Introduction
Polycomb group (PcG) genes are required for
maintenance of the correct spatial and temporal expression of
homeotic genes during development (1). They were originally identified in
Drosophila as transcriptional repressor genes, and
subsequently have been detected in numerous vertebrates and
invertebrates (1). Sex comb on
midleg (SCM) is a PcG gene, and encodes transcriptional repressors
required for appropriate development in flies and mammals (1–3). SCM is
required for the recruitment and repressive function of polycomb
repressive complex 1 (PRC1) and PRC2 (1), and contains two malignant brain tumor
(MBT) repeats, a domain of unknown function (DUF3588), an SPM [also
known as sterile α motif (SAM)] domain and two zinc fingers
(2,3). SCM exerts a repressive effect on target
genes through the actions of MBT and SPM domains, as do other PcG
proteins (4,5). Notably, abnormal SCM function may be
involved in tissue growth and certain cancers (6).
The sex comb on midleg like-2 (SCML2) gene is one of
the four homologs of Scm (the others being SCML1, SCM homolog 1 and
SCM-like with four MBT domains) in mammals (7–11). SCML2
has been identified as a human gene in the Xp22 region that encodes
a protein of 700 amino acids (7). In
previous proteomic studies in which possible markers of pancreatic
cancer were investigated (12–14), it
was incidentally observed, by immunohistology, that the SCML2
protein was specifically expressed in human polypeptide
hormone-producing tissues (pancreatic islet cells and islet-cell
carcinoma), but was not expressed in other pancreatic epithelial
cells. As a group, neuroendocrine tumors (NETs) secrete various
different peptide hormones, and the aforementioned observation
suggests that SCML2 could be a useful histologic marker for
NETs.
NETs are a heterogeneous group of tumors associated
with a wide variety of biological changes occurring in the cells of
the endocrine system (15). The
molecular genetic mechanism by which NETs develop is complex and
remains largely unknown (16). The
majority of NETs were once considered carcinoid tumors, but
recently the term ‘neuroendocrine’ has been accepted for use
instead of ‘carcinoid’ to more appropriately describe the malignant
potential of these tumors (17).
Although NETs may develop in almost any organ of the body, they
predominate within the pancreas and the gastrointestinal tract.
Gastroenteropancreatic (GEP)-NETs are considered to be rare, with
an incidence of 1 per 100,000 individuals for pancreatic tumors and
1.95–2.5 per 100,000 individuals for gastrointestinal tumors
(15). During the last three
decades, however, the reported incidence of GEP-NETs has increased
worldwide due to improvements in diagnostic tools and clinical
awareness of them (15). According
to the latest 2010 World Health Organization (WHO) classification
(18), GEP-NETs are divided into
three types, namely well-differentiated NET, poorly differentiated
neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma,
and their pathology can be further graded as G1 [<2 mitoses/10
high power fields (HPFs) and/or Ki-67 index ≤2%], G2 (2–20
mitoses/10 HPFs and/or Ki-67 index between 3 and 20%) and G3 (≥21
mitoses/10 HPFs and Ki-67 index >20%) (18–22).
Despite recent advances in the diagnosis and
treatment of GEP-NETs, their early diagnosis remains challenging as
the majority of patients lack typical symptoms (23). It is crucial to develop new markers
that are comparable with and even better than currently available
neuroendocrine markers, such as synaptophysin (Syn) and
chromogranin A (CgA), for use in the diagnosis and prognosis of
GEP-NETs. To contribute to the achievement of this goal, in the
present study, SCLM2 expression in GEP-NETs was detected using
immunohistochemistry, the diagnostic value of SCLM2 was compared
with that of Syn or CgA, and the correlations of SCLM2 with
clinicopathological variables and with the prognosis of GEP-NETs
were further investigated.
Patients and methods
Patients and tissue samples
A total of 64 paired tumor tissues and adjacent
non-tumorous tissues were obtained from paraffin-embedded tissues
of patients with GEP-NET (gastric, colorectal or pancreatic NET)
who had undergone surgical resection at the Affiliated Hospital of
Nantong University (Nantong, China) between January 2009 and
January 2014 and had been evaluated and classified according to the
WHO 2010 classification (18). The
tumor grading of these cases was based on proliferation and mitotic
count. Representative 1.5–2 mm tissue cores from each specimen were
selected for immunohistochemistry. Personal information and
clinicopathological data of the patients were obtained from
electronic hospital records and pathology reports. Patients with a
history of other cancers or who had received chemotherapy or
radiotherapy prior to surgery were excluded from the present study.
Follow-up information was collected by telephone interview or mail
survey, and used for patient survival analysis. For all patients
analyzed, the male/female ratio was 36:28, and the ages ranged from
17 to 86 years (median, 48 years). The personal information,
clinical variables and pathological findings of the patients are
summarized in Table I. In addition,
the tissue samples of 10 gastric adenocarcinoma, 10 colorectal
adenocarcinoma and 10 pancreatic adenocarcinoma cases, which had
been histologically documented according to the WHO histological
classifications of tumors, were used as controls. The present study
was approved by the Ethics Committee of the Affiliated Hospital of
Nantong University. Informed consent was obtained from all
individual participants included in the study.
 | Table I.Associations of sex comb on midleg
like-2 expression with clinicopathological parameters. |
Table I.
Associations of sex comb on midleg
like-2 expression with clinicopathological parameters.
|
| Grading |
|
|---|
|
|
|
|
|---|
| Parameters | n | Positive (%) | − | + | ++ | +++ | Z | P-value |
|---|
| Gender | 1.263 | 0.207 |
|
Male | 36 | 32 (88.9) | 4 | 16 | 12 | 4 |
|
|
|
Female | 28 | 26 (92.9) | 2 | 12 | 4 | 10 |
|
|
| Age (years) | 0.551 | 0.582 |
|
≤60 | 38 | 34 (89.5) | 4 | 16 | 12 | 6 |
|
|
|
>60 | 26 | 22 (84.6) | 2 | 12 | 4 | 8 |
|
|
| Tumor location | 0.400 | 0.690 |
|
Esophagus/stomach | 10 | 10 (100.0) | 0 | 4 | 0 | 6 |
|
|
|
Intestine | 40 | 34 (85.0) | 6 | 18 | 12 | 4 |
|
|
|
Pancreas | 14 | 14 (100.0) | 0 | 6 | 4 | 4 |
|
|
| Tumor diameter
(cm) | 0.501 | 0.616 |
| ≤3 | 50 | 44 (88.0) | 6 | 20 | 14 | 10 |
|
|
|
>3 | 14 | 14 (100.0) | 0 | 8 | 2 | 4 |
|
|
| Pathological
type | 2.370 | 0.020 |
|
NET | 36 | 30 (83.3) | 6 | 18 | 8 | 4 | 3.254a | 0.001a |
|
NEC | 26 | 26 (100.0) | 0 | 8 | 8 | 10 |
|
|
|
MANEC | 2 | 2 (100.0) | 0 | 2 | 0 | 0 |
|
|
| Pathological
grade | 4.320 | <0.001 |
| G1 | 24 | 18 (75.0) | 6 | 14 | 4 | 0 | 3.103b | 0.002b |
| G2 | 14 | 14 (100.0) | 0 | 6 | 4 | 4 | 4.277c |
<0.001c |
| G3 | 26 | 26 (100.0) | 0 | 8 | 8 | 10 |
| Depth of
invasion | 2.205 | 0.027 |
|
T1-T2 | 24 | 20 (83.3) | 4 | 12 | 6 | 2 |
|
|
|
T3-T4 | 40 | 38 (95.0) | 2 | 16 | 10 | 12 |
|
|
| Lymph node
metastasis | 1.798 | 0.072 |
|
Absent | 42 | 36 (85.7) | 6 | 18 | 12 | 6 |
|
|
|
Present | 22 | 22 (100.0) | 0 | 10 | 4 | 8 |
|
|
| Distant
metastasis | 0.684 | 0.494 |
|
Absent | 58 | 52 (89.7) | 6 | 24 | 14 | 14 |
|
|
|
Present | 6 | 6 (100.0) | 0 | 4 | 2 | 0 |
|
|
| TNM stage | 2.698 | 0.007 |
| I,
II | 28 | 22 (78.6) | 6 | 12 | 8 | 2 |
|
|
| III,
IV | 36 | 36 (100.0) | 0 | 16 | 8 | 12 |
|
|
Immunohistochemistry
All paraffin-embedded tissue samples were fixed in
4% paraformaldehyde solution for 24 h at room temperature and
embedded in paraffin, including 64 matched pairs of GEP-NET tissues
and adjacent non-tumorous tissues, 10 gastric adenocarcinoma
tissues, 10 colorectal adenocarcinoma tissues and 10 pancreatic
adenocarcinoma tissues, were sectioned to 4-µm thickness and
mounted on clean, charged microscope slides and then heated in a
tissue-drying oven for 45 min at 60°C. The sections were
deparaffinized in xylene, rehydrated through graded alcohol, and
then rinsed with deionized water. Endogenous peroxidase activity
was quenched with 0.3% hydrogen peroxide for 10 min at room
temperature and blocked with 5% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in PBS for 20 min at
room temperature. For antigen retrieval, the sections were heated
for 30 min in a microwave oven in a preheated 0.01 M citrate buffer
(pH 6.0, C6H8O7, H2O
0.378 g, Na3C6H5O7,
2H2O 2.412 g, and ddH2O to 1 l). The sections
were incubated overnight at 4°C with mouse monoclonal antibodies
against SCML2 (sc-271228), Syn (sc-398017) and CgA (sc-393941; all
1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
respectively. Afterwards, sections were further reacted with mouse
IgGκ binding protein-HRP (sc-516102; 1:25; Santa Cruz
Biotechnology, Inc.) for 30 min at room temperature. Slides were
stained with diaminobenzidine and counterstained with hematoxylin
as described previously (13,14).
Sections were observed under a light microscope. The evaluation
criteria of immunohistochemistry were as follows: Staining
intensity was scored as 0, negative; 1, weak; 2, medium; and 3,
strong; and staining extent was scored as 0, 0; 1, 1–25; 2, 26–50;
3, 51–75; and 4, 76–100% according to the percentage of the
positive staining areas in relation to the entire carcinoma area.
The final result was expressed as the sum of the intensity score
and the extent score, which was graded as: -, score 0–2; +, score 3
or 4; ++, score 5 or 6; and +++, score 7. Tumors with a final
staining score of ≥3 were considered positive. The
immunohistochemical results were evaluated independently by two
pathologists who were blinded to the patients' clinical and
pathological data.
Statistical analysis
SPSS v15.0 software (SPSS Inc., Chicago, IL, USA)
was used for statistical analysis. Materials with ranked data were
tested with the rank sum test. The correlations between SCML2, Syn
and CgA were tested by Spearman rank correlation. χ2
test or Fisher's exact test was used for any 2×2 tables. The
association between clinical parameters and SCML2 expression was
analyzed with a rank sum test. Survival analysis was performed
using Kaplan-Meier survival plots, and comparisons between groups
were made with the log-rank test. Multivariate analysis was
performed using Cox's proportional hazards model, and the risk
ratio and its 95% confidence interval were recorded for each
marker. P<0.05 was considered to indicate a statistically
significant result in all analyses.
Results
Expression of SCML2, Syn and CgA in
paired GEP-NET tissues and adjacent non-tumorous tissues
Using immunohistochemistry (Fig. 1), strong SCML2 staining was observed
predominantly in the cell nuclei of gastric, colorectal or
pancreatic NET tissues (Fig. 1Aa-1,
Aa-2, Ba-1, Ba-2, Ca-1 and Ba-2). By contrast, SCML2 staining
was negative in the adjacent non-tumorous tissues of gastric- and
colorectal-NET patients (Fig. 1Ab and
Bb), and in gastric or colorectal adenocarcinoma tissues
(Fig. 1Ac and Bc). Although SCML2
expression was low in islet cells, the final staining scores of
SCML2 expression were negative (scores <3) in the adjacent
non-tumorous tissues of patients with pancreatic NET (Fig. 1Cb) or pancreatic adenocarcinoma
(Fig. 1Cc). Furthermore, staining of
Syn and CgA was detected within the cytoplasm of NET cells (data
not shown). Following a comparison of the staining results, it was
noted that either the positive rate or the staining intensity of
SCML2 [90.6% (58/64), more than half of which were graded ++ and
+++] was higher compared with that of Syn [84.4% (54/64), the
majority of which were graded +] or than that of CgA [71.9%
(46/64), the majority of which were graded +] in 64 GEP-NET samples
(Z=4.179, P<0.001 and Z=5.449, P<0.001, respectively;
Table II).
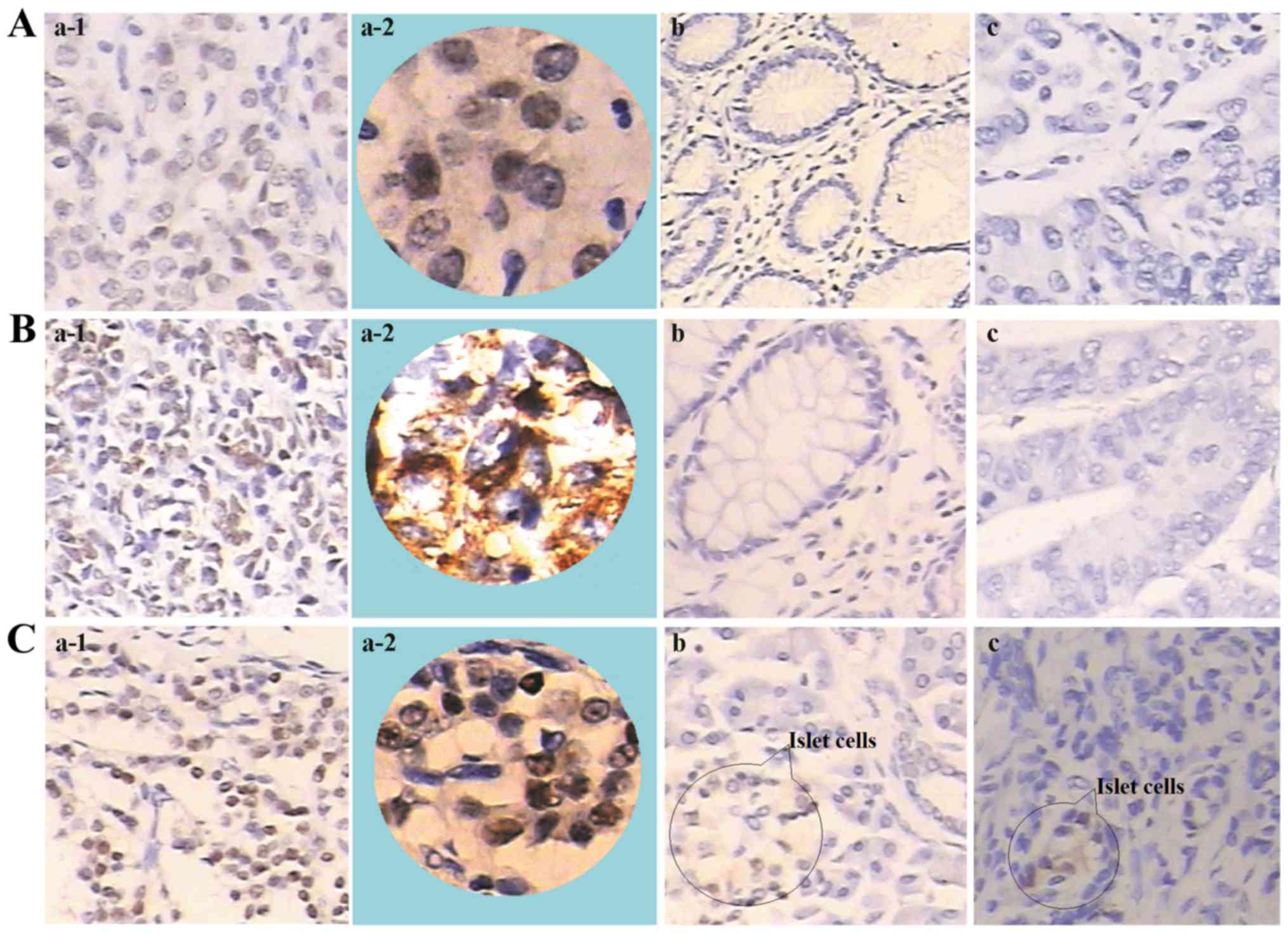 | Figure 1.Immunohistochemical staining of SCML2
in gastroenteropancreatic neuroendocrine tumors, adjacent
non-tumorous tissues and adenocarcinoma tissues. (A) Gastric
tissues: Gastric neuroendocrine tumor tissue at (a-1)
magnification, ×200 and (a-2) magnification, ×400, (b) adjacent
non-tumorous tissue and (c) gastric carcinoma (both magnification,
×200). (B) Colorectal tissues: Colorectal neuroendocrine tumor
tissue at (a-1) magnification, ×200 and (a-2) magnification, ×400,
(b) adjacent non-tumorous tissue and (c) colorectal carcinoma (both
magnification, ×200). (C) Pancreatic tissues: Pancreatic
neuroendocrine tumor tissue at (a-1) magnification, ×200 and (a-2)
magnification, ×400, (b) adjacent non-tumorous tissue and (c)
pancreatic carcinoma (both magnification, ×200). High expression of
SCML2 detected in gastric, colorectal and pancreatic neuroendocrine
tumors. No expression of SCML2 in the adjacent non-tumorous tissues
of gastric and colorectal neuroendocrine tumors and in gastric and
colorectal adenocarcinomas. Low expression of SCML2 in islet cells
was detected; however, the final staining scores of SCML2 were
negative (<3) in the adjacent non-tumorous tissues of pancreatic
neuroendocrine tumors and in pancreatic adenocarcinomas. SCML2, sex
comb on midleg like-2. |
 | Table II.Expression of SCML2, Syn and CgA in
GEP-NETs (n=64), adjacent non-tumorous tissues (n=64) and
adenocarcinoma tissues (n=30). |
Table II.
Expression of SCML2, Syn and CgA in
GEP-NETs (n=64), adjacent non-tumorous tissues (n=64) and
adenocarcinoma tissues (n=30).
|
| GEP-NETs | Adjacent
non-tumorous tissues | Adenocarcinoma |
|---|
|
|
|
|
|
|---|
| Marker | Positive | − | + | ++ | +++ | − | + | ++ | +++ | − | + | ++ | +++ |
|---|
| SCML2 | 58/64 | 6 | 28 | 16 | 14 | 64 | 0 | 0 | 0 | 30 | 0 | 0 | 0 |
| Syn | 54/64 | 10 | 48 | 4 | 2 | 64 | 0 | 0 | 0 | 30 | 0 | 0 | 0 |
| CgA | 46/64 | 18 | 44 | 2 | 0 | 64 | 0 | 0 | 0 | 30 | 0 | 0 | 0 |
Complementary value of SCML2, Syn and
CgA for diagnosis of GEP-NETs
Spearman rank correlation analysis was performed on
the expression of SCML2, Syn and CgA. The results demonstrated that
SCML2 was not correlated with either Syn (r=0.2132, P=0.091) or CgA
(r=0.0429, P=0.736), suggesting that these three markers are
complementary in the diagnosis of GEP-NETs. The sensitivity and
accuracy of GEP-NET diagnosis significantly increased due to the
combination of information on SCML2 and Syn or on SCML2 and CgA
(Table III). The sensitivity and
accuracy of each marker alone for the diagnosis of GEP-NETs was not
high, but the combination of SCML2 with Syn or with CgA increased
the sensitivity and accuracy to 100%.
 | Table III.Complementary value of SCML2, Syn and
CgA in the diagnosis of gastroenteropancreatic neuroendocrine
tumors. |
Table III.
Complementary value of SCML2, Syn and
CgA in the diagnosis of gastroenteropancreatic neuroendocrine
tumors.
| Marker |
Sensitivitya (%) | Specificity
(%) | Accuracy (%) |
|---|
| SCML2 | 58/64
(90.6)b | 94/94 (100) | 152/158
(96.2)b |
| Syn | 54/64
(84.4)b | 94/94 (100) | 148/158
(93.7)b |
| CgA | 46/64
(71.9)b | 94/94 (100) | 140/158
(88.6)b |
| SCML2 + Syn | 64/64 (100) | 94/94 (100) | 158/158 (100) |
| SCML2 + CgA | 64/64 (100) | 94/94 (100) | 158/158 (100) |
Associations of SCML2 expression with
clinicopathological parameters in GEP-NETs
Correlation analysis between SCML2 expression and
clinicopathological parameters (Table
I) indicated that SCML2 expression was independent of patient
gender, age, tumor location, tumor diameter, lymphatic or distant
metastasis (P=0.207, 0.582, 0.690, 0.616, 0.072 and 0.494,
respectively), but was significantly related to pathological type
(P=0.020), pathological grade (P<0.001), depth of invasion
(P=0.027) and TNM stage (P=0.007).
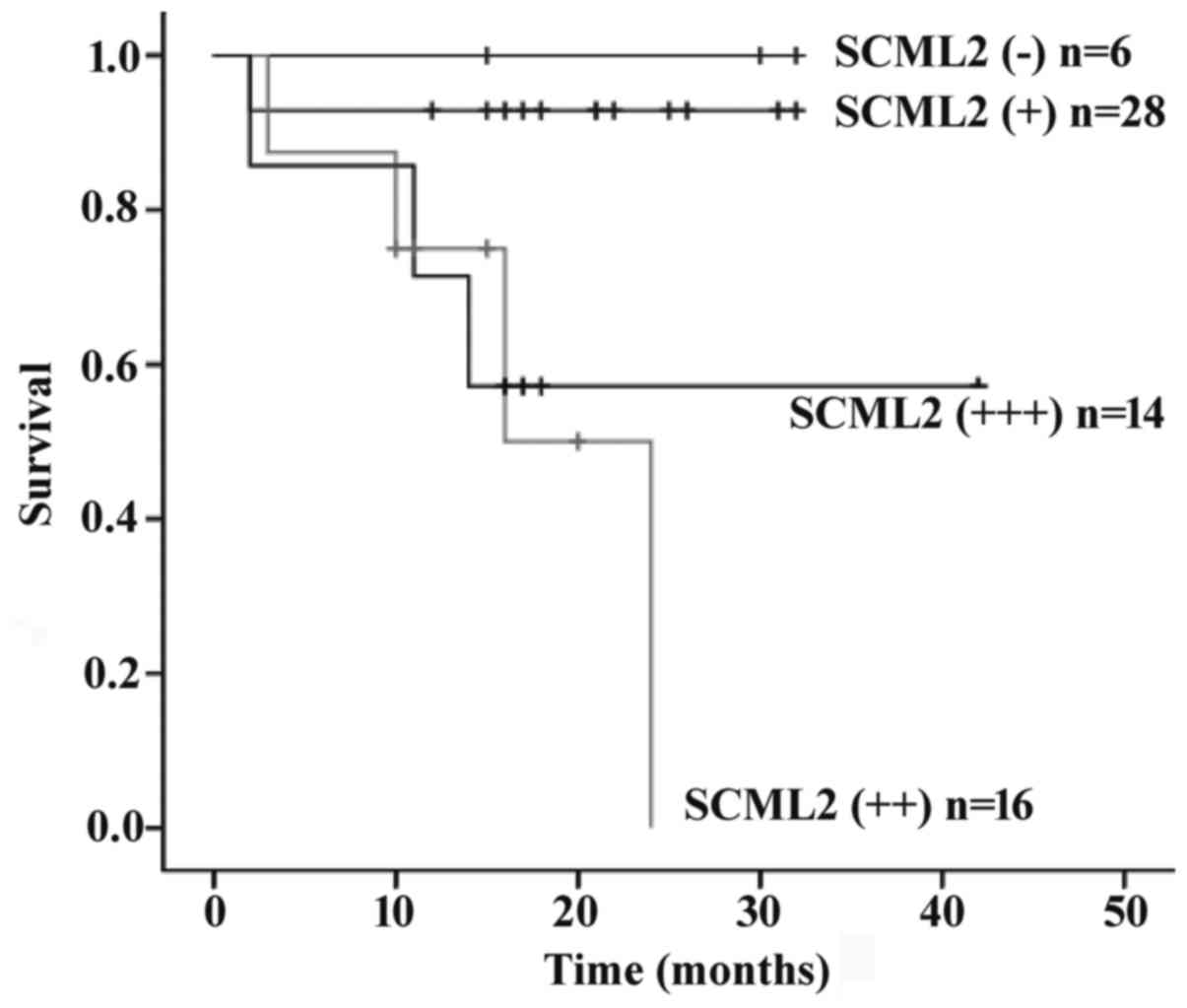
Survival analysis
At the end of follow-up, survival information was
available for all patients. The overall survival time was a median
of 1.42 years (range, 0.16–3.60 years), and 16 patients succumbed
to tumor progression during follow-up (25.0%). Univariate analysis
for overall survival using the log-rank test revealed that age
(P=0.003), pathological type (P<0.001), pathological grade
(P<0.001), depth of invasion (P=0.014), lymph node metastasis
(P<0.001), Syn expression (P<0.001), CgA expression
(P<0.001) or SCML2 expression (P=0.001) may serve as significant
prognostic predictors. Kaplan-Meier survival curves demonstrated
that SCML2 expression was associated with prognosis (Fig. 2). Multivariate analysis with the Cox
proportional hazards model revealed that with the exception of age
(P=0.006) and pathological grade (P=0.015), SCML2 (P=0.628) and
other prognostic markers tested by univariate analysis were not
independent predictors of the survival of patients with GET-NET
(Table IV).
 | Table IV.Univariate and multivariate analyses
of prognostic variables. |
Table IV.
Univariate and multivariate analyses
of prognostic variables.
|
|
Univariatea |
Multivariateb |
|---|
|
|
|
|
|---|
| Variables | χ2 | P-value | Risk ratio | 95% CI | Z | P-value |
|---|
| Gender,
male/female | 0.642 | 0.423 | – | – | – | – |
| Tumor location,
stomach/intestine/pancreas | 5.970 | 0.051 | – | – | – | – |
| Tumor diameter,
≤3/>3 cm | 3.457 | 0.063 | – | – | – | – |
| Distant metastasis,
absent/present | 0.239 | 0.566 | – | – | – | – |
| TNM stage, I,
II/III, IV | 3.760 | 0.053 | – | – | – | – |
| Age, ≤60/>60
years | 8.733 | 0.003 | 13.976 | 2.128–91.786 | 7.542 | 0.006 |
| Pathological type,
NET/NEC + MANEC | 15.678 | <0.001 | 0.216 | 0.024–1.974 | 1.844 | 0.175 |
| Pathological grade,
G1/G2/G3 | 15.648 | <0.001 | 20.591 | 1.814–233.712 | 5.956 | 0.015 |
| Depth of invasion,
T1,T2/T3,T4 | 6.040 | 0.014 | 0.393 | 0.035–4.395 | 0.574 | 0.449 |
| Lymph node
metastasis, absent/present | 12.275 | <0.001 | 1.564 | 0.264–9.284 | 0.243 | 0.622 |
| Syn,
-/+/++/+++ | 47.565 | <0.001 | 1.681 | 0.469–6.025 | 0.637 | 0.425 |
| CgA,
-/+/++/+++ | 20.991 | <0.001 | 0.402 | 0.137–1.184 | 2.731 | 0.098 |
| SCML2,
-/+/++/+++ | 16.271 | 0.001 | 0.824 | 0.378–1.799 | 0.235 | 0.628 |
Discussion
SCML2 is a gene with homologies to the
Drosophila Scm gene, and located in close proximity to
SCML1, forming a gene cluster in Xp22; in primates, this gene
cluster may have originated prior to primate divergence (9). SCML2 is specifically expressed in germ
cells of mice, and loss of SCML2 reduces sperm production. SCML2
also regulates the epigenetic state of sex chromosomes during male
meiosis (24–26). Human SCML2 gene encodes two protein
isoforms: SCML2A (chromatin-bound) and SCML2B (nucleoplasmic). The
former interacts with PRC1 and binds to non-coding RNAs in cultured
immortal or cancer cells (27,28),
whereas the latter regulates the cell cycle by binding to
cyclin-dependent kinase 2 (29).
Accordingly, SCML2 plays a role in modulating the cell-cycle
machinery and impacts the cellular activity when it is ectopically
expressed in transformed or cancer cells (28,29). In
addition, there is evidence suggesting that SCML2 may be involved
in human tumors, including malignant pediatric brain tumors, acute
myeloid leukemia and human hepatocellular carcinoma (30–36). The
above knowledge about SCML2, together with previous findings on
SCML2 expression in islet-cell carcinoma (12–14),
inspired the investigation of a more explicit linkage between SCML2
and GEP-NETs, a tumor with an increasing incidence worldwide, in
the present study.
SCML2 expression was detected in GEP-NET tissues
using immunohistochemical staining in the present study, and then
SCML2 was compared with existing markers in terms of diagnostic
value. At present, GEP-NETs are commonly diagnosed by the
immunostaining of Syn and CgA, which are widely accepted as classic
NET markers (37). In the present
study, the positivity rates of Syn and CgA in GEP-NETs were
calculated to be 84.4 and 71.9%, respectively, and GEP-NET tissues
exhibited weak positive staining with the majority of them being
rated +. The results were essentially consistent with previously
reported 76.19 and 72.62% positivity rates for Syn and CgA,
respectively, in 168 cases of GEP-NET (23,38). By
contrast, it was noted in the present study that the positive rate
of SCML2 in GEP-NETs was 90.6% and more than half of the staining
intensity was graded ++ or +++. Therefore, it could be suggested
that SCML2 was at least comparable to Syn or CgA and even somewhat
better than either of them for the diagnosis of GEP-NETs.
Furthermore, Spearman rank correlation analysis indicated that
SCML2 was not correlated with either Syn or CgA, implying the three
markers are complementary to one another for the diagnosis of
GEP-NETs. The combined use of SCML2 with Syn or with CgA increased
the diagnostic sensitivity and accuracy to 100%. Therefore, the
simultaneous detection of SCML2, Syn and CgA may be considered a
preferred method for the diagnosis of GEP-NETs.
The present study also investigated the association
between clinicopathological variables and the survival of patients.
The results demonstrated that SCML2 expression was significantly
correlated with pathological type, pathological grade, depth of
invasion and TNM stage. Although Cox regression analysis revealed
that age, pathological type, pathological grade, depth of invasion,
lymph node metastasis, Syn expression, CgA expression and SCML2
expression were not independent unfavorable prognostic factors in
GEP-NETs, they were consistently associated with survival time in
GEP-NET cases, suggesting that SCML2 may play a role in the
pathogenesis and development of GEP-NETs and its effect on survival
time may be synergistic with that of other clinicopathological
variables.
In summary, the present study provided original
findings suggesting the potential of SCML2 as a valuable marker for
GEP-NETs; however, the prognostic value of SCML2 may be poor
because it is ubiquitously expressed in the majority of GEP-NETs.
The joint use of SCML2 with Syn or CgA would clearly improve the
diagnostic efficiency for GEP-NETs. Therefore, simultaneous
measurement of SCML2 with Syn or CgA is recommended. Due to the
limited number of tumor samples examined in the present study, the
discriminating ability of markers was not validated, and further
large-scale studies are required to gain an improved understanding
of the role of SCML2 in GEP-NETs.
Acknowledgements
This study was supported by grants from the Natural
Youth Science Foundation of China (grant no. 81502055), the Natural
Science Foundation of Jiangsu Province (grant no. BK20161286), the
Health Project of Jiangsu Province (grant no. H201624) and the
Social Development Foundation of Nantong City (grant nos.
MS22016056, MS22015062, HS2014072 and MS22015044).
Glossary
Abbreviations
Abbreviations:
|
SCM
|
sex comb on midleg
|
|
SCML2
|
sex comb on midleg like-2
|
|
GEP-NETs
|
gastroenteropancreatic neuroendocrine
tumors
|
|
Syn
|
synaptophysin
|
|
CgA
|
chromogranin A
|
|
PcG
|
polycomb group
|
References
|
1
|
Wang L, Jahren N, Miller EL, Ketel CS,
Mallin DR and Simon JA: Comparative analysis of chromatin binding
by Sex Comb on Midleg (SCM) and other polycomb group repressors at
a Drosophila Hox gene. Mol Cell Biol. 30:2584–2593. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sathyamurthy A, Allen MD, Murzin AG and
Bycroft M: Crystal structure of the malignant brain tumor (MBT)
repeats in Sex Comb on Midleg-like 2 (SCML2). J Biol Chem.
278:46968–46973. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bornemann D, Miller E and Simon J: The
Drosophila Polycomb group gene Sex comb on midleg (Scm) encodes a
zinc finger protein with similarity to polyhomeotic protein.
Development. 122:1621–1630. 1996.PubMed/NCBI
|
|
4
|
Grimm C, de Ayala Alonso AG, Rybin V,
Steuerwald U, Ly-Hartig N, Fischle W, Müller J and Müller CW:
Structural and functional analyses of methyl-lysine binding by the
malignant brain tumour repeat protein Sex comb on midleg. EMBO Rep.
8:1031–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peterson AJ, Mallin DR, Francis NJ, Ketel
CS, Stamm J, Voeller RK, Kingston RE and Simon JA: Requirement for
sex comb on midleg protein interactions in Drosophila polycomb
group repression. Genetics. 167:1225–1239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo J and Jin D: A genetic screen in
Drosophila implicates Sex comb on midleg (Scm) in tissue overgrowth
and mechanisms of Scm degradation by Wds. Mech Dev. 136:1–7. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van de Vosse E, Walpole SM, Nicolaou A,
van der Bent P, Cahn A, Vaudin M, Ross MT, Durham J, Pavitt R,
Wilkinson J, et al: Characterization of SCML1, a new gene in Xp22,
with homology to developmental polycomb genes. Genomics. 49:96–102.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berger J, Kurahashi H, Takihara Y, Shimada
K, Brock HW and Randazzo F: The human homolog of Sex comb on midleg
(SCMH1) maps to chromosome 1p34. Gene. 237:185–191. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montini E, Buchner G, Spalluto C, Andolfi
G, Caruso A, den Dunnen JT, Trump D, Rocchi M, Ballabio A and
Franco B: Identification of SCML2, a second human gene homologous
to the Drosophila sex comb on midleg (Scm): A new gene cluster on
Xp22. Genomics. 58:65–72. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomotsune D, Takihara Y, Berger J, Duhl D,
Joo S, Kyba M, Shirai M, Ohta H, Matsuda Y, Honda BM, et al: A
novel member of murine Polycomb-group proteins, Sex comb on midleg
homolog protein, is highly conserved and interacts with RAE28/mph1
in vitro. Differentiation. 65:229–239. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Usui H, Ichikawa T, Kobayashi K and
Kumanishi T: Cloning of a novel murine gene Sfmbt, Scm-related gene
containing four mbt domains, structurally belonging to the Polycomb
group of genes. Gene. 248:127–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JH, Ni RZ, Xiao MB, Guo JG and Zhou
JW: Comparative proteomic analysis of differentially expressed
proteins in human pancreatic cancer tissue. Hepatobiliary Pancreat
Dis Int. 8:193–200. 2009.PubMed/NCBI
|
|
13
|
Xiao MB, Jiang F, Ni WK, Chen BY, Lu CH,
Li XY and Ni RZ: High expression of S100A11 in pancreatic
adenocarcinoma is an unfavorable prognostic marker. Med Oncol.
29:1886–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie L, Ni WK, Chen XD, Xiao MB, Chen BY,
He S, Lu CH, Li XY, Jiang F and Ni RZ: The expressions and clinical
significances of tissue and serum galectin-3 in pancreatic
carcinoma. J Cancer Res Clin Oncol. 138:1035–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mia-Jan K, Munkhdelger J, Lee MR, Ji SY,
Kang TY, Choi E and Cho MY: Expression of CD133 in neuroendocrine
neoplasms of the digestive tract: A detailed immunohistochemical
analysis. Tohoku J Exp Med. 229:301–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Öberg K: Genetics and molecular pathology
of neuroendocrine gastrointestinal and pancreatic tumors
(gastroenteropancreatic neuroendocrine tumors). Curr Opin
Endocrinol Diabetes Obes. 16:72–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gastrointestinal Pathology Study Group of
Korean Society of Pathologists, ; Cho MY, Kim JM, Sohn JH, Kim MJ,
Kim KM, Kim WH, Kim H, Kook MC, Park DY, et al: Current trends of
the incidence and pathological diagnosis of gastroenteropancreatic
neuroendocrine tumors (GEP-NETs) in Korea 2000–2009: Multicenter
study. Cancer Res Treat. 44:157–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jernman J, Välimäki MJ, Louhimo J, Haglund
CJ and Arola J: The novel WHO 2010 classification for
gastrointestinal neuroendocrine tumours correlates well with the
metastatic potential of rectal neuroendocrine tumours.
Neuroendocrinology. 95:317–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kloppel G: Classification and pathology of
gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat
Cancer. 18 Suppl 1:S1–S16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Şahan EK, Erdoğan N, Ulusoy I, Samet E,
İğdem AA and Gönüllü D: P53, KI-67, CD117 expression in
gastrointestinal and pancreatic neuroendocrine tumours and
evaluation of their correlation with clinicopathological and
prognostic parameters. Turk J Gastroenterol. 26:104–111. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rindi G, Klöppel H, Alhman M, Caplin M,
Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M,
Komminoth P, et al: TNM staging of foregut (neuro)endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
449:395–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rindi G, Klöppel A, Couvelard P, Komminoth
P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A,
et al: TNM staging of midgut and hindgut (neuro) endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
451:757–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YH, Yang QC, Lin Y, Xue L, Chen MH
and Chen J: Chromogranin A as a marker for diagnosis, treatment,
and survival in patients with gastroenteropancreatic neuroendocrine
neoplasm. Medicine (Baltimore). 93:e2472014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasegawa K, Sin HS, Maezawa S, Broering
TJ, Kartashov AV, Alavattam KG, Ichijima Y, Zhang F, Bacon WC,
Greis KD, et al: SCML2 establishes the male germline epigenome
through regulation of histone H2A ubiquitination. Dev Cell.
32:574–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo M, Zhou J, Leu NA, Abreu CM, Wang J,
Anguera MC, de Rooij DG, Jasin M and Wang PJ: Polycomb protein
SCML2 associates with USP7 and counteracts histone H2A
ubiquitination in the XY chromatin during male meiosis. PLoS Genet.
11:e10049542015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lecona E, Narendra V and Reinberg D: USP7
cooperates with SCML2 to regulate the activity of PRC1. Mol Cell
Biol. 35:1157–1168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bezsonova I: Solution NMR structure of the
DNA-binding domain from Scml2 (sex comb on midleg-like 2). J Biol
Chem. 289:15739–15749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonasio R, Lecona E, Narendra V, Voigt P,
Parisi F, Kluger Y and Reinberg D: Interactions with RNA direct the
Polycomb group protein SCML2 to chromatin where it represses target
genes. Elife. 3:e026372014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lecona E, Rojas LA, Bonasio R, Johnston A,
Fernández- Capetillo O and Reinberg D: Polycomb protein SCML2
regulates the cell cycle by binding and modulating CDK/CYCLIN/p21
complexes. PLoS Biol. 11:e10017372013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Old LJ: Cancer/testis (CT) antigens- a new
link between gametogenesis and cancer. Cancer Immun.
1:12001.PubMed/NCBI
|
|
31
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zendman AJ, Ruiter DJ and Van Muijen GN:
Cancer/testis-associated genes: Identification, expression profile
and putative function. J Cell Physiol. 194:272–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Branco MR, King M, Perez-Garcia V, Bogutz
AB, Caley M, Fineberg E, Lefebvre L, Cook SJ, Dean W, Hemberger M
and Reik W: Maternal DNA methylation regulates early trophoblast
development. Dev Cell. 36:152–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Northcott PA, Nakahara Y, Wu X, Feuk L,
Ellison DW, Croul S, Mack S, Kongkham PN, Peacock J, Dubuc A, et
al: Multiple recurrent genetic events converge on control of
histone lysine methylation in medulloblastoma. Nat Genet.
41:465–472. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grubach L, Juhl-Christensen C, Rethmeier
A, Olesen LH, Aggerholm A, Hokland P and Ostergaard M: Gene
expression profiling of Polycomb, Hox and Meis genes in patients
with acute myeloid leukaemia. Eur J Haematol. 81:112–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi L, Wang L, Huang J, Jiang M, Diao H,
Zhou H, Li X and Jiang Z: Activated amelogenin Y-linked (AMELY)
regulation and angiogenesis in human hepatocellular carcinoma by
biocomputation. Oncol Lett. 5:1075–1079. 2013.PubMed/NCBI
|
|
37
|
Wang Z, Li W, Chen T, Yang J, Luo L, Zhang
L, Sun B and Liang R: Retrospective analysis of the
clinicopathological characteristics of gastrointestinal
neuroendocrine neoplasms. Exp Ther Med. 10:1084–1088.
2015.PubMed/NCBI
|
|
38
|
Zhang X, Li M, Bao H, Zhang J, Wang Z and
Gong P: Clinical, pathological and prognostic characteristics of
gastroenteropancreatic neuroendocrine neoplasms in China: A
retrospective study. BMC Endocr Disord. 14:542014. View Article : Google Scholar : PubMed/NCBI
|