Introduction
Ischemia/reperfusion (I/R) injury of the kidneys
represents a challenge in the clinic and is commonly encountered in
renal transplantation, hemorrhagic shock, partial nephrectomy and
accidental or iatrogenic trauma, brining about serious injury to
multiple organs, including renal tubules, brain and heart (1). Therefore, it is urgent to find
effective therapies and elucidate molecular mechanisms by which
renal I/R injury may be attenuated. To date, a variety of signaling
pathways that are correlated with renal I/R injury have been
identified.
Oxidative stress has been validated to contribute to
the pathogenesis of renal I/R injury (2). Renal I/R-induced oxidative stress
generates high levels of reactive oxygen species (ROS).
Consequently, overproduction of ROS results in lipid peroxidation,
DNA mutation, apoptosis and necrosis, thus leading to cell death in
various ways (3,4). Anti-oxidants, such as febuxostat,
ligustrazine, sulforaphane, oxymatrine and gelsemine, have been
demonstrated to protect murine kidneys against I/R injury (3–7). All of
this evidence has indicated that renal I/R injury may be
ameliorated via targeting oxidative stress.
The signaling pathway previously found to be
associated with anti-oxidative stress is the nuclear factor
erythroid-2-related factor 2 (Nrf2)/heme oxygenase 1 (HO-1)
pathway. Nrf2, a transcription factor, has the potential to bind
with anti-oxidant response element (ARE), which is located at the
promoter regions of a battery of anti-oxidant and detoxifying
genes, including HO-1 (8).
Furthermore, the anti-oxidant N-acetylcysteine was found to have a
protective role in renal I/R injury via the Nrf2/HO-1 signaling
pathway (9). The present study aimed
to identify whether simvastatin is able to regulate oxidative
stress and the Nrf2/HO-1 signaling pathway to prevent I/R-induced
renal injury.
Statins, including simvastatin and pravastatin,
limit catalysis in cholesterol biosynthesis via suppressing the
activity of 3-hydroxyl-3-methyl coenzyme A (HMG-CoA) reductase
(10). In addition, short-term
pre-treatment with statins (HMG-CoA reductase inhibitors) was
demonstrated to reduce post-ischemic acute renal failure in a
uninephrectomized rat model, which may be achieved due to their
anti-inflammatory action (11). For
instance, atorvastatin was found to attenuate renal I/R injury in
rats via its anti-inflammatory action and reducing oxidative stress
(12), and improve renal function
via inhibiting active caspase-3 in rats (13). Simvastatin has the potential to lower
cholesterol in patients with cardiovascular disease (14,15). In
normocholesterolemic animals, simvastatin was reported to protect
against stroke mediated by endothelial nitric oxide synthase
(16), and to inhibit
leukocyte-endothelial cell interactions and vascular inflammatory
responses (17). In addition,
simvastatin was demonstrated to attenuate I/R injury in rat hearts
mediated by enhanced endothelial release of nitric oxide (18).
The present study aimed to investigate the effects
of simvastatin on renal I/R injury and to provide a molecular
foundation for the treatment of renal injury.
Materials and methods
Ethics statement and model
construction
A total of 24 male adult Sprague-Dawley (SD) rats (8
weeks old; weight, 220–250 g) were obtained from Animal Core
Facility of Nanjing Medical University (Nanjing, China), fed food
and water freely under pathogen-free conditions and kept at 22±2°C
with 60±5% humidity under a 12-h light/dark cycle. Rats were
randomly divided into 3 groups: Sham group, I/R group and I/R +
simvastatin group, with 8 rats in each group. After acclimatization
for approximately one week and fasting for 12 h, I/R injury was
performed. In brief, rats were anesthetized by intraperitoneal
injection of pentobarbital sodium (50 mg/kg, Sigma-Aldrich; Merck
KGaA; Darmstadt, Germany) and underwent right nephrectomy.
Subsequent occlusion of the left renal hilus lasted for 45 min,
followed by 24 h of reperfusion. Rats in the sham group were
subjected to the same procedure but without any vessel occlusion.
Rats in the I/R + simvastatin group received 2 ml simvastatin by
intragastric administration (2 mg/kg; Hangzhou Moshadong
Pharmaceutical Co. Ltd., Hangzhou, China) 60 min prior to
occlusion, while rats in the other two groups received the same
volume of 0.9% saline. The experimental protocol of the present
study was approved by the Animal Ethics Review Committee of Nanjing
Medical University (Nanjing, China). Procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health.
Assessment of renal function
Renal function was assessed by determining the
levels of blood urea nitrogen (BUN), serum creatinine (SCr) and
lactate dehydrogenase (LDH). In brief, at the end of reperfusion,
rats were sacrificed by intraperitoneal injection of pentobarbital
sodium (150 mg/kg). Then blood samples were collected by cardiac
puncture. Levels of SCr, BUN and LDH were evaluated by an automated
analyzer (Siemens ADVIA 2400; Siemens AG, Munich, Germany). Left
nephrectomy was performed and renal samples were fixed in 4%
paraformaldehyde (PFA) or frozen in liquid nitrogen for further
measurements.
Evaluation of oxidative stress in rat
renal tissues
As sensitive indicators of oxidative stress,
superoxide dismutase (SOD) activity and malondialdehyde (MDA)
content were estimated to demonstrate the anti-oxidative effects of
simvastatin in the tissues (19).
SOD activity and MDA concentration were determined by
commercialized assay kits (A001-1 and A003-1; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's instructions. The optical density at 535 nm was read
and results are presented in nm/mg.
Histological examination
Kidneys were fixed with 4% PFA, embedded in paraffin
and sectioned into 4-µm slices, followed by staining with
hematoxylin and eosin (HE). In brief, slices were de-waxed in
xylene, re-hydrated by a graded series of alcohols and rinsed with
distilled water with subsequent staining with hematoxylin for 3–5
min. Prior to change of solution from 70% alcohol to with 1% HCl,
slices were washed with distilled water for 15–30 min. Thereafter,
slices were stained with eosin for 1–4 min. Following dehydration
and differentiation in alcohol to remove the blue cytoplasm, making
nucleus more clear, slices were observed under a microscope
(Olympus, Tokyo, Japan). The degree of tubular damage was estimated
utilizing a semi-quantitative scale according to the following
criteria: 0, normal kidney tissue; 1, minimal damage (<5% area,
outer medulla or the cortex); 2, mild damage (5–25% area, outer
medulla or cortex); 3, moderate damage (25–75% area outer medulla
or cortex); and 4, severe damage (>75% area, outer medulla or
cortex).
Terminal deoxynucleotidyl transferase (TdT)-mediated
deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) assay.
At 24 h post-ischemia, apoptotic cells in kidney tissues were
evaluated by a TUNEL assay kit (11684817910; Roche Diagnostics,
Mannheim, Germany) in accordance with the manufacturer's
instructions. In brief, 4-mm slices were treated with 20 mg/ml
proteinase K, incubated in a nucleotide mixture of
fluorescein-12-dUTP and TdT. Cells were regarded as TUNEL-positive
if their nuclei were stained by 3,3-diaminobenzidine. In each
section, ten areas were randomly selected. TUNEL-positive cells
were counted in a double-blinded manner.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Kidneys were homogenized in TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total RNA was
immediately treated with DNaseI (Invitrogen; Thermo Fisher
Scientific, Inc.) and reversely transcribed into complementary DNA
(cDNA) by PrimeScript™ 1st Strand cDNA Synthesis Kit according to
the manufacturer's instructions (6110A; Takara Bio Inc., Otsu,
Japan). Reactions were performed using the SYBR® Premix
Ex Taq™ II (Perfect Real Time) Kit (DRR041A; Takara Bio,
Inc., Otsu, Japan) in an ABI Prism 7300 sequence detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). After an
initial denaturation at 95°C for 5 min, the PCR reaction conditions
were as follows: 35 cycles of denaturation at 95°C for 30 sec,
anneal at 55°C for 30 sec, and extension at 72°C for 30 sec. PCR
amplification of cDNA were performed in triplicate. GAPDH was used
as an endogenous control. The mean value of each sample was
expressed as the cycle threshold (ΔCt). Gene expression was
determined as the difference in ΔCt between target gene and GAPDH
(20). The primer sequences were as
follows: HO-1 forward, 5′-AAGATTGCCCAGAAAGCCCTGGAC-3′ and reverse,
5′-AACTGTCGCCACCAGAAAGCTGAG-3′; Nrf2 forward,
5′-AGTCGCTTGCCCTGGATATTC-3′ and reverse,
5′-GCCGGAGTCAGAGTCATTGAA-3′; GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′.
Western blot analysis
Rats were sacrificed with pentobarbital sodium (150
mg/kg), and the kidneys were removed, washed with ice-cold PBS and
homogenized in radioimmunoprecipitation assay lysis buffer (Roche
Diagnostics). Protein concentrations were assayed with a NanoDrop
instrument (Thermo Fisher Scientific, Inc., USA) and 40 µg of
protein was separated by 10% SDS-PAGE, and electro-transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked in 5% bovine serum albumin (BS043E;
Biosharp, Hefei, China) for 1 h at room temperature and then
incubated with primary antibody Nrf2 (1:1,000; sc-81342), HO-1
(1:1,000; sc-136256), β-actin (1:1,000; sc-58673; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. After
rinsing with Tris-buffered saline containing Tween 20 (TBST) for 3
times, membranes were incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody (A0216; 1:1,000; Beyotime
Institute of Biotechnology; Haimen, China) for 1 h at room
temperature followed by rinsing with TBST. Targeted proteins were
visualized with super signal west pico chemiluminescent substrate
(Thermo Fisher Scientific, Inc.). The membranes were visualized by
exposure to X-ray film in the dark. β-actin was used as an
endogenous control. Western blot analyses were performed 3 times in
triplicate.
Statistical analysis
Data were evenly distributed and expressed as the
mean ± standard deviation. Statistical analysis was performed by
one-way analysis of variance followed by a post-hoc analysis using
Dunnett's test. P<0.05 was considered to indicate a significant
difference.
Results
Simvastatin pre-treatment ameliorates
I/R-induced renal dysfunction
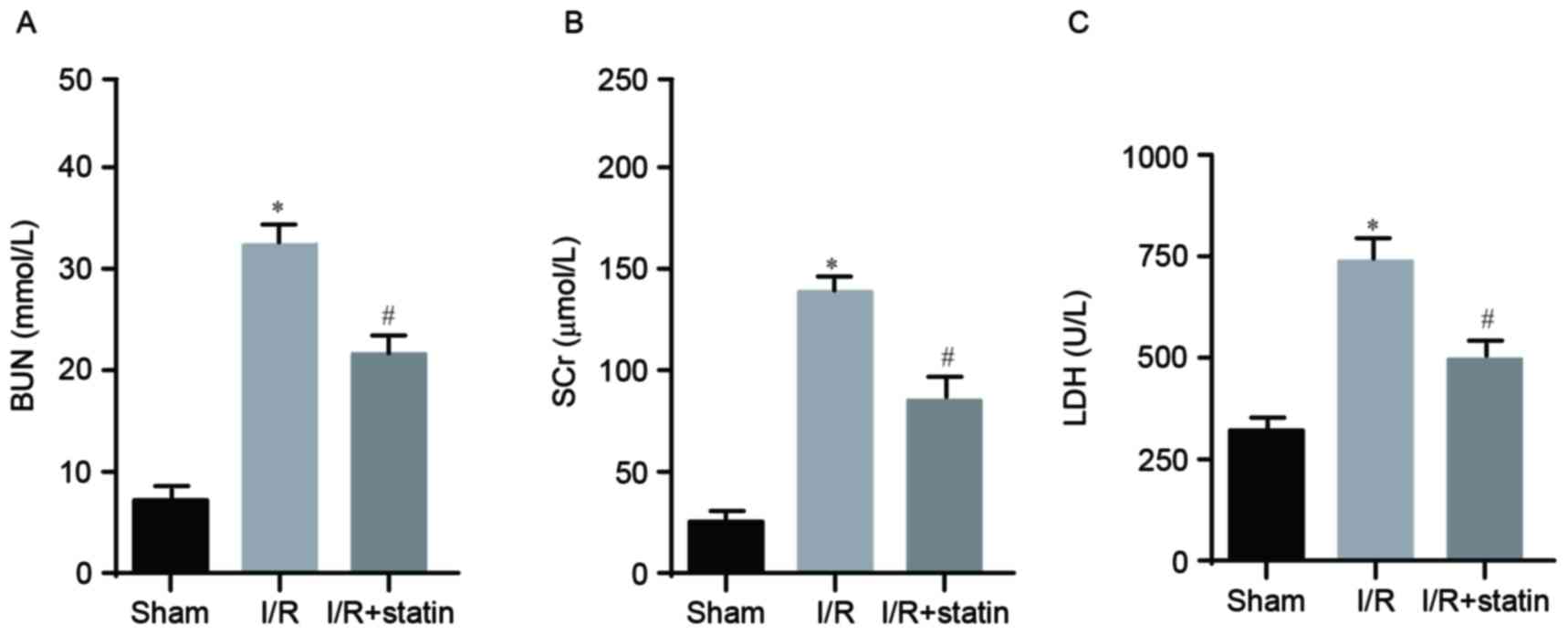
Renal function was assessed via the levels of BUN,
SCr and LDH, which were determined by an automated analyzer. In
comparison with the sham group, I/R significantly induced
upregulation of BUN (Fig. 1A), SCr
(Fig. 1B) and LDH (Fig. 1C), which were all notably repressed
by simvastatin pre-treatment. This inferred that simvastatin had a
protective role in I/R-induced renal dysfunction.
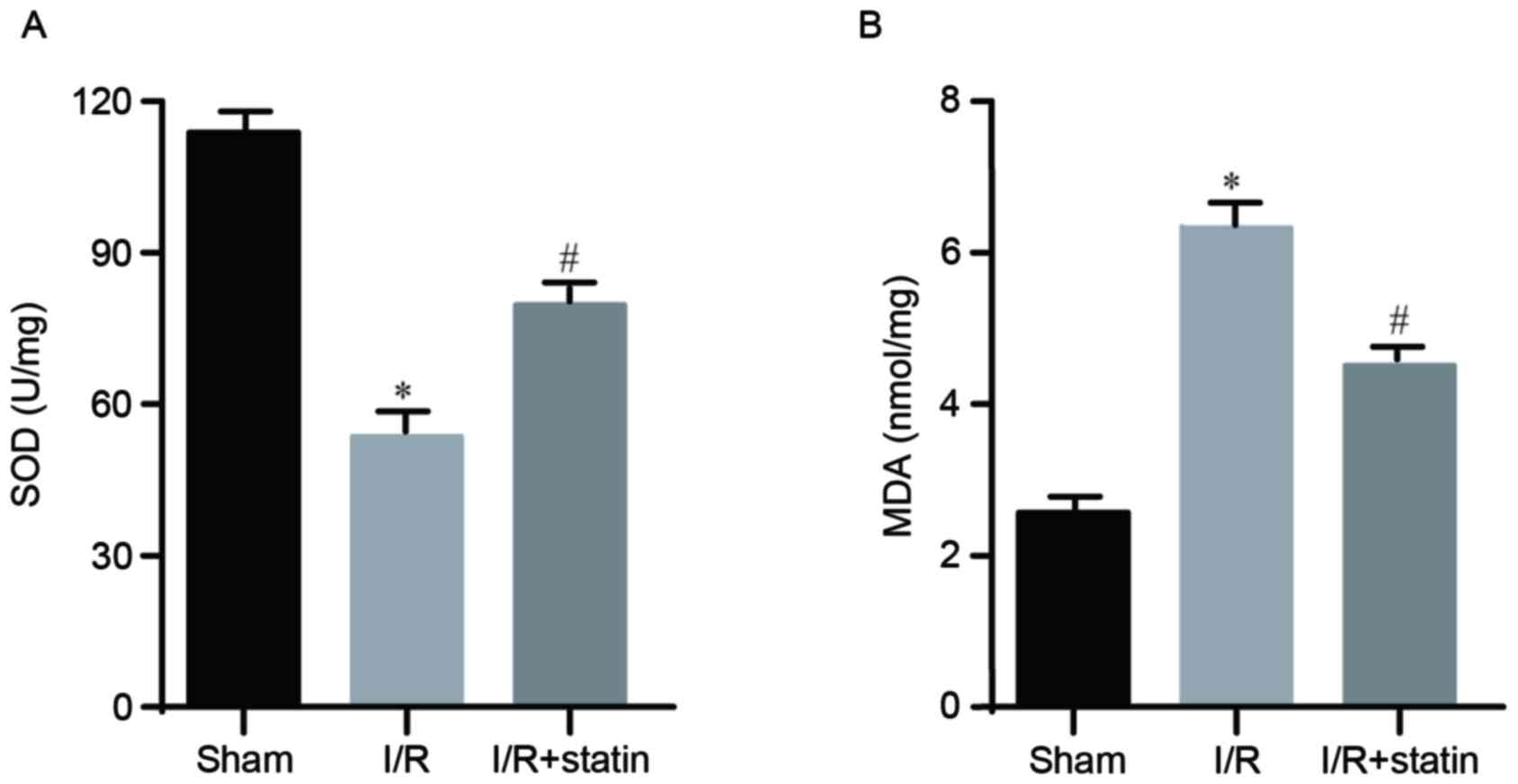
Simvastatin pre-treatment reduces
I/R-induced oxidative stress
The present study then assessed whether the
protective role of simvastatin relies on inhibition of oxidative
stress. As SOD activity and MDA concentration are markers for
oxidative stress, they were explored using commercial assay kits.
Compared with the sham group, I/R led to significantly lower SOD
activity, which was predominantly rescued by simvastatin
pre-treatment (Fig. 2A).
Furthermore, compared with the sham group, a significantly higher
MDA concentration was determined in the I/R group, which was
notably reduced by simvastatin (Fig.
2B). These data suggested that simvastatin indeed affected
oxidative stress in the kidney.
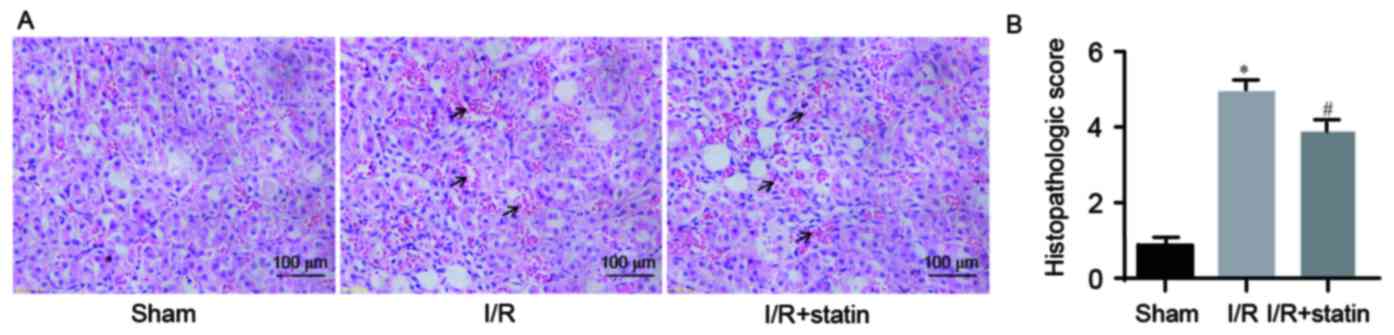
Simvastatin pre-treatment alleviates
I/R-induced renal histological injury
The present study then assessed whether simvastatin
influences the pathology of renal tubules. Morphological changes in
tissues were assessed by HE staining. In the I/R group, renal
tubules displayed severe pathological changes, including loss of
brush borders, congestion, tubular cell swelling and tubular
dilation. Of note, a marked amelioration was observed in the I/R +
simvastatin group (Fig. 3A). The
degree of tubular damage in the 3 groups was also estimated
(Fig. 3B). The data revealed that
simvastatin protected against renal I/R injury by preserving renal
tubule pathology.
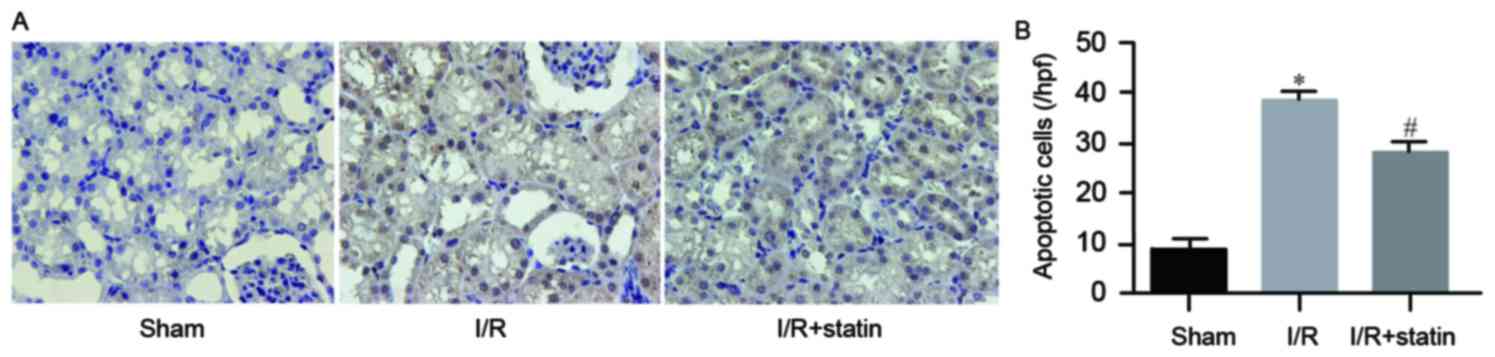
Simvastatin pre-treatment suppresses
I/R-induced tubular epithelial cell apoptosis
Furthermore, the present study explored whether
simvastatin affected tubular epithelial cell apoptosis. The TUNEL
assay revealed that the amount of apoptotic cells in the sham group
was low, while I/R resulted in a significantly elevated number of
apoptotic cells, which was significantly reduced by pre-treatment
with simvastatin (Fig. 4A).
Quantified rates of apoptotic cells are exhibited in Fig. 4B. These results demonstrated that
simvastatin prevented tubular epithelial cells from I/R
injury-induced apoptosis.
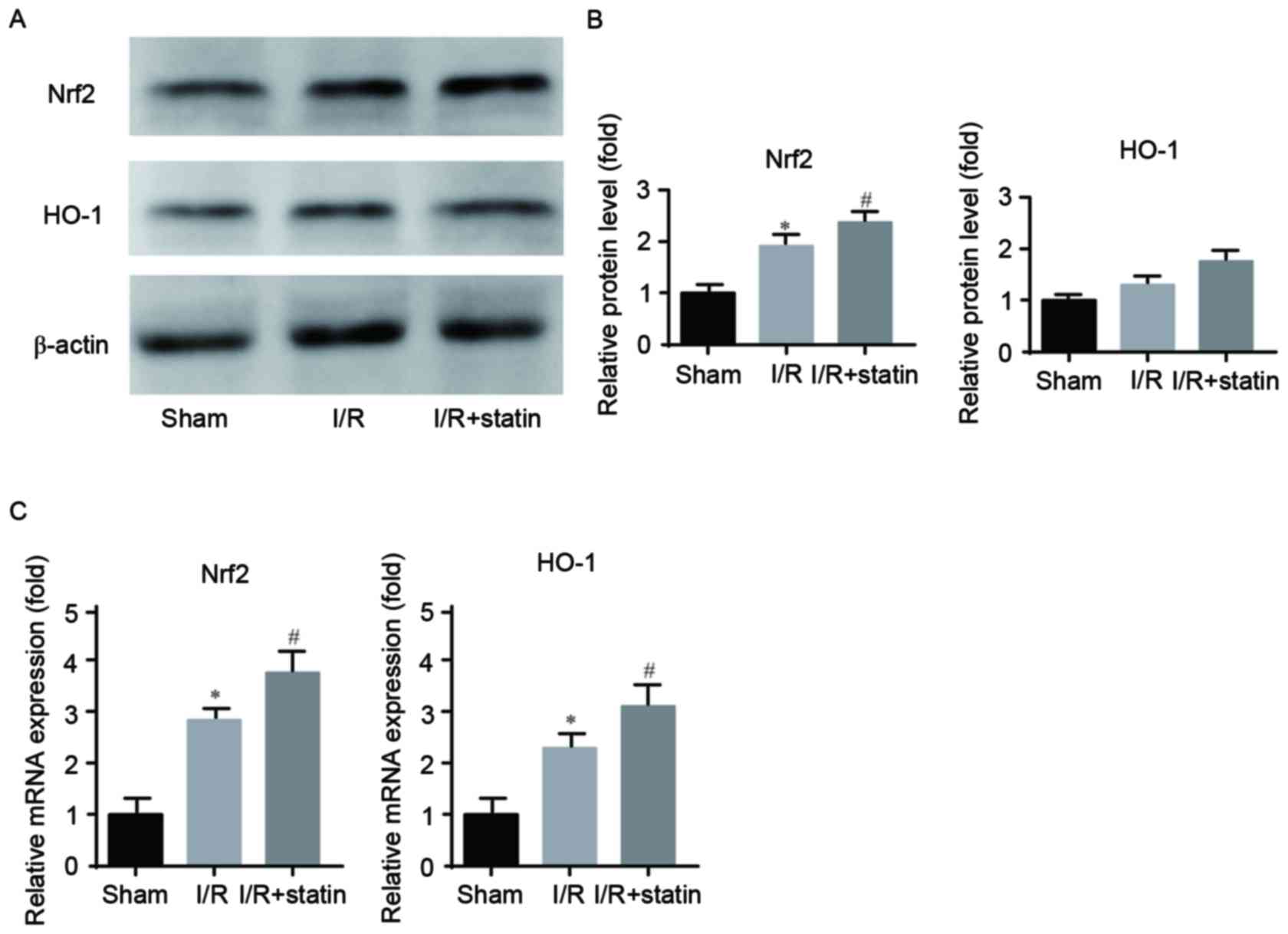
Simvastatin pre-treatment further
increases Nrf2/HO-1 levels after renal I/R
To elucidate the molecular mechanisms of action of
simvastatin in renal I/R injury, the present study assessed the
expression levels of Nrf2/HO-1 in ischemic renal tissues by RT-qPCR
and western blot analyses. Western blot analysis demonstrated that,
compared with that in the sham group, Nrf2 was upregulated in the
I/R group, while it was further increased in the simvastatin group;
however, no significant effect was noted among the 3 groups with
regard to HO-1 protein expression, while the same increasing trend
was observed (Fig. 5A and B).
RT-qPCR demonstrated that I/R induced an elevation of the mRNA
levels of Nrf2 and HO-1, which were further increased by
simvastatin treatment (Fig. 5C).
These results indicated that the Nrf2/HO-1 signaling pathway is
involved in the protective effects of simvastatin in renal I/R
injury.
Discussion
The present study found that simvastatin ameliorated
I/R-induced renal dysfunction and oxidative stress via activating
the Nrf2/HO-1 signaling pathway, thus providing a possible
molecular foundation for the effective treatment of renal
injury.
Multiple antioxidants, including febuxostat,
ligustrazine, sulforaphane, oxymatrine and gelsemine, have been
reported to protect murine kidneys against I/R injury by
attenuation of oxidative stress (3–7).
Similarly, statins have also been reported to
attenuate oxidative stress generated by I/R injury. For instance,
atorvastatin was found to attenuate rat renal I/R injury by
reducing oxidative stress (12) and
inhibiting active caspase-3 in rats (13). Simvastatin preserves microvascular
barrier function by inhibiting the ischemia-induced release of
vasoactive angiopoietin-2 and endothelin-1 as well as the tubule
interstitial injury markers kidney injury molecule-1 and SCr
following I/R (21). The acute
protective effects of simvastatin pre-treatment in rat renal I/R
injury were also proved by Todorovic et al (22).
The present study aimed to investigate a possible
molecular foundation of simvastatin pre-treatment for the
attenuation of renal injury. BUN, SCr and LDH are pivotal indexes
for kidney function. In the sham group, the concentrations of BUN,
SCr and LDH were relatively low, while I/R induced a significant
elevation of these parameters, which was notably reduced by
simvastatin pre-treatment, suggesting a protective role of
simvastatin in renal I/R injury. However, whether any other
indicators were affected by simvastatin remains elusive.
Inordinate or aberrant ROS is implicated in the
pathogenesis of tissue injury (23,24). As
an organ with the ability to generate ROS, the kidney has long been
studied and is also vulnerable to damage caused by ROS (25,26).
Furthermore, reperfusion has the ability to produce
excess ROS, while downregulation of SOD leads to oxidative stress
in the kidney (16,27). As the most crucial endogenous
antioxidant enzyme, SOD scavenges oxygen free radicals and prevents
mitochondria from damage through cytotoxic agents. The improved
activities of these endogenous antioxidant enzymes provide
protection against oxidative stress. As acknowledged, SOD activity
and MDA content reflect the anti-oxidative ability of tissues
(28). As one of the products of
lipid peroxidation, MDA is generated by the reaction of ROS with
polyunsaturated fatty acids. Therefore, the present study examined
SOD activity and the MDA concentration in kidney tissues of the
three different groups. The results demonstrated that SOD activity
and MDA concentration were normal in the sham group, and that
simvastatin pre-treatment predominantly rescued I/R-induced
reduction of SOD activity and upregulation of MDA. Based on these
results, it is not difficult to infer that simvastatin exerts its
protective effect on renal I/R injury via suppressing the oxidative
stress response.
ROS was reported to induce apoptosis during
reperfusion (29). Apoptosis, which
has been frequently observed in animal models of I/R-induced kidney
injury and in human acute tubular necrosis, is crucial in the
initiation of I/R-induced tissue injury (30). The present study evaluated changes in
apoptotic rates in kidneys from different treatment groups. The
results demonstrated a small amount of apoptotic tubular epithelial
cells in the sham group, and the number of tubular epithelial cells
with TUNEL-positive nuclei in the simvastatin-pre-treated group was
significantly lower than that in the I/R group, which suggested
that simvastatin ameliorated apoptosis. Furthermore, decreased
production of MDA may have also contributed to the protection
against tubular epithelial cell apoptosis. The corresponding
signaling pathways were then further explored.
Signaling pathways associated with renal I/R injury
have remained to be fully elucidated. Under physiological
conditions, the Nrf2 signaling pathway is sequestered by virtue of
binding with Kelch-like ECH-associated protein 1 (Keap 1) (31). However, the pathological conditions
of oxidative stress may lead to the dissociation of the Nrf2-Keap 1
complex and the nuclear translocation of Nrf2, where it may bind
with ARE and HO-1 to ultimately offset cellular oxidative stress
(8,32). The Nrf2/HO-1 signaling pathway was
reported to be tightly associated with ROS scavenging during the
process of oxidative stress and the attenuation thereof. Of note,
the Nrf2/HO-1 pathway has been reported to represent a therapeutic
target in renal I/R injury (9).
However, the effects of simvastatin on the
expression of Nrf2 and HO-1 in ischemic kidneys have remained to be
elucidated. In the present study, western blot and RT-qPCR analyses
were utilized to investigate their levels. As for the protein
levels, simvastatin pre-treatment predominantly further elevated
I/R-induced elevation of Nrf2, but no significant difference was
noted among the 3 groups with regard to HO-1 protein expression. At
the mRNA level, I/R-induced upregulation of Nrf2 and HO-1 were
further enhanced by simvastatin, which strongly suggested that
simvastatin pre-treatment provided renoprotection by activating
Nrf2 and its target gene HO-1.
Simvastatin has also been reported to activate
Nrf2-associated pathways in other cells types. For instance,
simvastatin was discovered to lower ROS levels by activating Nrf2
via the phosphoinositide-3 kinase (PI3K)/Akt pathway in ST-2 cells
(33), to induce HO-1 via Nrf2
activation through the extracellular signal-regulated kinase and
PI3K/Akt pathway in the HCT116 and HT-29 colon cancer cell lines
(34), and to activate Keap1/Nrf2
signaling in rat liver primary hepatocytes (35).
Taken together, it is possible to draw the
conclusion that simvastatin upregulates HO-1 mRNA levels through
activating the Nrf2 signaling pathway to ultimately protect the
kidney from the I/R injury-associated oxidative stress response,
renal tubule pathology and tubular epithelial cell apoptosis.
However, three questions remain unanswered: i) While
the Nrf2 pathway was activated in the I/R + simvastatin group in
comparison with the sham or I/R group, resulting in upregulation of
HO-1, SOD activity was reduced in the I/R and IR + simvastatin
groups compared with that in the sham group. One possible
explanation may be that the SOD activity or its expression is
impaired by ROS. ii) The effect on other molecules downstream of
the Nrf2 pathway, such as glutamate-cysteine ligase catalytic
subunit and quinine oxidoreductase 1 in the experimental groups
remains to be clarified. iii) The exact involvement of the
Nrf2/HO-1 signaling pathway in the protection of the kidney from
I/R injury after treatment with simvastatin. These questions will
be addressed in future studies.
References
|
1
|
Anaya-Prado R, Toledo-Pereyra LH, Lentsch
AB and Ward PA: Ischemia/reperfusion injury. J Surg Res.
105:248–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nath KA and Norby SM: Reactive oxygen
species and acute renal failure. Am J Med. 109:665–678. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsuda H, Kawada N, Kaimori JY, Kitamura H,
Moriyama T, Rakugi H, Takahara S and Isaka Y: Febuxostat suppressed
renal ischemia-reperfusion injury via reduced oxidative stress.
Biochem Biophys Res Commun. 427:266–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng L, Ke N, Cheng F, Guo Y, Li S, Li Q
and Li Y: The protective mechanism of ligustrazine against renal
ischemia/reperfusion injury. J Surg Res. 166:298–305. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shokeir AA, Barakat N, Hussein AM,
Awadalla A, Harraz AM, Khater S, Hemmaid K and Kamal AI: Activation
of Nrf2 by ischemic preconditioning and sulforaphane in renal
ischemia/reperfusion injury: A comparative experimental study.
Physiol Res. 64:313–323. 2015.PubMed/NCBI
|
|
6
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin L, Zheng J, Zhu W and Jia N:
Nephroprotective effect of gelsemine against cisplatin-induced
toxicity is mediated via attenuation of oxidative stress. Cell
Biochem Biophys. 71:535–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Zhu Z, Liu J, Zhu Z and Hu Z:
Protective effect of N-acetylcysteine (NAC) on renal
ischemia/reperfusion injury through Nrf2 signaling pathway. J
Recept Signal Transduct Res. 34:396–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gueler F, Rong S, Park JK, Fiebeler A,
Menne J, Elger M, Mueller DN, Hampich F, Dechend R and Kunter U:
Postischemic acute renal failure is reduced by short-term statin
treatment in a rat model. J Am Soc Nephrol. 13:2288–2298. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu K, Lei W, Tian J and Li H: Atorvastatin
treatment attenuates renal injury in an experimental model of
ischemia-reperfusion in rats. BMC Nephrol. 15:142014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haylor JL, Harris KP, Nicholson ML, Waller
HL, Huang Q and Yang B: Atorvastatin improving renal ischemia
reperfusion injury via direct inhibition of active caspase-3 in
rats. Exp Biol Med (Maywood). 236:755–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Randomized trial of cholesterol lowering
in 4444 patients with coronary heart disease: The scandinavian
simvastatin survival study (4S). Lancet. 344:1383–1389.
1994.PubMed/NCBI
|
|
15
|
Levine GN, Keaney JF Jr and Vita JA:
Cholesterol reduction in cardiovascular disease. Clinical benefits
and possible mechanisms. N Engl J Med. 332:512–521. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Endres M, Laufs U, Huang Z, Nakamura T,
Huang P, Moskowitz MA and Liao JK: Stroke protection by
3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated
by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A.
95:8880–8885. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pruefer D, Scalia R and Lefer AM:
Simvastatin inhibits leukocyte-endothelial cell interactions and
protects against inflammatory processes in normocholesterolemic
rats. Arterioscler Thromb Vasc Biol. 19:2894–2900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lefer AM, Campbell B, Shin YK, Scalia R,
Hayward R and Lefer DJ: Simvastatin preserves the
ischemic-reperfused myocardium in normocholesterolemic rat hearts.
Circulation. 100:178–184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCord JM: The evolution of free radicals
and oxidative stress. Am J Med. 108:652–659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tuuminen R, Nykänen AI, Saharinen P,
Gautam P, Keränen MA, Arnaudova R, Rouvinen E, Helin H, Tammi R and
Rilla K: Donor simvastatin treatment prevents ischemia-reperfusion
and acute kidney injury by preserving microvascular barrier
function. Am J Transplant. 13:2019–2034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Todorovic Z, Nesic Z, Stojanović R,
Basta-Jovanović G, Radojevic-Skodrić S, Velicković R, Chatterjee
PK, Thiemermann C and Prostran M: Acute protective effects of
simvastatin in the rat model of renal ischemia-reperfusion injury:
It is never too late for the pretreatment. J Pharmacol Sci.
107:465–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McCord JM: Oxygen-derived free radicals in
postischemic tissue injury. N Engl J Med. 312:159–163. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Halliwell B and Gutteridge J.M.C: Free
radicals, other reactive species and diseaseFree Radicals in
Biology and Medicine. Third. Oxford University Press; Oxford: pp.
617–783. 1999
|
|
25
|
Guidet BR and Sudh SV: In vivo generation
of hydrogen peroxide by rat kidney cortex and glomeruli. Am J
Physiol. 256:F158–F164. 1989.PubMed/NCBI
|
|
26
|
Andreucci VE and Fine LG: Reactive oxygen
species and renal injuryInternational Yearbook of Nephrology. 1st.
Kluwer Academic Press; New York, NY: pp. 47–69. 1991
|
|
27
|
Szeto HH: Mitochondria-targeted
cytoprotective peptides for ischemia-reperfusion injury. Antioxid
Redox Signal. 10:601–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tartibian B and Maleki BH: The effects of
honey supplementation on seminal plasma cytokines, oxidative stress
biomarkers and antioxidants during 8 weeks of intensive cycling
training. J Androl. 33:449–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daemen MA, van de Ven MW, Heineman E and
Buurman WA: Involvement of endogenous interleukin-10 and tumor
necrosis factor-alpha in renal ischemia-reperfusion injury.
Transplantation. 67:792–800. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Daemen MA, van 't Veer C, Denecker G,
Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P and Buurman WA:
Inhibition of apoptosis induced by ischemia-reperfusion prevents
inflammation. J Clin Invest. 104:541–549. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HJ and Vaziri ND: Contribution of
impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in
chronic renal failure. Am J Physiol Renal Physiol. 298:F662–671.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chartoumpekis D, Ziros PG, Psyrogiannis A,
Kyriazopoulou V, Papavassiliou AG and Habeos IG: Simvastatin lowers
reactive oxygen species level by Nrf2 activation via PI3K/Akt
pathway. Biochem Biophys Res Commun. 396:463–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jang HJ, Hong EM, Kim M, Kim JH, Jang J,
Park SW, Byun HW, Koh DH, Choi MH, Kae SH and Lee J: Simvastatin
induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2)
activation through ERK and PI3K/Akt pathway in colon cancer.
Oncotarget. 7:46219–46229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Habeos IG, Ziros PG, Chartoumpekis D,
Psyrogiannis A, Kyriazopoulou V and Papavassiliou AG: Simvastatin
activates Keap1/Nrf2 signaling in rat liver. J Mol Med (Berl).
86:1279–1285. 2008. View Article : Google Scholar : PubMed/NCBI
|