Introduction
Lymphatic malformations (LMs), previously termed
lymphangioma, are benign congenital malformations that stem from a
malformation of the lymphatic vessels in the soft tissues (1). Although LMs have been studied, no
consensus has been found regarding their cause or treatment
(2). They primarily occur in the
head and neck, accounting for 75% of all cases (3), and grow proportional to a patient's
body growth (4). In approximately
half of the cases, the LMs are obvious at birth and as many as 90%
are diagnosed by the end of the second year of life due to clinical
symptoms (5). LMs can be solitary or
multifocal and have a variety of clinical presentations based on
their size and location (6).
Bleeding, trauma and/or infection may rapidly increase the cyst's
size, leading to respiratory obstruction, swallowing difficulties
and speech problems. In a small number of cases, rapid tumor growth
may occur as a result of increasing lymphatic flow and the sudden
closure of drainage channels due to infection and/or the
inflammatory process.
LM can be divided into three morphological types:
Microcystic, macrocystic and combined (combination of microcystic
and macrocystic components) (7).
Various types may coexist in the same lesion. Macrocystic LMs were
historically referred to as ‘cystic hygroma’, while microcystic LMs
were referred to as ‘capillary and cavernous lymphangioma’. The
previous classification of lymphangioma into capillary, cavernous
and cystic types, however, has little clinical value and should be
abandoned (8). A dominating
histological feature of LMs is endothelial-lined lymphatic channels
separated by connective tissues (9).
Clinically, the treatment of LMs has remained a
challenge. Current major treatments for LMs include surgical
excision and sclerotherapy. Due to the tendency of LMs to gradually
increase and compress the surrounding tissues, surgical resection
has been recommended by numerous clinicians as the first-line
treatment. For macrocystic LMs, surgical excision has been the
first choice of treatment, but total excision is not easy due to
their infiltration of the surrounding nerves and muscles. Complete
excision without damage to vital structures is possible in most
cases; however, improper surgical treatment may result in
disfigurement, scarring or injury to vital structures such as the
recurrent laryngeal nerve, cranial nerves and carotid artery. The
present study reported on cervical macrocystic LMs in 68 infants
encountered over a 5-year period as well as their treatment.
Materials and methods
Patient data
A total of 68 infants (aged between 3 and 36 months)
with macrocystic LMs in the head and neck region were included in
the present study and underwent surgical resection between January
2009 and December 2015 at the Department of Otolaryngology and Head
and Neck Surgery of Kunming Children's Hospital (Kunming, China).
The present study was performed in accordance with the declaration
of Helsinki and with approval from the Ethics Committee of Kunming
Children's Hospital (Kunming, China). Written informed consent was
obtained from the guardians of all participants. For all patients,
the diagnosis was confirmed by histological examination after the
resection. The LMs had grown slowly, a compressible mass being the
only sign. The majority of the lesions were elliptical or
irregularly shaped, soft, multiloculated and well-circumscribed
masses with sound trans-illuminance. Exclusion criteria for the
present study consisted of patients that had not undergone surgery
and/or exhibited anesthetic contraindication diseases.
Patient treatment
Imaging examinations, including ultrasonography
(Philips IU22; Royal Philips Electronics, Holland), computed
tomography (CT; GE Optima CT660; General Electric Company, Boston,
MA, USA) and magnetic resonance imaging (MRI; GE HDi 1.5T; General
Electric Company) were performed. Uncooperative patients were given
10% chloral hydrate (0.5 ml/kg) orally prior to the examination.
Ultrasonography was routinely performed in the axial and coronal
views. The transducer frequency was 5 MHz. Lesion size, internal
echo, morphology and correlation with the surrounding structures
were noted. Blood supply to the lesions was observed using color
Doppler imaging. The CT scan was performed from the skull to the
root of the neck. When necessary, it was extended to the superior
mediastinum, with a layer thickness of 5-mm, interval of 5-mm,
pitch of 1.0, tube voltage of 120 kV and tube current of 240 mA.
Contrast enhancement was performed via iohexol administration. The
MRI scanning sequence was spin-echo (SE) T1-weighted imaging
(T1WI), SE T2WI and T2WI with fat suppression.
Therapeutic decisions were individualized based on
clinical features, imaging appearance and parental preference.
Meticulous planning was required for surgical excision of the
lesions. In all patients, the surgery was performed under general
anesthesia with endotracheal intubation. Soft tissue masses were
excised as completely as possible with particular attention paid to
the identification and preservation of vital structures such as the
carotid sheath, hypoglossal nerve, glossopharyngeal nerve, vagus
nerve, accessory nerve, branch of the facial nerve, and sublingual
and submandibular glands. A negative pressure drainage tube was
placed after the operation. All lesions were pathologically
confirmed. After the operation, side effects and complications,
including fever, infection, pain, hematoma, injury to the facial
nerve and respiratory difficulties were monitored and recorded.
Results
Clinical features
The patients included 35 males and 33 females with a
gender ratio of approximately 1:1, and no significant difference in
the incidence of complications was observed with regard to gender.
Forty of the subjects were newborns (0–1 years), while the other 28
were infants (1–3 years). The mean age of the patients was 15
months (range, 3 months to 3 years) at their first treatment. The
mean follow-up period was 27.8±10.5 months (range, 3–60 months),
including two patients who were lost to follow-up (Table I).
 | Table I.Clinical features of cervical
macrocystic lymphatic malformations in infants (n=68). |
Table I.
Clinical features of cervical
macrocystic lymphatic malformations in infants (n=68).
| Characteristic | Number | Percentage of
patients (%) |
|---|
| Age at diagnosis,
months |
|
|
| Mean | 15 | – |
|
Range | 3–36 | – |
| Gender |
|
|
| Male | 35 | 51.5 |
|
Female | 33 | 48.5 |
| Age, months |
|
|
| 3–12 | 40 | 58.8 |
|
12–36 | 28 | 41.2 |
| Follow-up period,
months |
|
|
| Mean | 27.8 | 97a |
|
Range | 3–60 |
|
| Distribution |
|
|
| Left
region | 28 | 41.2 |
| Right
region | 36 | 52.9 |
| Cervical
midline | 4 | 5.9 |
| Location |
|
|
|
Suprahyoid region | 24 | 35.3 |
| Subhyoid
region | 44 | 64.7 |
| Shape |
|
|
|
Elliptical | 24 | 35.3 |
|
Lobulated | 44 | 64.7 |
Distribution and location
The anatomical locations of LMs included the left
cervical region (28/68 cases), right cervical region (36/68 cases)
and median cervical region (4/68 cases). A total of 24 were located
in the suprahyoid region, while 44 were located in the subhyoid
region (Table I). Six patients
previously underwent resection in other hospitals but experienced
local recurrence within 5 months. In four cases, the LMs had
extended into the parotid region and infiltrated the facial nerve.
Lesion size ranged from 2.8×3.2 to 6×7.8 cm (mean, 4.5×6.2 cm). The
disease course was 3–12 months.
Imaging features and diagnosis
By ultrasonography, 24 LMs appeared monolocular and
44 were multilocular; 16 LMs had no echo, 20 had a uniform
low-level echo and 32 had a non-uniform level echo. Their boundary
with the surrounding tissues was usually clear and the lesion
tension was low. Ultrasonograms of monolocular LMs showed
elliptical and circular dark space. The lesion wall was thin,
smooth or slightly coarse and accompanied by posterior wall
acoustic enhancement. The ultrasonic manifestation of multilocular
LMs was a lobulated lesion with irregular fine septation (Table I). Loose alveolar tissue was
characteristic of multilocular LMs. Color Doppler imaging
demonstrated no blood flow signal within the mass. Solid components
were not seen in any case.
A total of 36 patients underwent CT examinations.
The CT features of cervical LMs were non-enhancing,
well-circumscribed lesions showing no calcifications and varying
attenuation values. CT attenuation values were 10–45 HU (mean, 26
HU). Most of the LMs (32/36) had low densities, while the densities
of the remaining four were identical. Eight patients underwent MRI
examinations. On MRI, the lymphanigomas appeared as irregular,
well-defined lesions with signs of vessel encasement. Compared with
the adjacent muscles, the LMs had low signal intensity on T1WI and
a high signal intensity on T2WI. Six cases had been misdiagnosed as
branchial cysts, four as hemangioma and two as cervical abscesses
by their local hospitals.
Treatment
Complete resection was possible in 56 patients
(82.4%), while subtotal resection was performed in eight and
partial resection was performed in four. For the subtotal resection
cases, the lesions had extended toward the mouth floor and beneath
the mandible and their complete removal was not possible. In the
four partial resection cases, the LMs had wrapped around the
parotid gland, including the posterior lobe. To avoid injury to the
facial nerve trunk, partial resection was performed. Two
complications were observed: A reversible paresis of the marginal
mandibular branch of the facial nerve and a local infection.
After the resection, a histopathological examination
showed that in all cases, the lesions had formed from the
fibromuscular walls and lymphatic vessels with endothelial cells.
No signs of malignancy were noted. Only two patients who underwent
partial resection had recurrences at ~2 months after the operation
(Table II). For those patients, a
sclerotherapy injection with pingyangmycin (PYM; a widely used
sclerosing agent for the treatment of venous malformations) was
performed under general anesthesia with ultrasonographic guidance
to accurately localize the cysts. The solution was prepared by
dissolving 8 mg PYM in 8 ml isotonic sodium chloride. Under
ultrasonographic guidance, a 22-gauge needle was inserted into the
cystic space under sterile conditions. The cystic fluid was
aspirated and the PYM solution was instilled into the cyst at a
dose of 0.2–0.3 mg/kg. In all patients, the treatment with PYM
injection achieved satisfactory results.
 | Table II.Type of treatment and outcome of
cervical macrocystic lymphatic malformations in infants (n=68). |
Table II.
Type of treatment and outcome of
cervical macrocystic lymphatic malformations in infants (n=68).
| Treatment | Number | Percentage of
patients (%) |
|---|
| Surgical
procedure |
|
|
| Complete
resection | 56 | 82.4 |
| Subtotal
resection | 8 | 11.8 |
| Partial
resection | 4 | 5.8 |
| Outcome |
|
|
|
Cured | 66 | 97.1 |
|
Recurrence | 2 | 2.9 |
Case presentations
Case 1
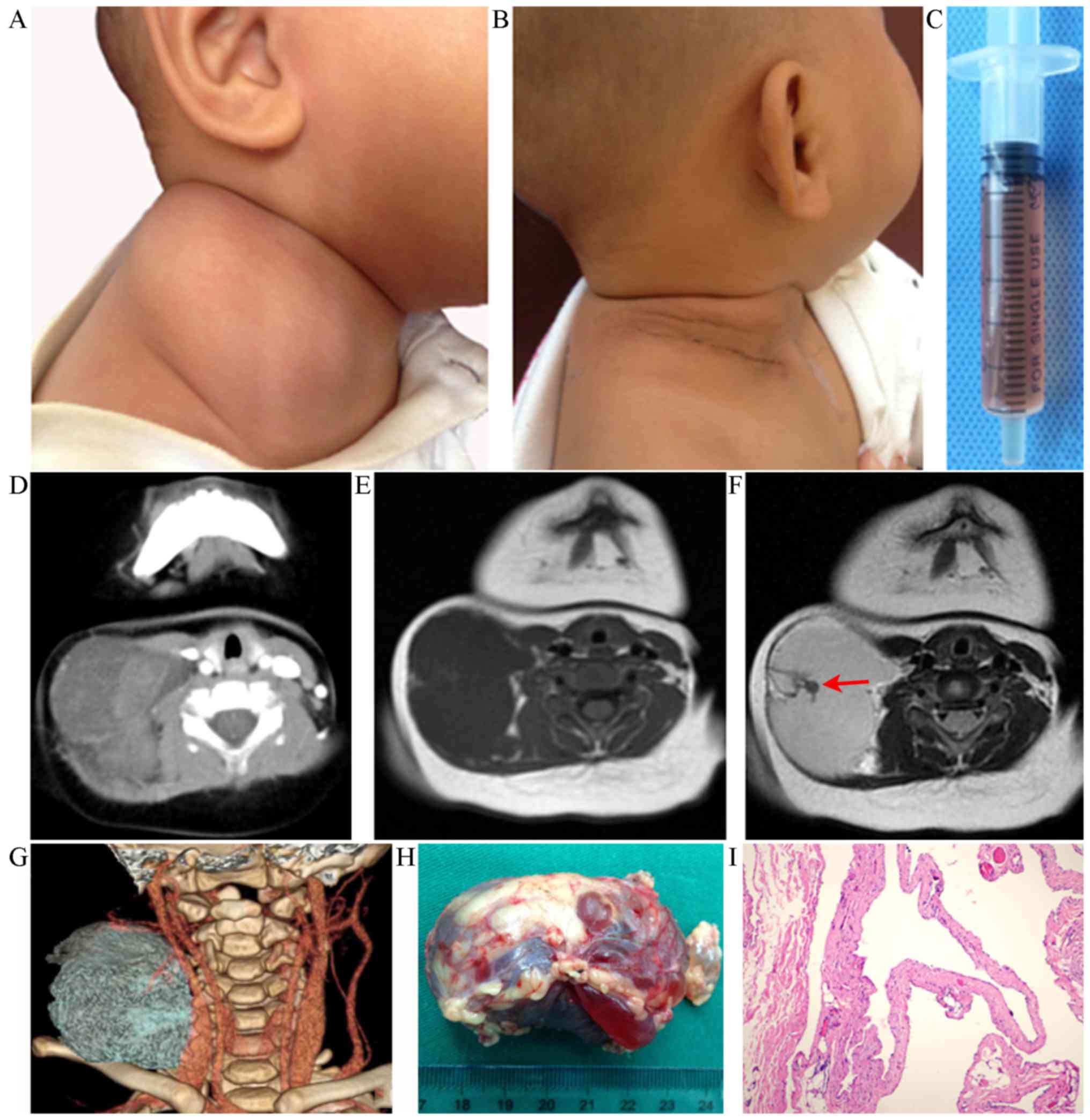
An 8-month-old male patient presented with a giant
macrocystic LM in the right lateral cervical region (Fig. 1A) which was subsequently removed
(Fig. 1B). The mass was noted at
birth as a small lesion and he had no obvious clinical symptoms.
Over time, the mass gradually enlarged but remained painless. Ten
days before the patient presented at the clinic, the size of the
lesion had rapidly increased. Fine-needle aspiration obtained a
slightly bloody fluid (Fig. 1C). The
lesion was diagnosed as an LM with hemorrhage and was 5.9×4.8 cm in
size (Fig. 1D-G). The patient
underwent radical surgery under general anesthesia. The course of
anesthesia and surgery was smooth. The wound healed satisfactorily
within reasonable time (Fig. 1H). No
complications occurred. Histopathological examination after the
resection demonstrated a macrocystic LM (Fig. 1I). The patient was followed up with
ultrasonography for 1 year and no sign of recurrence was noted.
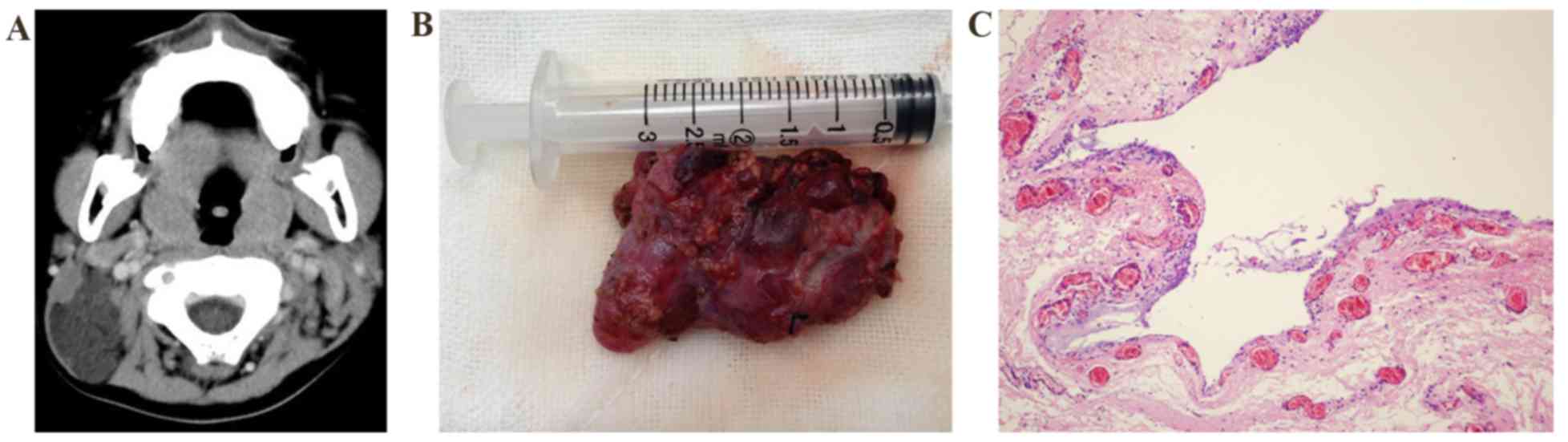
Case 2
A 2-year-old boy presented with a mass that was
found in the left sternocleidomastoid 1 year previously. Upon
admission, ultrasonography showed a cystic mass of 3.8×2.5 cm on
the deep surface of the left sternocleidomastoid with an
ill-defined border. The CT attenuation value was 10–20 HU (Fig. 2A). Ultrasound-guided centesis
obtained a pale-yellow liquid suggestive of left submandibular LM.
The patient underwent LM resection under general anesthesia. During
the operation, the mass was observed to be attached to the
surrounding muscular tissues and to have infiltrated the deep
surface of the internal jugular vein, and complete removal was
therefore not possible. For this reason, subtotal resection was
performed (Fig. 2B). The surgery
proceeded smoothly and no notable post-operative complications
occurred. After the resection, the wound was rinsed with bleomycin.
Histopathological examination after the resection demonstrated a
macrocystic LM (Fig. 2C). The
patient was followed up for 1 year with no significant
recurrence.
Discussion
LMs are rare congenital malformations of the
lymphatic system that are generally thought to be malformations of
segregated lymphatic tissues that have failed to develop into
lymphatic tissues with normal communications with the regional
lymphatic drainage, resulting in the dilatation of abnormal
channels. The incidence of LMs is 4/10,000 to 1/16,000 at birth,
accounting for 5–6% of all benign types of soft-tissue tumor in
children. No significant sex or racial differences have been noted
in the incidence of LMs (10). An
estimated 50% of LMs are correctly diagnosed prior to the patients
reaching an age of 2 years. In the cohort of the present study, 32
cases (88.23%) were identified at birth, eight were diagnosed by 1
year, and 20 were diagnosed by reaching 2 years of age.
The clinical manifestations of faciocervical LMs
vary in size and location. At birth, the lesions were usually small
and patients had no obvious symptoms. Over time, clinical
manifestations may gradually present, with a painless mass being
the only sign. Due to the elasticity of the faciocervical skin and
subcutaneous tissue, large LMs do not always cause serious
symptoms. Rapid growth of the LMs may occur and cause a
faciocervical deformity to interfere with breathing and/or
swallowing. This may be attributed to lymphatic vessel obstruction
and intracystic hemorrhage due to upper respiratory infection
and/or trauma.
In the cohort of the present study, the only
clinical manifestation observed in the majority of patients (56/68)
was a painless mass accompanied by a cosmetic deformity of varying
degrees. One infant had faciocervical motor dysfunction, another
had airway obstruction and another had dysphagia. The LMs
(macrocystic LMs in particular) were usually diagnosed in the
clinic and had a certain degree of light transmittance. The
aspirated cystic fluid had a straw-yellow color, and was
transparent and apt to solidification.
Ultrasonography, CT and MRI have been widely used to
assess the size and extent of LMs. In the present case series, a
pre-operative ultrasound examination was routinely performed.
Ultrasonography typically showed a multiloculated cystic lesion
with cystic cavities divided by septa of variable thickness, but
certain cases also showed an irregular or calcified appearance.
Doppler imaging demonstrated no blood flow within the lesions. CT
features of macrocystic LMs have included a well-circumscribed
lesion showing no calcifications and varied attenuation values
therein (11). Macrocystic LMs may
present as low-intensity signals on T1WI and high-intensity signals
on T2WI but signal intensity may be variable due to varying amounts
of protein and/or hemorrhage (12).
Each of the two techniques was helpful for determining the extent
and nature of the cysts and their correlation with the surrounding
structures. Such imaging techniques were also essential for the
identification of LMs and differentiating them from other vascular
malformations, branchial and thyroglossal cysts, lipomas, abscesses
or thyroid masses (13).
In the present case series, six patients had been
misdiagnosed with branchial cysts, which included four hemangiomas
and two cervical abscesses, at external hospitals. Although
lymphangiomas are benign, their treatment is difficult due to their
propensity to infiltrate and extend to important adjacent
structures. The mainstays of treatment have included the injection
of sclerosing agents and surgical excision. Balakrishnan et
al (14) found that
sclerotherapy and initial surgery for head and neck LMs were
similar in effectiveness in a multisite comparison, including total
hospital days, total intensive care unit days and higher likelihood
of subsequent tracheostomy. Sclerotherapy appears accurate,
minimally invasive, safe, low-cost and reliable without
complications, particularly for cervicofacial LMs in infants and
children (7). However, most cases
may require repeated injections with intervals varying from weeks
to months. Certain patients may have poor reactions and/or serious
allergic reactions to the sclerosing agents. Therefore, this
strategy must be abandoned in certain cases. Moreover, this does
not necessarily exclude later surgical treatment.
In 2007, Okazaki et al (15) reported their experience of treating
128 cases with lymphangioma with OK-432 and surgery. They found
that sclerotherapy may cause fever, infection, upper respiratory
tract obstruction or anaphylactic shock and suggested that
sclerotherapy with OK-432 was not as effective as previously
reported, although it may be efficient for a relatively long time.
In the past 20 years, intralesional laser therapy (carbon dioxide
or neodym-yttrium-aluminium garnet laser) has also been introduced
for the treatment of LMs (16).
Laser ablation is suitable for superficial mucosal lesions.
However, in most LMs, the cysts are often connected with the
expansion of a deep cyst connected to the muscular tissue. Due to
their pathological features and the limitations of laser
penetration, deep lesions are difficult to eradicate and prone to
relapse.
In 2012, Swetman et al (17) reported a marked regression of severe
LMs in three children after the treatment with orally administered
sildenafil. The authors suggested that the drug may act by relaxing
smooth muscles, resulting in decompression of the cyst. This
relaxation may also allow the secondary lymphatic space to reopen.
Sildenafil may also normalize endothelial dysfunction of the
lymphatic system.
Surgery has retained an important role in the
treatment of head and neck LMs in children (18). Surgical excision achieves higher cure
rates and may be an appropriate first-line therapy for giant
macrocystic LMs involving the cervicofacial region. The surgical
results depend on the location and extent of the lesion. Lesions
located in low-risk regions, such as the posterior cervical
triangle, should be subjected to surgery as soon as possible, the
outcomes of which are usually satisfactory. Secure surgery may be
achieved for lesions located in the submaxillary and parotid
regions. Should complete excision jeopardize vital structures
and/or functions, partial resection should be performed.
In general, isolated neck lesions have the highest
rate of complete resection. In the present case series, total
resection was achieved in 56 of 68 patients, while eight patients
underwent subtotal resection and four underwent partial resection.
In two cases, post-operative complications were observed, namely
reversible paresis of the marginal mandibular branch of the facial
nerve and local wound infection. Each of the two cases was treated
conservatively.
Chen et al (19) reported their experience of surgical
excision of cervicofacial giant macrocystic LMs in infants and
children. In 89.4% of the cases, complete resection was achieved,
with minor complications occurring in 19.1%. Surgical excision was
considered safe and yielded satisfactory esthetic and functional
results.
Intubation and nursing of the airway may also be a
clinical challenge. The infantile throat has a fragile mucosa and
narrow airway, particularly at the glottis and cricoid; therefore,
it is prone to laryngeal edema. The prophylactic use of
corticosteroids prior to intubation is necessary. Laryngoscopy and
intubation should be performed gently. Intubation under light
anesthesia may induce swallowing and choking, causing friction of
the catheter with cricoid mucosa and resulting in post-operative
laryngeal edema. Children with huge cervical LMs may also
experience tracheal cartilage softening as a result of long-term
compression. As the tracheal stent formed by the surrounding tissue
may disappear after the resection, tracheal collapse may occur,
resulting in airway obstruction. Therefore, tube withdrawal should
occur in steps after the surgery once the patients have become
fully awake. Extubation should be performed in 1-cm steps. If no
sign of airway obstruction is present, the tube is withdrawn for
another 1 cm each time until it is completely removed.
During the first year of life, observation is
necessary, since the lesions may cause severe deformity and/or
complications. A previous study has suggested that spontaneous
regression may occur (20). Kennedy
et al (21) suggested that
the surgery should be performed when the patient is >2 years of
age when the cervical mass is the only sign. For such patients, the
minimum age at surgery should be 3 months. The results of the
present study suggest that patient age is not a crucial factor
regarding treatment. The operation may be delayed until the patient
is at least 12 months of age, when the cervicofacial tissue has
fully developed and the patients can better tolerate general
anesthesia. The possibility of complete excision, risk of
post-operative complications, injury to adjacent structures as well
as scarring should be carefully evaluated.
Surgeons who operate on large macrocystic LMs must
be familiar with the complex anatomy of the blood vessels, nerves
and muscles (19). Important
structures such as nerves, muscles and vessels surrounding the
adjacent masses should not be injured. The general goal of surgical
treatment is to remove the tissue involved without sacrificing any
vital structures. The complications of surgical excision depend on
the location of the LM and the affected structures. The most common
complications include damage to neurovascular structures such as
the cranial nerves, chylous fistula, chylothorax, hemorrhage and
recurrence (22).
In conclusion, cervical LMs may manifest with a
range of clinical features. Ultrasonography, CT and MRI are
effective methods for their pre-operative diagnosis and safety
evaluation. Therapeutic decisions should be individualized and
based on clinical features, imaging and a multidisciplinary
consultation. To achieve optimal treatment, a problem-based
approach should be adopted. LM location, size and clinical
manifestations may significantly influence the treatment plan.
Surgical excision remains the mainstay of treatment. Complete
resection is the standard approach and can be achieved in most
cases. Should the lesions be too large and their neighboring
structures prone to injury, complete resection should not be
attempted. Surgeons should be aware of the limitations and
potential complications. Leaving a small amount of residual lesion
to avoid complications is acceptable.
Acknowledgements
The present study was supported by a grant from the
High-level Health and Family Planning Technical Personnel Training
Projects of Yunnan province (grant no. D201637).
References
|
1
|
Mulliken JB, Burrows PE and Fishman SJ:
Mulliken and Young's vascular anomalies: Hemangiomas and
malformations. Oxford: Oxford University Press; 2013, View Article : Google Scholar
|
|
2
|
Cho BC, Kim JB, Lee JW, Choi KY, Yang JD,
Lee SJ, Kim YS, Lee JM, Huh S and Chung HY: Cervicofacial lymphatic
malformations: A retrospective review of 40 cases. Arch Plast Surg.
43:10–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vlahovic A, Gazikalovic A and Adjic O:
Bleomycin sclerotherapy for lymphatic malformation after
unsuccessful surgical excision: Case report. Acta Otorhinolaryngol
Ital. 35:365–367. 2015.PubMed/NCBI
|
|
4
|
Wiegand S and Werner JA: Lymphatic
malformations in the head and neck area. HNO. 64:133–142. 2016.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Werner JA, Dünne AA, Folz BJ, Rochels R,
Bien S, Ramaswamy A and Lippert BM: Current concepts in the
classification, diagnosis and treatment of hemangiomas and vascular
malformations of the head and neck. Eur Arch Otorhinolaryngol.
258:141–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farnoosh S, Don D, Koempel J, Panossian A,
Anselmo D and Stanley P: Efficacy of doxycycline and sodium
tetradecyl sulfate sclerotherapy in pediatric head and neck
lymphatic malformations. Int J Pediatr Otorhinolaryngol.
79:883–887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Sun M, Ma Q, Cheng X, Ao J, Tian
L, Wang L and Lei D: Bleomycin A5 sclerotherapy for cervicofacial
lymphatic malformations. J Vasc Surg. 53:150–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ameh EA, Caouette-Laberge L and Laberge
JM: Lymphangiomas. Ameh EA, Bickler SW, Lakhoo K, Nwomeh BC and
Poenaru D: Paediatric surgery: A comprehensive text for Africa.
GLOBAL HELP Organization Seattle. 649–654. 2011.
|
|
9
|
Ates LE, Kapran Y, Erbil Y, Barbaros U and
Dizdaroglu F: Cystic lymphangioma of the right adrenal gland.
Pathol Oncol Res. 11:242–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zadvinskis DP, Benson MT, Kerr HH, Mancuso
AA, Cacciarelli AA, Madrazo BL, Mafee MF and Dalen K: Congenital
malformations of the cervicothoracic lymphatic system: Embryology
and pathogenesis. Radiographics. 12:1175–1189. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zakaria RH, Barsoum NR, El-Basmy AA and
El-Kaffas SH: Imaging of pericardial lymphangioma. Ann Pediatr
Cardiol. 4:65–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaabouni A, Rebai N, Fourati M, Rekik S,
Chabchoub K, Slimen MH, Bahloul A and Mhiri MN: Cystic lymphangioma
of the kidney: Diagnosis and management. Int J Surg Case Rep.
3:587–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fung K, Poenaru D, Soboleski DA and Kamal
IM: Impact of magnetic resonance imaging on the surgical management
of cystic hygromas. J Pediatr Surg. 33:839–841. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balakrishnan K, Menezes MD, Chen BS, Magit
AE and Perkins JA: Primary surgery vs primary sclerotherapy for
head and neck lymphaticmal formations. JAMA Otolaryngol Head Neck
Surg. 140:41–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okazaki T, Iwatani S, Yanai T, Kobayashi
H, Kato Y, Marusasa T, Lane GJ and Yamataka A: Treatment of
lymphangioma in children: Our experience of 128 cases. J Pediatr
Surg. 42:386–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balakrishnan A and Bailey CM: Lymphangioma
of the tongue: A review of pathogenesis, treatment and the use of
surface laser photocoagulation. J Laryngol Otol. 105:924–930. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swetman GL, Berk DR, Vasanawala SS,
Feinstein JA, Lane AT and Bruckner AL: Sildenafil for severe
lymphatic malformations. N Engl J Med. 366:384–386. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boardman SJ, Cochrane LA, Roebuck D,
Elliott MJ and Hartley BE: Multimodality treatment of pediatric
lymphatic malformations of the head and neck using surgery and
sclerotherapy. Arch Otolaryngol Head Neck Surg. 136:270–276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen WL, Zhang B, Wang JG, Ye HS, Zhang DM
and Huang ZQ: Surgical excision of cervicofacial giant macrocystic
lymphatic malformations in infants and children. Int J Pediatr
Otorhinolaryngol. 73:833–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perkins JA, Maniglia C, Magit A, Sidhu M,
Manning SC and Chen EY: Clinical and radiographic findings in
children with spontaneous lymphatic malformation regression.
Otolaryngol Head Neck Surg. 138:772–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kennedy TL, Whitaker M, Pellitteri P and
Wood WE: Cystic hygroma/lymphangioma: A rational approach to
management. Laryngoscope. 111:1929–1937. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar V, Kumar P, Pandey A, Gupta DK,
Shukla RC, Sharma SP and Gangopadhyay AN: Intralesional bleomycin
in lymphangioma: An effective and safe non-operative modality of
treatment. J Cutan Aesthet Surg. 5:133–136. 2012. View Article : Google Scholar : PubMed/NCBI
|