Introduction
Ulcerative colitis (UC), one of the primary forms of
inflammatory bowel disease (IBD), is a non-specific, chronic
inflammatory disorder of the colonic mucosa. Its primary features
are abdominal pain, diarrhoea, bloody mucopurulent stool and
alternating periods of exacerbation and remission of clinical
symptoms (1). The incidence and
prevalence of UC is increasing globally: The highest annual
incidence of UC was 24.3 in 100,000 individuals/year in Europe, 6.3
in 100,000 individuals/year in Asia and the Middle East and 19.2 in
100,000 individuals/year in North America (2). UC tends to persist for long durations,
and patients are prone to relapse and exhibit a higher risk of
developing colorectal cancer (CRC) than the general population
(3). In addition, the detection rate
of UC-associated CRC (UC-CRC), one of the most serious
complications, has also increased. The probability of CRC in
patients with UC increases with the duration of disease; 1.6% at 10
years after onset, 8.3% at 20 years and 18.4% at 30 years after
onset (4). Owing to the delayed
healing and lifetime debilitating physical symptoms (urgent
diarrhoea, rectal bleeding, vomiting, anorexia and lethargy),
patients typically suffer poor psychosocial well-being (5).
At present, the precise cause of UC remains poorly
understood; however, its pathophysiology has been demonstrated to
be a complex result of epithelial barrier defects, commensal
microflora, antigen recognition, dysregulation of immunological
responses, leukocyte recruitment and genetic factors (1), of which the balance between anti- and
pro-inflammatory signals serves a vital role. Furthermore,
inflammation resulting from a failure to maintain this balance is
evident in patients with immune dysregulation (6).
The CRC involves continuous intestinal inflammation,
non-dysplastic mucosa, indefinite dysplasia, low-grade dysplasia,
high-grade dysplasia and finally invasive adenocarcinoma (7). Furthermore, the progression from
inflammation to atypical hyperplasia to cancer in patients with UC
is more rapid than the progression of adenoma to adenocarcinoma in
the general population (8,9). Thus, as an independent risk factor,
intestinal inflammation is the initial step in UC-CRC development,
and the risk of CRC increases with the severity of inflammation in
patients with atypical hyperplasia or chronic UC. Furthermore, the
length of the disease course is a key factor for cancer in patients
with UC. The average incidence rate of cancer increases
exponentially with the disease lasting >10 years (10). Therefore, as the control of
inflammation ameliorates UC, to enhance the quality of life and
work efficiency, it also prevents the development of CRC and
reduces its incidence.
Although research on UC has progressed a great deal,
effective treatment approaches remain to be elucidated. The
treatment of UC aims to relieve the symptoms and complications, in
addition to preventing recurrence and improving the patients'
quality of life (11). UC treatment
includes glucocorticosteroids, aminosalicylates, immunomodulators,
biopharmaceuticals, surgery and diet therapy. Among these,
aminosalicylates and glucocorticosteroids are currently the
first-line agents for mild-moderate and moderate-severe UC
(12), respectively. Use of these
agents, however, has a number of problems, including lack of
tolerance to the drug, side effects, prolonged treatment duration
and high recurrence rates. In addition, the efficacy and safety of
immunomodulators and antibiotics, which are currently in the
clinical trial stages, require further evaluation (13). Therefore, there is an increasing
requirement for the development of more effective and less toxic
agents for UC treatment.
Indigo naturalis (IN), which is a traditional
Chinese medicinal composition comprising the dried powder of
processed leaves or stems of Baphicacanthus cusia (Nees)
Bremek, Polygonum tinctorium or Isatis indigotica
Fort, has been demonstrated to be effective in treating UC
(14). The primary active
ingredients are indigo, indirubin, tryptanthrin, and qingdainone.
The clinical disease activity indexes (DAIs) and endoscopic Matts
grades have been demonstrated to significantly decrease following
oral administration of IN powder in patients with UC who are
unresponsive to 5-aminosalicylic acid (5-ASA), prednisolone and
infliximab treatment (15). The
majority of patients were relieved of hormone dependence.
Furthermore, IN has a powerful scavenging effect on hydroxyl
radicals in patients with UC. IN extract has been successfully used
to treat clinical psoriasis (16)
and induce the apoptosis and autophagy of acute lymphoblastic
leukaemia cell lines (17).
The present study aimed to provide an experimental
basis for the use of IN as a potential therapeutic agent for UC by
investigating the possible protective mechanism of IN in dextran
sulphate sodium (DSS)-induced UC rats on the basis of colonic
mucosal injuries and inflammation. The effects of IN were
investigated in comparison with the bowel-specific aminosalicylate
drug mesalazine.
Materials and methods
Preparation of IN and mesalazine slow
release (SR) granules
IN granules comprising crude IN were provided by the
Pharmacy Department of Dongfang Hospital, Beijing University of
Chinese Medicine (Beijing, China). IN was administered orally at
high (INH), medium (INM) and low (INL) doses (16.8, 8.4 and 4.2
g/kg, respectively) of the crude drug (in terms of medicinal
material quality in rats). Mesalazine SR granules were provided by
Shanghai Ethypharm Pharmaceutical Co., Ltd., (Shanghai, China).
Animals and treatment
A total of 48 healthy male Sprague-Dawley rats (age,
7 weeks; weight, 180–220 g) were supplied by Vital River
Laboratories Co., Ltd. [Beijing, China; license no. SCXK (Jing)
2012-0001]. The rats were maintained under a 12-h light/dark cycle
under constant temperature of 22±2°C and 50–60% humidity, with
ad libitum access to water. All experimental procedures were
approved by the Animal Ethics Committee of Beijing University of
Chinese Medicine under the guidelines issued by the Regulations of
Beijing Laboratory Animal Management. IN or mesalazine granules
were dissolved in 100 ml normal saline and stored at 2–8°C until
use. The rats were provided ad libitum access to 3.5% DSS
solution (36–50 kDa; MP Biomedical, LLC, Santa Ana, CA, USA) for
seven days to prepare the acute experimental UC rat model, as
described previously (18). The
animals in the INL (4.2 g/kg/day), INM (8.4 g/kg/day), INH (16.8
g/kg/day), and mesalazine (Mes; 400 mg/kg/day) groups (n=8 in all
groups) were administered the respective doses at the beginning of
3.5% DSS feeding. Normal saline (10 ml/kg/day) was administered
orally to chow-fed rats (chow, n=8) and the model control (model,
n=8). Chow-fed rats were provided to access to water ad
libitum, whereas rats in the model group were provided with
ad libitum access to DSS solution. All groups were
administered their respective treatment or saline orally for seven
days.
Detection of DAI
Rat stool samples were harvested and evaluated every
day, and fecal occult blood test papers (BaSO Biotechnology, New
Taipei, Taiwan) were used to test for fecal occult blood following
the manufacturer's protocol. The scoring method of Hamamoto
(19) was used to calculate the rat
DAI score and evaluate disease activity. The DAI score includes
three aspects: Stool, faecal occult blood, and body weight loss,
scored on a 0–4-point system. For body mass, points were
distributed as follows; 0, no drop in body mass; 1, 1–5% drop in
body mass; 2, 5–10% drop in body mass; 3, 10–15% drop in body mass;
and 4, >15% drop in body mass. For stool, points were
distributed as follows; 0, normal stool; 2, loose stool; and 4,
diarrhoea. For fecal occult blood, points were distributed as
follows; 0, no blood; 2, positive; and 4, naked bloody stool.
Normal stool was well formed or shaped; loose stool, not adhered to
the anus, pasty, or semi-formed; and diarrhoea, shapeless and
adhering to the anus. DAI was evaluated daily in a blinded manner
by non-project team members as follows: DAI=(weight loss
score+stool consistency score+fecal occult blood score)/3 (20).
Colon pathology analysis in rats
After blood (10 ml) was sampled from the abdominal
aorta, all rats were anesthetized with 10% chloral hydrate (300
mg/kg, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and
sacrificed by decapitation. Colon tissues were harvested, fixed in
10% formalin for 24 h at 22°C, embedded in paraffin and cut into 4
µm sections. Haematoxylin and eosin staining was performed using
paraffin sections. The histological score (HS) was determined by
microscopically under a light microscope (Olympus Corp., Tokyo,
Japan) examining stained paraffin sections using the scoring
criteria of colonic histological activity index (21–23).
Each sample was randomly selected from five perspectives to
calculate the total score (0–10 points), including the infiltration
degree of inflammatory cells, ulcers and lesion level (Table I). The HS assessment was completed by
non-project team members under the guidance of a pathologist and
blindly reviewed by specific members.
 | Table I.Histological activity index
scoring. |
Table I.
Histological activity index
scoring.
| Score | Inflammation | Ulcer | Layer |
|---|
| 0 | A small amount of
inflammatory cells in the lamina propria | None | None |
| 1 | More neutrophil
granulocytes in the lamina propria | 1–2 ulcers | Mucosa |
| 2 | Infiltration of
inflammatory cells to the submucosa | 3–4 ulcers | Submucosa |
| 3 | Permeability of
inflammatory cells infiltration to the whole layers | Wide range or
continuous ulcer | Muscle |
| 4 | – | – | Serosa |
Detection of colon myeloperoxidase
activity
Colonic myeloperoxidase (MPO) activity was detected
according to a previously described method (24). Colonic tissue was dissected 1 cm
above the anus, washed with ice-cold saline and weighed.
Subsequently, 1 ml hexadecyl trimethyl ammonium bromide (HTAB)
buffer (0.5% HTAB 50 mmol; l PBS, pH 6.0; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was used for tissue homogenisation. The
tissue homogenate was centrifuged at 60,000 × g for 15 min at 4°C.
The absorbance optical density (OD) 1 of 0.1 ml supernatant added
with 2.9 ml 50 mmol/l phosphate buffered saline (PBS; containing
0.167 mg/ml o-dianisidine and 0.0005%
H2O2) was immediately measured at 460 nm
using a spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and the absorbance OD2 was measured 1 min later. MPO activity
was calculated as (OD2-OD1)/total protein (OD/mg protein).
Detection of cytokines
The supernatants of fresh colon tissue and serum
were carefully prepared and analysed following the protocol of a
commercial liquid-phase chip kit (RECYMAG65K04; Merck KGaA). Serum
cytokines [interleukin (IL)-1α, IL-1β, ILl-18, epidermal growth
factor (EGF) and vascular endothelial growth factor] were expressed
as pg/ml, and those in the tissues were expressed as pg/mg
protein.
Western blot analysis to detect
occludin
Proteins in 100 mg colonic tissue homogenate were
extracted using ice-cold radioimmunoprecipitation assaay lysis
buffer with a final concentration of 1 mM PMSF at 4°C in an
electric tissue homogenizer (ULTRA-TURRAX; IKA Industrie- und
Kraftfahrzeugausrüstung GmbH, Konigswinter, Germany) at 250 × g for
5 sec, 5 times at 5 sec intervals, and incubated on ice for 20 min
following homogenization. The supernatant was extracted by
centrifugation at 12,000 × g for 20 min at 4°C. Protein
concentrations were determined by using a bicinchoninic acid
protein assay kit (Promega, Madison, WI, USA). Samples (30 µg) were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. The membranes were then incubated in 5%
skimmed milk powder for 2 h prior to the addition of primary rabbit
anti-occludin polyclonal antibody (ab31721; 1:2,000; Abcam,
Cambridge, MA, USA) and GAPDH rat antibody (TDY042, 1:20,000;
Beijing TDY Biotech Co., Ltd., Beijing, China) and incubation at
4°C overnight. Membranes were subsequently incubated with
peroxidase-conjugated goat anti-rabbit secondary antibody
(1:20,000; cat. no. S001; Beijing TDY Biotech, Co., Ltd.) at room
temperature for 2 h, and an ECL detection system (horseradish
peroxidase substrate; WBKLS0500; EMD Millipore, Billerica, MA, USA)
was used according to routine methods (25). The intensities of the protein bands
were analysed using Gel-Pro 3.2 software (Media Cybernetics, Inc.,
Rockville, MD, USA). GAPDH protein was used as the internal control
to normalize the protein loading. The experiment was performed in
triplicate.
Immunohistochemistry of occludin
Following conventional dehydration, embedding
(26) and tissue slicing (thickness,
4 µm) of colon tissues, the paraffin sections were dewaxed and
hydrated (26). The diluent primary
rabbit anti-human occludin antibody (ab31721; 1:100; Abcam) was
used after the tissue antigens were repaired in a high pressure
cooker, >120°C. Secondary antibody incubation was performed
according to the instructions of the PV9000 secary antibody kit
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China), followed by 3,3′-diaminobenzidine staining. Five images
(mucosal layer) were randomly collected in each tissue slice at a
magnification of ×400 under a light microscope for analysis using
Image Plus 6.0 software (Media Cybernetics, Inc.). The OD was used
to represent protein expression, and the total stained area was
used to express the distribution and total expression area of the
target protein.
Determination of liver enzymes in
rats
An automatic biochemical analyser (CX4 Pro; Beckman
Coulter, Inc., Brea, CA, USA) (27)
was used to detect serum alanine transaminase (ALT) and aspartate
transaminase (AST) to assess the safety of IN.
Statistical analysis
All data are expressed as the mean ± standard
deviation, unless otherwise indicated, and were analysed with SPSS
17.0 statistical software (SPSS, Inc., Chicago, IL, USA). The data
that met the normal distribution and homogeneity of variance
criteria were subjected to one-way analysis of variance and
pairwise comparison was performed with the least significant
difference test. The data that fulfilled normal distribution but
did not meet the heterogeneity of variance criteria were subjected
to Tamhane's T2 test for pairwise comparison. P<0.05 was
considered to indicate a statistically significant difference. The
data that did not meet the normal distribution or homogeneity of
variance criteria were subjected to non-parametric testing, with
P<0.008 following correction considered to indicate a
statistically significant difference.
Results
DAI
All rats tolerated the experimental procedures well,
and none succumbed to mortality during the study. Following ad
libitum access to 3.5% DSS for two days, positive fecal occult
blood began to appear in the DSS group; however, no marked change
was observed in hair colour or weight (which are indicative of the
health of rats). With an increase in DSS consumption, haematochezia
was exacerbated and gross blood began to appear in the stool,
together with the lack of hair colour lustre, slowing of activity
and reaction to stimulus. The maximum DAI score was observed at 7
days. Compared with the chow group, DAI scores in the model group
were significantly increased (P<0.01; Fig. 1). Furthermore, Mes treatment and all
the IN treatments significantly attenuated the elevated DAI
compared with the model group (P<0.05; Fig. 1).
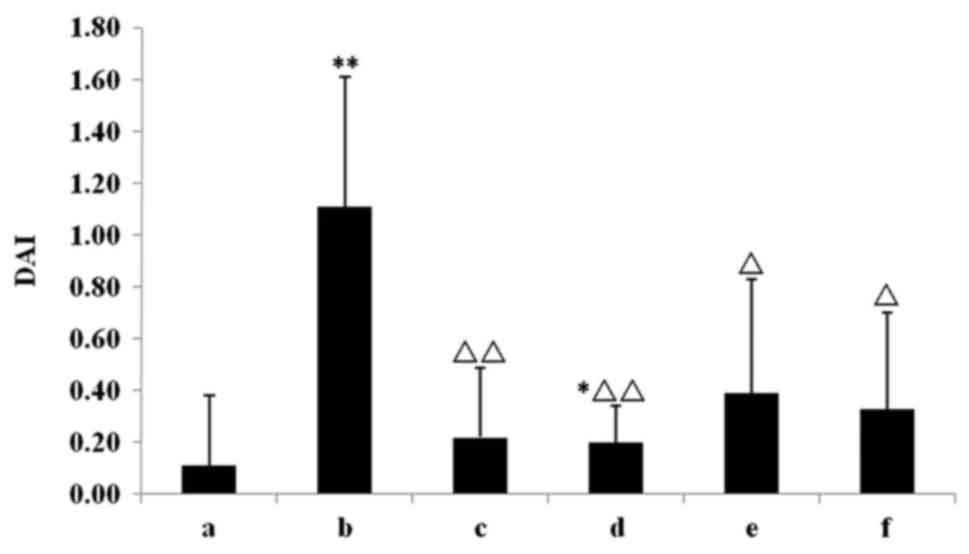 | Figure 1.Effect of IN on the detection of DAI.
The Mes treatment and all the IN treatments significantly
attenuated the elevated DAI. *P<0.05, **P<0.01 vs. chow
group; ΔP<0.05, ΔΔP<0.01 vs. model
group. Data are presented as mean ± standard deviation. IN, indigo
naturalis; DAI, disease activity index; Mes, mesalazine; a, chow
group; b, model group; c, Mes group; d, IN high-dose group; e, IN
medium-dose group; f, IN low-dose group. |
Mucosal histopathology changes in
rats
Histological analysis demonstrated few inflammatory
cells in the mucosa, and normal crypts and epithelial integrity in
the chow group (Fig. 2Aa). In the
DSS model group, light microscopy of the colonic mucosa indicated
congestion, edema and excessive infiltration of inflammatory cells,
which were primarily neutrophils. There were very few goblet cells
and the mucosa was continuously broken. Ulceration was observed
deep into the submucosa, and the lesions were primarily
concentrated from the mucosal layer to the submucosal layer
(Fig. 2Ab). Conversely, all
treatment groups exhibited reduced congestion, oedema and
infiltration of inflammatory cells (Fig.
2Ac-f). Compared with the chow group, the colonic
histopathological score of the model group was significantly higher
(P<0.01; Fig. 2B). The mesalazine
group and all IN groups exhibited significantly reduced colonic
histopathologic scores compared with the model group (P<0.01;
Fig. 2B).
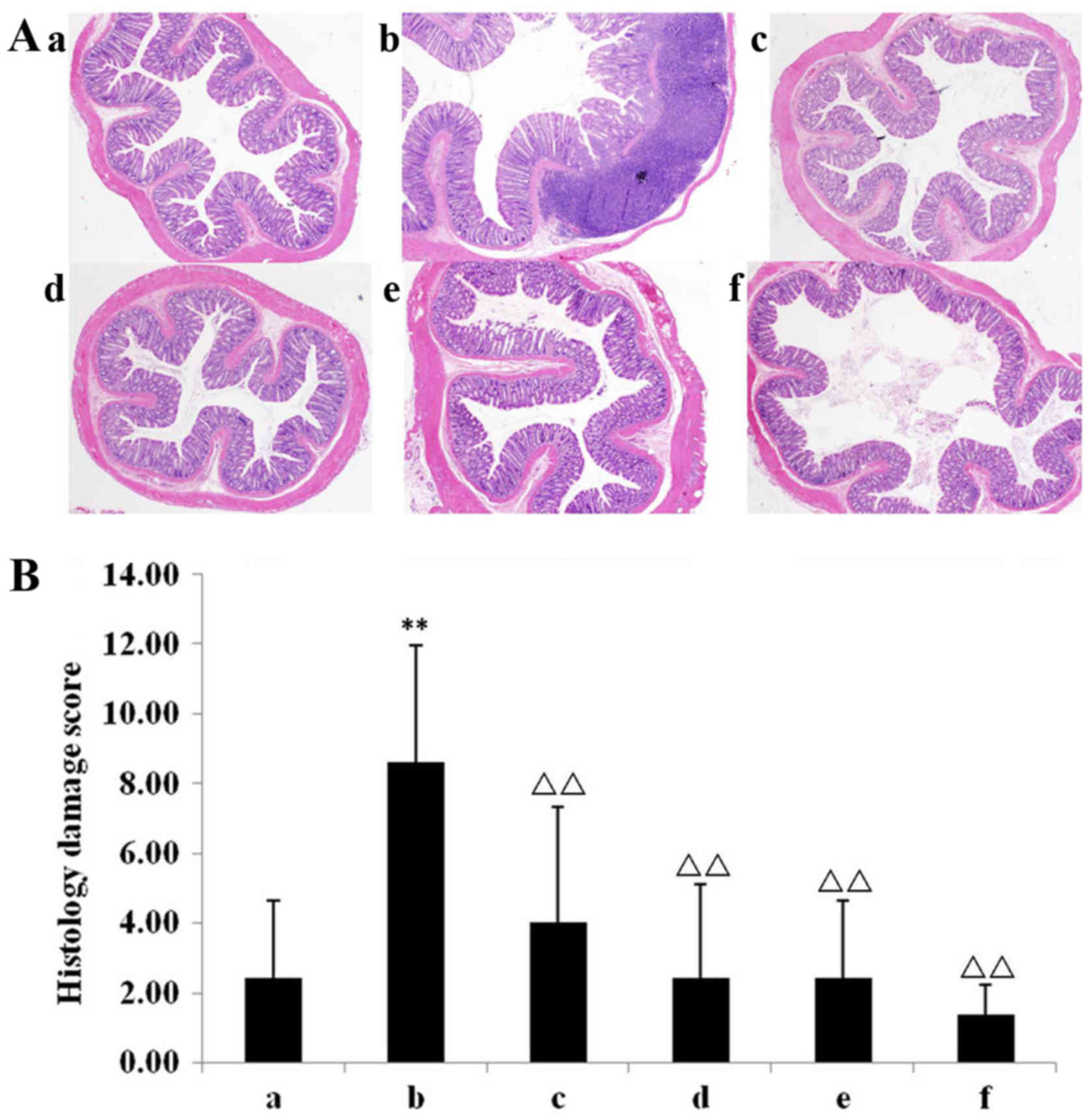 | Figure 2.Mucosal histopathology changes in
rat. (A) Haematoxylin and eosin stained paraffin sections of the
(Aa) chow, (Ab) model, (Ac) Mes, (Ad) IN high-dose, (Ae) IN
medium-dose, and (Af) IN low-dose groups. In the dextran sulphate
sodium model group, the colonic mucosa indicates congestion, oedema
and excessive infiltration of inflammatory cells. Magnification,
×40. (B) All treatment groups demonstrate reduced congestion,
oedema and infiltration of inflammatory cells. Compared with the
chow group, the colonic histopathological score of the model group
was significantly higher. The Mes group and all IN groups had lower
colonic histopathologic scores compared with the model group.
**P<0.01 vs. chow group; ΔΔP<0.01 vs. model group.
Data are presented as mean ± standard deviation. Mes, mesalazine;
IN, indigo naturalis; INH, IN high-dose; INM, IN medium-dose; INL,
IN low-dose; a, chow group; b, model group; c, Mes group; d, IN
high-dose group; e, IN medium-dose group; f, IN low-dose group. |
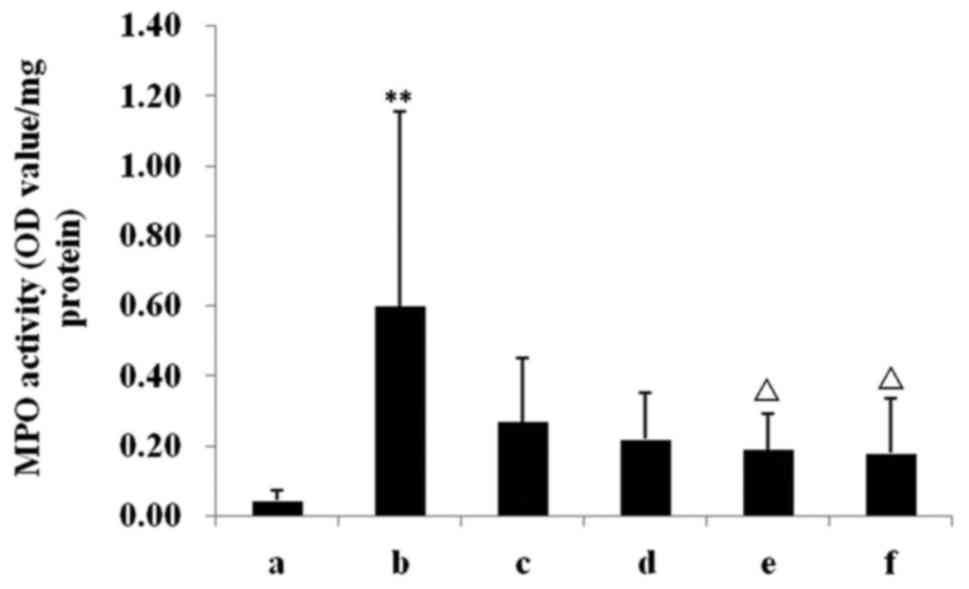
Activities of colon MPO
Compared with the chow group, the MPO activity in
the colon was significantly higher in the DSS model group
(P<0.01; Fig. 3). INM and INL
treatments significantly decreased the MPO activities compared with
the model group (P<0.05; Fig. 3).
As presented in Fig. 3, although the
MPO in INH and Mes groups decreased, this was not statistically
significant.
Level of inflammatory cytokines
Compared with the chow group, the level of serum
IL-1α in the model group increased, although this was not
statistically significant (Fig. 4A).
The level of IL-1α and IL-1β in the colonic tissue of the model
group, however, was increased significantly compared with the chow
group (P<0.01; Fig. 4B), whereas
levels of IL-1β were significantly increased in the model group
compared with the chow group in both the serum (P<0.05; Fig. 4C) and colonic tissue (P<0.01;
Fig. 4D). IL-18 levels were
significantly increased in the model group compared with the chow
group in the serum (P<0.05; Fig.
4E) and markedly in colonic tissue (Fig. 4F). All IN treatments reduced the
level of IL-1α IL-1β and IL-18 in the colonic tissue. In INH, a
significant reduction was observed in the serum IL-1β (P<0.05;
Fig. 4C) and IL-18 (P<0.01;
Fig. 4E) levels, in addition to
colonic IL-1α (P<0.05; Fig. 4B),
IL-1β (P<0.05; Fig. 4D) and IL-18
(P<0.01; Fig. 4F) levels, in
comparison with the model group. In INM, the serum levels of IL-1β
(P<0.01; Fig. 4C) and IL-18
(P<0.05; Fig. 4E) were
significantly reduced, while the colonic tissue exhibited
significant reductions in IL-1α (P<0.01; Fig. 4B) and IL-1β (P<0.01; Fig. 4D) levels, compared with the model
group. In INL, compared with the model group, the serum IL-18 level
was significantly reduced (P<0.05; Fig. 4E), whereas the levels of IL-1α
(P<0.01; Fig. 4B), IL-1β
(P<0.05; Fig. 4D) and IL-18
(P<0.01; Fig. 4F) in the colonic
tissue were significantly reduced. In the Mes group, although there
was no significant difference in the serum levels of IL-1α, IL-1β
or IL-18, the colonic tissue IL-1α (P<0.01; Fig. 4B), IL-1β (P<0.01; Fig. 4D) and IL-18 (P<0.05; Fig. 4F) levels were significantly reduced
compared with the model group.
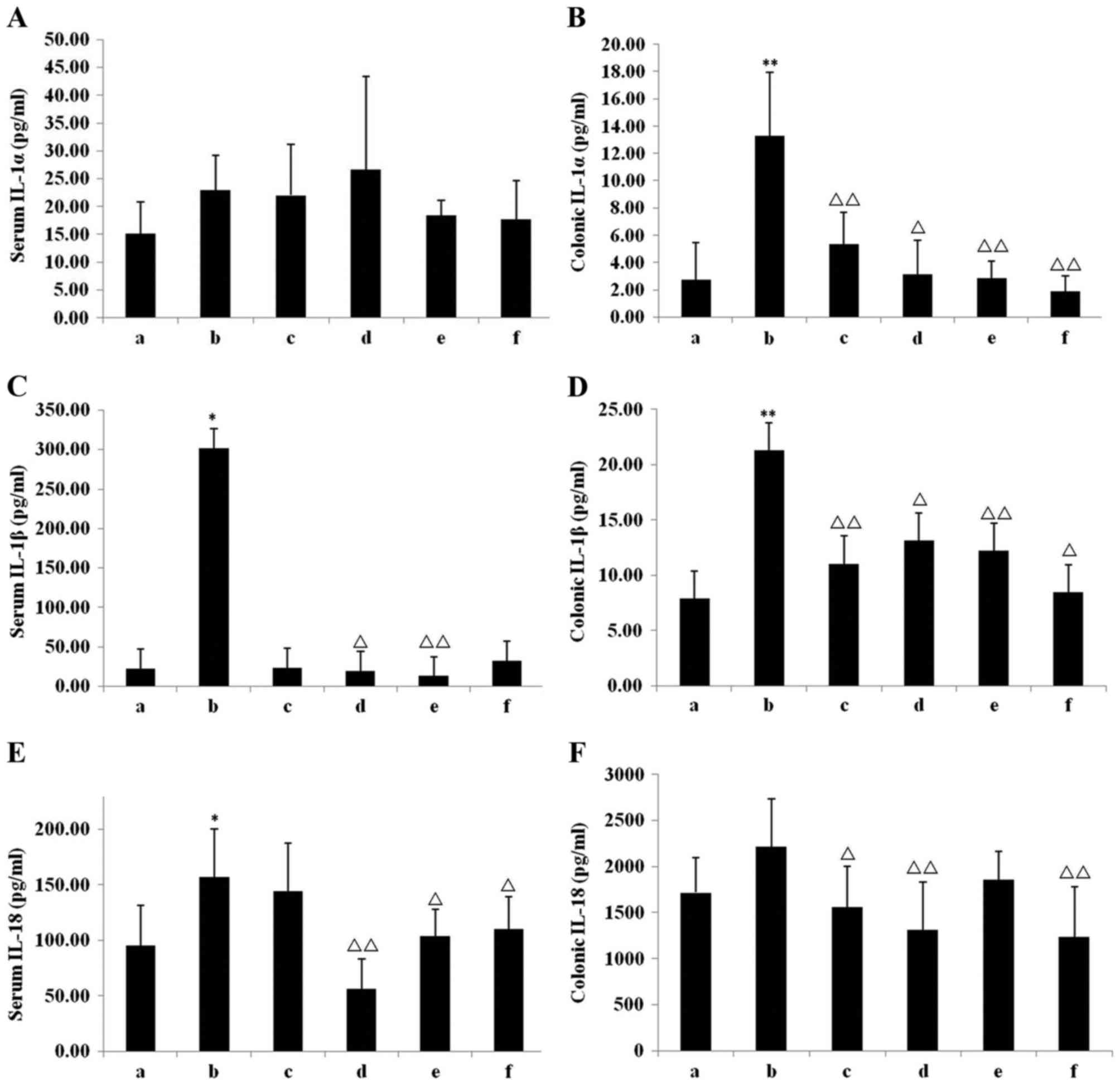 | Figure 4.Inflammatory cytokine levels in serum
and colonic tissue. (A) Serum IL-1α, (B) colonic IL-1α, (C) serum
IL-1β, (D) colonic IL-1β, (E) serum IL-18, and (F) colonic IL-18 in
the 6 groups. *P<0.05, **P<0.01 vs. chow group;
ΔP<0.05, ΔΔP<0.01 vs. model group. Data
are presented as mean ± standard deviation. IL, interleukin; a,
chow group; b, model group; c, mesalazine group; d, IN high-dose
group; e, IN medium-dose group; f, IN low-dose group; IN, indigo
naturalis. |
Repair of colonic mucosal damage
Compared with the chow group, EGF and VEGF in serum
and colonic tissues of the model group were decreased (Fig. 5), with significant decreases observed
in the serum EGF (P<0.05; Fig.
5A) and VEGF (P<0.05; Fig.
5C), and VEGF in the colonic tissue (P<0.01; Fig. 5D). Different doses of IN exhibited
different effects in repairing colonic mucosal damage. Compared
with the model group, the serum levels of EGF (P<0.05; Fig. 5A) and VEGF (P<0.05; Fig. 5C), and VEGF in the colonic tissue
(P<0.01; Fig. 5D) in the INH
group were significantly increased, whereas the INM group only
exhibited a significant increase in serum VEGF levels (P<0.05;
Fig. 5C) but not in serum EGF. The
level of serum VEGF (P<0.05; Fig.
5C), colonic EGF (P<0.01; Fig.
5B) and VEGF (P<0.05; Fig.
5D) in the INL group were significantly increased compared with
the model group. In the Mes group, the serum levels of EGF
(P<0.05; Fig. 5A) and VEGF
(P<0.01; Fig. 5C), and VEGF in
the colonic tissue (P<0.01; Fig.
5D), were significantly increased compared with the model
group.
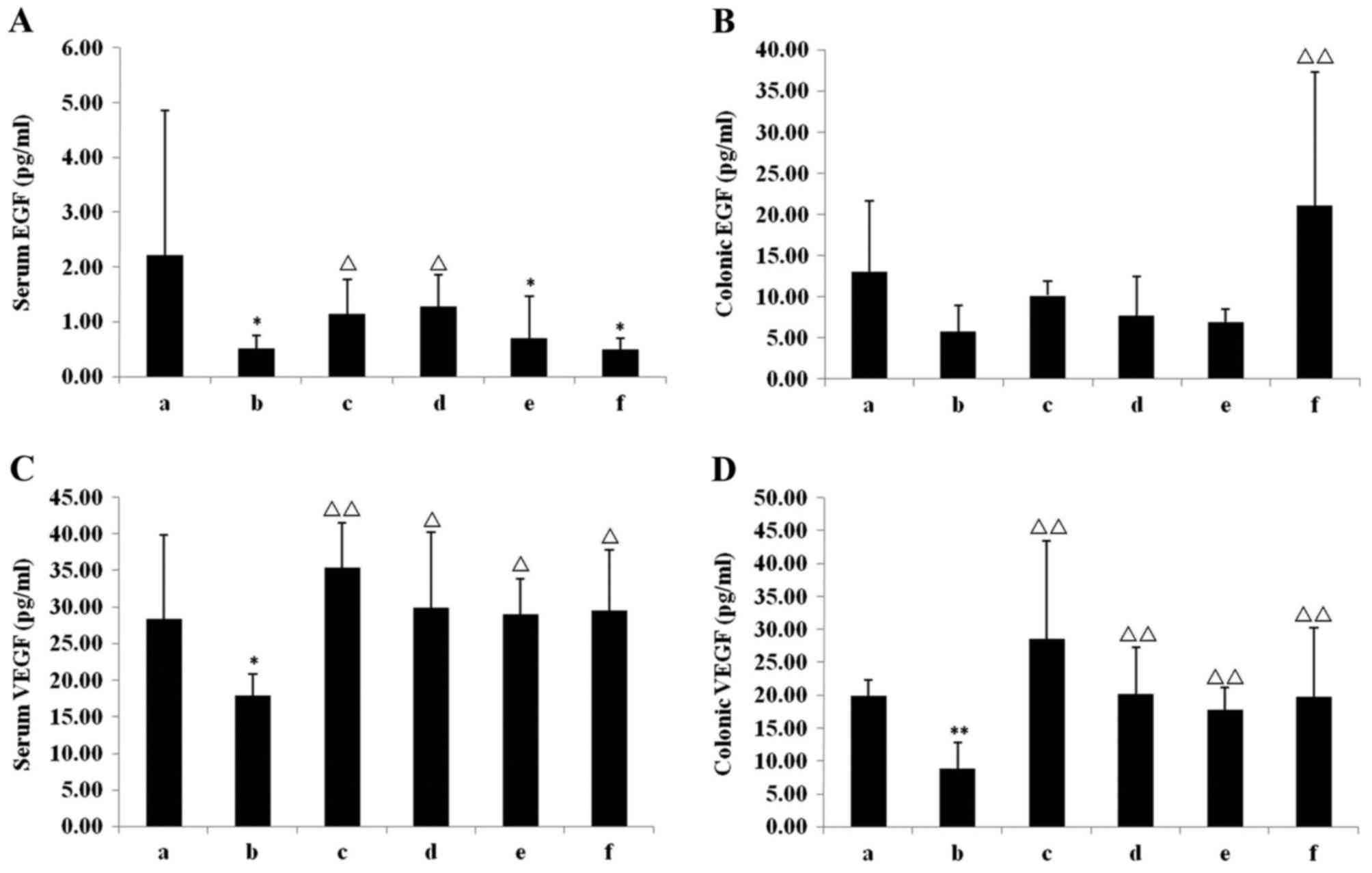 | Figure 5.EGF and VEGF levels in serum and
colonic tissue. (A) Serum EGF, (B) colonic EGF, (C) serum VEGF and
(D) colonic VEGF levels in the six groups. *P<0.05, **P<0.01
vs. chow group; ΔP<0.05, ΔΔP<0.01 vs.
model group. Data are presented as mean ± standard deviation. EGF,
epidermal growth factor; VEGF; vascular endothelial growth factor;
a, chow group; b, model group; c, mesalazine group; d, IN high-dose
group; e, IN medium-dose group; f, IN low-dose group; IN, indigo
naturalis. |
The quantification of immunohistochemical results
indicated that the occludin content in the model group was
significantly lower than the chow group (P<0.05; Fig. 6A). The occludin content was
significantly higher in the INH (P<0.05) and Mes groups
(P<0.01) compared with the model group, whereas there was no
significant increase in the INL group (Fig. 6A).
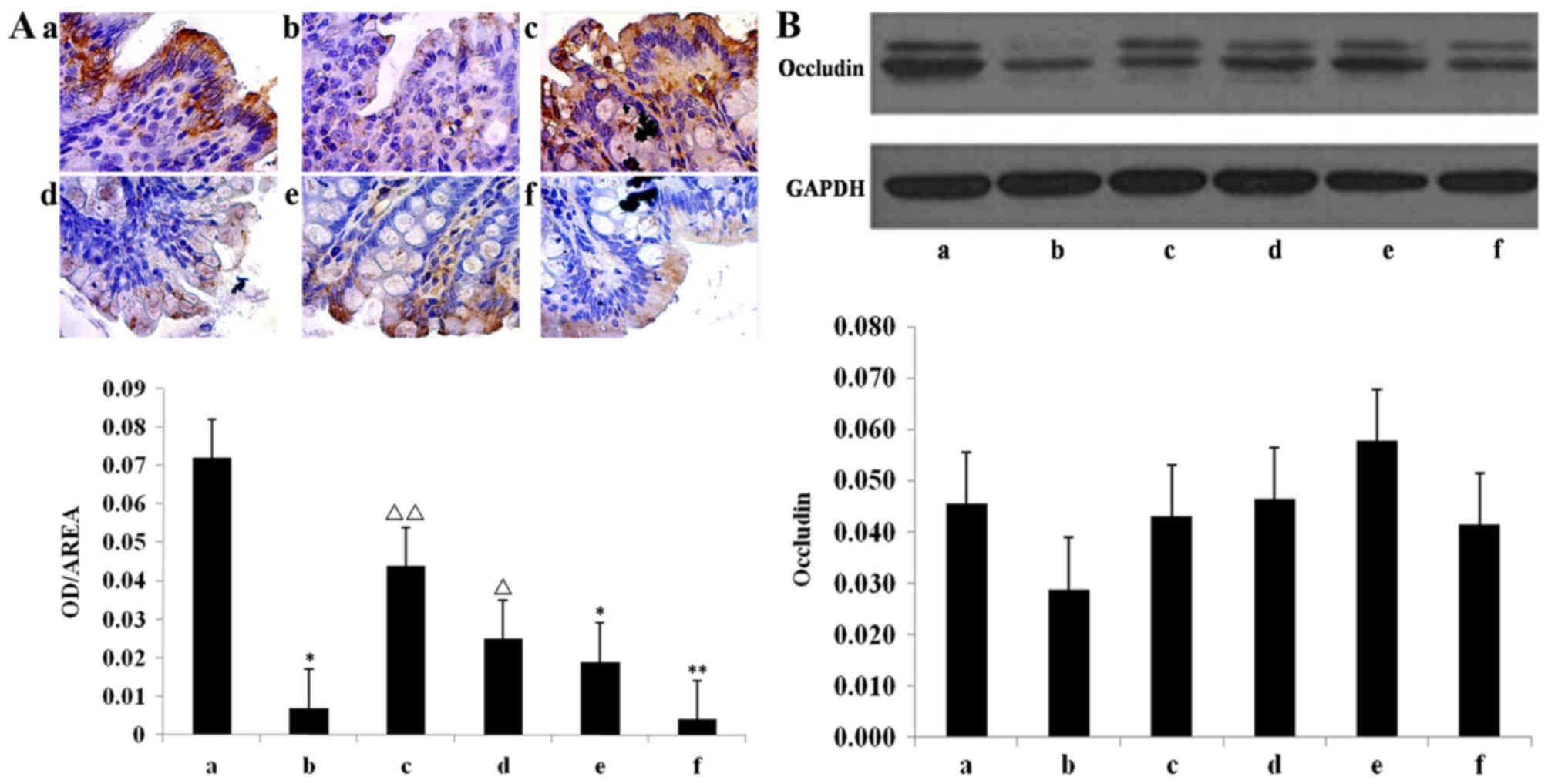 | Figure 6.Expression of occludin protein.
Assessed using (A) immunohistochemistry in the the (Aa) chow, (Ab)
model, (Ac) mesalazine, (Ad) IN high-dose, (Ae) IN medium-dose, and
(Af) IN low-dose groups, and (B) western blot analysis
quantification. Thank you for your response, please add your
reference to the manuscript. Make sure to add the reference to the
appropriate point in the references list, carefully updating all
subsequent references and in-text citations accordingly.
*P<0.05, **P<0.01 vs. chow group; ΔP<0.05,
ΔΔP<0.01 vs. model group. Data are presented as mean
± standard deviation. IN, indigo naturalis; a, chow group; b, model
group; c, mesalazine group; d, IN high-dose group; e, IN
medium-dose group; f, IN low-dose group; OD, optical density. |
The model and chow groups did not significantly
differ with regard to occludin protein expression, nor did IN and
Mes groups, although increasing trends were observed (Fig. 6B).
Liver injuries
To elucidate IN safety, serum AST and ALT were also
assessed. In the model group, the serum AST and ALT increased, but
no statistically significant difference was observed when compared
with the chow group. All IN treatments demonstrated a good safety
range, specifically the INH group, in which the AST and ALT were
highly significantly reduced (both P<0.01, Fig. 7) compared with the model group. In
the Mes group, the serum AST was significantly reduced (P<0.05,
vs. model group; Fig. 7B), whereas
no statistically significant difference was observed in ALT.
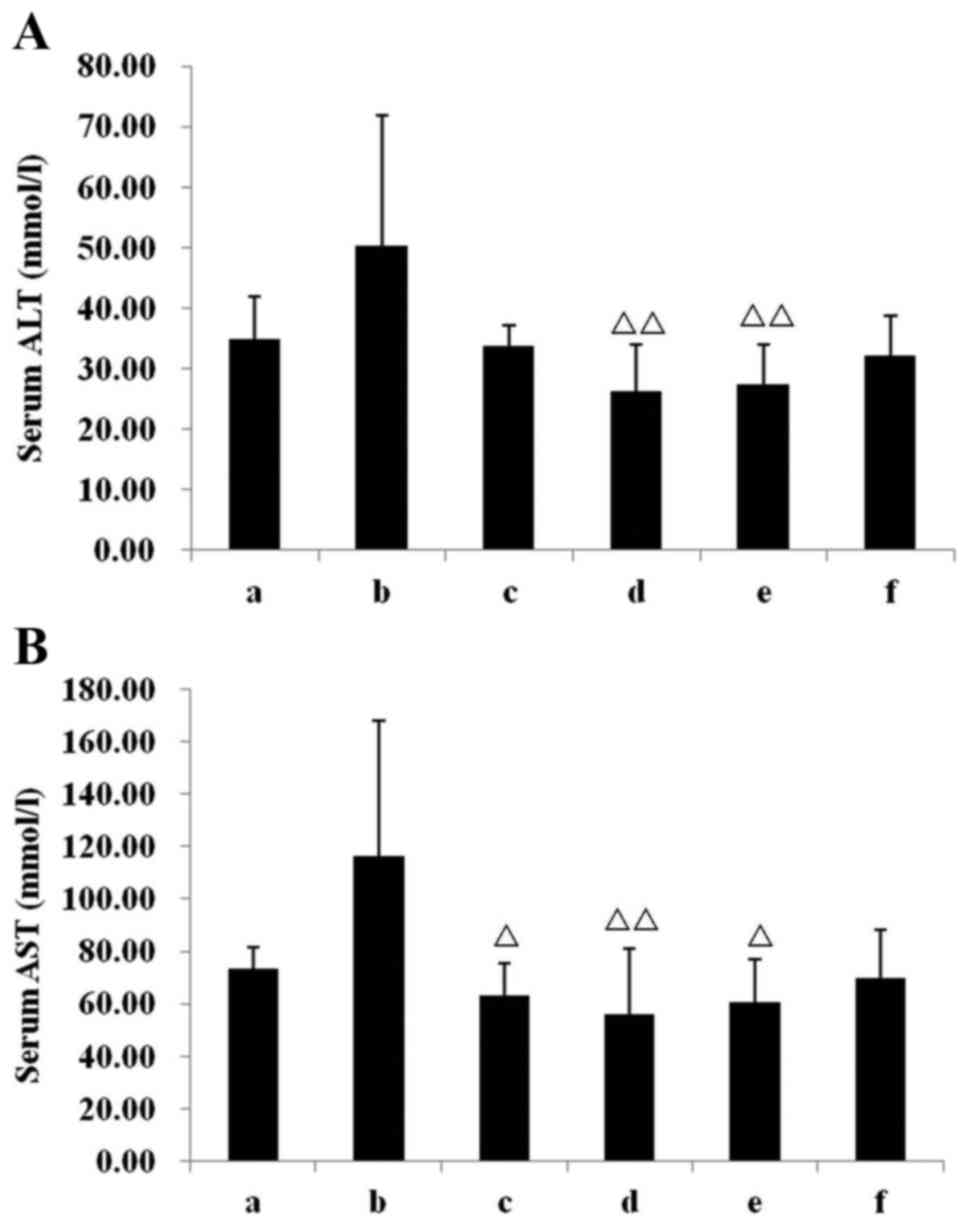 | Figure 7.Effect of IN on the serum
concentration of ALT and AST. Concentration of serum (A) ALT and
(B) AST in the six groups. ΔP<0.05,
ΔΔP<0.01 vs. model group. Data are presented as mean
± standard deviation. ALT, alanine transaminase; AST, aspartate
transaminase; a, chow group; b, model group; c, mesalazine group;
d, IN high-dose group; e, IN medium-dose group; f, IN low-dose
group; IN, indigo naturalis. |
Discussion
UC is a chronic IBD (28) and its symptoms include fatigue, a
constant need to defecate, nausea, diarrhoea, rectal bleeding and
abdominal pain, all of which markedly affect the quality of life
for the patient (29). It is a
chronic lifelong condition characterized by alternating flare-ups
and remission; therefore, patients with UC have a higher incidence
of colon cancer than the general population (30). Male sex, young age at UC diagnosis,
and extensive colitis are risk factor for the development of colon
cancer (3).
Multifactorial genetic basis, environmental factors,
microbiota and immune system have all been implicated to serve
important roles in the pathogenesis of IBD, which is driven by an
exaggerated immune response towards the gut microbiome in a
genetically susceptible host (31,32).
According to a previous study, the number of IBD association loci
is as high as 201, of which 27 loci contribute specifically to the
development of UC, including innate immunity, autophagy and
inflammatory response such as IL-23 receptor expression (33), other immune-mediated diseases
(STAT3), and susceptibility to Mycobacterium infection
(IL-12B) (34). IBD is associated
with an immunological imbalance of the intestinal mucosa, primarily
associated with adaptive immune system cells, which respond to
self-antigens and produce chronic inflammatory conditions in
patients (35). In UC, there is a
marked increase in the secretion of IL-13, which is the primary
interleukin responsible for inflammation and chronicity (36). Furthermore, despite Th1 involvement,
patients with UC also present a Th2 response, with increased
secretion of IL-4, IL-5 and IL-9 (36). IL-1 genes are highly upregulated in
the inflamed colonic mucosa of UC (37–39).
IL-1 has been demonstrated to serve a pivotal role during acute
phase response, wherein the α form of IL-1 is associated with
exacerbations in Crohn's disease and the β form of IL-1 is
associated with UC flares during the treatment of IBD patients
(40). Furthermore, mononuclear
cells in the lamina propria of UC patients may secrete more IL-1β
(41).
EGF, which consists of a class of polypeptides
secreted by salivary and duodenal glands, effectively promotes
mitosis and ameliorates injuries (42). EGF enema has been reported to
effectively treat left hemicolonic UC (43). In preliminary studies in humans,
topical EGF improved the healing of skin wounds (44) and systemic EGF helped resolve
necrotizing enterocolitis in neonates (45). VEGF, a type of protein that promotes
angiogenesis, may be expressed in colonic epithelial cells,
endothelial cells and the muscular layer, promoting the healing and
regeneration of vascular injuries (46). A marked increase in VEGF gene
expression and genes encoding the receptor Flt-1 has been observed
in patients with active UC and these increased VEGF levels in serum
and plasma in active UC patients may reflect VEGF overexpression in
intestinal inflammatory tissue (47). In addition, neutralizing anti-VEGF
antibody is able to significantly ameliorate experimental UC in
rats, partly by reducing excessive vascular permeability and
decreasing inflammatory cell infiltration via the Src-dependent
mechanism (48). Injuries of the
intestinal mucosal barrier, which is constituted by colonic
epithelial tight junction proteins, are important pathological
factors of UC, and the tight junction transmembrane protein
occludin has an important role in maintaining this barrier
(49). The occludin protein content
in the colonic mucosa of patients with active UC was identified to
be lower than those of healthy individuals and patients with UC in
the remission stage. This suggests that occludin protein serves a
major role in the pathogenesis of UC (50).
Current therapies primarily involve 5-ASA agents,
corticosteroids and immunosuppressive agents, of which the
5-aminosalicylates have a potent inhibitory effect on a number of
pro-inflammatory mediators released by the mucosa. These mediators
include leukotrienes, IL-1 and tumour necrosis factor-α (51–53),
with a response rate of 30–80%, depending on the endpoint used
(54). However, although the
mechanisms of 5-ASA action are numerous and not fully understood,
they are of limited benefits. As for corticosteroids, despite a
number of observed benefits, patient resistance to and dependency
on corticosteroids are persisting problems (55). Immunosuppressive agents such as
azathioprine are beneficial but may have serious side effects
(56). Therefore, novel therapeutic
approaches are required (43),
necessitating the identification of novel agents to counter the
limitations of current UC medications. In the present study,
mesalazine was used as a positive control drug in order to compare
between the groups in terms of different effects, e.g., mucosal
histopathology changes, activities of colon MPO, levels of
inflammatory cytokines and liver injuries.
Chinese herbal medicine has been demonstrated to be
effective in treating UC diseases and has a broad scope for drug
safety (57,58). In the present study, DSS-induced rats
were used as UC animal models. The rats exhibited morphological
changes in stool and blood in stool. Colonic pathology indicated
marked congestion, edema, ulcer and erosion, in addition to
infiltration of inflammatory cells and disappearance of crypts,
replicating the onset of human UC (59). The activity of MPO, which is
primarily located in inflammatory cells, specifically in the
cytoplasm of neutrophils, reflects the degree of tissue
infiltration of inflammatory cells and is recognized as an
important indicator of inflammation (24). Colon MPO activities of DSS-induced UC
rats were reduced by all doses of IN used, which is consistent with
the fact that IN may significantly ameliorate DSS-induced
pathological injuries. The infiltration levels of colonic
inflammatory cells and severity and inflammation ranges were
significantly reduced by IN treatment. Cytokines, including
inflammatory factors and EGF and VEGF, in serum and colonic
tissues, were detected and the concentration of IL-1 concentrations
in the IN treatment group decreased. The changing trends in serum
and colonic tissue levels were consistent with more prominent
changes in the colon, as mononuclear cells in the lamina propria of
patients with UC secrete more IL-1β (41). Concentrations of IL-18 in the IN
treatment groups were significantly reduced, with more marked
changes observed in the serum. IL-18 has been demonstrated to
markedly increase in the serum of patients with UC and serum IL-18,
rather than colonic mucosal IL-18, may be an effective indicator to
assess the disease activity of UC (60). However, changes in serum IL-1α in
DSS-induced UC rats were not significant, although the levels were
significantly increased in the colon. The primary reason for this
may be that in DSS-induced UC rats, IL-1α is predominantly produced
by colonic epithelial cells, whereas IL-1β originates in myeloid
cells (61). IN increased the
expression of serum EGF, in addition to serum and colonic VEGF, but
the effect on colonic EGF was not significant. Immunohistochemistry
and western blot analysis indicated that a high dose of IN enhanced
the occludin content in the colonic mucosa, indicating that IN may
be repairing tight junction proteins, promoting EGF and VEGF
expression in the colon and serum and repairing colonic mucosal
damage, thereby reversing disease activity. Although
dose-dependency of IN treatment was observed in some aspects,
further studies are required to make this clearer.
The historical use of IN over 1,000 years attests
its safety, reliability and tolerability. This was confirmed by
examining liver enzyme levels in response to IN treatment. All
doses of IN evaluated exhibited a good safety range, specifically
the INH group, in which the AST and ALT were significantly reduced
compared with the model group.
In conclusion, IN had a positive effect on
experimental UC by improving the inflammation by means of
regulating pro-inflammatory factors and ameliorating colonic
mucosal damage by repairing tight junction proteins. The present
study demonstrated that IN may be an optimal agent for UC treatment
due to its anti-inflammatory and colonic mucosal damage repair
properties. Further experiments should focus on the in-depth
investigation of the mechanisms of IN that modulate cytokines and
tight junctions, in addition to the association between intestinal
flora and the inflammatory immune response.
Acknowledgments
The present study was supported by G20 Project
Support, The Beijing Municipal Science and Technology Plan (grant
no. Z151100003815011; The Beijing municipal Science And Technology
Commission, Beijing, China) and the Youth Fund of National Science
Foundation of China (grant no. 81403369; State Natural Science
Funds Commission, Beijing, China).
References
|
1
|
Ordás I, Eckmann L, Talamini M, Baumgart
DC and Sandborn WJ: Ulcerative colitis. Lancet. 380:1606–1619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW and Kaplan GG: Increasing incidence and prevalence of the
inflammatory bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54, e42; quiz e30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jess T, Rungoe C and Peyrin-Biroulet L:
Risk of colorectal cancer in patients with ulcerative colitis: A
meta-analysis of population-based cohort studies. Clin
Gastroenterol Hepatol. 10:639–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: A meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neovius M, Arkema EV, Blomqvist P, Ekbom A
and Smedby KE: Patients with ulcerative colitis miss more days of
work than the general population, even following colectomy.
Gastroenterology. 144:536–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yashiro M: Ulcerative colitis-associated
colorectal cancer. World J Gastroenterol. 20:16389–16397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ording AG, Horváth-Puhó E, Erichsen R,
Long MD, Baron JA, Lash TL and Sørensen HT: Five-year mortality in
colorectal cancer patients with ulcerative colitis or Crohn's
disease: A nationwide population-based cohort study. Inflamm Bowel
Dis. 19:800–805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuoka H, Ikeuchi H, Uchino M, Bando T,
Takesue Y, Nishigami T and Tomita N: Clinicopathological features
of ulcerative colitis-associated colorectal cancer pointing to
efficiency of surveillance colonoscopy in a large retrospective
Japanese cohort. Int J Colorectal Dis. 28:829–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ullman T, Odze R and Farraye FA: Diagnosis
and management of dysplasia in patients with ulcerative colitis and
Crohn's disease of the colon. Inflamm Bowel Dis. 15:630–638. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dignass A, Eliakim R, Magro F, Maaser C,
Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire
S, et al: Second European evidence-based consensus on the diagnosis
and management of ulcerative colitis part 1: Definitions and
diagnosis. J Crohns Colitis. 6:965–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daperno M, Sostegni R, Rocca R, Rigazio C,
Scaglione N, Castellino F, Ercole E and Pera A: Review article:
Medical treatment of severe ulcerative colitis. Aliment Pharmacol
Ther. 16 Suppl 4:S7–S12. 2002. View Article : Google Scholar
|
|
13
|
Torres J, Boyapati RK, Kennedy NA, Louis
E, Colombel JF and Satsangi J: Systematic review of effects of
withdrawal of immunomodulators or biologic agents from patients
with inflammatory bowel disease. Gastroenterology. 149:1716–1730.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao HT, Peng J, Hu DD, Lin CY, Du B,
Tsang SW, Lin ZS, Zhang XJ, Lueng FP, Han QB and Bian ZX: Qing-dai
powder promotes recovery of colitis by inhibiting inflammatory
responses of colonic macrophages in dextran sulfate sodium-treated
mice. Chin Med. 10:292015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki H, Kaneko T, Mizokami Y, Narasaka
T, Endo S, Matsui H, Yanaka A, Hirayama A and Hyodo I: Therapeutic
efficacy of the Qing Dai in patients with intractable ulcerative
colitis. World J Gastroenterol. 19:2718–2722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin YK, See LC, Huang YH, Chang YC, Tsou
TC, Lin TY and Lin NL: Efficacy and safety of Indigo naturalis
extract in oil (Lindioil) in treating nail psoriasis: A randomized,
observer-blind, vehicle-controlled trial. Phytomedicine.
21:1015–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee MY, Liu YW, Chen MH, Wu JY, Ho HY,
Wang QF and Chuang JJ: Indirubin-3′-monoxime promotes autophagic
and apoptotic death in JM1 human acute lymphoblastic leukemia cells
and K562 human chronic myelogenous leukemia cells. Oncol Rep.
29:2072–2078. 2013.PubMed/NCBI
|
|
18
|
Hao Y, Nagase K, Hori K, Wang S, Kogure Y,
Fukunaga K, Kashiwamura S, Yamamoto S, Nakamura S, Li J, et al:
Xilei san ameliorates experimental colitis in rats by selectively
degrading proinflammatory mediators and promoting mucosal repair.
Evid Based Complement Alternat Med. 2014:5695872014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamamoto N, Maemura K, Hirata I, Murano M,
Sasaki S and Katsu K: Inhibition of dextran sulphate sodium
(DSS)-induced colitis in mice by intracolonically administered
antibodies against adhesion molecules (endothelial leucocyte
adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1
(ICAM-1)). Clin Exp Immunol. 117:462–468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasaki S, Hirata I, Maemura K, Hamamoto N,
Murano M, Toshina K and Katsu K: Prostaglandin E2 inhibits lesion
formation in dextran sodium sulphate-induced colitis in rats and
reduces the levels of mucosal inflammatory cytokines. Scand J
Immunol. 51:23–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dieleman LA, Palmen MJ, Akol H, Bloemena
E, Peña AS, Meuwissen SG and Van Rees EP: Chronic experimental
colitis induced by dextran sulphate sodium (DSS) is characterized
by Th1 and Th2 cytokines. Clin Exp Immunol. 114:385–391. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laroui H, Ingersoll SA, Liu HC, Baker MT,
Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV and Merlin
D: Dextran sodium sulfate (DSS) induces colitis in mice by forming
nano-lipocomplexes with medium-chain-length fatty acids in the
colon. PLoS One. 7:e320842012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rashidian A, Mehrzadi S, Ghannadi AR,
Mahzooni P, Sadr S and Minaiyan M: Protective effect of ginger
volatile oil against acetic acid-induced colitis in rats: A light
microscopic evaluation. J Integr Med. 12:115–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bradley PP, Priebat DA, Christensen RD and
Rothstein G: Measurement of cutaneous inflammation: Estimation of
neutrophil content with an enzyme marker. J Invest Dermatol.
78:206–209. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vink EI, Yondola MA, Wu K and Hearing P:
Adenovirus E4-ORF3-dependent relocalization of TIF1α and TIF1γ
relies on access to the Coiled-Coil motif. Virology. 422:317–325.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gaifulina R, Maher AT, Kendall C, Nelson
J, Rodriguez-Justo M, Lau K and Thomas GM: Label-free Raman
spectroscopic imaging to extract morphological and chemical
information from a formalin-fixed, paraffin embedded rat colon
tissue section. Int J Exp Pathol. 97:337–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen T, Gu D, Zhu Y, Shi J, Xu D and Cao
X: The value of eosinophil VCS parameters in predicting
hepatotoxicity of antituberculosis drugs. Int J Lab Hematol.
38:514–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yashiro M: Ulcerative colitis-associated
colorectal cancer. World J Gastroenterol. 20:16389–16397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yarlas A, Yen L and Hodgkins P: The
relationship among multiple patient-reported outcomes measures for
patients with ulcerative colitis receiving treatment with MMX ®
formulated delayed-release mesalamine. Qual Life Res. 24:671–683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abraham BP: Cancer surveillance in
ulcerative colitis and Crohn's disease: New strategies. Curr Opin
Gastroenterol. 32:32–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coskun M: Intestinal epithelium in
inflammatory bowel disease. Front Med (Lausanne).
1:242014.PubMed/NCBI
|
|
32
|
Akiho H, Yokoyama A, Abe S, Nakazono Y,
Murakami M, Otsuka Y, Fukawa K, Esaki M, Niina Y and Ogino H:
Promising biological therapies for ulcerative colitis: A review of
the literature. World J Gastrointest Pathophysiol. 6:219–227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duerr RH, Taylor KD, Brant SR, Rioux JD,
Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M,
Griffiths A, et al: A genome-wide association study identifies
IL23R as an inflammatory bowel disease gene. Science.
314:1461–1463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bianco AM, Girardelli M and Tommasini A:
Genetics of inflammatory bowel disease from multifactorial to
monogenic forms. World J Gastroenterol. 21:12296–12310. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Mattos BR, Garcia MP, Nogueira JB,
Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM
and Simioni PU: Inflammatory bowel disease: An overview of immune
mechanisms and biological treatments. Mediators Inflamm.
2015:4930122015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu ZJ, Yadav PK, Su JL, Wang JS and Fei
K: Potential role of Th17 cells in the pathogenesis of inflammatory
bowel disease. World J Gastroenterol. 15:5784–5788. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamamoto-Furusho JK, Santiago-Hernández
JJ, Pérez-Hernández N, Ramírez-Fuentes S, Fragoso JM and
Vargas-Alarcón G: Interleukin 1 β (IL-1B) and IL-1 antagonist
receptor (IL-1RN) gene polymorphisms are associated with the
genetic susceptibility and steroid dependence in patients with
ulcerative colitis. J Clin Gastroenterol. 45:531–535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Planell N, Lozano JJ, Mora-Buch R,
Masamunt MC, Jimeno M, Ordás I, Esteller M, Ricart E, Piqué JM,
Panés J and Salas A: Transcriptional analysis of the intestinal
mucosa of patients with ulcerative colitis in remission reveals
lasting epithelial cell alterations. Gut. 62:967–976. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Román J, Planell N, Lozano JJ, Aceituno M,
Esteller M, Pontes C, Balsa D, Merlos M, Panés J and Salas A:
Evaluation of responsive gene expression as a sensitive and
specific biomarker in patients with ulcerative colitis. Inflamm
Bowel Dis. 19:221–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andus T, Daig R, Vogl D, Aschenbrenner E,
Lock G, Hollerbach S, Köllinger M, Schölmerich J and Gross V:
Imbalance of the interleukin 1 system in colonic mucosa-association
with intestinal inflammation and interleukin 1 receptor antagonist
[corrected] genotype 2. Gut. 41:651–657. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reinecker HC, Steffen M, Witthoeft T,
Pflueger I, Schreiber S, MacDermott RP and Raedler A: Enhanced
secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by
isolated lamina propria mononuclear cells from patients with
ulcerative colitis and Crohn's disease. Clin Exp Immunol.
94:174–181. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heitz PU, Kasper M, van Noorden S, Polak
JM, Gregory H and Pearse AG: Immunohistochemical localisation of
urogastrone to human duodenal and submandibular glands. Gut.
19:408–413. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sinha A, Nightingale J, West KP,
Berlanga-Acosta J and Playford RJ: Epidermal growth factor enemas
with oral mesalamine for mild-to-moderate left-sided ulcerative
colitis or proctitis. N Engl J Med. 349:350–357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brown GL, Nanney LB, Griffen J, Cramer AB,
Yancey JM, Curtsinger LJ III, Holtzin L, Schultz GS, Jurkiewicz MJ
and Lynch JB: Enhancement of wound healing by topical treatment
with epidermal growth factor. N Engl J Med. 321:76–79. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sullivan PB, Brueton MJ, Tabara ZB,
Goodlad RA, Lee CY and Wright NA: Epidermal growth factor in
necrotising enteritis. Lancet. 338:53–54. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kanazawa S, Tsunoda T, Onuma E, Majima T,
Kagiyama M and Kikuchi K: VEGF, basic-FGF, and TGF-beta in Crohn's
disease and ulcerative colitis: A novel mechanism of chronic
intestinal inflammation. Am J Gastroenterol. 96:822–828. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Frysz-Naglak D, Fryc B, Klimacka-Nawrot E,
Mazurek U, Suchecka W, Kajor M, Kurek J and Stadnicki A:
Expression, localization and systemic concentration of vascular
endothelial growth factor (VEGF) and its receptors in patients with
ulcerative colitis. Int Immunopharmacol. 11:220–225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tolstanova G, Khomenko T, Deng X, Chen L,
Tarnawski A, Ahluwalia A, Szabo S and Sandor Z: Neutralizing
anti-vascular endothelial growth factor (VEGF) antibody reduces
severity of experimental ulcerative colitis in rats: Direct
evidence for the pathogenic role of VEGF. J Pharmacol Exp Ther.
328:749–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Al-Sadi R, Khatib K, Guo S, Ye D, Youssef
M and Ma T: Occludin regulates macromolecule flux across the
intestinal epithelial tight junction barrier. Am J Physiol
Gastrointest Liver Physiol. 300:G1054–G1064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yamamoto-Furusho JK, Mendivil EJ and
Fonseca-Camarillo G: Differential expression of occludin in
patients with ulcerative colitis and healthy controls. Inflamm
Bowel Dis. 18:E19992012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nielsen OH, Verspaget HW and Elmgreen J:
Inhibition of intestinal macrophage chemotaxis to leukotriene B4 by
sulphasalazine, olsalazine, and 5-aminosalicylic acid. Aliment
Pharmacol Ther. 2:203–211. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kaiser GC, Yan F and Polk DB: Mesalamine
blocks tumor necrosis factor growth inhibition and nuclear factor
kappaB activation in mouse colonocytes. Gastroenterology.
116:602–609. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Caprilli R, Cesarini M, Angelucci E and
Frieri G: The long journey of salicylates in ulcerative colitis:
The past and the future. J Crohns Colitis. 3:149–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hanauer SB: Medical therapy of ulcerative
colitis. Lancet. 342:412–417. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gionchetti P, Rizzello F, Annese V,
Armuzzi A, Biancone L, Castiglione F, Comberlato M, Cottone M,
Danese S, Daperno M, et al: Use of corticosteroids and
immunosuppressive drugs in inflammatory bowel disease: Clinical
practice guidelines of the Italian Group for the Study of
Inflammatory Bowel Disease. Dig Liver Dis. pii:S1590–8658.
2017.
|
|
56
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wan P, Chen H, Guo Y and Bai AP: Advances
in treatment of ulcerative colitis with herbs: From bench to
bedside. World J Gastroenterol. 20:14099–14104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo M, Ding S, Zhao C, Gu X, He X, Huang
K, Luo Y, Liang Z, Tian H and Xu W: Red ginseng and semen coicis
can improve the structure of gut microbiota and relieve the
symptoms of ulcerative colitis. J Ethnopharmacol. 162:7–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Perse M and Cerar A: Dextran sodium
sulphate colitis mouse model: Traps and tricks. J Biomed
Biotechnol. 2012:7186172012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wiercinska-Drapalo A, Flisiak R,
Jaroszewicz J and Prokopowicz D: Plasma interleukin-18 reflects
severity of ulcerative colitis. World J Gastroenterol. 11:605–608.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bersudsky M, Luski L, Fishman D, White RM,
Ziv-Sokolovskaya N, Dotan S, Rider P, Kaplanov I, Aychek T,
Dinarello CA, et al: Non-redundant properties of IL-1α and IL-1β
during acute colon inflammation in mice. Gut. 63:598–609. 2014.
View Article : Google Scholar : PubMed/NCBI
|