Introduction
Diabetes leads to vascular changes and dysfunction
with the most critical factor of insulin resistance, and morbidity
and mortality in diabetic patients are mainly caused by diabetic
complications (1). According to the
International Diabetes Federation Atlas in 2014, the estimated
diabetes prevalence for 2015 has risen to 387 million, representing
8.3% of the world's adult population, and it has been predicted
that by 2035 the number of people with diabetes will have risen to
592 million (2–4).
Sirtuin 1 (SIRT1), a nicotinamide adenine
dinucleotide (NAD+)-dependent histone deacetylase, is
involved in the regulation of metabolism, cell survival,
differentiation and longevity (5),
and exerts beneficial effects on glucose-lipid homeostasis and
insulin sensitivity in diabetes in both animal studies and clinical
research (6,7). This suggests that SIRT1 may be a
promising novel therapeutic target for diabetic complications and
recognized as a key regulator of vascular endothelial homeostasis,
controlling angiogenesis, endothelial senescence and dysfunction
(8). It is reported that the
activation of SIRT1 prevents hyperglycemia-induced vascular cell
senescence and protects against vascular dysfunction in mice with
diabetes, which suggests a protective role of SIRT1 in the
pathogenesis of diabetic vasculopathy (9). According to another study,
mitochondrial biogenesis can be enhanced by resveratrol through the
5′-adenosine monophosphate-activated kinase/SIRT1 pathway in muscle
and liver, resulting in extension of life span or amelioration of
high-fat diet-induced metabolic impairment, including obesity and
insulin resistance (10).
Recently, it has been indicated that SIRT1 regulates
endothelial nitric oxide synthase (eNOS), which generates
endothelial nitric oxide (NO) (11).
Furthermore, another study demonstrated that the production of NO,
stimulated by caloric restriction, increases SIRT1 expression,
which implies that eNOS may be involved in regulation of the
expression of SIRT1 in murine white adipocytes (12). Therefore, it may be hypothesized that
there is an interaction between eNOS and SIRT1. The aim of the
present study was to investigate the potential effects of
overexpressed eNOS on cell proliferation and apoptosis with SIRT1
activation in the mouse pancreatic β cell line, Min6. The results
of the present study indicated that SIRT1 expression was
significantly upregulated following eNOS recombinant plasmid
transfection, which induced cell proliferation and decreased cell
apoptosis. Furthermore, we explored the underlying mechanisms
between eNOS and SIRT1, which demonstrated that there was a strong
interaction between these two proteins.
Materials and methods
Plasmid construction
The mouse eNOS cDNA (BC052636.1) was cloned into the
eukaryotic expression vector pcDNA3.0 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) using NotI and
HindIII restriction sites. The forward primer was
5′-ATAAGAATGCGGCCGCATGGGCAACTTGAAGAGTGTGG-3′ (NotI site
underlined) and the reverse primer was
5′-CCCAAGCTTTCAGGAACCAGGTGTTTCTTGGG-3′ (HindIII site
underlined). Subsequently, the recombinant vector was amplified in
DH5α Escherichia coli (Sangon Biotech Co., Ltd., Shanghai,
China) and plasmid DNA was purified with an endotoxin-free plasmid
purification kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's instructions. Each segment was amplified by
polymerase chain reaction (PCR) with Takara LA Taq or Primestar
(Takara Bio Inc., Otsu, Japan) and cloned into the pcDNA3.0 vector.
Furthermore, all joints in the constructs were confirmed by
sequencing (Sangon Biotech Co., Ltd.). The present study was
approved by the Ethics Committee of Guilin Medical University
(Guilin, China).
Cell line culture and treatment
The mouse pancreatic β cell line Min6 (American Type
Culture Collection, Manassas, VA, USA) was maintained in Dulbecco's
modified Eagle's medium with high glucose, 10% heat inactivated
newborn calf serum, and 1% antibiotic-antimycotic solution
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere of 95% air and 5% CO2. Min6 cells
were treated with pcDNA3.0-eNOS plasmid (1 µg) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 24, 48, 72
and 96 h. Empty pcDNA3.0 vector (1 µg) was used as a negative
control.
Cell proliferation activity
Min6 cells were seeded at a density of
1.0×105 cells/ml in 6-well plates in order to achieve
~50% confluence the next day, and were then transfected with
pcDNA3.0-eNOS (50 µM). Thereafter, 100 µl cell counting kit-8
(CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
solution was added to each well and were incubated with the cells
for 1 h. The absorbance was then measured at 450 nm using a
microplate reader.
Apoptosis assay
Following plasmid transfection for 24 h, the
apoptotic cells were quantified using an Annexin V/propidium iodide
(PI) apoptosis kit (Multi Sciences Biotech Co., Ltd., Hangzhou,
China). Min6 cells were collected, washed with PBS and resuspended
in 200 µl binding buffer containing 5 µl Annexin V (10 µg/ml) for
10 min in the dark. The cells were then incubated with 10 µl PI (20
µg/ml), and the samples were immediately analyzed using flow
cytometry (Beckman Coulter, Inc., Brea, CA, USA). Data acquisition
and analysis was performed using CellQuest software (CellQuest Pro,
version 5.1; BD Biosciences, Franklin Lakes, NJ, USA).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using a UNIQ-10 column and
TRIzol total RNA isolation kit (Sangon Biotech Co., Ltd.). In
total, 3 µg total RNA was used for reverse transcription in a
reaction volume of 20 µl using Cloned AMV Reverse Transcriptase
(Invitrogen; Invitrogen; Thermo Fisher Scientific, Inc.). In
addition, 3 µl cDNA was used for qPCR using a Takara Ex Taq RT-PCR
version 2.1 kit (Takara Bio, Inc.). Gene-specific PCR primers for
eNOS, SIRT1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
are listed in Table I, and PCR
signals were detected with a DNA Engine Opticon 2 Continuous
Fluorescence Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). PCR was monitored for 45 cycles using an
annealing temperature of 60°C. At the end of the PCR cycles, melt
curve analysis and 2% agar electrophoresis was performed in order
to assess the purity of the PCR products. Negative control
reactions (no template) were routinely included to monitor
potential contamination of reagents. Relative quantities of eNOS
mRNA and SIRT1 mRNA were normalized to the quantity of GAPDH mRNA
using the 2−∆∆Cq method (13).
 | Table I.Sequences of the primers used for eNOS
and SIRT1 detection by quantitative polymerase chain reaction. |
Table I.
Sequences of the primers used for eNOS
and SIRT1 detection by quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| eNOS | Forward:
TTCCTGGACATCACTTCCCC |
|
| Reverse:
CTTCCATTCTTCGTAGCGCC |
| SIRT1 | Forward:
TGCCATCATGAAGCCAGAGA |
|
| Reverse:
AACATCGCAGTCTCCAAGGA |
| GAPDH | Forward:
CGGAGTCAACGGATTTGGTCGTAT |
|
| Reverse:
AGCCTTCTCCATGGTGGTGAAGAC |
Protein isolation and western blot
analysis
The concentration of protein in extracts from mice
pancreatic β cells was determined using a bicinchoninic acid assay
kit (Pierce; Thermo Fisher Scientific, Inc.). Protein lysates were
prepared using NP-40 buffer (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) on ice for 20 min before centrifuging at 30, 000 ×
g for 30 min at 4°C. The supernatant was subsequently
separated by 10% SDS-PAGE (20 µg protein/lane) followed by transfer
onto nitrocellulose membranes. Western blot analysis was performed
as previously described (14), and
the signals were detected using an enhanced chemiluminescence (ECL)
system (Millipore, Billerica, MA, USA). Antibodies used in the
present study included anti-mouse eNOS (cat. no. SA-201-0100;
1:4,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-mouse SIRT1 (cat. no. sc-74465; 1:4,000; Santa Cruz
Biotechnology, Inc.) and anti-mouse GAPDH (cat. no. sc-365062;
1:20,000; Santa Cruz Biotechnology, Inc.). The blots were
subsequently incubated with a secondary antibody (horseradish
peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG; cat. no.
111-035-003; 1:10,000; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA) at room temperature for 2 h. and exposed to
ECL reagent according to the manufacturer's protocol for the
detection of protein expression.
Co-immunoprecipitation (Co-IP)
assay
Protein-protein interactions were analyzed by co-IP
experiments. The cells were collected, and the proteins were
solubilized in IP buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 1%
protease inhibitor mixture]. Co-IP was performed according to the
standard protocols previously described (15,16).
Briefly, the Min6 cells were washed with ice-cold PBS twice and
lysed in ice-cold radioimmunoprecipitation assay buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology, Haimen, China). The
supernatant was incubated with 10 µl mouse anti-eNOS (cat. no.
SA-201-0100; 1:100; Santa Cruz Biotechnology, Inc.) or mouse
anti-SIRT1 (cat. no. sc-74465; 1:100; Santa Cruz Biotechnology,
Inc.) monoclonal antibody and agarose ligand (Catch and Release
v2.0; EMD Millipore, Billerica, MA, USA), followed by incubation at
4°C for 1 h. The immune complexes were washed, eluted by boiling in
2X SDS sample buffer, separated by 10% SDS-PAGE and transferred
onto membranes. The blots were then incubated with a
horseradish-peroxidase-conjugated secondary antibody (cat. no.
BHR101-1; 1:10,000; Bersee, Biomart company, Beijing, China) at
room temperature for 1 h and exposed to ECL reagent for the
detection of protein expression according to the manufacturer's
protocol.
Statistical analysis
Differences between each group were expressed as the
mean ± standard deviation. Statistical significance was assessed by
Student's t-test and one-way analysis of variance followed by a
Tukey post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Validation assay of eNOS
overexpression at the mRNA and protein levels
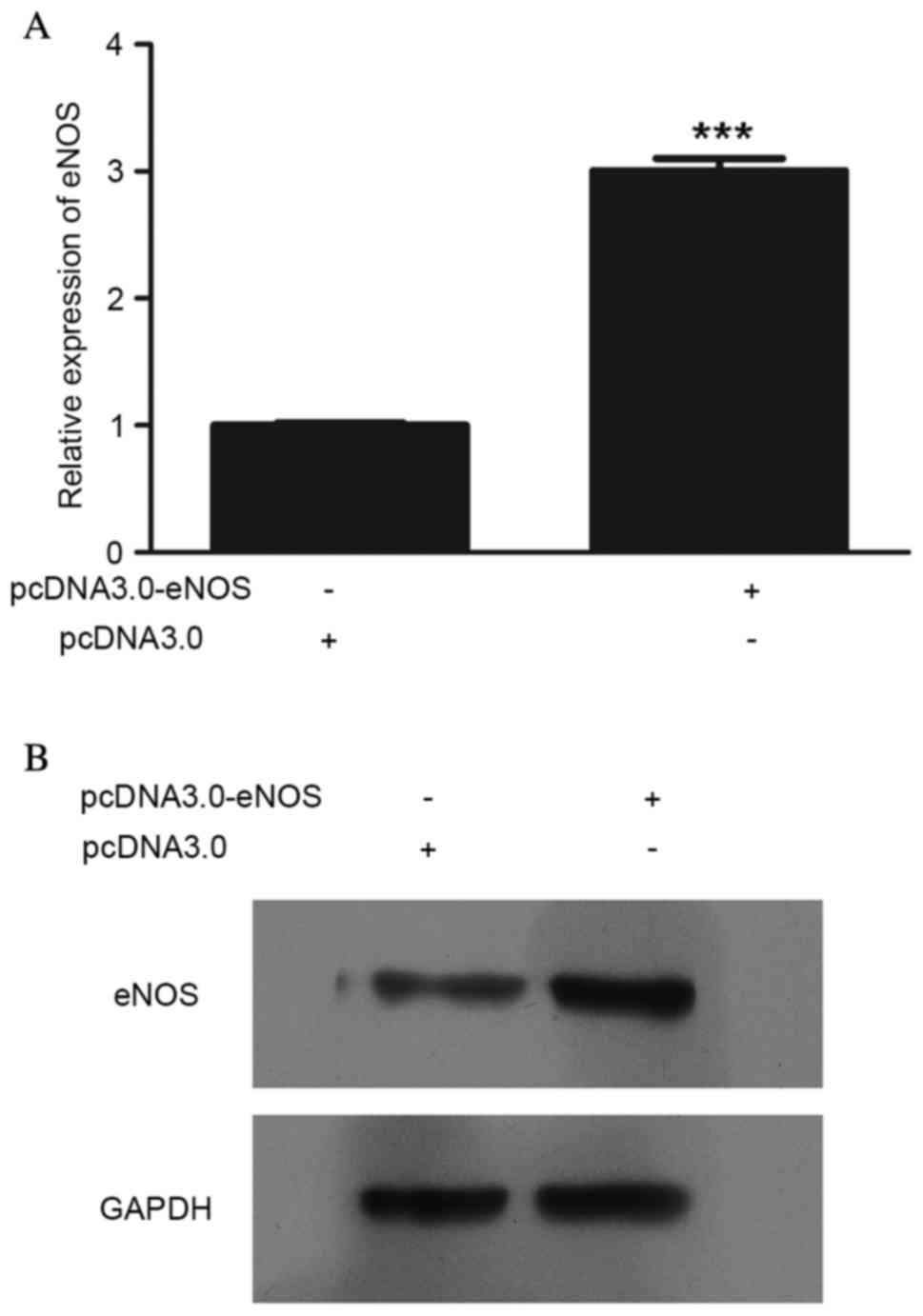
eNOS expression was upregulated following
pcDNA3.0-eNOS transfection (Fig. 1).
The mRNA (P<0.001; Fig. 1A) and
protein (Fig. 1B) levels of eNOS in
Min6 cells were clearly increased following pcDNA3.0-eNOS
transfection for 24 h.
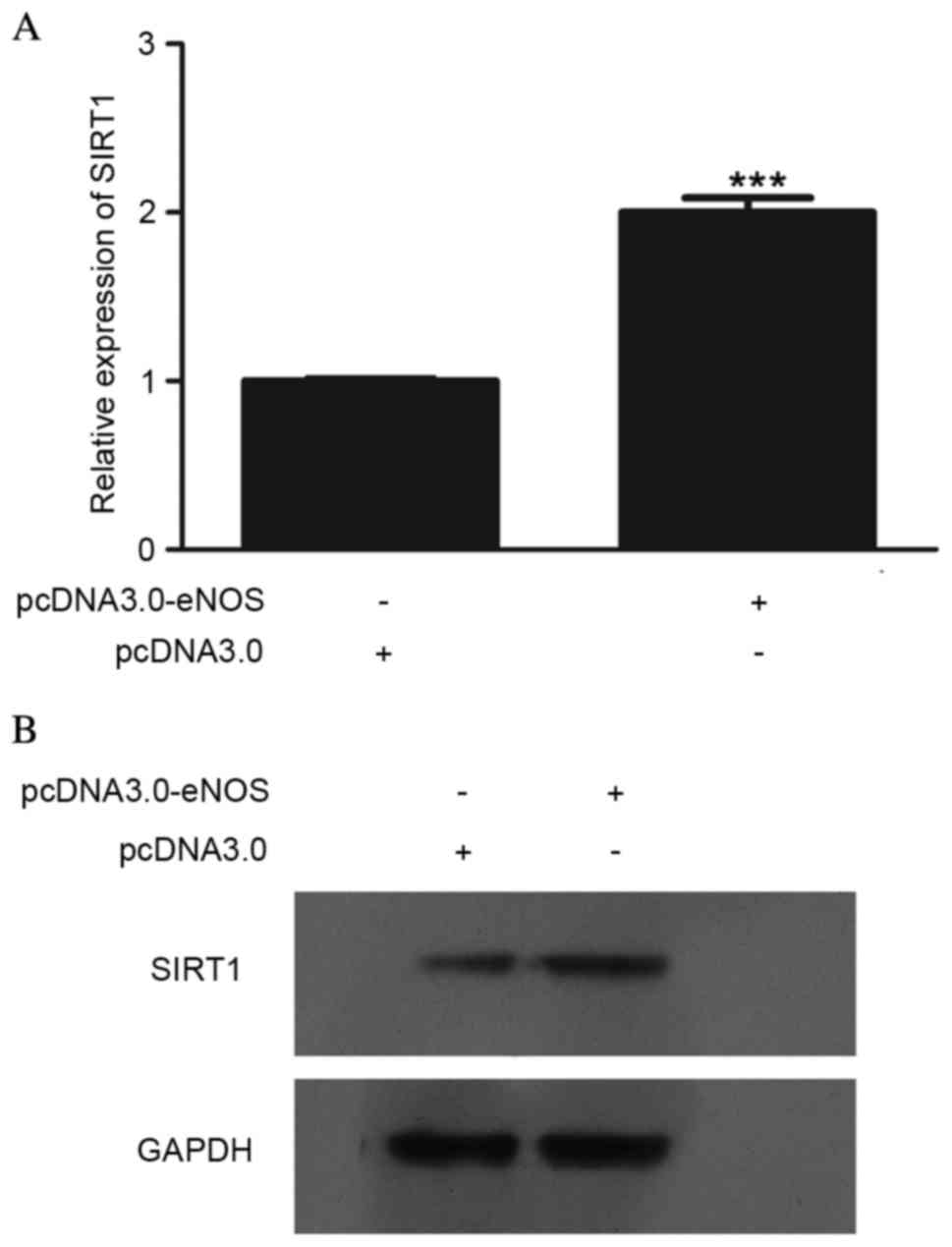
Effect of overexpressed eNOS on SIRT1
expression at the mRNA and protein levels
In order to determine the effect of eNOS on SIRT1,
SIRT1 expression at the mRNA and protein levels was detected, as
shown in Fig. 2. The mRNA
(P<0.001; Fig. 2A) and protein
(Fig. 2B) levels in Min6 cells were
clearly upregulated following transfection with pcDNA3.0-eNOS for
24 h.
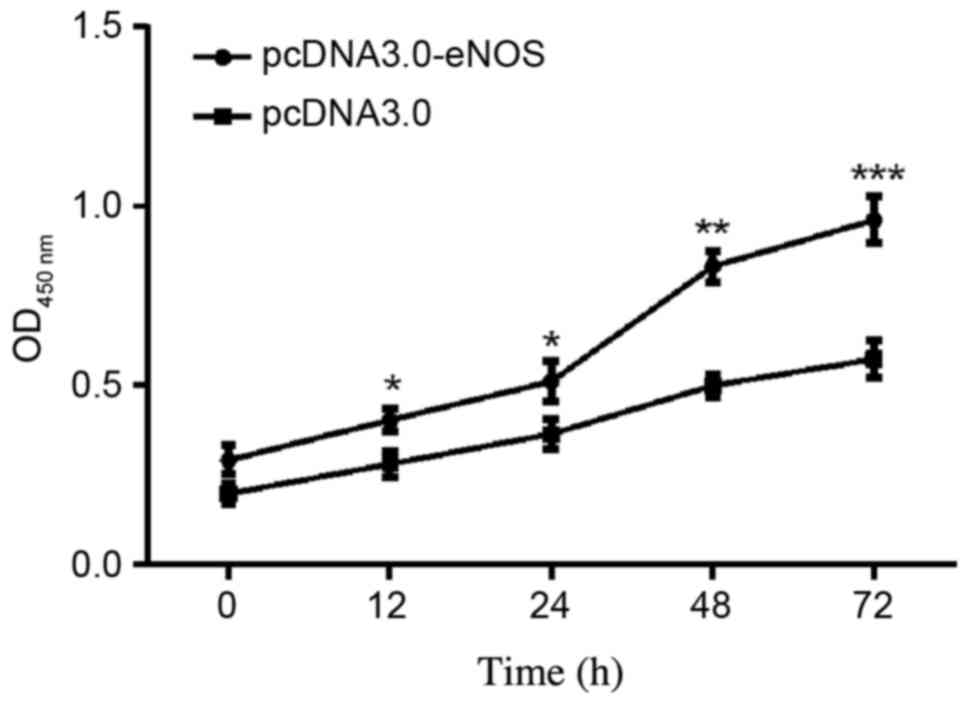
Effect of overexpressed eNOS on Min6
cell proliferation
The effects of eNOS overexpression on Min6 cell
proliferation were examined. As shown in the CCK-8 assay results in
Fig. 3, the cellular population was
increased time-dependently in the pcDNA3.0-eNOS transfection group
compared with the negative control (pcDNA3.0) group, particularly
at 48 h (P<0.01) and 72 h (P<0.001).
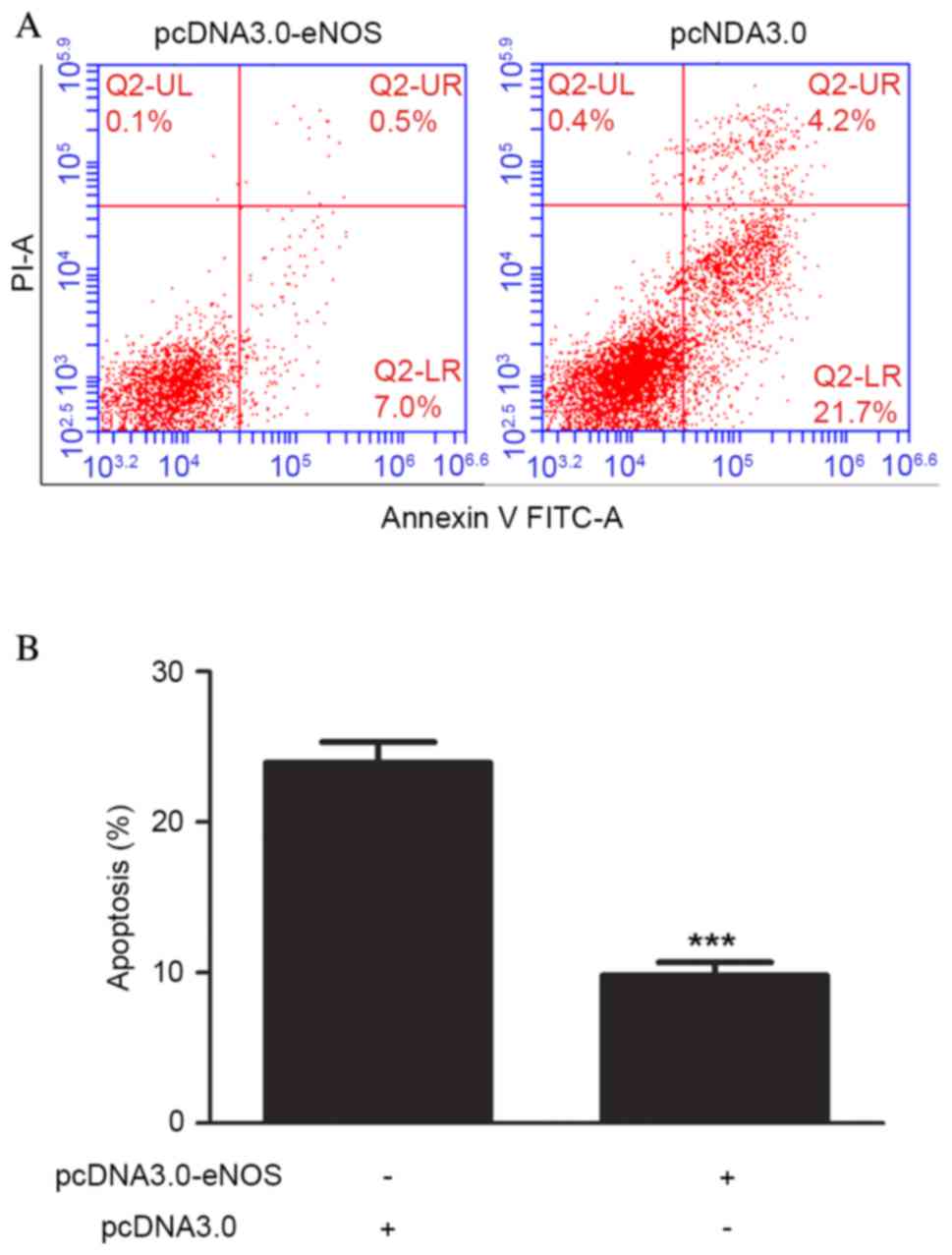
Overexpression of eNOS reduced
apoptosis of the Min6 cell line
Cell apoptosis was analyzed using flow cytometry
following pcDNA3.0-eNOS transfection for 24 h based on the CCK-8
results. Exposure of Min6 cells to the recombinant eNOS plasmid
inhibited the apoptosis of the cells compared with that in the
negative control group. Furthermore, the occurrence of apoptosis
was significantly lower (P<0.001) in the pcDNA3.0-eNOS group
compared with the pcDNA3.0 group (negative control), as shown in
Fig. 4.
Interaction between eNOS and
SIRT1
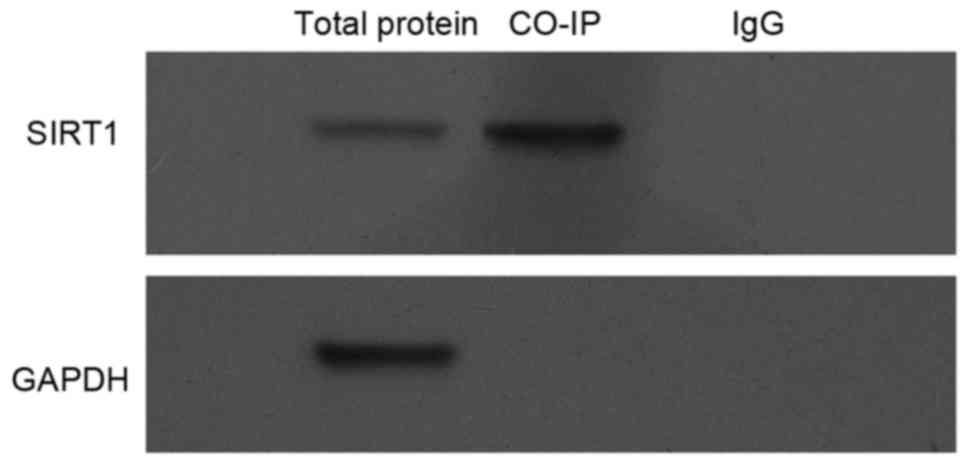
Finally, the possibility that eNOS interacts with
SIRT1 was investigated. To this end, Min6 cell lysates were
harvested, and then were subjected to Co-IP and the results in
Fig. 5 clearly indicated that there
is an interaction between exogenous SIRT1 and eNOS proteins.
Discussion
In the present study, eNOS has been indicated to be
a regulator of SIRT1 in the mouse pancreatic β cell line Min6. This
conclusion is based on several novel observations. Firstly,
evidence that SIRT1 was activated by overexpressed eNOS was
provided, which was achieved through recombinant plasmid
transfection. Secondly, overexpressed eNOS promoted mouse
pancreatic β cell proliferation and protected mouse pancreatic β
cells from cell apoptosis. Thirdly, a strong protein-protein
interaction between eNOS and SIRT1 was demonstrated. Furthermore,
the present study implied that overexpressed eNOS may induce SIRT1
activation, which is indicated to have a protective role in Min6
cells.
NO is produced by three isoforms of NO synthase
(NOS): Neuronal nNOS (NOS I), inducible iNOS (NOS II) and
endothelial eNOS (NOS III) (17).
Under physiological conditions, vascular NO is mostly produced by
eNOS. It is known that NO reduces oxidative stress and the
progression of atherosclerosis (18), and exerts cardioprotective and
vasoprotective effects in endothelial cells though a regulatory
effect for inhibition of platelet aggregation, blood flow and
inflammatory cell adhesion (19,20),
while SIRT1 has previously been identified as a critical regulator
of vascular endothelial homeostasis, controlling angiogenesis,
endothelial senescence and dysfunction (8,21). A
recent study has shown that SIRT1 is an endogenous protective
molecule and a promising novel therapeutic target against
myocardial ischemia/reperfusion (MI/R)-induced injury, which
reduces oxidative stress and diabetes-exacerbated injury via the
activation of eNOS in diabetic rats (11). As Lemarie et al (22) reported, some effective antioxidants,
including resveratrol, have been shown to act via the stimulation
of endothelial SIRT1, which regulates endothelium-dependent
vasodilation and bioavailable NO, stimulates eNOS activity and
increases endothelial NO. However, eNOS-mediated NO also regulates
SIRT1 expression during the aging of endothelial cells; the
uncoupling of eNOS results in decreased expression of SIRT1, and
ultimately to increased stress-induced senescence (22). It has also been reported that the
interaction of SIRT1 with eNOS is important in the augmentation of
the protective effect of statins against endothelial senescence, as
it was shown that testosterone induced eNOS activity, and
subsequently increased SIRT1 expression in endothelial cells
(23). In type 2 diabetic rats, a
reduction in the cellular redox status and an increase in oxidant
stress may work together to reduce vascular SIRT1 expression
(24–26). Furthermore, eNOS expression levels
have also been shown to be low within cerebral arteries, which
implies a connection between SIRT1 and eNOS (27).
In conclusion, the aim of the present study was to
explore the interaction of SIRT1 and eNOS and elucidate the
mechanism and potential therapeutic targets in diabetes. The
results showed that eNOS was upregulated significantly through
recombinant plasmid transfection, and subsequently increased SIRT1
expression through direct protein-protein interaction in the mouse
pancreatic β cell line, Min6, which may assist future research.
Further research may also focus on the identification of an
effective drug playing a protective and therapeutic role for
diabetes through the targeting of eNOS and regulation of SIRT1.
Acknowledgements
The present study was supported by the Science and
Technology Research Projects of Guangxi Universities (grant no.
YB2014266) and the National Natural Science Foundation of China
(grant nos. 81460164 and 31060161).
References
|
1
|
Pernický M, Papinčák J, Reptová A, Kiňová
S and Murín J: What may cause diabetes. Vnitr Lek. 61:447–450.
2015.PubMed/NCBI
|
|
2
|
Linnenkamp U, Guariguata L, Beagley J,
Whiting DR and Cho NH: The IDF Diabetes Atlas methodology for
estimating global prevalence of hyperglycaemia in pregnancy.
Diabetes Res Clin Pract. 103:186–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan JC, Cho NH, Tajima N and Shaw J:
Diabetes in the Western Pacific Region - past, present and future.
Diabetes Res Clin Pract. 103:244–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
IDF Diabetes Atlas Group, . Update of
mortality attributable to diabetes for the IDF Diabetes Atlas:
Estimates for the year 2013. Diabetes Res Clin Pract. 109:461–465.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Codocedo JF, Allard C, Godoy JA,
Varela-Nallar L and Inestrosa NC: SIRT1 regulates dendritic
development in hippocampal neurons. PLoS One. 7:e470732012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitada M and Koya D: SIRT1 in type 2
diabetes: Mechanisms and therapeutic potential. Diabetes Metab J.
37:315–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitada M, Kume S, Kanasaki K,
Takeda-Watanabe A and Koya D: Sirtuins as possible drug targets in
type 2 diabetes. Curr Drug Targets. 14:622–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ota H, Eto M, Ogawa S, Iijima K, Akishita
M and Ouchi Y: SIRT1/eNOS axis as a potential target against
vascular senescence, dysfunction and atherosclerosis. J Atheroscler
Thromb. 17:431–435. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orimo M, Minamino T, Miyauchi H, Tateno K,
Okada S, Moriya J and Komuro I: Protective role of SIRT1 in
diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol.
29:889–894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiota A, Shimabukuro M, Fukuda D, Soeki
T, Sato H, Uematsu E, Hirata Y, Kurobe H, Maeda N, Sakaue H, et al:
Telmisartan ameliorates insulin sensitivity by activating the
AMPK/SIRT1 pathway in skeletal muscle of obese db/db mice.
Cardiovasc Diabetol. 11:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding M, Lei J, Han H, Li W, Qu Y, Fu E, Fu
F and Wang X: SIRT1 protects against myocardial
ischemia-reperfusion injury via activating eNOS in diabetic rats.
Cardiovasc Diabetol. 14:1432015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lempiainen J, Finckenberg P, Mervaala EE,
Sankari S, Levijoki J and Mervaala EM: Caloric restriction
ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS
pathway and enhanced autophagy. Acta Physiol (Oxf). 208:410–421.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan X, Hao Q, Mu Y, Timani KA, Ye L, Zhu Y
and Wu J: Nucleocapsid protein of SARS-CoV activates the expression
of cyclooxygenase-2 by binding directly to regulatory elements for
nuclear factor-kappa B and CCAAT/enhancer binding protein. Int J
Biochem Cell Biol. 38:1417–1428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alessi DR, Cuenda A, Cohen P, Dudley DT
and Saltiel AR: PD 098059 is a specific inhibitor of the activation
of mitogen-activated protein kinase kinase in vitro and in vivo. J
Biol Chem. 270:27489–27494. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohr T, Van Soeren M, Graham TE and Kjaer
M: Caffeine ingestion and metabolic responses of tetraplegic humans
during electrical cycling. J Appl Physiol (1985). 85:979–985.
1998.PubMed/NCBI
|
|
17
|
Eghbalzadeh K, Brixius K, Bloch W and
Brinkmann C: Skeletal muscle nitric oxide (NO) synthases and
NO-signaling in ‘diabesity’-what about the relevance of exercise
training interventions? Nitric Oxide. 37:28–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strawn WB: Pathophysiological and clinical
implications of AT(1) and AT(2) angiotensin II receptors in
metabolic disorders: Hypercholesterolaemia and diabetes. Drugs 62.
1:31–41. 2002.(In French). View Article : Google Scholar
|
|
19
|
Schafer A and Bauersachs J: Endothelial
dysfunction, impaired endogenous platelet inhibition and platelet
activation in diabetes and atherosclerosis. Curr Vasc Pharmacol.
6:52–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Monti M, Solito R, Puccetti L, Pasotti L,
Roggeri R, Monzani E, Casella L and Morbidelli L: Protective
effects of novel metal-nonoates on the cellular components of the
vascular system. J Pharmacol Exp Ther. 351:500–509. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paschalaki KE, Starke RD, Hu Y, Mercado N,
Margariti A, Gorgoulis VG, Randi AM and Barnes PJ: Dysfunction of
endothelial progenitor cells from smokers and chronic obstructive
pulmonary disease patients due to increased DNA damage and
senescence. Stem Cells. 31:2813–2826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lemarie CA, Shbat L, Marchesi C, Angulo
OJ, Deschênes ME, Blostein MD, Paradis P and Schiffrin EL: Mthfr
deficiency induces endothelial progenitor cell senescence via
uncoupling of eNOS and downregulation of SIRT1. Am J Physiol Heart
Circ Physiol. 300:H745–H753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ota H, Akishita M, Akiyoshi T, Kahyo T,
Setou M, Ogawa S, Iijima K, Eto M and Ouchi Y: Testosterone
deficiency accelerates neuronal and vascular aging of SAMP8 mice:
Protective role of eNOS and SIRT1. PLoS One. 7:e295982012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tajbakhsh S, Aliakbari K, Hussey DJ, Lower
KM, Donato AJ and Sokoya EM: Differential telomere shortening in
blood versus arteries in an animal model of type 2 diabetes. J
Diabetes Res. 2015:1538292015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gencoglu H, Tuzcu M, Hayirli A and Sahin
K: Protective effects of resveratrol against streptozotocin-induced
diabetes in rats by modulation of visfatin/sirtuin-1 pathway and
glucose transporters. Int J Food Sci Nutr. 66:314–320. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tajbakhsh N and Sokoya EM: Sirtuin 1 is
upregulated in young obese Zucker rat cerebral arteries. Eur J
Pharmacol. 721:43–48. 2013. View Article : Google Scholar : PubMed/NCBI
|