Introduction
Breast cancer is one of the most common malignant
tumors and the second most common cause of cancer-related fatality
in the United States (1). Although
the death rate of breast cancer has decreased with advances in
prevention, surgical resection and adjuvant therapies, there were
~232,340 new cases of breast cancer and 39,620 associated
fatalities in the United States in 2013 (1). Metastasis to vital organs such as lung,
brain and bone is a major cause of mortality resulting from breast
cancer (2). Therefore, it is
essential to study the molecular mechanism of breast cancer and
identify further effective diagnostic and treatment methods.
CCAAT enhancer binding protein β (C/EBPβ), a type of
trans-acting factor, is one of the important members of the C/EBP
family (including C/EBPα, β, γ, δ, ε and ζ) (3). C/EBPβ is able to bind to the DNA
specific regulatory region and is involved in multiple cell
processes, such as metabolism, hematopoiesis, adipogenesis, immune
response and morphogenesis (4,5).
Additionally, C/EBPβ serves as a key factor in neuronal
differentiation and apoptosis (6)
and is involved in inflammatory processes and brain injury by
regulating the expression levels of several genes, such as GRO1/KC,
24p3/LCN2 and TM4SF1/L6 (7). As
observed in C/EBPβ-null mice by Zhu et al (8), reduced levels of C/EBPβ result in cell
apoptosis, and thus these mice display resistance to
7,12-dimethylbenz[a]anthracene-induced skin tumorigenesis (8). Moreover, C/EBPβ has been shown to
promote cell survival downstream of DNA damage by repressing p53
expression and activity (9).
Considerable research has demonstrated that C/EBPβ
is an essential mediator of breast tumorigenesis. C/EBPβ has been
indicated to be overexpressed at late stages of breast
carcinogenesis (10), suggesting its
potential role in the metastatic progression of breast cancer.
C/EBPβ also has an important role in the evasion of metastatic
breast cancer cells from the cytostatic effects of transforming
growth factor (TGF)-β (11). The
loss of C/EBPβ promotes epithelial-mesenchymal transition (EMT) and
invasion in breast cancer (12).
Although C/EBPβ has been reported to be deregulated in breast
cancer, the underlying mechanisms of the effects of C/EBPβ on
breast cancer cells remain far from clear and require further
elucidation.
C/EBP functionally and physically interacts with
TGF-β1 signaling factors in astrocytes (13). TGF-β1 has a key role in tumor
pathogenesis, contributes to cell growth, invasion and metastasis,
and inhibits host antitumor immune responses (14). A previous study indicated that the
TGF-β pathway may be considered a therapeutic target for tumor
diseases (15). TGF-β super family
ligands bind to serine/threonine kinase receptors type II, which
phosphorylate receptor type I (16).
The receptor type I phosphorylates Smad2/3 (R-Smads), which
combines with coSmad-Smad4 and R-Smad/coSmad complexes and
subsequently shuttles into the nucleus to regulate the expression
of their downstream genes (16).
Several studies have suggested that activation of TGF-β-Smad
signaling has a deteriorative effect on glioblastoma, and that
inhibition of TGF-β signaling reduces the growth and invasion of
gliomas (17–19). However, studies concerning the
interactions of C/EBPβ and the TGF-β signaling pathway are
limited.
The aim of the present study was to investigate
whether C/EBPβ contributes to the development of breast cancer via
the regulation of TGF-β1-Smad3 signaling. In this study, a
recombinant lentiviral vector containing the C/EBPβ gene was
constructed and the effect of C/EBPβ on cell viability, cell cycle,
cell apoptosis and TGF-β1-Smad3 signaling in the MDA-MB-468 human
breast cancer cell line was investigated.
Materials and methods
Cell lines
The human breast cancer cell line, MDA-MB-468, was
purchased from The Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco, Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere
containing 5% CO2.
Construction of lentiviral vector
Human C/EBPβ gene was synthesized by Shanghai Sangon
Biotech Co., Ltd. (Shanghai, China) and was cloned into pCDH
lentiviral vector (System Biosciences, Mountain View, CA, USA). In
the pCDH lentiviral vector, green fluorescent protein was a single
transcript under the control of a CMV promoter and expressed after
the transcription of the C/EBPβ gene. To knockdown C/EBPβ
expression, the selected interfering [short hairpin (SH)] sequence
5′-CCTTTAGACCCATGGAAGTTT-3′ was cloned into pLKO.1 vector
(Sigma-Aldrich; merck KGgA, Darmstadt, Germany) after the
oligonucleotides were annealed.
Packaging and infection of lentivirus
vector
The lentiviral vectors pCDH-C/EBPβ and
pLKO.1-shC/EBPβ were co-transfected with the corresponding helper
plasmids into 293T cells (Cell bank, Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences, Shanghai, China)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) After 6 h incubation at 37°C in a humidified atmosphere
containing 5% CO2, DMEM was exchanged for complete
medium (containing 10% FBS, Gibco; Thermo Fisher Scientific, Inc.).
The supernatant was harvested after culturing for 48 h and
concentrated by ultrafiltration. MDA-MB-468 cells were infected
with recombinant lentivirus pCDH-C/EBPβ, lentivirus pCDH,
lentivirus pLKO.1-shC/EBPβ and the negative control (NC) lentivirus
pLKO.1-shNC, respectively. MDA-MB-468 cells without infection
served as the blank group. Medium was replaced with fresh medium 24
h post-infection and cells were collected 72 h post-infection for
subsequent analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were washed three times with PBS and total RNA
was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was treated with DNase to remove genomic DNA
contamination. The Revert Aid First-Strand RT-PCR kit (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to synthesize cDNA from
250 ng of each extracted RNA sample. cDNA was amplified in a 20-µl
reaction mixture containing 10 µl of SYBR-Green PCR Supermix
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 ng of cDNA
template and selected primers (200 nM; Table I). Each transcript was normalized to
the amplification levels of GAPDH, which served as control. C/EBPβ
mRNA levels were quantified by qPCR amplification. The following
conditions were used: Pre-denaturing at 95°C for 5 min followed by
40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 10 sec,
and finally elongation at 72°C for 10 min. Data analyses were
conducted with the 2−ΔΔCq method (20). Each experiment was performed in
triplicate.
 | Table I.The primers for reverse
transciption-quantitative polymerase chain reaction. |
Table I.
The primers for reverse
transciption-quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| C/EBPβ |
CCTCGCAGGTCAAGAGCAAG |
GAACAAGTTCCGCAGGGTG |
| GAPDH |
TGTTGCCATCAATGACCCCTT |
CTCCACGACGTACTCAGCG |
MTT assay
1×104 cells were seeded into each well of
a 96-well plate. On the second day, the cells were infected with
the lentiviruses pCDH, pCDH-C/EBPβ, pLKO.1-shNC and
pLKO.1-shC/EBPβ, respectively, to form the pCDH, pCDH-C/EBPβ,
pLKO.1-shNC and pLKO.1-shC/EBPβ groups. Subsequently, the cells
were incubated for 24, 48 or 72 h at 37°C in a humidified
atmosphere containing 5% CO2. MTT (10 µl, 5 mg/ml;
Shanghai Sangon Biotech Co., Ltd.) was added into each well at the
same time of each day and the cells were then incubated for 4 h.
Dimethyl sulfoxide (DMSO; 100 µl; Shanghai Sangon Biotech Co.,
Ltd.) was added to each well to solubilize the formazan crystals.
Zero (DMEM, MTT and DMSO) and blank wells were established. The
absorbance of each well was read at 570 nm using a microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Flow cytometry
For cell cycle detection, infected cells cultured in
DMEM from all groups were digested with 0.25% trypsin (Invitrogen;
Thermo Fisher Scientific, Inc.), collected by centrifuging at 377 ×
g for 6 min at 4°C and washed once with phosphate-buffered saline
(PBS; Shanghai Sangon Biotech Co.). Cells were fixed with ice-cold
75% ethanol at 4°C overnight. Subsequently, cells were centrifuged
(377 × g for 6 min at 4°C) and ethanol was removed by washing with
PBS three times. Cells were slightly resuspended with 300 µl PBS
and treated with 50 µg/ml RNase A (Shanghai Sangon Biotech Co.,
Ltd.) for 30 min at 37°C. Cells were stained with propidium iodide
(PI; BioLegend, Inc., San Diego, CA, USA) in the dark for 15 min at
4°C and detected using a flow cytometer (BD Biosciences, San Jose,
CA, USA). Data were analyzed by FCS Express 4 (De Novo Software,
Los Angeles, CA, USA).
Annexin V-APC Apoptosis Detection kit (BD
Biosciences) was used to detect cell apoptosis. Infected cells were
digested with 0.25% trypsin-EDTA (Invitrogen; Thermo Fisher
Scientific, Inc.) and collected by centrifuging at 377 × g for 6
min at 4°C, then washed once with PBS. Cells were added to
APC-Annexin V and PI in the dark for 15 min at 25°C after being
slightly resuspended with 1X Binding Buffer. A total of 400 µl 1X
Binding Buffer was added and the cells were detected using a flow
cytometer.
Cell migration and wound healing
assay
Cell motility was measured using a wound healing
assay. Cells from all groups were seeded onto 60-mm plates and
incubated in serum-free DMEM overnight at 37°C. A P200 pipette tip
was used to create an artificial wound by scratching the confluent
cell monolayer. Immediately, a photomicrograph was taken (time 0
h). Subsequently, at 24, 48 and 72 h post wounding, images were
captured to observe the migrating cells and closure of the scratch
wound. The wound areas were quantified using Muscale analysis
software (Muscale LLC, Scottsdale, AZ, USA).
Western blotting
Cells from all groups were collected into 1.5-ml
tubes, washed twice with PBS, and then placed on ice for 30 min in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing 1 mM phenylmethylsulfonyl
fluoride (Shanghai Sangon Biotech Co., Ltd.). Supernatant were
acquired by centrifuging at 18894 × g for 15 min at 4°C.
Subsequently, BCA protein quantitative assay was used to determine
the protein concentration (Shanghai Sangon Biotech Co., Ltd.). A
sample containing 40 µg total protein was separated using 12%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes,
which were blocked in 5% non-fat milk for 1 h. The membranes were
incubated overnight at 4°C with mouse anti-human β-actin monoclonal
antibody (1:1,000; sc-58673), rabbit anti-human C/EBPβ polyclonal
antibody (1:500; sc-56637), mouse anti-human TGFβ1 monoclonal
antibody (1:500; sc-146), rabbit anti-human Smad3 polyclonal
antibody (1:800; sc-8332; all Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and rabbit anti-human P-Smad3 polyclonal antibody
(1:1,000; ab52903; Abcam, Cambridge, MA, USA). Membranes were
washed three times with PBS and then incubated with secondary
antibodies goat anti-mouse IgG(H+L)-HRP (1:5,000) or goat
anti-rabbit IgG (H+L)-HRP (1:5,000; both Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for 2 h at room
temperature, respectively. Proteins were detected using enhanced
chemiluminescence (ECL; EMD Millipore, Billerica, MA, USA).
Statistical analysis
Statistical analysis in the present study was
performed using SPSS 12.0 statistical analysis software (SPSS Inc.,
Chicago, IL, USA). All determinations were performed in triplicate.
Data are expressed as the mean ± standard deviation and analyzed by
one-way analysis of variance and multiple comparisons between
groups were performed using Student-Newman-Keuls method. P<0.05
was considered to indicated a statistically significant
difference.
Results
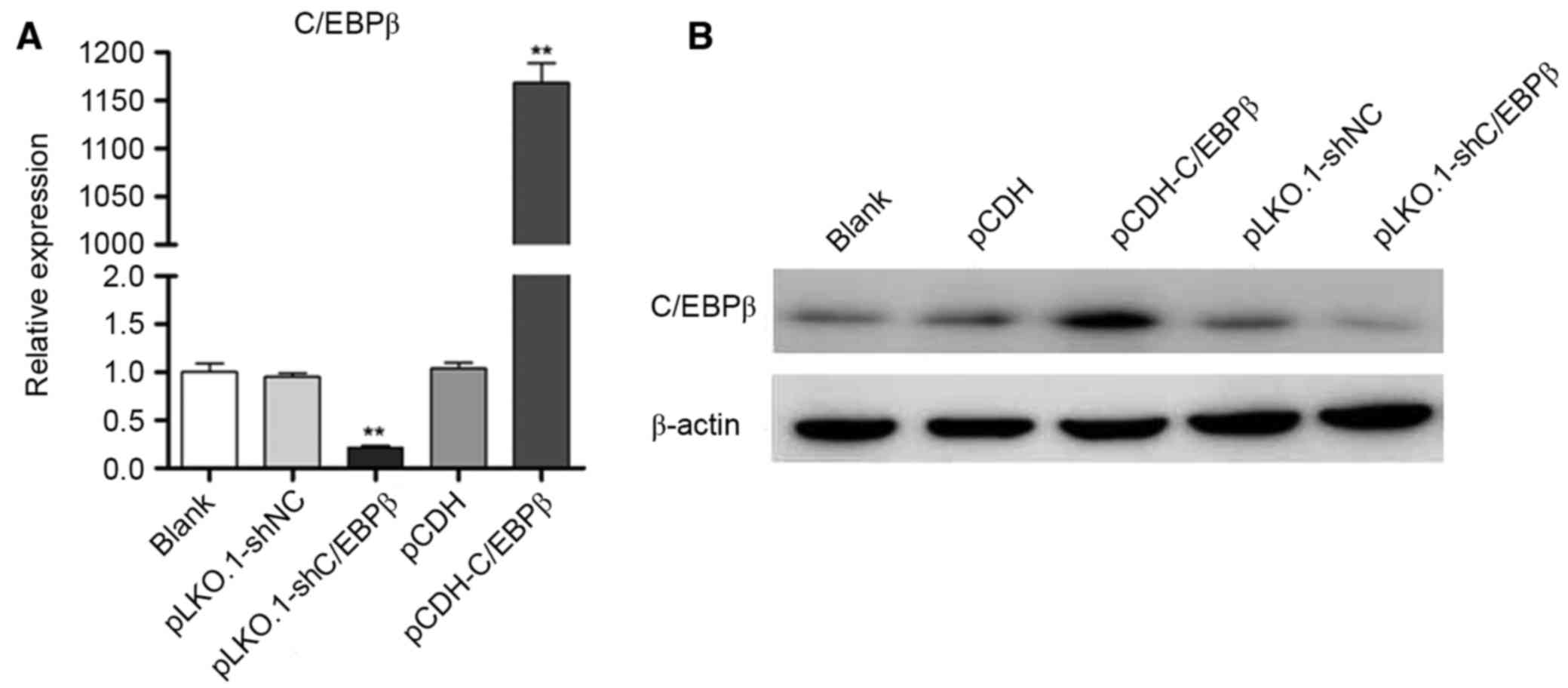
Identification of lentiviral vector
pCDH-C/EBPβ
Recombinant lentiviruses pCDH-C/EBPβ and
pLKO.1-shC/EBPβ were efficiently infected into MDA-MB-468 cells
(Fig. 1A), respectively. Western
blotting indicated that the protein expression level of C/EBPβ was
markedly increased in the pCDH-C/EBPβ group, whereas the protein
expression level of C/EBPβ was markedly decreased in the
pLKO.1-shC/EBPβ group when compared with the blank control
(Fig. 1B). These results
demonstrated that lentiviral vector pCDH-C/EBPβ and pLKO.1-shC/EBPβ
were successfully constructed and that the lentiviruses (including
pCDH-C/EBPβ, pCDH, pLKO.1-shC/EBPβ and pLKO.1-shNC) had a high
efficiency of infection.
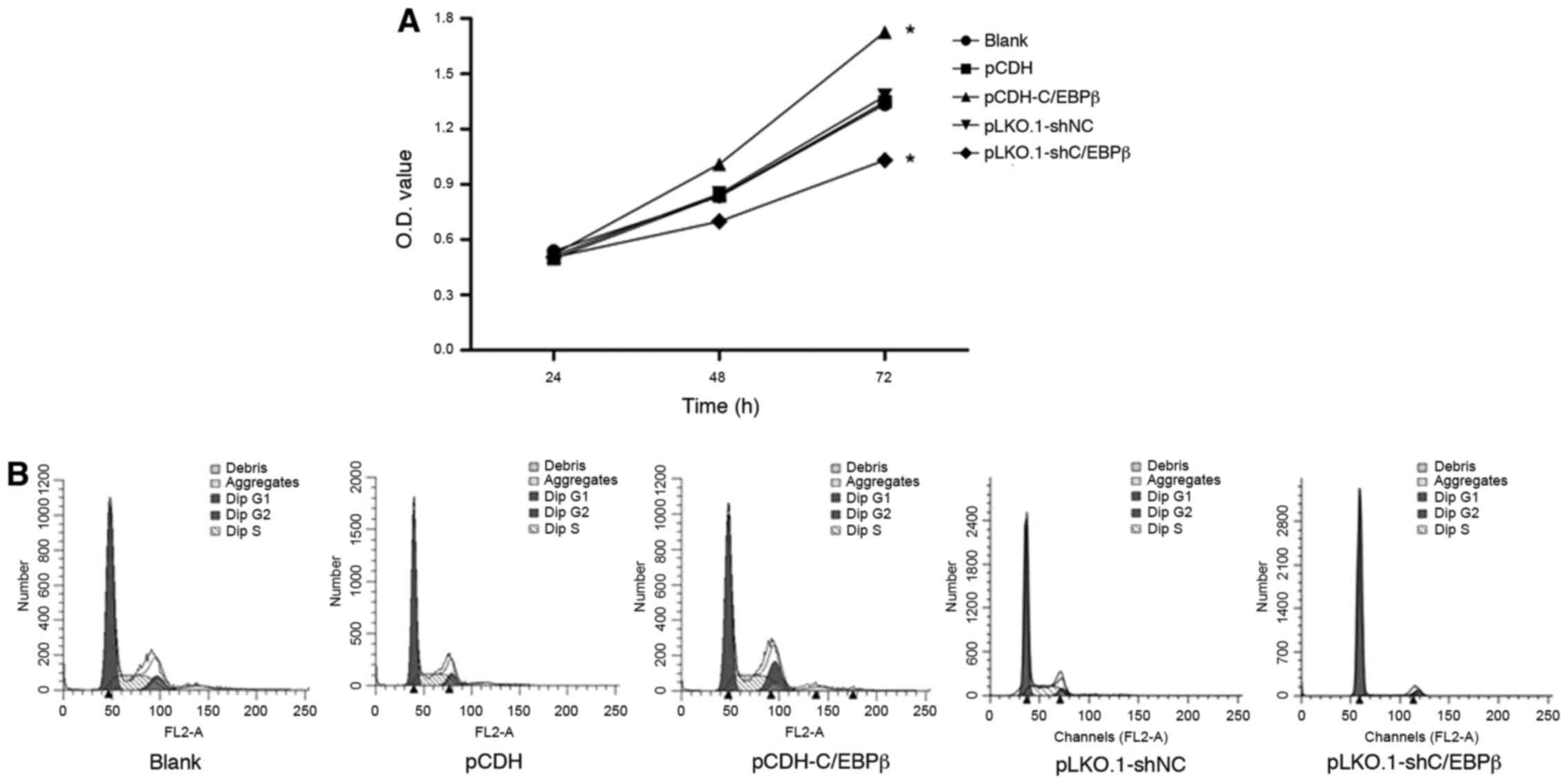
Effect of C/EBPβ on cell viability and
cell cycle in MDA-MB-468 cells
MTT analysis was performed to observe the cell
viability after infection. As Fig.
2A indicates, the absorbance of the MDA-MB-468 cells was
significantly increased in the pCDH-C/EBPβ group at 48 and 72 h
compared with that of the pCDH and blank groups (P<0.05).
Conversely, cell viability was significantly diminished in the
pLKO.1-shC/EBPβ group compared with that in the pLKO.1-shNC and
blank groups (P<0.05). These data suggest that C/EBPβ has an
important role in cell viability.
Cell cycle analysis was performed using flow
cytometry, and the results demonstrated that the percentage of
MDA-MB-468 cells in the G1 phase was significantly decreased and
that in the G2 phase was significantly increased in the pCDH-C/EBPβ
group compared with the blank and pCDH groups (P<0.05; Fig. 2B and Table II). Furthermore, the percentage of
cells in the G1 phase in the pLKO.1-shC/EBPβ group was
significantly increased compared with that in the blank and
pLKO.1-shNC groups (P<0.05; Fig.
2B and Table II). These results
suggest that cells in the pLKO.1-shC/EBPβ group were blocked at the
G1 boundary, which concomitantly reduced the proportion of cells in
the S phase (Fig. 2B and Table II).
 | Table II.Cell cycle analysis in the blank,
pCDH, pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ groups. |
Table II.
Cell cycle analysis in the blank,
pCDH, pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ groups.
| Group | G1 phase (%) | S phase (%) | G2 phase (%) |
|---|
| Blank | 62.79±1.06 | 27.87±1.36 |
9.31±1.09 |
| pCDH | 64.83±1.69 | 28.18±1.35 |
8.42±0.75 |
| pCDH-C/EBPβ |
55.13±1.13a,b | 26.21±1.95 |
18.67±0.73a,b |
| pLKO.1-shNC | 66.08±3.50 | 27.91±1.02 |
5.75±0.62 |
|
pLKO.1-shC/EBPβ |
91.62±2.83a,c |
4.21±0.23a,c |
4.13±0.29 |
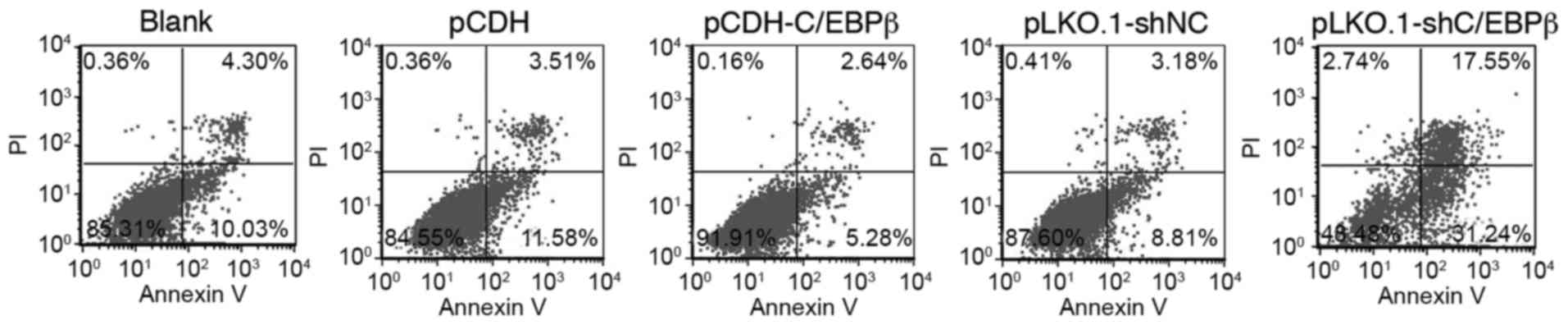
Effect of C/EBPβ on cell apoptosis in
MDA-MB-468 cells
The apoptotic ratio of the cells was quantitatively
analyzed using flow cytometry. As shown in Fig. 3 and Table III, the apoptotic cell ratio was
significantly decreased in the pCDH-C/EBPβ group compared with the
blank and pCDH groups (P<0.05); however, the apoptotic cell
level was significantly increased in the pKLO.1-shC/EBPβ group
compared with the blank and pLKO.1-shNC groups (P<0.05; Fig. 3 and Table III). These results suggest that the
expression of C/EBPβ decreases cell apoptosis.
 | Table III.Cell apoptosis in the blank, pCDH,
pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ groups. |
Table III.
Cell apoptosis in the blank, pCDH,
pCDH-C/EBPβ, pLKO.1-shNC and pLKO.1-shC/EBPβ groups.
| Group | Apoptotic cell
ratio (%) |
|---|
| Blank | 14.46±0.89 |
| pCDH | 15.32±1.86 |
| pCDH-C/EBPβ |
7.49±0.51a,b |
| pLKO.1-shNC | 12.14±0.94 |
|
pLKO.1-shC/EBPβ |
48.21±1.97a,c |
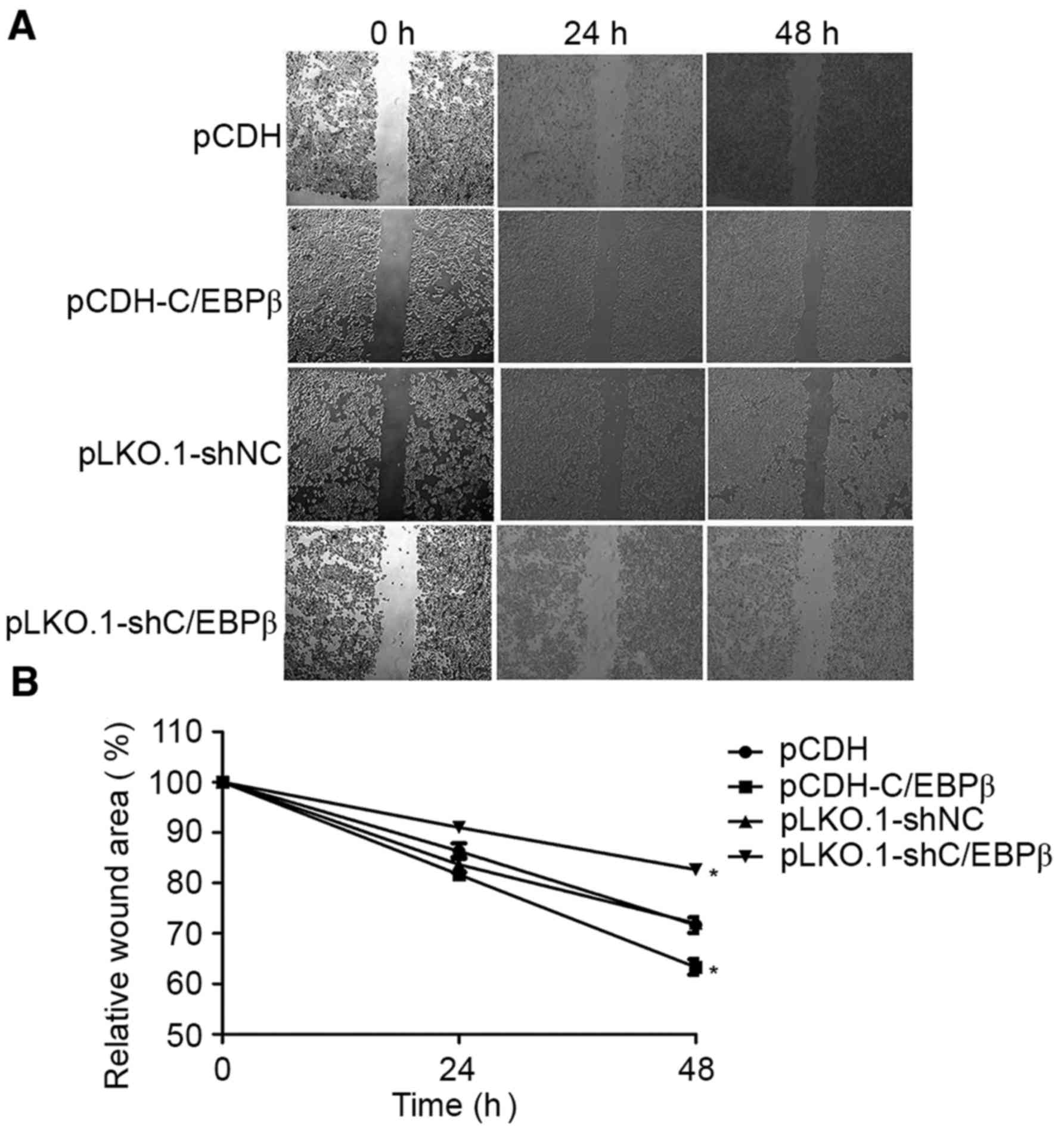
C/EBPβ depletion inhibits MDA-MB-468
cell motility
Scratch-wound assays were performed to assess the
role of C/EBPβ in MDA-MB-468 cell motility. These assays showed
that MDA-MB-468 cells with overexpressed C/EBPβ exhibited
significantly increased motility, whereas the cells depleted of
C/EBPβ exhibited significantly decreased motility as they did not
fill in the scratch as extensively as did cells from the control
groups (P<0.05; Fig. 4).
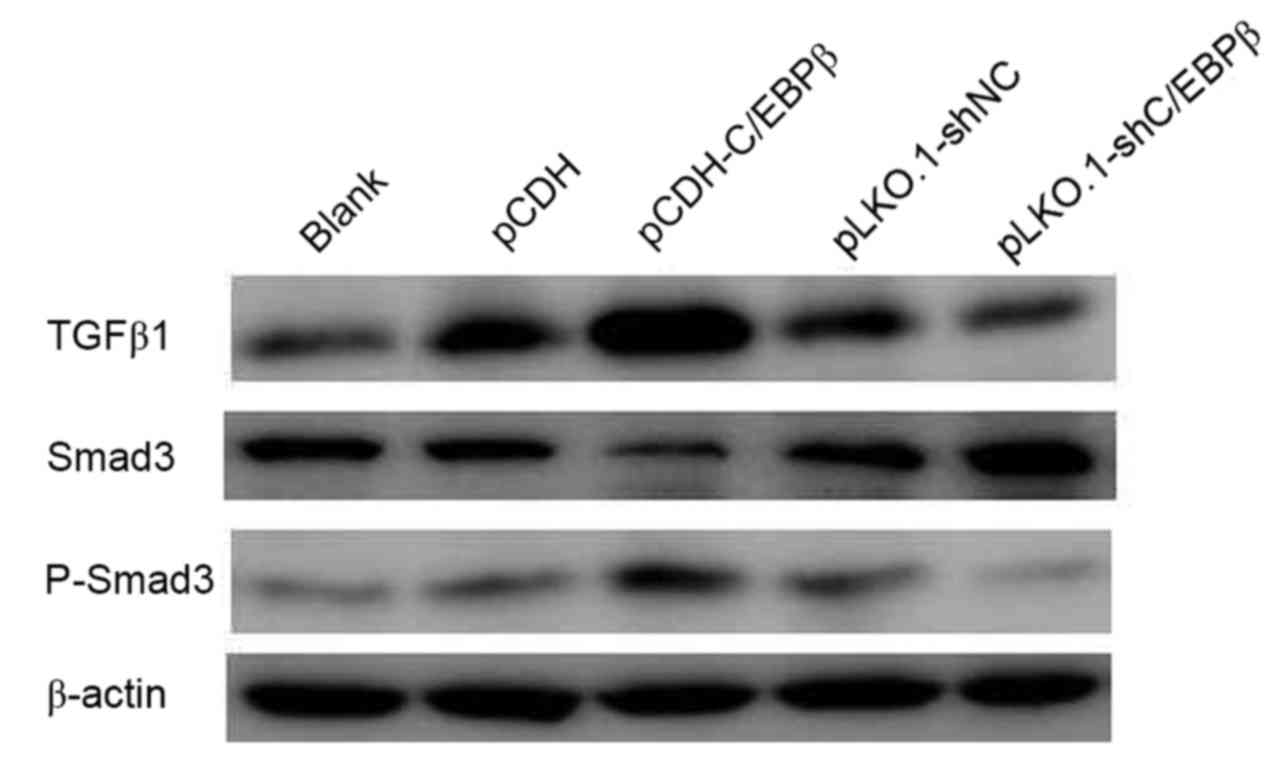
Effect of overexpression of C/EBPβ on
TGFβ1-Smad3 signaling in MDA-MB-468 cells
To investigate the mechanism by which C/EBPβ affects
MDA-MB-468 cells, the protein expression levels of TGFβ1, P-Smad3
and Smad3 were detected. Western blotting indicated that TGFβ1 and
P-Smad3 protein expression levels were increased in the pCDH-C/EBPβ
group and decreased in pLKO.1-shC/EBPβ group when compared with
those in the respective control groups (Fig. 5). Conversely, a marked reduction in
the protein expression level of Smad3 was observed in the
pCDH-C/EBPβ group, whereas Smad3 expression was notably increased
in the pLKO.1-shC/EBPβ group compared with the respective control
groups (Fig. 5).
Discussion
In recent years, the morbidity of breast cancer has
increased, despite considerable achievements being made in tumor
therapy (21). It has been reported
that C/EBPβ expression may be used to predict the overall survival
in breast cancer patients, since it affects tumor growth and
metastasis formation in mice (22).
In the present study, the effect of C/EBPβ on a breast cancer cell
line and the molecular mechanism of its effect were investigated.
The present results showed that overexpression of C/EBPβ
significantly increased breast cancer cell viability and
concomitantly decreased the cell apoptosis rate. Western blotting
results suggested that overexpression of C/EBPβ increased the
protein expression levels of TGFβ1 and P-Smad3 and repressed the
expression of Smad3.
The present study demonstrated that the
overexpression of C/EBPβ promoted viability and inhibited
apoptosis, and knockdown of C/EBPβ inhibited cell viability and
promoted apoptosis in MDA-MB-468 cells. The role of C/EBPβ in the
regulation of cell viability and apoptosis in the present study is
partly consistent with previous studies in other cancer cell lines.
For example, Buck et al (23)
observed that the expression of C/EBPβ had an important role in the
survival of hepatic stellate cells with DNA damage caused by
CCl4-induced free radicals. The ability of cancer cells
to invade into surrounding tissue is affected by their motility as
well as their ability to penetrate through tissue barriers, such as
the extracellular matrix. Wound assay results in the present study
suggested that the overexpression of C/EBPβ increased cell
motility, suggesting the potential role of C/EBPβ in cancer
metastasis.
Previous studies have suggested that C/EBPβ has
antiproliferative effects in various normal cells. For example,
C/EBPβ was demonstrated to inhibit cell proliferation through
interacting with the retinoblastoma protein family to suppress the
expression of E2F target genes (S-phase genes) in primary
fibroblasts (24). Furthermore,
decreased expression of C/EBPβ enabled primary keratinocytes to
resist calcium-induced growth arrest (25). The results of the present study are
consistent with other data. In a previous study, knockdown of
C/EBPβ significantly inhibited glioblastoma cell proliferation and
invasion, and also prolonged survival in a murine brain tumor model
(26). Additionally, another study
demonstrated that C/EBPβ−/− mice were completely
resistant to tumorigenic agents applied to the skin due to the
Ras-dependent promotion of apoptosis (8). Also, growth-promoting activity of
C/EBPβ has been observed in mammary epithelial cells (27) and hepatic cells (28). By consideration of the previous and
present study results, it may be proposed that C/EBPβ has an
accelerative role in breast cancer development by controlling cell
proliferation and apoptosis.
The present study investigated the molecular
mechanism of C/EBPβ and indicated that C/EBPβ promoted cell
viability in MDA-MB-468 cells. Furthermore, the results indicated
that it affected the expression levels of proteins associated with
the TGF-β1-Smad3 signaling pathway. A previous study showed that
increased C/EBPβ expression elevated transcription activity of the
TGF-β1 promoter in human primary astrocytes and microglial cells
(29). The present results
demonstrated that overexpression of C/EBPβ increased the expression
of TGF-β1 and P-Smad3, suggesting that C/EBPβ promoted TGF-β-Smad3
signaling by activating the TGFβ1 promoter in breast cancer.
Notably, the present study also revealed that the protein
expression levels of Smad3 were strongly inhibited in the
pCDH-C/EBPβ group. Smad3 has previously been demonstrated to
inhibit the activity of C/EBPβ on transcribing monocyte
chemoattractant protein-1, inducible nitric oxide synthase and
haptoglobin promoter by interacting with C/EBPβ (30–32).
Additionally, the MH2 domain of Smad3 has been shown to combine
with C/EBPβ, and subsequently decrease the association between
C/EBPβ and TGF-β1 promoter, suggesting that the increased
expression of Smad3 inhibited the activity of C/EBPβ on the TGF-β1
promoter (29,33). Thus, it may be speculated that
inhibited Smad3 expression further promoted the activity of C/EBPβ
on the TGF-β1 promoter via a positive feedback mechanism. A
previous study indicated that TGFβ had an antiproliferative effect
on epithelial cells, astrocytes and immune cells; however, in
certain malignant tumors the capacity of TGFβ to inhibit
proliferation is selectively lost (34) and TGF-β1 is considered as an
oncogenic factor (16). The present
results indicated that C/EBPβ may promote MDA-MB-468 cell growth
through activating TGF-β signaling.
In conclusion, the present study demonstrated that
C/EBPβ has a crucial role in regulating breast cancer cell growth
through the activation of TGF-β-Smad3 signaling. These findings
suggest that C/EBPβ may be a potential therapeutic target for
breast cancer, although in vivo studies in animal models are
required to confirm this.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osada S, Yamamoto H, Nishihara T and
Imagawa M: DNA binding specificity of the CCAAT/enhancer-binding
protein transcription factor family. J Biol Chem. 271:3891–3896.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poli V: The role of C/EBP isoforms in the
control of inflammatory and native immunity functions. J Biol Chem.
273:29279–29282. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramji DP and Foka P:
CCAAT/enhancer-binding proteins: Structure, function and
regulation. Biochem J. 365:561–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cortés-Canteli M, Pignatelli M, Santos A
and Perez-Castillo A: CCAAT/enhancer-binding protein beta plays a
regulatory role in differentiation and apoptosis of neuroblastoma
cells. J Biol Chem. 277:5460–5467. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cortes-Canteli M, Wagner M, Ansorge W and
Pérez-Castillo A: Microarray analysis supports a role for
ccaat/enhancer-binding protein-beta in brain injury. J Biol Chem.
279:14409–14417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu S, Yoon K, Sterneck E, Johnson PF and
Smart RC: CCAAT/enhancer binding protein-beta is a mediator of
keratinocyte survival and skin tumorigenesis involving oncogenic
Ras signaling. Proc Natl Acad Sci USA. 99:pp. 207–212. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ewing SJ, Zhu S, Zhu F, House JS and Smart
RC: C/EBPbeta represses p53 to promote cell survival downstream of
DNA damage independent of oncogenic Ras and p19 (Arf). Cell Death
Differ. 15:1734–1744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zahnow CA: CCAAT/enhancer binding proteins
in normal mammary development and breast cancer. Breast Cancer Res.
4:113–121. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomis RR, Alarcón C, Nadal C, Van Poznak C
and Massagué J: C/EBPbeta at the core of the TGFbeta cytostatic
response and its evasion in metastatic breast cancer cells. Cancer
Cell. 10:203–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johansson J, Berg T, Kurzejamska E, Pang
MF, Tabor V, Jansson M, Roswall P, Pietras K, Sund M, Religa P and
Fuxe J: MiR-155-mediated loss of C/EBPβ shifts the TGF-β response
from growth inhibition to epithelial-mesenchymal transition,
invasion and metastasis in breast cancer. Oncogene. 32:5614–5624.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coyle-Rink J, Sweet T, Abraham S, Sawaya
B, Batuman O, Khalili K and Amini S: Interaction between TGFbeta
signaling proteins and C/EBP controls basal and Tat-mediated
transcription of HIV-1 LTR in astrocytes. Virology. 299:240–247.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaminska B, Wesolowska A and Danilkiewicz
M: TGF beta signalling and its role in tumour pathogenesis. Acta
Biochim Pol. 52:329–337. 2005.PubMed/NCBI
|
|
15
|
Seoane J: The TGFBeta pathway as a
therapeutic target in cancer. Clin Transl Oncol. 10:14–19. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wrana JL, Attisano L, Cárcamo J, Zentella
A, Doody J, Laiho M, Wang XF and Massagué J: TGF beta signals
through a heteromeric protein kinase receptor complex. Cell.
71:1003–1014. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang P, Yu J, Yin Q, Li W, Ren X and Hao
X: Rosiglitazone suppresses glioma cell growth and cell cycle by
blocking the transforming growth factor-beta mediated pathway.
Neurochem Res. 37:2076–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eichhorn PJ, Rodón L, Gonzàlez-Juncà A,
Dirac A, Gili M, Martínez-Sáez E, Aura C, Barba I, Peg V, Prat A,
et al: USP15 stabilizes TGF-β receptor I and promotes oncogenesis
through the activation of TGF-β signaling in glioblastoma. Nat Med.
18:429–435. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaminska B, Kocyk M and Kijewska M: TGF
beta signaling and its role in glioma pathogenesis. Adv Exp Med
Biol. 986:171–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parks RM and Cheung KL: Patient pathway
for breast cancer: Turning points and future aspirations. Future
Oncol. 11:1059–1070. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurzejamska E, Johansson J, Jirström K,
Prakash V, Ananthaseshan S, Boon L, Fuxe J and Religa P: C/EBPβ
expression is an independent predictor of overall survival in
breast cancer patients by MHCII/CD4-dependent mechanism of
metastasis formation. Oncogenesis. 3:e1252014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buck M, Poli V, Hunter T and Chojkier M:
C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase
inhibitory box critical for cell survival. Mol Cell. 8:807–816.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sebastian T, Malik R, Thomas S, Sage J and
Johnson PF: C/EBPbeta cooperates with RB:E2F to implement
Ras(V12)-induced cellular senescence. EMBO J. 24:3301–3312. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu S, Oh HS, Shim M, Sterneck E, Johnson
PF and Smart RC: C/EBPbeta modulates the early events of
keratinocyte differentiation involving growth arrest and keratin 1
and keratin 10 expression. Mol Cell Biol. 19:7181–7190. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aguilar-Morante D, Cortes-Canteli M,
Sanz-Sancristobal M, Santos A and Perez-Castillo A: Decreased
CCAAT/enhancer binding protein β expression inhibits the growth of
glioblastoma cells. Neuroscience. 176:110–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bundy LM and Sealy L: CCAAT/enhancer
binding protein beta (C/EBPbeta)-2 transforms normal mammary
epithelial cells and induces epithelial to mesenchymal transition
in culture. Oncogene. 22:869–883. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buck M, Poli V, van der Geer P, Chojkier M
and Hunter T: Phosphorylation of rat serine 105 or mouse threonine
217 in C/EBP beta is required for hepatocyte proliferation induced
by TGF alpha. Mol Cell. 4:1087–1092. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abraham S, Sweet T, Khalili K, Sawaya BE
and Amini S: Evidence for activation of the TGF-beta1 promoter by
C/EBPbeta and its modulation by Smads. J Interferon Cytokine Res.
29:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abraham S, Sweet T, Sawaya BE, Rappaport
J, Khalili K and Amini S: Cooperative interaction of C/EBP beta and
Tat modulates MCP-1 gene transcription in astrocytes. J
Neuroimmunol. 160:219–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feinberg MW, Watanabe M, Lebedeva MA,
Depina AS, Hanai J, Mammoto T, Frederick JP, Wang XF, Sukhatme VP
and Jain MK: Transforming growth factor-beta1 inhibition of
vascular smooth muscle cell activation is mediated via Smad3. J
Biol Chem. 279:16388–16393. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zauberman A, Lapter S and Zipori D: Smad
proteins suppress CCAAT/enhancer-binding protein (C/EBP) beta- and
STAT3-mediated transcriptional activation of the haptoglobin
promoter. J Biol Chem. 276:24719–24725. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wotton D and Massagué J: Smad
transcriptional corepressors in TGF beta family signaling. Curr Top
Microbiol Immunol. 254:145–164. 2001.PubMed/NCBI
|
|
34
|
Seoane J: Escaping from the TGFbeta
anti-proliferative control. Carcinogenesis. 27:2148–2156. 2006.
View Article : Google Scholar : PubMed/NCBI
|