Introduction
Chronic hepatitis C virus (HCV) infection may cause
chronic hepatitis and is associated with liver cirrhosis and
hepatocellular carcinoma (1).
Although HCV infection primarily affects the liver, the virus is
capable of evading the immune response of the host by suppressing
specific T and B cell responses (2).
Furthermore, a number of autoimmune diseases have been identified
in patients with chronic hepatitis C (CHC) (3), indicating that HCV infection disrupts
the normal activity of the immune system. Therefore, improving
understanding regarding immune-associated abnormalities in patients
with CHC may help to control HCV progression and lead to the
development of more effective treatment strategies.
Normal B cell development contributes to an
effective immune response to remove pathogens, whereas disturbance
of B cell differentiation and activation leads to the disruption of
B cell homeostasis in chronic infection and autoimmunity disease
(4–6). Previous studies have demonstrated that
B cell disorders mediated by HCV infection are not only associated
with B cell subset skewing, with an increased proportion of
immature B cells and a decreased proportion of memory B cells
(7–10), but are also associated with the
increased activation of naive and memory B cells (11–13).
Immunoglobulin (Ig) M+B cells are responsible for the production of
IgM antibodies and are involved in the pathogenesis of viral
infections and autoimmunity diseases (14–16).
Roughan et al (17)
determined that there is a higher frequency of circulating IgM+B
memory subsets in the peripheral blood of patients with CHC;
however the phenotypic features of different IgM+B cell subsets and
associated risk factors in patients with CHC remain unknown.
The present study aimed to identify the effects of
HCV infection on IgM+B cell subsets. The percentage,
differentiation and activation status of peripheral IgM+B cell
subsets was evaluated in patients with CHC using flow cytometry. In
addition, the association between IgM+B cell subsets and different
clinical parameters was investigated in patients with CHC.
Patients and methods
Patients and controls
The study population consisted of 27 patients with
CHC with genotype 1b, including 11 male and 16 female patients
(mean age 54.8 years), and 20 age- and sex-matched healthy controls
(HCs; 9 male and 11 female patients; mean age 50 years). Patients
with CHC were recruited in November 2013 from Guan county (China),
in which the majority of patients are infected with HCV genotype 1b
(18). Inclusion criteria of
patients with chronic HCV infection were: Positive HCV antibodies
and HCV RNA levels (>2,000 IU/ml) in the past 6 months.
Exclusion criteria were: Co-infection with hepatitis B virus or
human immunodeficiency virus. The HCs were tested negative for HCV
antibodies. In addition, liver damage induced by HCV infection was
monitored via measuring serum alanine aminotransferase (ALT). The
baseline characteristics of all study subjects are presented in
Table I. The present study was
approved by the Ethics Committee of Peking University People's
Hospital (Beijing, China) and all participants provided written
informed consent.
 | Table I.Baseline characteristics of
subjects. |
Table I.
Baseline characteristics of
subjects.
| Parameters | CHC | HC | P-values |
|---|
| Subjects (n) | 27 | 20 | N/A |
| Age (years) | 54.8±10.4 | 50.0±5.7 |
0.06a |
| Sex, male/female | 11/16 | 9/11 | 0.77 |
| ALT | 45.9±36.6 |
26.7±15.4 |
0.03a |
| Genotype (1b), n | 27 | N/A | N/A |
| HCV-RNA (log10
IU/ml) | 6.9±0.8 | N/A | N/A |
Clinical tests and peripheral blood
mononuclear cell (PBMC) preparation
Levels of HCV antibodies, HCV RNA and HCV genotypes
were determined as previously described (18). ALT was detected using a Hitachi 7600
automated biochemical analyzer (Hitachi, Ltd., Tokyo, Japan). PBMCs
were isolated using density gradient centrifugation with
Ficoll-Paque Plus (GE Healthcare Life Sciences, Uppsala, Sweden),
as described previously (19). PBMCs
were kept in liquid nitrogen until analysis.
Flow cytometric analysis
To measure the phenotypic features of peripheral
IgM+B cell subsets, 1×106 PBMCs were incubated in 200 µl
blocking solution consisting of 2% bovine serum albumin (Genview
Corp., Houston, TX, USA) in PBS for 30 min at room temperature to
block non-specific binding. Cells were stained with different
fluorescent conjugated anti-human antibodies: Peridinin chlorophyll
protein (PerCP)-conjugated anti-cluster of differentiation (CD)19
antibody (catalogue no. 340421; 1:100), allophycocyanin
(APC)-conjugated anti-CD5 antibody (catalogue no. 340583; 1:100),
APC-conjugated anti-IgD antibody (catalogue no. 561303; 1:100),
APC-conjugated anti-CD10 antibody (catalogue no. 340923; 1:100) and
phycoerythrin (PE)-conjugated anti-CD21 antibody (catalogue no.
555422; 1:100) were purchased from BD Pharmingen (BD Biosciences,
San Jose, CA, USA). Fluorescein isothiocyanate (FITC)-conjugated
anti-CD27 antibody (catalogue no. 11-0279; 1:100), APC-conjugated
anti-CD38 antibody (catalogue no. 17-0389; 1:100), APC-conjugated
anti-IgM antibody (catalogue no. 17-9998; 1:100), PE-conjugated
anti-IgM antibody (catalogue no. 12-9998; 1:100), PE-conjugated
anti-CD86 antibody (catalogue no. 12-0869; 1:100) and PE-conjugated
anti-CD95 (catalogue no. 12-0959; 1:100) antibody were purchased
from eBioscience (Thermo Fisher Scientific, Inc., Waltham MA, USA).
Cells were incubated with these antibodies for 20 min at room
temperature in the dark.
In addition, PBMCs were incubated with
isotype-matched control antibodies listed below for 20 min at room
temperature in the dark to determine background levels of staining.
PerCP Mouse IgG1 κ Isotype Control (catalogue no. 559425; 1:100),
APC Mouse IgG1, κ Isotype Control (catalogue no. 550854; 1:100) and
APC Mouse IgG2b κ Isotype Control (catalogue no. 555745; 1:100)
were purchased from BD Biosciences. Mouse IgG1 K isotype control
FITC (catalogue no. 11-4714; 1:100), Mouse IgG2b K isotype control
PE (catalogue no. 12-4732; 1:100), and Mouse IgG1 K isotype control
PE (catalogue no. 12-4714; 1:100) were purchased from eBioscience
(Thermo Fisher Scientific, Inc.).
Following washing twice with PBS, the cells were
fixed with 1% paraformaldehyde in the dark at 4°C for 12 h. Then
the expression of surface molecules on cells were assayed using the
BD FACSCalibur (BD Biosciences) and data were analyzed using FlowJo
7.6 software (Tree Star, Inc., Ashland, OR, USA). Based on
expression of CD5, B cells were divided into CD5+B cells (B1 cells)
and CD5-B cells (B2 cells) (20).
Depending on the expression of CD27, a distinct memory B cell
marker, IgM+B cells were divided into naive subsets (CD27-IgM+B
cells) and memory subsets (CD27+IgM+B cells) (17). In addition, CD21 was used to identify
activated subsets (CD21-cells) and resting subsets (CD21+cells) in
naïve B cells and memory B cells (7).
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons in all experiments were performed using Student's
t-test or two-sample Wilcoxon rank-sum (Mann-Whitney) tests,
according to data distribution measured using a Kolmogorow-Smirnov
test with SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). The
linear correlation between variables was analyzed using GraphPad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Frequency of CD27+IgM+B memory subsets
in total B cells of patients with CHC
To characterize IgM+B cells (CD19+IgM+B cells) in
patients infected with HCV, the frequency of IgM+B cells in
patients with CHC and HCs was detected using flow cytometry
(Fig. 1A). There were no significant
differences between the frequency of total IgM+B cells in patients
with CHC and HCs (Fig. 1B). The
percentage of IgM+B naive subsets (CD27-IgM+B cells) and IgM+B
memory subsets (CD27+IgM+B cells) were further assessed in both
groups (Fig. 1A). As presented in
Fig. 1B, there was no significant
difference between the frequency of CD27-IgM+B cells in patients
with CHC and HCs. However, the frequency of CD27+IgM+B cells was
significantly higher in patients with CHC than in HCs (P<0.05;
Fig. 1B). This suggests that
infection with HCV may disrupt the homeostasis of IgM+B cell
subsets.
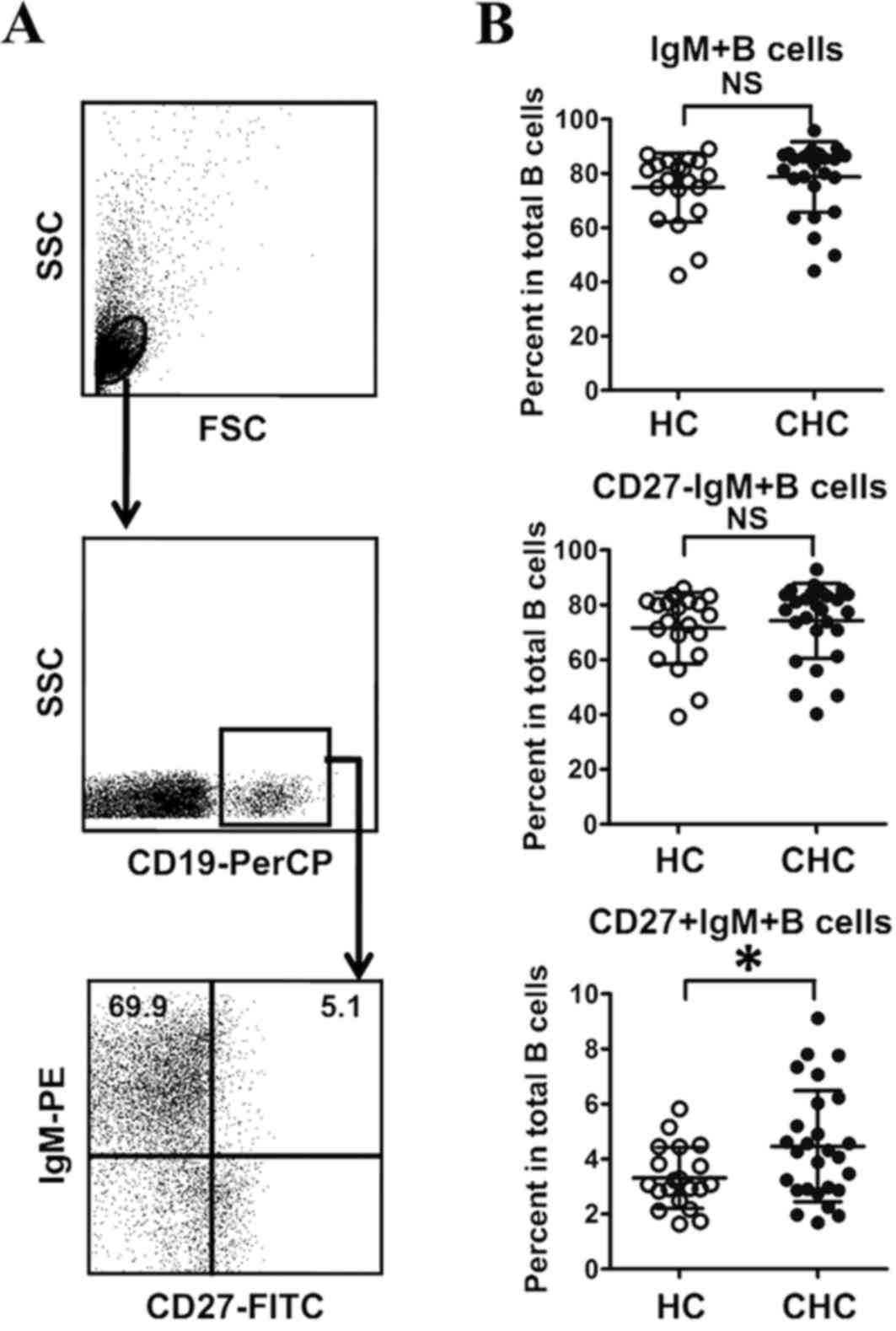 | Figure 1.Frequency of CD27+IgM+B cells in
patients with CHC and HCs. (A) Gating strategy for IgM+B cell
subsets by flow cytometry. (B) Frequencies of IgM+B, CD27-IgM+B and
CD27+IgM+B cells in total B cells. *P<0.05. PerCP, peridinin
chlorophyll; PE, phycoerythrin; CHC, chronic hepatitis C; HC,
healthy control; NS, no significant difference; FSC, forward
scatter; SSC, side scatter; Ig, immunoglobulin; FITC, Fluorescein
isothiocyanate; CD, cluster of differentiation. |
Percentage of CD5+B cells in
CD27-IgM+B cells of patients with CHC
Previous research has demonstrated that expansion of
CD5+B cells is associated with HCV infection (21), whereas the association between CD5+B
cells and IgM+B cell subsets is unclear in patients with HCV
infection. In the current study, the percentage of CD5+B cells in
total B cells was significantly higher in patients with CHC
compared with HCs (P<0.05; Fig.
2), which was consistent with a previous report (21). The percentage of CD5+B cells in IgM+B
cell subsets was also measured in the current study. The results
indicated that the frequency of CD5+B cells was significantly
higher in CD27-IgM+B cells from patients with CHC compared with HCs
(P<0.05; Fig. 2), while there was
no significant difference in the frequency of CD5+B in CD27+IgM+B
cells between the two groups (Fig.
2). This suggests that only CD27-IgM+B cells are associated
with expansion of CD5+B cells in HCV infection.
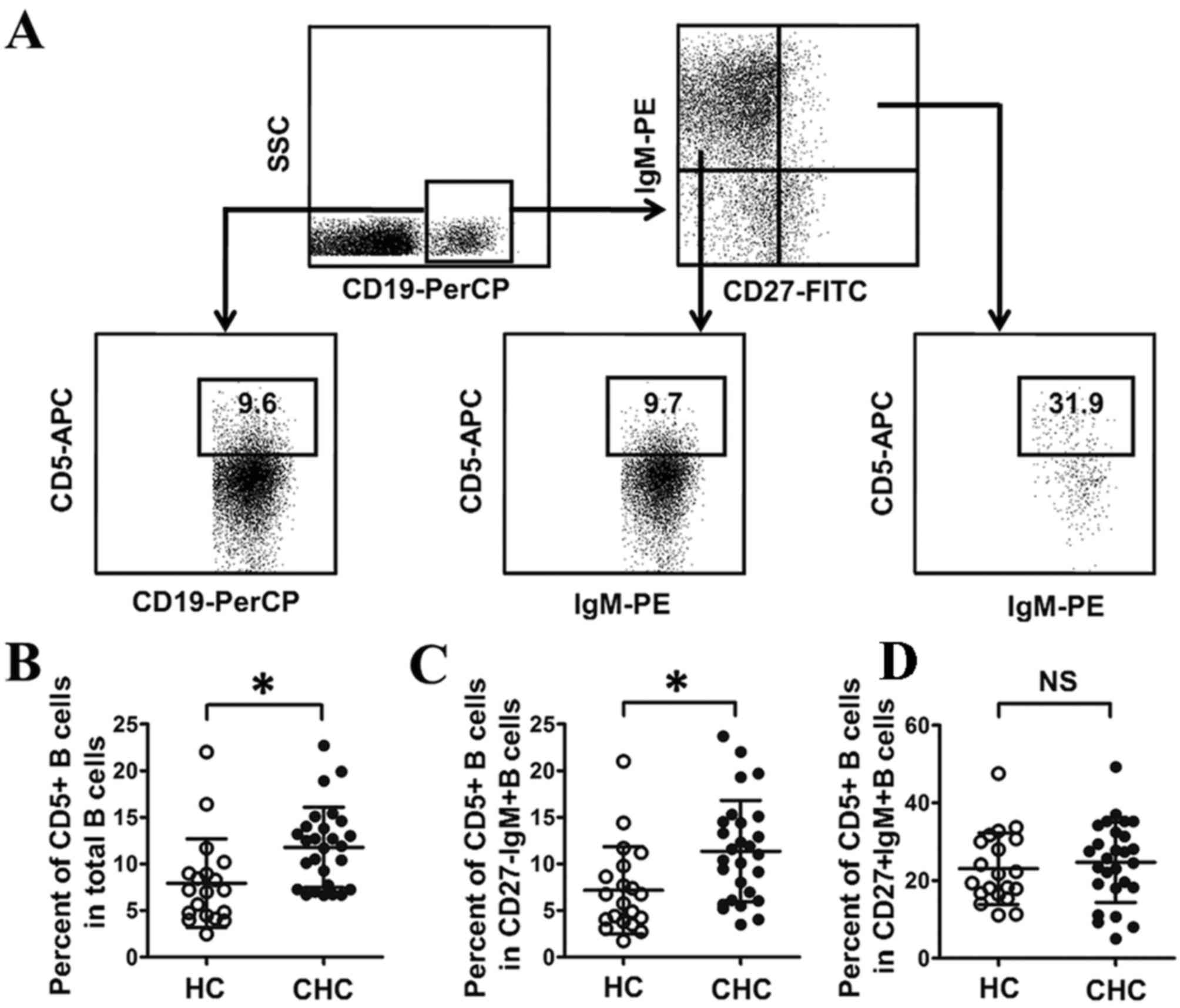 | Figure 2.Frequency of CD5+B cells in patients
with CHC. (A) Gating strategy for measuring the frequency of CD5+B
cells in different B cell subsets by flow cytometry. (B) Frequency
of CD5+B cells in total B cells. (C) Frequency of CD5+B cells in
CD27-IgM+B cells. (D) Frequency of CD5+B cells in CD27+IgM+B cells.
*P<0.05. CHC, chronic hepatitis C; HC, healthy control; NS, no
significant difference; SSC, side scatter; PE, phycoerythrin; FITC,
fluorescein isothiocyanate; PerCP, peridinin chlorophyll; APC,
allophycocyanin; Ig, immunoglobulin; CD, cluster of
differentiation. |
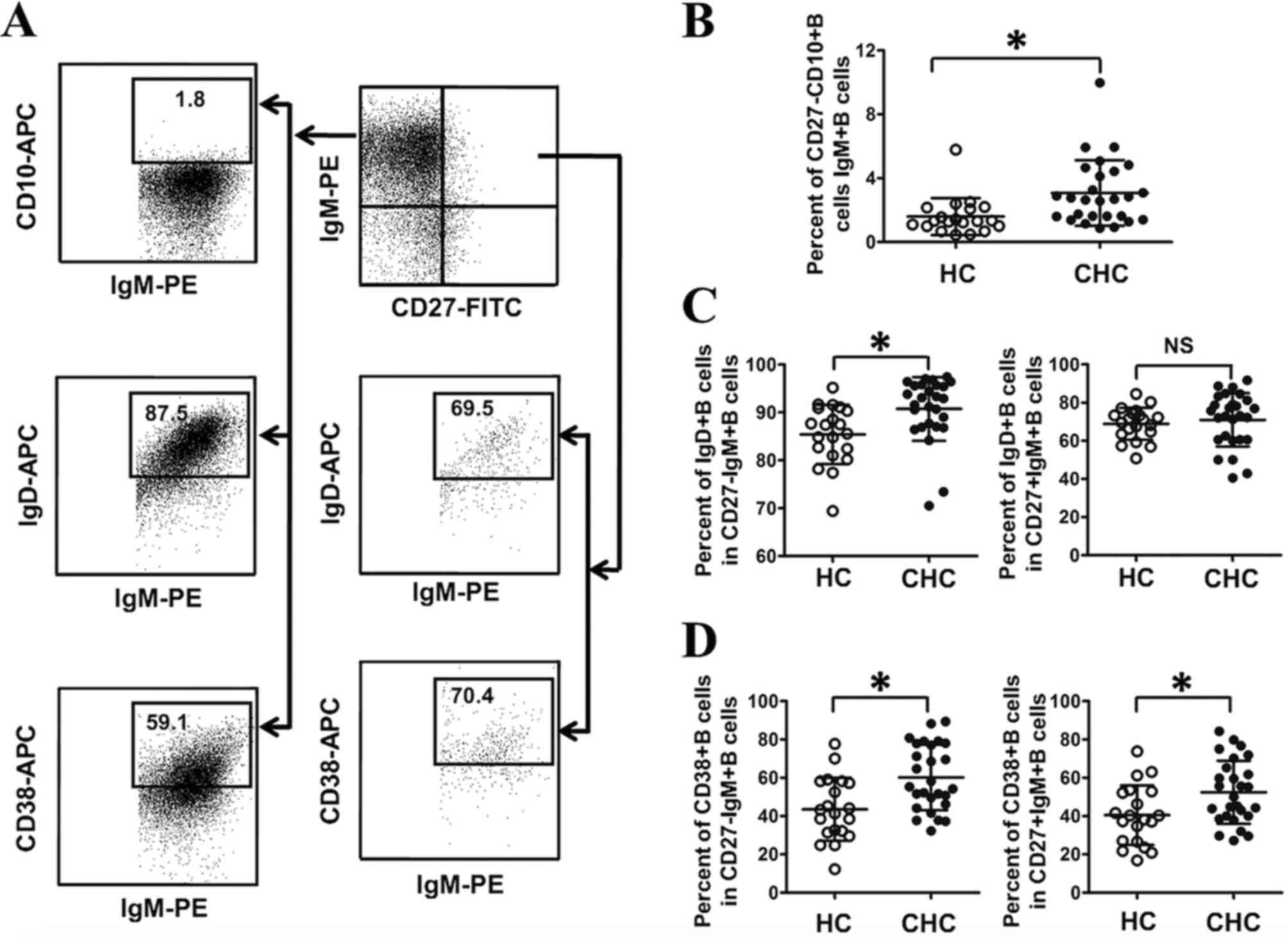
Abnormal differentiation of CD27-IgM+B
and CD27+IgM+B subsets in patients with CHC
B cell homeostasis is primarily dependent on B cell
differentiation and activation (22,23).
Thus, the differentiation of IgM+B cells in both IgM+B cell subsets
was evaluated in the current study. IgM+B cells at the
differentiation stage transition from immature B cells to
plasmablasts (22,23). In the current study, immature B cells
were CD27-CD10+B cells (Fig. 3A).
The percentage of CD27-CD10+B cells in IgM+B cells was determined
in both groups, and the results demonstrated that the frequency of
CD27-CD10+IgM+B cells in IgM+B cells was significantly higher in
patients with CHC compared with HCs (P<0.05; Fig. 3B). At naïve B cells differentiate
into plasmablasts, B cell differentiation is associated with
alterations in IgD and CD38 expression (22,23) and
the current study measured the expression of IgD and CD38 in IgM+B
cell subsets. As presented in Fig.
3C, a significantly higher percentage of IgD+B cells in
CD27-IgM+B cells was identified in patients with CHC compared with
HCs (P<0.05), whereas there were no significant differences in
the frequency of IgD+B cells in CD27+IgM+B cells between the two
groups. Furthermore, a significantly higher percentage of CD38+B
cells was observed in CD27-IgM+B and CD27+IgM+B cells in patients
with CHC compared with HCs (P<0.05; Fig. 3D). Together, these results indicate
that HCV infection is associated with the abnormal differentiation
of CD27-IgM+B and CD27+IgM+B cells.
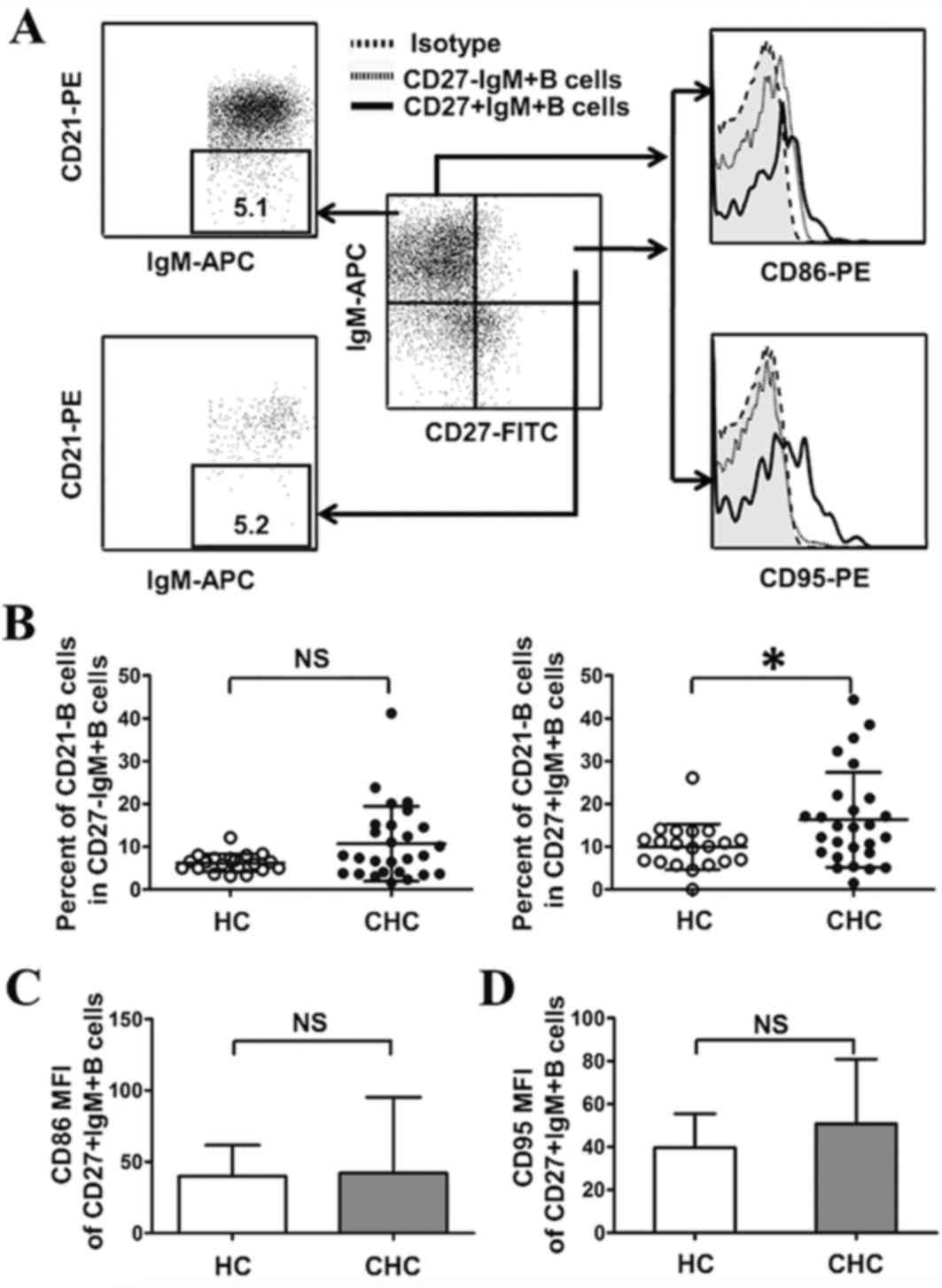
Activation of CD27+IgM+B subsets in
patients with CHC
The activation of IgM+B subsets in both groups was
investigated (Fig. 4A). As shown in
Fig. 4B, compared with HCs, the
number of activated CD27+IgM+B cells (CD27+IgM+CD21-B cells)
increased significantly in patients with CHC (P<0.05); however,
there were no significant differences between the number of
activated CD27-IgM+B cells (CD27-IgM+CD21-B cells) in patients with
CHC compared with HCs (Fig. 4B).
Activated B cells are associated with high levels of
the co-stimulatory molecules CD86 and CD95, and the abnormal
expression of these molecules in B cells is associated with chronic
diseases such as autoimmune hepatitis and rheumatoid arthritis
(24,25). Based on this, levels of CD86 and CD95
were evaluated in CD27+IgM+B cells, using mean fluorescence
intensity to represent molecule expression. The results
demonstrated that the expression of CD86 and CD95 in CD27+IgM+B
cells was similar in patients with CHC and HCs (Fig. 4C and D). Taken together, these
results suggest that HCV infection is associated with the
activation of CD27+IgM+B cell subsets but is not associated with
the expression of CD86 and CD95 in CD27+IgM+B cells.
Correlation of HCV RNA and ALT with
IgM+B cell subsets in patients with CHC
In order to evaluate the association between IgM+B
cell subsets and clinical parameters in patients with CHC, it was
investigated whether IgM+B subsets were correlated with HCV RNA and
the liver cell damage marker ALT. As presented in Fig. 5A, the percentages of CD27-IgD+IgM+B
cells (P<0.01, r2=0.49), CD27-CD38+IgM+B cells
(P<0.01, r2=0.34) and CD27+CD38+IgM+B cells (P=0.01,
r2=0.23) were negatively correlated with viral load in
patients with CHC. However, no significant correlations between any
IgM+B cell subsets and ALT were detected in patients with CHC
(Fig. 5B). Thus, these data indicate
that there is a HCV but not ALT is negatively correlated with the
abnormal expression of certain IgM+B cell subsets.
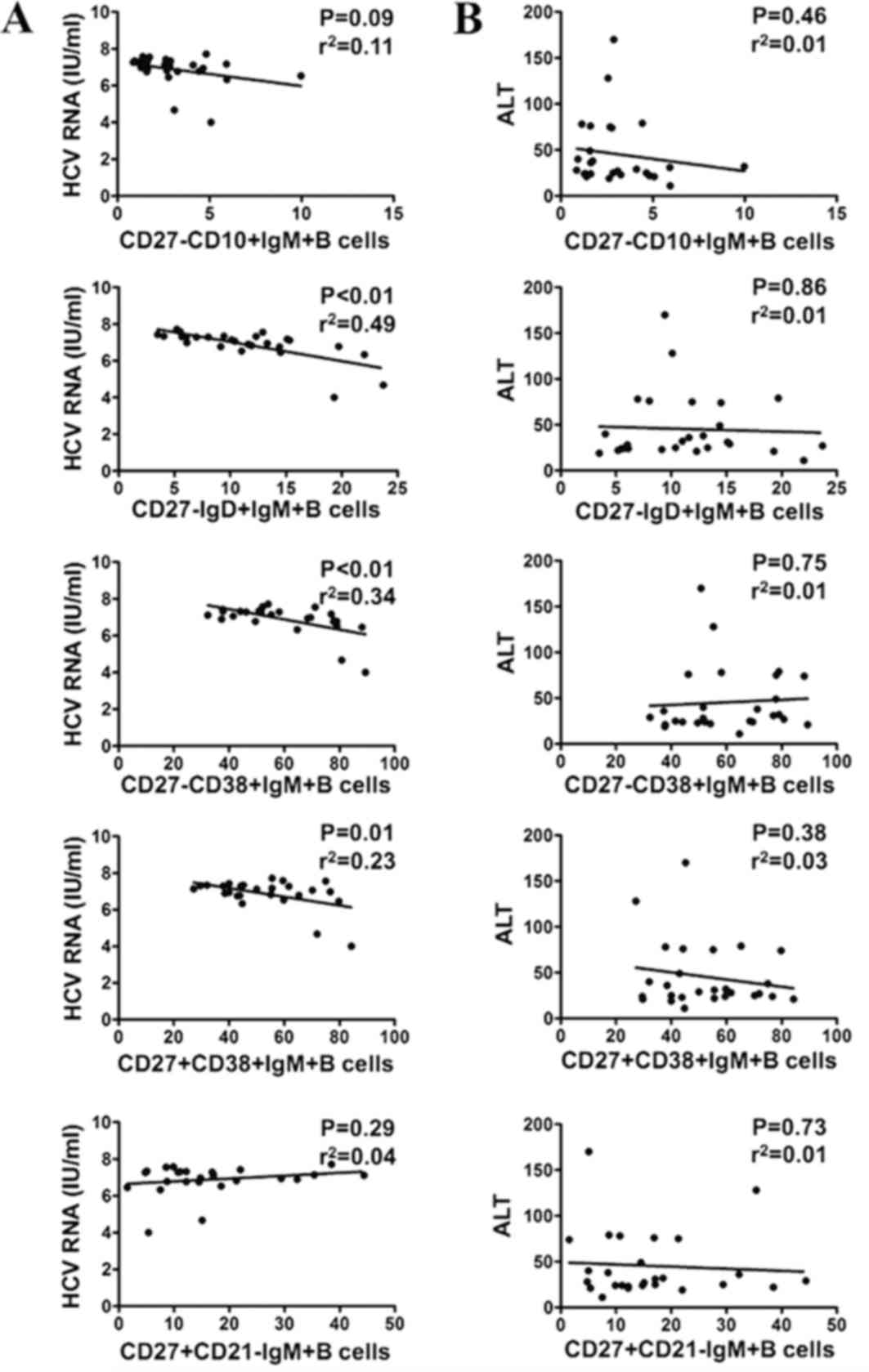 | Figure 5.Correlation analysis of HCV RNA and
ALT with different IgM+B cell subsets in patients with CHC. (A)
Correlation of HCV RNA with CD27-CD10+IgM+B, CD27-IgD+IgM+B,
CD27-CD38+IgM+B cells, CD27+CD38+IgM+B and CD27+CD21-IgM+B cells.
(B) Correlation of ALT with CD27-CD10+IgM+B cells, CD27-IgD+IgM+B
cells, CD27-CD38+IgM+B cells, CD27+CD38+IgM+B cells, and
CD27+CD21-IgM+B cells. HCV, hepatitis C virus; ALT, alanine
transaminase; CD, cluster of differentiation; Ig,
immunoglobulin. |
Discussion
It has been demonstrated that abnormalities of
peripheral B cell subsets are associated with HCV infection
(7–13). However, the effect of HCV infection
on the differentiation and activation of IgM+B cell subsets has not
been fully elucidated. The current study aimed to characterize
IgM+B cell subsets in patients with CHC and the results indicated
that HCV infection was responsible for abnormalities in the
percentage, differentiation and activation of IgM+B cell subsets.
In addition, negative correlations between viral load and
CD27-IgD+IgM+B cells, CD27-CD38+IgM+B cells and CD27+CD38+IgM+B
cells were detected in patients with CHC.
Roughan et al (17) demonstrated that IgM+ memory B cells
were expanded in patients with CHC, and identified that the unusual
polyclonal expansion of the IgM+ memory B cell subset was made up
of autoreactive B cells. Consistent with a previous report
(17), the present study
demonstrated that an increase in CD27+IgM+B cells was associated
with HCV infection. The frequency of CD27-IgM+B cells in patients
with CHC was similar to that in HCs. However, the percentage of
CD5+B cells, which are not only characterized by the production of
low-affinity IgM that recognizes a variety of self-antigens but
also serve an important role in the pathogenesis of autoimmunity
disease (26), was higher in the
CD27-IgM+B cells of patients with CHC than in HCs. This implies
that CD27-IgM+B cells may be involved in the reaction to
autoantigens in patients with CHC. Taken together, these results
suggest that abnormalities of CD27-IgM+B and CD27+IgM+B cells may
be associated with autoreactivity and increased susceptibility to
autoimmune diseases in patients with CHC.
Alterations in B cell differentiation and activation
are frequently noted in pathological conditions (4–6). On this
basis, the current study evaluated the differentiation status of
IgM+B cell subsets in patients with CHC. Based on the immature B
cell marker CD10, it was determined that the percentage of
CD27-CD10+IgM+B cells was increased in patients with CHC. Previous
results have demonstrated that immature B cells are CD10+CD27-IgM+B
cells (27) and the current results
are consistent with those from a previous study, which identified
that HCV infection was associated with an increase in immature B
cells (8). IgD and CD38 are
associated with the development of B cells (22,23). In
the present study, it was observed that the frequency of CD27-IgD+B
cells in IgM+B cells was increased in patients with CHC compared
with HCs. The increased frequency of this subpopulation in IgM+B
cells may be associated with chronic HCV stimulation and immune
dysfunction mediated by CD27-IgD-IgM+B cells. CD38 is a
multifunctional ectoenzyme, which participates in lymphocyte
activation and terminal differentiation (28). The increased percentage of
CD27-CD38+IgM+B cells and CD27+CD38+IgM+B cells imply that chronic
HCV infection may induce the activation and terminal
differentiation of certain IgM+B cell subsets.
Previous studies have demonstrated that HCV
infection induces the activation of naïve and memory B cell subsets
(11,12,29). In
the current study, the activation of IgM+B cell subsets was
assessed based on the expression of the activation marker CD21. It
was determined that the activation of CD27+IgM+B cells was
increased in patients with CHC. Furthermore, the expression of
distinct molecules associated with B cell activation was evaluated
in CD27+IgM+B cells. CD86 is a critical co-stimulatory molecule to
facilitate the interaction of T cells with B cells (30). CD95 is a death receptor, which
initiates extrinsic pathways of apoptosis to remove activated
immune cells (31). Although HCV
infection was associated with an increase in the number of
activated CD27+IgM+B cells, these activated CD27+IgM+B cell subsets
were not correlated with CD86 and CD95 expression. This implies
that the activation of CD27+IgM+B cells may not be associated with
T cell stimulation or apoptosis. Further studies are required to
determine the underlying mechanisms that result in increased
numbers of activated CD27+IgM+B subsets in the peripheral
circulation.
In the present study, the association between IgM+B
cell subsets and clinical parameters was investigated in patients
with CHC. Negative correlations were identified between HCV RNA and
CD27-IgD+IgM+B, CD27-CD38+IgM+B and CD27+CD38+IgM+B cells. Although
the relative expression of CD27-IgD+IgM+B, CD27-CD38+IgM+B and
CD27+CD38+IgM+B cells was higher in patients with CHC compared with
HCs, the exact roles of these IgM+B cell subsets remain unknown. It
is possible that chronic HCV antigen stimulation disrupts the
immune response by increasing these IgM+B cell subsets, which, in
turn, helps to inhibit HCV replication to some extent in
pathological conditions. Further studies are required to determine
the roles of these IgM+B cell subsets in patients with CHC.
In conclusion, the current study identified the
phenotypic abnormalities of CD27-IgM+B and CD27+IgM+B cells in
patients with CHC and demonstrated that the frequencies of IgM+B
cell subsets were negatively correlated with viral load. The
alterations in IgM+B cell subset populations may be associated with
the disruption of the B cell immune response and improved
understanding of the association between IgM+B cells and HCV
infection will be required in order to identify potential targets
for HCV treatment. Despite various limitations of the current
study, including the relatively small number of patients with CHC
enrolled and a lack of functional analysis of IgM+B cell subsets in
the pathogenic process of HCV infection, the results of the current
study lay a foundation for evaluating the roles and associated
mechanisms of certain IgM+B cell subsets in patients with HCV.
Acknowledgements
The authors wish to thank all patients with CHC for
providing blood samples. The current study was funded by the
National Science and Technology Major Project for Infectious
Diseases Control during the 12th Five-Year Plan Period (grant no.
2012ZX10002003).
References
|
1
|
Manns MP, Buti M, Gane E, Pawlotsky JM,
Razavi H, Terrault N and Younossi Z: Hepatitis C virus infection.
Nat Rev Dis Primers. 3:170062017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park SH and Rehermann B: Immune responses
to HCV and other hepatitis viruses. Immunity. 40:13–24. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrari SM, Fallahi P, Mancusi C, Colaci
M, Manfredi A, Ferri C and Antonelli A: HCV-related autoimmune
disorders in HCV chronic infection. Clin Ter. 164:e305–e312.
2013.PubMed/NCBI
|
|
4
|
Moir S and Fauci AS: B cells in HIV
infection and disease. Nat Rev Immunol. 9:235–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sang A, Zheng YY and Morel L:
Contributions of B cells to lupus pathogenesis. Mol Immunol.
62:329–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perry HM, Bender TP and McNamara CA: B
cell subsets in atherosclerosis. Front Immunol. 3:3732012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugalski JM, Rodriguez B, Moir S and
Anthony DD: Peripheral blood B cell subset skewing is associated
with altered cell cycling and intrinsic resistance to apoptosis and
reflects a state of immune activation in chronic hepatitis C virus
infection. J Immunol. 185:3019–3027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holz LE, Yoon JC, Raghuraman S, Moir S,
Sneller MC and Rehermann B: B cell homeostasis in chronic hepatitis
C virus-related mixed cryoglobulinemia is maintained through naïve
B cell apoptosis. Hepatology. 56:1602–1610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Racanelli V, Frassanito MA, Leone P,
Galiano M, De Re V, Silvestris F and Dammacco F: Antibody
production and in vitro behavior of CD27-defined B-cell subsets:
Persistent hepatitis C virus infection changes the rules. J Virol.
80:3923–3934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuochi T, Ito M, Saito K, Kasai M,
Kunimura T, Morohoshi T, Momose H, Hamaguchi I, Takai K, Iino S, et
al: Possible recruitment of peripheral blood CXCR3+ CD27+ CD19+ B
cells to the liver of chronic hepatitis C patients. J Interferon
Cytokine Res. 30:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosa D, Saletti G, De Gregorio E, Zorat F,
Comar C, D'Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G and
Abrignani S: Activation of naïve B lymphocytes via CD81, a
pathogenetic mechanism for hepatitis C virus-associated B
lymphocyte disorders. Proc Natl Acad Sci USA. 102:pp. 18544–18549.
2005; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oliviero B, Cerino A, Varchetta S, Paudice
E, Pai S, Ludovisi S, Zaramella M, Michelone G, Pugnale P, Negro F,
et al: Enhanced B-cell differentiation and reduced proliferative
capacity in chronic hepatitis C and chronic hepatitis B virus
infections. J Hepatol. 55:53–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oliviero B, Mantovani S, Ludovisi S,
Varchetta S, Mele D, Paolucci S, Baldanti F and Mondelli MU: Skewed
B cells in chronic hepatitis C virus infection maintain their
ability to respond to virus-induced activation. J Viral Hepat.
22:391–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valle L Della, Dohmen SE, Verhagen OJ,
Berkowska MA, Vidarsson G and van der Schoot C Ellen: The majority
of human memory B cells recognizing RhD and tetanus resides in IgM+
B cells. J Immunol. 193:1071–1079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D'Orsogna LJ, Krueger RG, McKinnon EJ and
French MA: Circulating memory B-cell subpopulations are affected
differently by HIV infection and antiretroviral therapy. AIDS.
21:1747–1752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carsetti R, Rosado MM, Donnanno S, Guazzi
V, Soresina A, Meini A, Plebani A, Aiuti F and Quinti I: The loss
of IgM memory B cells correlates with clinical disease in common
variable immunodeficiency. J Allergy Clin Immunol. 115:412–417.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roughan JE, Reardon KM, Cogburn KE,
Quendler H, Pockros PJ and Law M: Chronic hepatitis C virus
infection breaks tolerance and drives polyclonal expansion of
autoreactive B cells. Clin Vaccine Immunol. 19:1027–1037. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rao H, Wei L, Lopez-Talavera JC, Shang J,
Chen H, Li J, Xie Q, Gao Z, Wang L, Wei J, et al: Distribution and
clinical correlates of viral and host genotypes in Chinese patients
with chronic hepatitis C virus infection. J Gastroenterol Hepatol.
29:545–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Z, Zhang H, Rao H, Jiang D, Cong X,
Feng B, Wang J, Wei L and Chen H: DCs pulsed with novel
HLA-A2-restricted CTL epitopes against hepatitis C virus induced a
broadly reactive anti-HCV-specific T lymphocyte response. PLoS One.
7:e383902012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hardy RR: B-1 B cell development. J
Immunol. 177:2749–2754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Curry MP, Golden-Mason L, Nolan N, Parfrey
NA, Hegarty JE and O'Farrelly C: Expansion of peripheral blood CD5+
B cells is associated with mild disease in chronic hepatitis C
virus infection. J Hepatol. 32:121–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perez-Andres M, Paiva B, Nieto WG, Caraux
A, Schmitz A, Almeida J, Vogt RF Jr, Marti GE, Rawstron AC, Van
Zelm MC, et al: Human peripheral blood B-cell compartments: A
crossroad in B-cell traffic. Cytometry B Clin Cytom. 78 Suppl
1:S47–S60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bemark M, Holmqvist J, Abrahamsson J and
Mellgren K: Translational Mini-Review Series on B cell subsets in
disease. Reconstitution after haematopoietic stem cell
transplantation-revelation of B cell developmental pathways and
lineage phenotypes. Clin Exp Immunol. 167:15–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma L, Qin J, Ji H, Zhao P and Jiang Y: Tfh
and plasma cells are correlated with hypergammaglobulinaemia in
patients with autoimmune hepatitis. Liver Int. 34:405–415. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma
L and Jiang Y: High frequencies of activated B cells and T
follicular helper cells are correlated with disease activity in
patients with new-onset rheumatoid arthrits. Clin Exp Immunol.
174:212–220. 2013.PubMed/NCBI
|
|
26
|
Youinou P and Renaudineau Y: CD5
expression in B cells from patients with systemic lupus
erythematosus. Crit Rev Immunol. 31:31–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dörner T, Giesecke C and Lipsky PE:
Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther.
13:2432011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deterre P, Berthelier V, Bauvois B,
Dalloul A, Schuber F and Lund F: CD38 in T- and B-cell functions.
Chem Immunol. 75:146–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong FY, Feng B, Zhang HH, Rao HY, Wang
JH, Cong X and Wei L: CD4+CXCR5+ T cells activate CD27+IgG+ B cells
via IL-21 in patients with hepatitis C virus infection.
Hepatobiliary Pancreat Dis Int. 15:55–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lim TS, Goh JK, Mortellaro A, Lim CT,
Hämmerling GJ and Ricciardi-Castagnoli P: CD80 and CD86
differentially regulate mechanical interactions of T-cells with
antigen-presenting dendritic cells and B-cells. PLoS One.
7:e451852012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strasser A, Jost PJ and Nagata S: The many
roles of FAS receptor signaling in the immune system. Immunity.
30:180–192. 2009. View Article : Google Scholar : PubMed/NCBI
|