppressive drugs, patients who had
received intra-articular sodium hyaluronate injection treatment,
patients who had previous operations for OA or trauma, or patients
with other combined inflammatory or neurodegenerative disease.
Patients with a Stoller grade I MI were also excluded.
From January 2010 to December 2014, 91 patients were
enrolled for the present study, 51 with OA and 40 with MI. All the
patients underwent anterior-posterior and lateral X-ray, and MRI of
the knee. The classic MRI images of OA and MI are shown in Figs. 1 and 2. Medical and surgical histories were
surveyed. Synovial fluid was collected from each patient via
arthrocentesis immediately before the first injection in the
current course of sodium hyaluronate treatment or before a
scheduled operation.
Synovial fluid studies
Arthrocentesis of the affected knee was performed on
all the patients to collect 2 ml synovial fluid samples prior to
the first therapeutic sodium hyaluronate injection of the current
treatment course or prior to operation. Centrifugation at at 1,200
× g for 5 min was implemented on the samples and then they were
stored at a temperature of −80°C. Synovial fluid samples were
assayed for VEGF, tumor necrosis factor-α (TNF-α), MMP-13,
interleukin (IL)-1, IL-6, IL-8, IL-10 and collagenase 2 using
commercial ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's instructions.
Statistical analysis
Demographic characteristics of OA and MI patients
were analyzed. Range and median were obtained for continuous
variables, and the percentage and frequency were shown for
categorical variables. Assessment of OA was carried out using X-ray
K-L classification score (14),
while assessment of MI was carried out with the use of a
semi-quantitative scoring method based on MRI (15). The association between K-L score and
biochemical markers, including VEGF, TNF-α, MMP-13, IL-10, IL-8,
IL-6, IL-1 and collagenase 2, were evaluated by ordinal logistic
(cumulative logistic) regression analysis (16), adjusting for sex, disease duration
and operation history. Similarly, the association between MRI score
and biochemical markers were assessed. Linear regression analysis
was then used to examine the mean difference in values of
biochemical markers between the OA and MI patients, adjusting for
sex, disease duration and operation history. Since biochemical
marker values were heavily skewed, they were log-transformed when
we performed association/difference analysis. Analyses were
performed using the statistical software SPSS version 20 (IBM SPSS,
Armonk, NY, USA).
Results
Table IA and B shows
the characteristics of OA and MI patients. The gender and age
distributions between the two patient groups were notably
different, the OA group was older and included more women.
 | Table I.Characteristics of patients with OA
and MI. |
Table I.
Characteristics of patients with OA
and MI.
| A, Patients with OA
(N=51) |
|---|
|
|---|
| Characteristics | Values |
|---|
| Age (years), median
(range) | 63 (44–79) |
| Male, N (%) | 14 (27) |
| Disease duration
(months), median (range) | 12 (8–240) |
| Biochemical markers,
median (range) |
| VEGF
(pg/ml) | 0.71 (0.30–1.18) |
| TNF-α
(pg/ml) | 0.44 (0.15–0.83) |
| MMP-13
(ng/ml) | 0.19 (0.15–0.43) |
| IL-10
(pg/ml) | 0.13 (0.08–0.72) |
| IL-8
(pg/ml) | 0.27 (0.13–1.89) |
| IL-6
(pg/ml) | 0.75 (0.23–3.04) |
| IL-1
(pg/ml) | 0.09 (0.06–0.34) |
|
Collagenase 2 (ng/ml) | 3.14 (1.90–3.49) |
| Previous operation, N
(%) |
| Yes | 0 (0) |
| No | 51 (100) |
| K-L score, N (%) |
| I | 18 (35) |
| II | 17 (33) |
| III | 16 (32) |
|
| B, Patients with MI
(N=40) |
|
| Age (years), median
(range) | 32 (16–40) |
| Male, N (%) | 18 (45) |
| Disease duration
(months), median (range) | 9 (1–19) |
| Biochemical markers,
median (range) |
| VEGF
(pg/ml) | 0.51 (0.16–1.23) |
| TNF-α
(pg/ml) | 0.42 (0.15–0.73) |
| MMP-13
(ng/ml) | 0.19 (0.16–0.40) |
| IL-10
(pg/ml) | 0.17 (0.08–0.97) |
| IL-8
(pg/ml) | 0.21 (0.10–1.43) |
| IL-6
(pg/ml) | 0.48 (0.13–3.00) |
| IL-1
(pg/ml) | 0.09 (0.06–0.66) |
|
Collagenase 2 (ng/ml) | 3.13 (1.39–3.53) |
| Previous operation, N
(%) |
| Yes | 5 (13) |
| No | 35 (87) |
| MRI level, N (%) |
| I | 2 (5) |
| II | 14 (35) |
| III | 24 (60) |
As shown in Table
II, after adjusting for sex, disease duration, and operation
history, no biochemical markers were found to be significantly
associated with the K-L score in OA patients. The biochemical
marker TNF-α was significantly correlated with MRI score in MI
patients (Table III). Table IV shows the mean difference in
log-transformed biochemical marker values between OA and MI
patients, adjusting for sex, disease duration and operation
history. We found that the mean log-transformed IL-6 value
significantly differed between OA and MI patients (P<0.05).
Specifically, OA patents had significantly greater IL-6 value. In
addition, the OA patents had marginally significantly smaller IL-10
values.
 | Table II.Estimates from the ordinal logistic
regression of biochemical marker values (log-transformed) to K-L
score for OA patients, with higher B-value indicating tendency
towards higher K-L score. |
Table II.
Estimates from the ordinal logistic
regression of biochemical marker values (log-transformed) to K-L
score for OA patients, with higher B-value indicating tendency
towards higher K-L score.
| Biochemical
markers | B-value | SE | P-value |
|---|
| VEGF (pg/ml) | 0.13 | 0.97 | 0.89 |
| TNF-α (pg/ml) | −0.81 | 0.73 | 0.27 |
| MMP-13 (ng/ml) | −0.25 | 1.33 | 0.85 |
| IL-10 (pg/ml) | −0.65 | 0.81 | 0.42 |
| IL-8 (pg/ml) | 0.83 | 0.86 | 0.34 |
| IL-6 (pg/ml) | −0.32 | 0.52 | 0.54 |
| IL-1 (pg/ml) | 0.89 | 1.13 | 0.43 |
| Collagenase 2
(ng/ml) | −0.31 | 3.25 | 0.92 |
 | Table III.Statistical results of the ordinal
logistic regression between synovial fluid levels of biochemical
markers and K-L scores for patients with OA. |
Table III.
Statistical results of the ordinal
logistic regression between synovial fluid levels of biochemical
markers and K-L scores for patients with OA.
| Biochemical
markers | B-value | SE | P-value |
|---|
| VEGF (pg/ml) | −2.65 | 1.49 | 0.07 |
| TNF-α (pg/ml) | 2.92 | 1.36 |
0.03a |
| MMP-13 (ng/ml) | 0.78 | 2.17 | 0.72 |
| IL-10 (pg/ml) | 0.54 | 1.15 | 0.64 |
| IL-8 (pg/ml) | 0.84 | 1.10 | 0.44 |
| IL-6 (pg/ml) | 0.77 | 1.04 | 0.46 |
| IL-1 (pg/ml) | 0.74 | 2.01 | 0.71 |
| Collagenase 2
(ng/ml) | −12.20 | 7.08 | 0.09 |
 | Table IV.Statistical results of the ordinal
logistic regression between synovial fluid levels of biochemical
markers and MOAKS for patients with meniscus injury. |
Table IV.
Statistical results of the ordinal
logistic regression between synovial fluid levels of biochemical
markers and MOAKS for patients with meniscus injury.
| Biochemical
markers | Mean
difference | SE | P-value |
|---|
| VEGF (pg/ml) |
0.33 | 0.09 | 0.24 |
| TNF-α (pg/ml) |
0.01 | 0.09 | 0.13 |
| MMP-13 (ng/ml) |
0.00 | 0.05 | 0.43 |
| IL-10 (pg/ml) | −0.15 | 0.10 | 0.10 |
| IL-8 (pg/ml) |
0.13 | 0.11 | 0.19 |
| IL-6 (pg/ml) |
0.55 | 0.15 | 0.02a |
| IL-1 (pg/ml) | −0.06 | 0.07 | 0.38 |
| Collagenase 2
(ng/ml) |
0.03 | 0.03 | 0.97 |
Discussion
The present study revealed that patients with OA had
significantly higher VEGF and IL-6 levels, and lower IL-10 levels
in their synovial fluid as compared to those with MI. VEGF is a
potent proangiogenic factor. An in vivo study has shown that
primary human chondrocytes incubated in synovial fluids of patients
with OA actively secrete VEGF (14),
suggesting a role for inflammation and hypoxia in OA pathogenesis.
In addition, intra-articular VEGF injection in healthy mice led to
symptoms including synovial hyperplasia, calcification in articular
cartilage, as well as bone sclerosis, followed by cartilage
degradation, indicative of OA (17).
However, in animal models of meniscal lesions, the local
application of VEGF did not increase angiogenesis in avascular or
vascular regions of meniscus or improve meniscal healing (18). Consistent with these findings, a high
level of VEGF was observed in synovial fluids from patients with OA
which was not observed in that from patients with MI.
IL-6 is a protein involved in cartilage degradation
in vitro and stimulates nociceptors in patients with OA
(19). Recent findings have
demonstrated that patients with end-stage knee OA have
significantly higher levels of IL-6 in synovial fluids as compared
with control donors (20),
supporting the hypothesis that inflammatory processes are involved
in OA. Similarly, another study has shown that the levels of IL-6
in the synovial fluid from patients with OA and those with
symptomatic cartilage defects are identical, and this level is
significantly higher than those of healthy volunteers (21). More IL-6 is produced in cartilage
regeneration by osteoarthritic chondrocytes compared to healthy and
defective chondrocytes. Furthermore, adding IL-6 to healthy
chondrocytes in vitro increased glycosaminoglycan
production, but adding IL-6 to osteoarthritic chondrocytes led to
decreased production (22). These
results suggest IL-6 as a key player in cartilage matrix
production, and that suppressing IL-6 in synovial fluids may
improve cartilage repair in patients who have OA or symptomatic
cartilage defects. Our finding that the levels of IL-6 in synovial
fluid from patients with OA are higher than those in patients with
MI adds to our current knowledge regarding the levels of IL-6 in
the synovial fluid from patients with MI.
IL-10, serving as a pleiotropic immunoregulatory
cytokine, has chondroprotective effects, and the promoting effect
on chondrocyte proliferation and on hypertrophic or chondrogenic
differentiation by means of activating the bone morphogenetic
protein (BMP) signaling pathway. In OA, the level of IL-10 in
cartilage and the synovium are elevated (23). Studies on synovial fluid levels of
IL-10 in patients with MI are limited, and our study shows that
patients with MI have higher levels of IL-10 in their synovial
fluids in relation to patients with OA. In our study, MI patients
were younger than OA patients. It is possible higher IL-10 reflects
better control of joint inflammation in younger populations.
However, the underlying mechanism of how IL-10 relates to OA and MI
remains to be elucidated.
In our study, any significant correlation between
the K-L score of patients with OA or any of the investigated
biomarkers (VEGF, TNF-α, MMP-13, IL-10, IL-8, IL-6, IL-1 and
collagenase 2) was not found. However, a significant correlation
was observed between the MRI Osteoarthritis Knee Score (MOAKS)
value and TNF-α level in MI patients. These results suggest that
levels of these biomarkers cannot reliably differentiate the
severity of OA and MI, which explains the lack of reported
associations.
One possible limitation of our study is single
sampling of biomarkers in synovial fluid. The wide range in the
levels of biomarkers suggests that future studies should rely on
multiple sampling to avoid possible false results. Our study was
also limited by patient number, which may not have been sufficient
to detect true differences in levels of cytokine or growth factor
in synovial fluid between OA and MI.
In conclusion, this study compared a series of
synovial fluid biomarkers from patients with OA and MI. Patients
with OA had higher VEGF and IL-6 levels, and lower levels of IL-10
than that found in patients with MI, indicating different biomarker
patterns in these pathologies.
Acknowledgements
The authors would like to thank Dr Jie Mao for
editing the manuscript. The study was supported by Chinese National
Nature Science Foundation (grant 81171861).
References
|
1
|
Benazzo F, Perticarini L, Padolino A,
Castelli A, Gifuni P, Lovato M, Manzini C and Giordan N: A
multi-centre, open label, long-term follow-up study to evaluate the
benefits of a new viscoelastic hydrogel (Hymovis®) in the treatment
of knee osteoarthritis. Eur Rev Med Pharmacol Sci. 20:959–968.
2016.PubMed/NCBI
|
|
2
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: an update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maldonado M and Nam J: The role of changes
in extracellular matrix of cartilage in the presence of
inflammation on the pathology of osteoarthritis. Biomed Res Int.
2013:2848732013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fox AJ, Wanivenhaus F, Burge AJ, Warren RF
and Rodeo SA: The human meniscus: a review of anatomy, function,
injury, and advances in treatment. Clin Anat. 28:269–287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Badlani JT, Borrero C, Golla S, Harner CD
and Irrgang JJ: The effects of meniscus injury on the development
of knee osteoarthritis: data from the osteoarthritis initiative. Am
J Sports Med. 41:1238–1244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iannone F and Lapadula G: The
pathophysiology of osteoarthritis. Aging Clin Exp Res. 15:364–372.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buma P, Ramrattan NN, van Tienen TG and
Veth RPH: Tissue engineering of the meniscus. Biomaterials.
25:1523–1532. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balakrishnan L, Nirujogi RS, Ahmad S,
Bhattacharjee M, Manda SS, Renuse S, Kelkar DS, Subbannayya Y, Raju
R, Goel R, et al: Proteomic analysis of human osteoarthritis
synovial fluid. Clin Proteomics. 11:62014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karsdal MA, Nielsen MJ, Sand JM, Henriksen
K, Genovese F, Bay-Jensen AC, Smith V, Adamkewicz JI, Christiansen
C and Leeming DJ: Extracellular matrix remodeling: the common
denominator in connective tissue diseases. Possibilities for
evaluation and current understanding of the matrix as more than a
passive architecture, but a key player in tissue failure. Assay
Drug Dev Technol. 11:70–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karsdal MA, Woodworth T, Henriksen K,
Maksymowych WP, Genant H, Vergnaud P, Christiansen C, Schubert T,
Qvist P, Schett G, et al: Biochemical markers of ongoing joint
damage in rheumatoid arthritis - current and future applications,
limitations and opportunities. Arthritis Res Ther. 13:2152011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mabey T, Honsawek S, Saetan N, Poovorawan
Y, Tanavalee A and Yuktanandana P: Angiogenic cytokine expression
profiles in plasma and synovial fluid of primary knee
osteoarthritis. Int Orthop. 38:1885–1892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rubin DA and Paletta GA Jr: Current
concepts and controversies in meniscal imaging. Magn Reson Imaging
Clin N Am. 8:243–270. 2000.PubMed/NCBI
|
|
13
|
Tanishi N, Yamagiwa H, Hayami T, Mera H,
Koga Y, Omori G and Endo N: Relationship between radiological knee
osteoarthritis and biochemical markers of cartilage and bone
degradation (urine CTX-II and NTX-I): the Matsudai Knee
Osteoarthritis Survey. J Bone Miner Metab. 27:605–612. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hunter DJ, Guermazi A, Lo GH, Grainger AJ,
Conaghan PG, Boudreau RM and Roemer FW: Evolution of
semi-quantitative whole joint assessment of knee OA: MOAKS (MRI
Osteoarthritis Knee Score). Osteoarthritis Cartilage. 19:990–1002.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoff P, Buttgereit F, Burmester GR,
Jakstadt M, Gaber T, Andreas K, Matziolis G, Perka C and Röhner E:
Osteoarthritis synovial fluid activates pro-inflammatory cytokines
in primary human chondrocytes. Int Orthop. 37:145–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCullagh P and Nelder JA: Generalized
Linear Models. 37. 2nd. Chapman & Hall; New York, NY: 1989,
View Article : Google Scholar
|
|
17
|
Ludin A, Sela JJ, Schroeder A, Samuni Y,
Nitzan DW and Amir G: Injection of vascular endothelial growth
factor into knee joints induces osteoarthritis in mice.
Osteoarthritis Cartilage. 21:491–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kopf S, Birkenfeld F, Becker R, Petersen
W, Stärke C, Wruck CJ, Tohidnezhad M, Varoga D and Pufe T: Local
treatment of meniscal lesions with vascular endothelial growth
factor. J Bone Joint Surg Am. 92:2682–2691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee AS, Ellman MB, Yan D, Kroin JS, Cole
BJ, van Wijnen AJ and Im HJ: A current review of molecular
mechanisms regarding osteoarthritis and pain. Gene. 527:440–447.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beekhuizen M, Gierman LM, van Spil WE, Van
Osch GJ, Huizinga TWJ, Saris DBF, Creemers LB and Zuurmond AM: An
explorative study comparing levels of soluble mediators in control
and osteoarthritic synovial fluid. Osteoarthritis Cartilage.
21:918–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuchida AI, Beekhuizen M, Rutgers M, van
Osch GJ, Bekkers JE, Bot AG, Geurts B, Dhert WJ, Saris DB and
Creemers LB: Interleukin-6 is elevated in synovial fluid of
patients with focal cartilage defects and stimulates cartilage
matrix production in an in vitro regeneration model. Arthritis Res
Ther. 14:R2622012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M, Shen J, Jin H, Im HJ, Sandy J and
Chen D: Recent progress in understanding molecular mechanisms of
cartilage degeneration during osteoarthritis. Ann N Y Acad Sci.
1240:61–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung YK, Kim GW, Park HR, Lee EJ, Choi JY,
Beier F and Han SW: Role of interleukin-10 in endochondral bone
formation: anabolic effect via BMP/Smad pathway. Arthritis Rheum.
65:3153–3164. 2013. View Article : Google Scholar : PubMed/NCBI
|