Introduction
Irritable bowel syndrome (IBS) is a complex disorder
that is associated with chronic, functional gastrointestinal (GI)
disorder, chronic abdominal pain, altered bowel movements and
affects approximately 10–15% of the world's population. Clinically,
there is a subset of IBS patients termed as postinfectious or
post-inflammatory IBS (PI-IBS); in these patients, IBS symptoms
occur after an initial episode of acute GI infection. PI-IBS is
characterized by loose stool with urgency, accelerated colonic
transit, and decreased pain threshold (1–3).
The mechanism of visceral hypersensitivity in PI-IBS
patients is not fully understood. Recent studies have indicated
that altered enterochromaffin (EC) cells and/or 5-HT can result in
GI dysmotility, visceral hypersensitivity, and secretomotor
abnormalities (4). In addition, it
has been thought that receptors of 5-HT such as 5-HT3
and 5-HT4 may play an important role in conveying
visceral sensation from the GI. These findings suggest that altered
EC cells or 5-HT might be one pathophysiologic mechanism
contributing to visceral pain in PI-IBS. More than 90% of 5-HT in
the body is secreted from EC cells which are located within the
mucosal mucosa, and the tryptophan hydroxylase (TPH) which in EC
cells is the rate-limiting enzyme in the 5-HT synthesis process
(5–7). Moreover, recent studies indicated that
CD4+ T cells played an important role in the development
of colonic EC cell hyperplasia in intestinal infection, and EC
cells were influenced by helper T-cell subtype 1 (Th1) or subtype 2
(Th2) cytokine predominant environments (8,9).
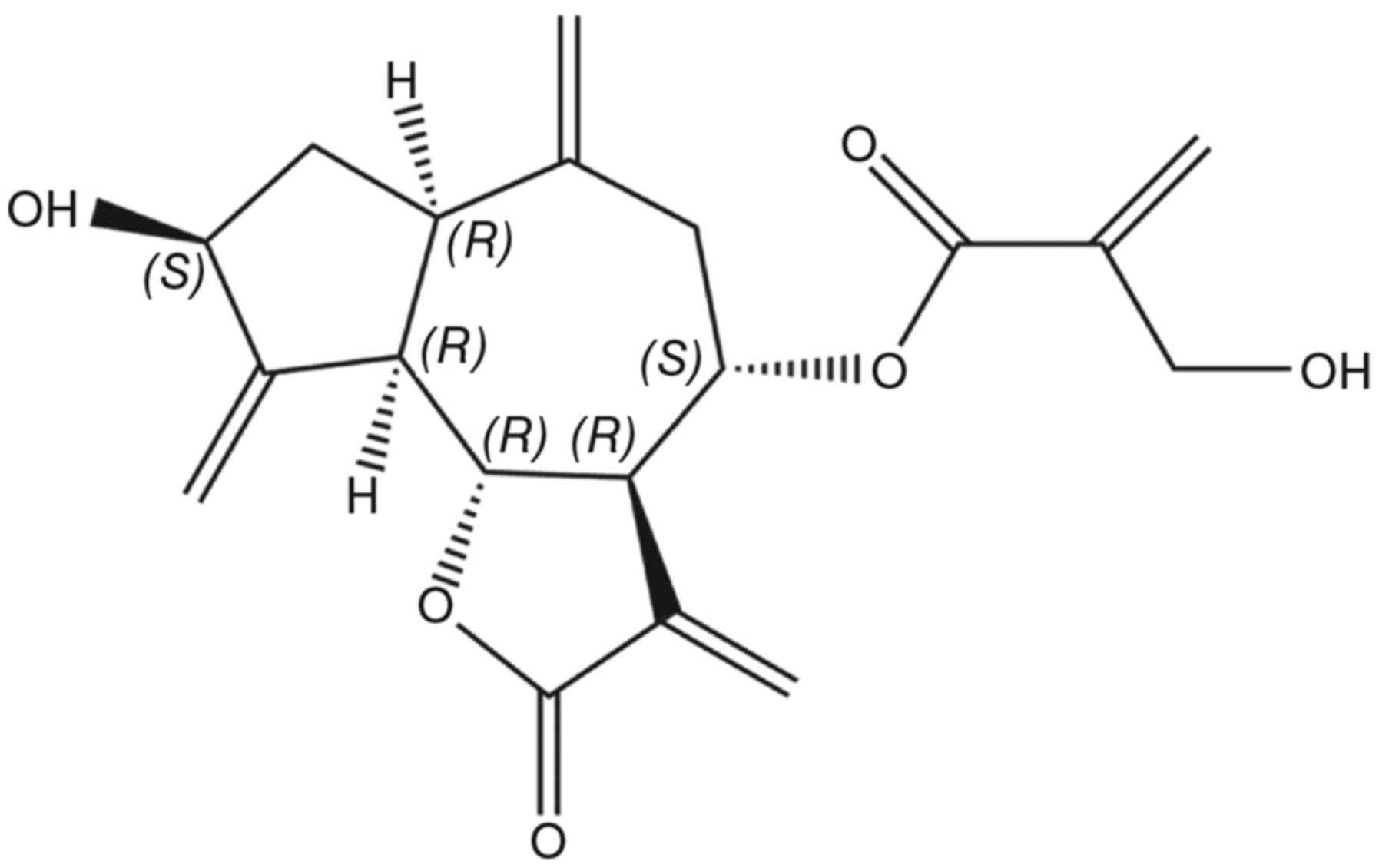
Cynaropicrin (Fig. 1)
is a sesquiterpene lactone of a guaianolide type. Sesquiterpene
lactones are the most biologically significant class of secondary
metabolites (10). Cynaropicrin has
been shown to possess various biological activities and has
demonstrated extraordinary pharmacologic properties such as
anti-parasitic, anti-spasmodic and anti-inflammatory properties on
suppression of NF-ĸB (11).
Especially, cynaropicrin possesses a marked effect on mucosal
injuries, preventing acute gastritis and it is also a promising
antispasmodic agent (12). In
addition, cynaropicrin is beneficial to the gastrointestinal
actions and use to ameliorate dyspeptic symptoms (13). To our knowledge, there are no studies
indicating that the administration of cynaropicrin has been tested
in a PI-IBS visceral hyperalgesia model.
This study was aimed at evaluating the analgesic
activity of cynaropicrin on PI-IBS visceral hyperalgesia. An
experimental PI-IBS visceral hyperalgesia rat model was induced by
administering trinitrobenzene sulfonic (TNBS) as reported
previously. Then, TNBS-induced PI-IBS visceral hyperalgesia rats
were treated by administration of cynaropicrin, and the effects on
visceral sensation, colonic 5-HT content, colonic TPH expression,
EC cell number and colonic cytokines levels of TNBS-induced PI-IBS
visceral hyperalgesia rats were investigated.
Materials and methods
Materials
Cynaropicrin was obtained from Wako (Tokyo, Japan).
Fontana-Masson staining kit and anti-tryptophan hydroxylase
antibody were purchased from Abcam (Cambridge, UK). 5-HT
enzyme-linked immunosorbent assay (ELISA) kit, 5-HIAA ELISA
kit, TNF-α ELISA kit and IL-6 ELISA kit were obtained from
MyBioSource, Inc., (San Diego, CA, USA). TNBS and
para-chlorophenylalanine (pCPA) were purchased from Sigma-Aldrich
(Tokyo, Japan). SABC rabbit IgG kit and DAB coloring reagent kit
were obtained from Boster Inc., (Wuhan, China).
Animals
One hundred and twenty male Sprague-Dawley rats
(weighing ~220 g) were housed under environmentally controlled
conditions (21±3°C and maintained on a light-dark cycle with the
lights on from 6:00 a.m.-7:00 p.m. in sawdust-lined transparent
plastic cages with free access to chow pellets and tap water). All
experiments were performed in compliance with the Shaanxi
administration rules and guidelines for laboratory animals and
approved by the Laboratory Animal Ethics Committee at the Shaanxi
University of Chinese Medicine (no. 2280109/2015).
Experimental TNBS-induced PI-IBS
visceral hyperalgesia in a rat model
After fasting for 24 h, the rats were deeply
anesthetized with chloral hydrate (350 mg/kg, i.p.). A plastic
catheter (external diameter approximately 0.95 mm) was inserted
into the descending colon at a depth of 8 cm from anus, and then,
the rats in the control group were colorectally instilled with 0.9%
saline solution, and the PI-IBS visceral hyperalgesia rats were
colorectally instilled with TNBS in 50% ethanol (TBNS 5 mg/rat).
After TNBS administration for 4 weeks, the animal model of PI-IBS
visceral hyperalgesia was determined by measuring visceral pain
threshold pressure. The rats with acquired visceral hyperalgesia
(pain threshold pressure below 30 mmHg) were selected as the PI-IBS
visceral hypersensitive rats (14).
Experimental design
In the present study, the experiments were divided
into 2 series. The first series was aimed at investigating whether
the cynaropicrin can attenuate visceral hyperalgesia in
TNBS-induced PI-IBS rats. This was done by using AWR testing and
EMG recording. The second series was aimed at evaluating the
effects of cynaropicrin on EC cell number, colonic TPH expression
and 5-HT metabolization in PI-IBS rats.
Therefore, 6 groups of 20 rats were used. The normal
rats in control group were treated with water (control group,
gavage administration), and the PI-IBS visceral hyperalgesia rats
in others 5 groups were treated with water (TNBS Group), pCPA (pCPA
Group, 150 mg/kg/d, i.p. for 3 days), and cynaropicrin (group 4–6,
at the dose of 5, 10, and 20 mg/kg/d, gavage administration for 3
days).
After the treatment, 10 rats from each group were
randomly chosen for AWR testing. Subsequently, the rats were
sacrificed for sample collection. A 6 cm long piece of proximal
colon (1–2 cm from caecum) was harvested for the evaluation of
colonic 5-HT content, colonic TPH expression, EC cell number and
the cytokine levels. The rest of 10 rats from each group were just
used for EMG recording.
Abdominal withdrawal reflex (AWR)
test
Ten rats from each group were used for AWR testing
by random choice. Each rat was lightly anesthetized with ether, and
inserted a balloon into the descending colon, and the end of the
balloon was secured at least 1 cm proximal to the anal verge. Then,
the rat was housed in a small lucite box (20×8×8 cm) and allowed to
wake up and recuperate for 1 h. The colorectal distension was
applied in increments of 5 mmHg until a visible contraction of
abdominal muscles was observed by an investigator blinded. The
Al-chaer's AWR score 3 was defined as the pain threshold pressure
here according to the behavioral response of the rat lifting its
abdomen off the platform (15).
Electromyography (EMG) recording
The rest of 10 rats from each group were used for
EMG recording. After intraperitoneal anesthesia with nembutal, the
rats were put into supine position and on the constant temperature
mat, keeping the temperature at about 37°C. The surface of
hypogastrium was sterilized, then, cut along the surface of the
medioventral line, isolating the subcutaneous fascia layer on one
side of the rats, exposing the musculus obliquus externus
abdominis, then inserting the insulated silver electrode into the
left external abdominal oblique muscles, with intervals of 0.5–1
cm. The other end of the electrode was got out of the back
subcutaneously. The incision was treated with 1% lidocaine gel for
pain relief. After one week recuperation, the rat was lightly
anesthetized with ether, and inserted a balloon into the descending
colon. The EMG signal was amplified and filtered (50-5,000 Hz).
Total three cycles of graded colorectal distention (20, 40, 60, and
80 mmHg; 20 sec duration; 2 min inter-stimulus intervals) were
applied to each rat. The changes of the AUC (the area under the
curve) during the 20 sec distention period over the preceding 20
sec baseline of each rat were calculated and analyzed by Axconscope
software (16,17).
EC cell counting
The method of EC cell counting was performed as
previously described (18). Briefly,
the colon was harvested and fixed in 4% paraformaldehyde and
embedded in paraffin. And then, tissue sections (6 µm thick, 6
sections for each rat) were deparaffinized and rehydrated for
Fontana-Masson staining. The sections were incubated in ammoniacal
silver solution (1 h, 60°C), gold chloride solution (0.2%, 30 sec),
sodium thiosulfate solution (5%, 2 min, room temperature), and
nuclear fast red solution successively. Finally, the sections were
dehydrated with alcohol and mounted in synthetic resin. Five random
fields at 200× magnifications were counted in each section by a
researcher blinded to the treatment; the number of EC cells per
mm2 of mucosa was quantified using ImageJ NIH
software.
Western blot analysis
Western blot analysis was performed as previously
described (19). Western blot
analysis was used for the detection of tryptophan hydroxylase, and
the β-actin was chosen as the loading control. Briefly, the total
protein of the colon was extracted and quantified. Then the samples
containing 30 µg of protein were boiled for 5 min and subjected to
SDS-PAGE electrophoresis and then transferred to PVDF membranes.
The PVDF membranes were incubated in blocking buffer, and then
incubated with anti-tryptophan hydroxylase antibody or anti-beta
actin antibody for 1 night at 4°C. Subsequently, the PVDF membranes
were incubated with secondary antibodies labeled alkaline
phosphatase. The immunoblots were detected by western blue and
quantified using the ImageJ program.
ELISA
The content of 5-HT and the cytokine levels of TNF-α
and IL-6 in the colon tissue were assayed by ELISA (20). The samples were measured according to
the manufacturer's protocol. Briefly, the colon tissue was
harvested and homogenized. Then the samples were diluted with PBS
(0.02 mol/l, pH 7.2). After centrifuging of the samples, the 5-HT
content and cytokine levels were measured using ELISA kits.
Absorbance at 450 nm in each well was measured using a
spectrophotometer.
Statistical analysis
All data are presented as mean ± standard error of
the mean (SEM). Statistical analysis was conducted using SPSS 15.0
Software (SPSS Inc., Chicago, IL, USA). The data of visceral pain
threshold pressure were analyzed by comparing the values before and
after treatment for each group using a paired t-test, and the
differences between before and after treatment in a group using a
one-way analysis of variance (ANOVA). After testing for homogeneity
of variance, data of EMG recording, EC cell counting, TPH, 5-HT and
5-HIAA in the colon were compared using one-way ANOVA and
Student-Newman-Keuls (SNK) method post-hoc testing. P<0.05 was
considered to indicate a statistically significant difference.
Results
Analgesic effect of cynaropicrin on
TNBS-induced PI-IBS visceral hyperalgesia rats
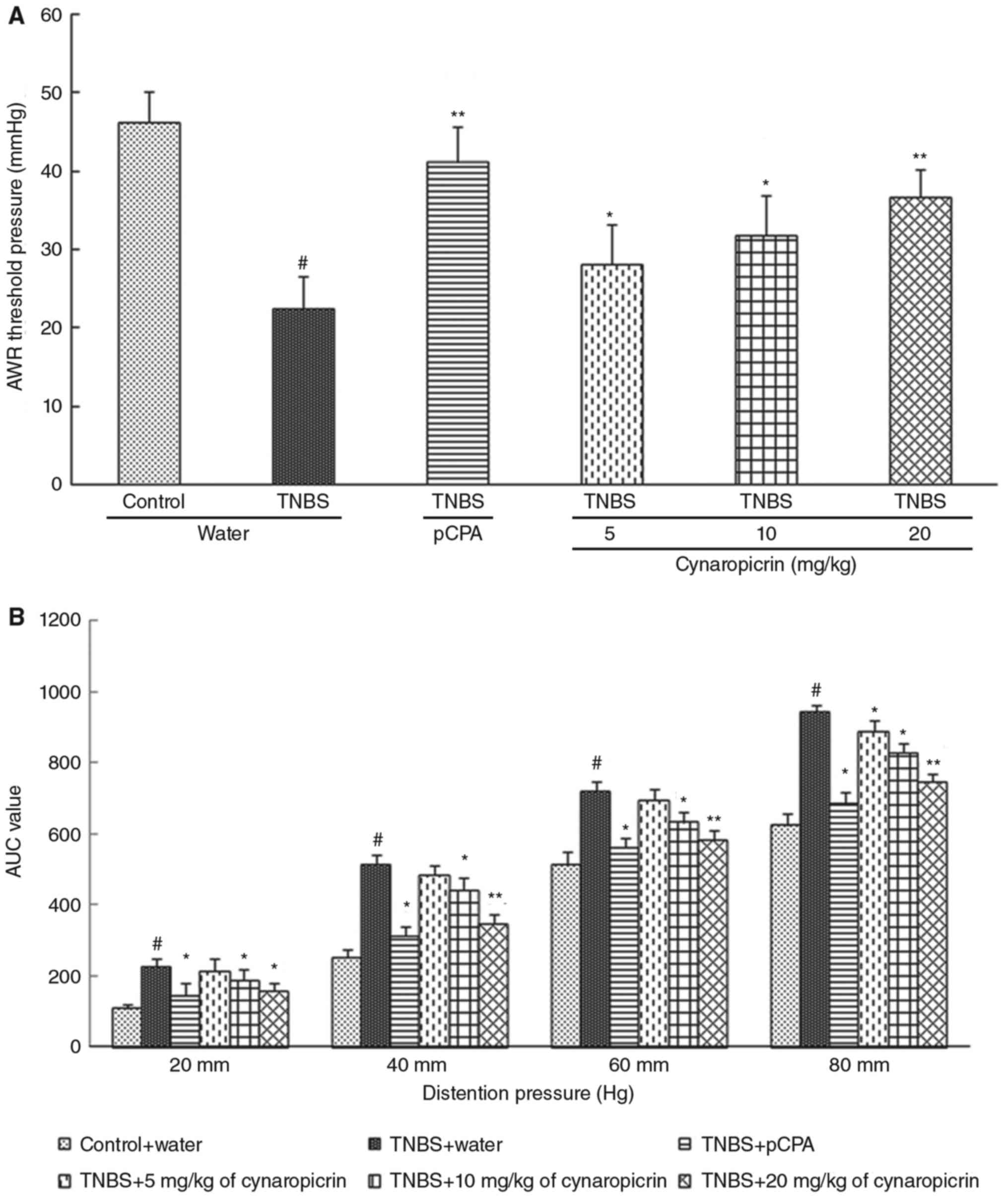
As shown in Fig. 2A,
the pain threshold pressures of the rats (TNBS group, 22.51±4.15
mmHg, P<0.05, n=10) administered with TNBS were significantly
decreased when compared to that of the rats (control group,
46.27±3.84 mmHg) administered with saline. After treatment with
cynaropicrin, pain threshold pressures of the rats in group 4–6
(28.15±5.09 mmHg, P<0.05 in group 4; 31.85±5.15 mmHg, P<0.05
in group 5; 36.64±3.56 mmHg, P<0.01 in group 6; n=10) were
significantly and dose-dependently elevated when compared to that
of the rats in the TNBS group. After treatment with pCPA, one of
the TPH inhibitors that inhibits the production of 5-HT, the pain
threshold pressures of the rats in the pCPA group (41.15±4.56 mmHg,
P<0.01, n=10) were significantly elevated when compared to that
of the control group.
Consistent with the results from AWR tests, as shown
in Fig. 2B, the results of EMG
recording showed that the visceral motor responses to graded
colorectal distension of the rats in TNBS group (228.09±18.73,
P<0.05; 516.15±24.77, P<0.05; 722.58±25.82, P<0.05;
942.48±21.72, P<0.05; for the pressures 20, 40, 60, 80 mmHg,
n=10) were significantly increased when compared to that of the
rats in the control group (108.86±12.84, 252.86±23.16,
514.35±34.45, 624.34±28.05 for the pressures 20, 40, 60, 80 mmHg).
Also, the visceral motor responses to graded colorectal distension
were decreased significantly in the rats treated with pCPA (pCPA
group, 146.46±32.7, P<0.05; 313.57±24.43, P<0.05;
561.76±25.92, P<0.05; 687.33±28.05, P<0.05; for the pressures
20, 40, 60, 80 mmHg, n=10) when compared to that of the rats in the
TNBS group. Also, the pain threshold pressures of the rats in group
4 (214.3±32.47; 484.91±27.75; 694.43±30.7; 890.06±31.03, P<0.05;
for the pressures 20, 40, 60, 80 mmHg, n=10), group 5
(187.32±31.63, P<0.05; 422.58±34.15, P<0.05; 632.98±27.67,
P<0.05; 828.42±24.41, P<0.05; for the pressures 20, 40, 60,
80 mmHg, n=10), and group 6 (156.44±24.2, P<0.05; 348.45±22.87,
P<0.01; 582.72±25.12, P<0.01; 747.68±22.85, P<0.01; for
the pressures 20, 40, 60, 80 mmHg, n=10) were significantly and
dose-dependently elevated after treatment with cynaropicrin.
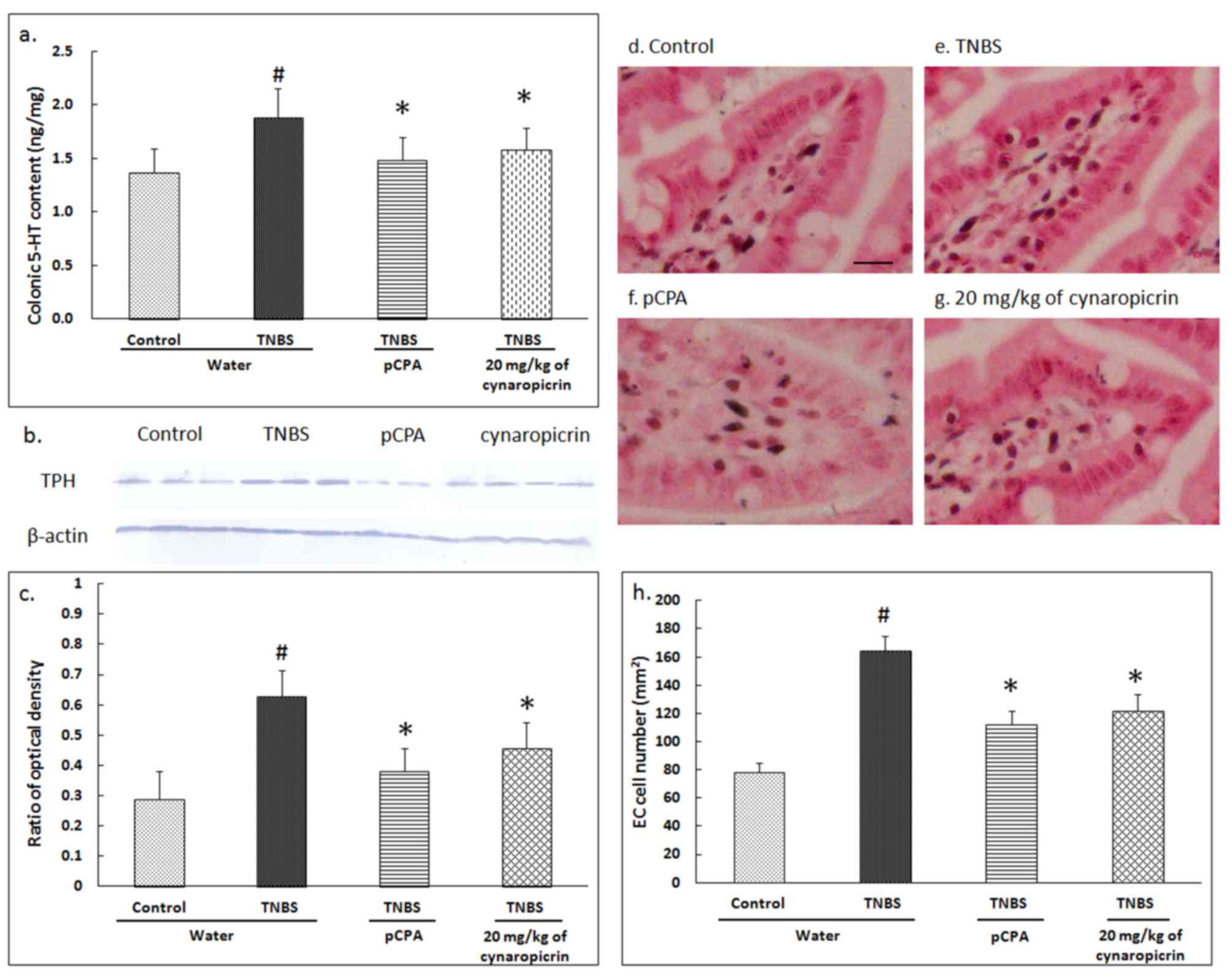
Effects of cynaropicrin on 5-HT
content, TPH expression and EC cell number in the colon
The 5-HT content in the colon of the rats in TNBS
group (1.88±0.27 ng/mg, P<0.05, n=10) was significantly
increased when compared to that of the rats from the control group
(1.37±0.22 ng/mg) (Fig. 3A).
Compared to the rats in the TNBS group, the 5-HT content was
significantly reduced in the rats of the pCPA group (1.48±0.22
ng/mg, P<0.05, n=10), as well as significantly reduced in group
6 (1.58±0.21 ng/mg in group 6, P<0.05, n=10).
TPH expression in the rats of TNBS group
(0.628±0.085, P<0.05, n=10) was significantly increased compared
to the TPH expression in rats from the control group (0.289±0.092)
(Fig. 3B and C). When the data was
compared to that of the rats in TNBS group, the TPH expression was
significantly reduced in the rats by the treatment with pCPA
(0.382±0.074, P<0.05, n=10) and cynaropicrin (0.456±0.087,
P<0.05, n=10).
The number of colonic EC cells were significantly
increased in the rats of the TNBS group (164.4±10.6 per
mm2, P<0.05, n=10) when compared to that of the rats
in the control group (78.4±6.2 per mm2) (Fig. 3D-H). This result suggests that EC
cell hyperplasia occurs in PI-IBS visceral hyperalgesic rats. After
treatment with pCPA or cynaropicrin, the EC cell hyperplasia was
reduced significantly in the rats of the pCPA group (112.4±9.2 per
mm2, P<0.05, n=10) and group 6 (121.5±12.4 per
mm2, P<0.05, n=10).
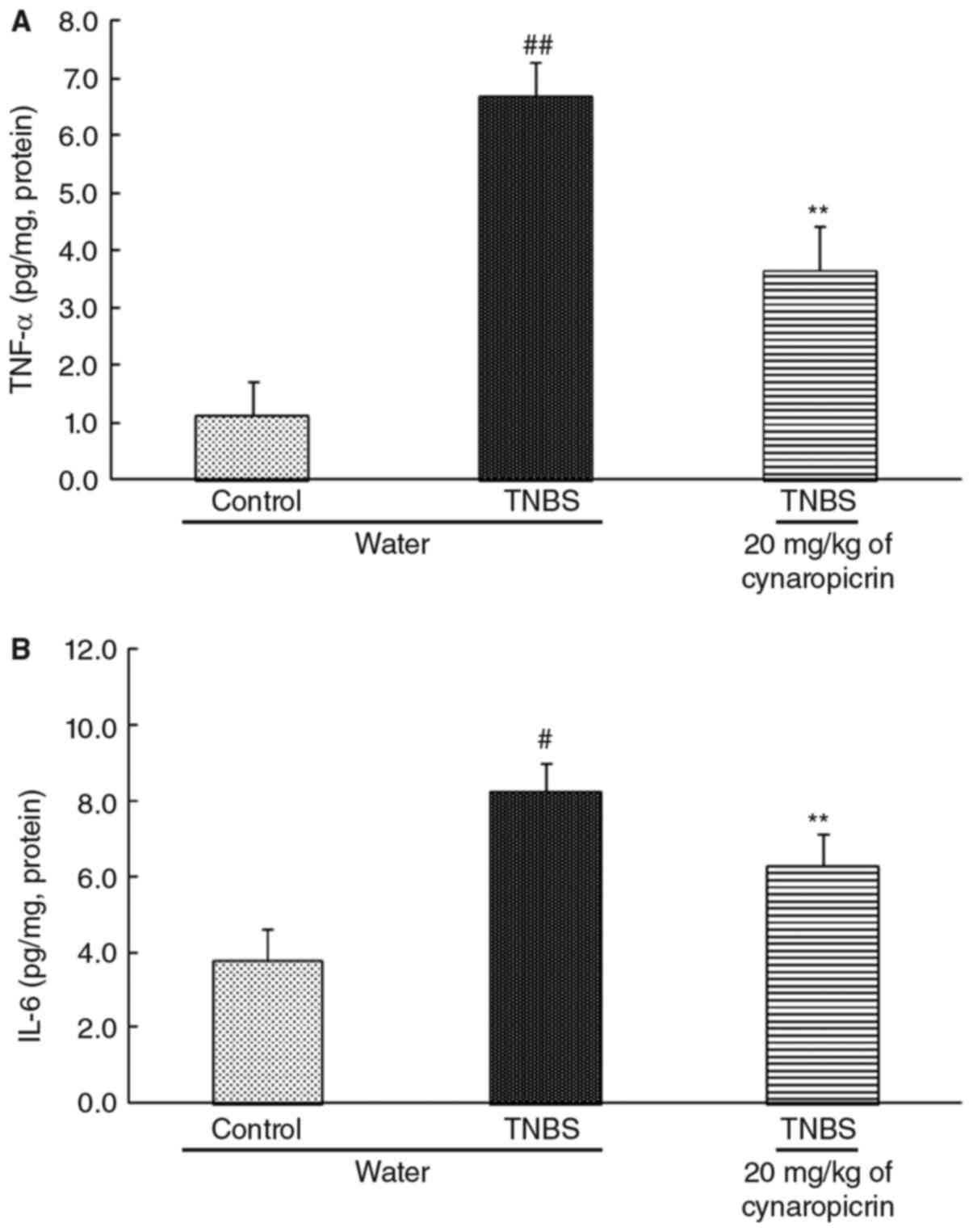
Effects of cynaropicrin on the levels
of cytokines in the colon
The levels of TNF-α and IL-6 in the colon of the
rats in TNBS group (6.71±0.58 pg/mg, P<0.01, for TNF-α and
8.24±0.77 pg/mg, P<0.05, for IL-6, n=10) were all significantly
increased when compared to that of the rats in control group
(1.12±0.61 pg/mg and 3.77±0.82 pg/mg for TNF-α and IL-6) (Fig. 4). Cynaropicrin significantly improved
the levels of the cytokines in the colon of the rats in group 6
(3.66±0.76 pg/mg, P<0.01, for TNF-αand 6.28±0.84 pg/mg,
P<0.01, for IL-6, n=10).
Discussion
In the present study, the results of AWR tests and
EMG recordings indicated a significant analgesic effect on PI-IBS
visceral hyperalgesia by cynaropicrin. Meanwhile, the changes of
colonic 5-HT content, colonic TPH expression, EC cell number and
colonic cytokines levels that induced by cynaropicrin were also
indicated here.
As a neurotransmitter, 5-HT plays an important role
in the GI tract. 5-HT stimulates the serotonergic receptors such as
5-HT3 and 5-HT4 receptors which are located
on primary afferent neurons of both splanchnic and vagal fibers,
thereby modulating both sensory and motor responses. In previous
studies, antagonists such as Alosetron, Granisetron and Odansetron,
have been shown to reduce the visceral hypersensitivity and rectal
sensitivity in IBS patients, by blocking the transmission of the
afferent signal, after occupying the 5-HT3 receptor
combining site (5–7). It is suggested that 5-HT played an
important role in the development of visceral hypersensitivity. As
an inhibitor, pCPA acts as a selective and irreversible inhibitor
of TPH. The results in this study indicated that the colonic 5-HT
contents in TNBS-induced PI-IBS visceral hyperalgesia rats were
decreased by treatment with pCPA. Similarly, the colonic 5-HT
contents in TNBS-induced PI-IBS visceral hyperalgesia rats were
decreased by treatment with cynaropicrin. Similar results from AWR
testing and EMG recording indicate a significant analgesic effect
accompanied by a decrease of 5-HT content on the TNBS-induced
PI-IBS visceral hyperalgesia rats treated with pCPA or
cynaropicrin.
In addition, results from this study showed that
5-HT content was decreased dramatically and depleted seriously in
TNBS-induced PI-IBS visceral hyperalgesia rats by treatment with
pCPA. But the results of AWR tests and EMG recordings showed that
no differences were found in visceral pain threshold pressure
between the rats that were treated with pCPA and the rats in the
control group. This result indicated that even below the normal
level of 5-HT content may be enough to meet the minimum requirement
of invoking the sensory reflex (21–23).
Past studies indicated that the excessive
availability of 5-HT mainly come from increases of EC cell number
(24), and TPH which in EC cells is
the rate-limiting enzyme in the 5-HT synthesis process. Alleviating
visceral hyperalgesia may be mediated via decreasing hyperplastic
colonic EC cell number. Results from this study indicated that
colonic TPH expression and EC cell number were decreased by the
treatment with cynaropicrin. The underlying mechanisms of EC cell
hyperplasia in PI-IBS are unknown, but they are considered to have
close correlation with CD4+ T lymphocytes, especially
the Th1/Th2 balance. TNF-α has been shown to downregulate
CD4+ T-cell responses, while deficiency of TNF-α leads
to enhanced expansion of CD4+ T cells. IL-6 initiates
maturation of Th2 cells from Th0 in conjunction with IL-4 (25–27).
Therefore, Th1/Th2 balance is influenced by the cytokines. Results
from this study, show that the levels of TNF-α and IL-6 in the
colon were changed by treatment with cynaropicrin. Therefore, the
decreases of colonic TPH expression and EC cell number by
cynaropicrin were mediate via the decreases of the cytokines
levels.
Unlike the cynaropicrin, the analgesic effect in
TNBS-induced PI-IBS visceral hyperalgesia rats by treatment of pCPA
is mediated via selective and irreversible inhibition of TPH. Many
serotonergenic receptors have been found on various immune cells
such as B and T lymphocytes, monocytes, macrophage, and dentritic
cells (28). EC cell hyperplasia is
considered to have close correlation with T lymphocytes. Therefore,
5-HT content can influence the EC cell hyperplasia. Consequently,
the phenomenon of the EC cell number decrease by treatment with
pCPA may be mediated via reducing colonic 5-HT content. The TPH
inhibitors that were developed for the selective inhibition of 5-HT
biosynthesis are expected to be used in the treatment of GI
diseases such as IBS and IBD. Besides its well characterized
function as a neurotransmitter, 5-HT has been reported to be a
potent immunoregulator (29–34). 5-HT has been reported to be a potent
regulator of cytokine secretion in different kinds of cells. Human
monocytes release different cytokines and chemokines, mainly via
5-HT3, 5-HT4 and 5-HT7 activation
(35). Past studies also showed that
5-HT increase production of the pro-inflammatory cytokine IL-6 in
mature Dendritic cells (DCs) via 5-HT3, 5-HT4
and 5-HT7 (8,9). And DCs are known to produce different
chemokines thereby regulating the traffic of Th1 and Th2 cells into
inflamed tissue (36). Results from
this study showed that pCPA significantly improved the levels of
the cytokines in the colon of the PI-IBS rats. It is not hard to
understand that pCPA, as an inhibitor of TPH, improved the levels
of the cytokines in the colon via reducing colonic 5-HT content.
The shortcoming of most of these inhibitors is that the blockade of
the enzyme or the 5-HT depletion produced is relatively
short-lasting. And the side effects of some TPH inhibitors such as
pCPA have been impeded by the central adverse effects of inhibition
of brain 5-HT synthesis with consequent affective disorders. Unlike
the pCPA, cynaropicrin reduced colonic 5-HT content was mediated
via the decreases of the cytokines levels and thus reducing
putative side effects.
In conclusion, this study demonstrated that
cynaropicrin can attenuate visceral hyperalgesia on TNBS-induced
PI-IBS visceral hyperalgesia rats. The analgesic activity of
cynaropicrin on TNBS-induced PI-IBS visceral hypersensitive rats
was mediated via reducing the cytokines levels. Thus, cynaropicrin
as a promising bioactive natural product will offer therapeutic
avenues for visceral hypersensitivity in IBS.
Acknowledgements
We acknowledge the group of Laboratory for
Functional Glycomics, Northwest University for their help in the
present study.
References
|
1
|
Crowell MD: Role of serotonin in the
pathophysiology of the irritable bowel syndrome. Br J Pharmacol.
141:1285–1293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dunlop SP, Coleman NS, Blackshaw E,
Perkins AC, Singh G, Marsden CA and Spiller RC: Abnormalities of
5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin
Gastroenterol Hepatol. 3:349–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Salhy M, Gundersen D, Hatlebakk JG,
Gilja OH and Hausken T: Abnormal rectal endocrine cells in patients
with irritable bowel syndrome. Regul Pept. 188:60–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta V, Khan AA, Sasi BK and Mahapatra
NR: Molecular mechanism of monoamine oxidase A gene regulation
under inflammation and ischemia-like conditions: Key roles of the
transcription factors GATA2, Sp1 and TBP. J Neurochem. 134:21–38.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozlowski CM, Green A, Grundy D,
Boissonade FM and Bountra C: The 5-HT(3) receptor antagonist
alosetron inhibits the colorectal distention induced depressor
response and spinal c-fos expression in the anaesthetised rat. Gut.
46:474–480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schikowski A, Thewissen M, Mathis C, Ross
HG and Enck P: Serotonin type-4 receptors modulate the sensitivity
of intramural mechanoreceptive afferents of the cat rectum.
Neurogastroenterol Motil. 14:221–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slater BJ, Plusa SM, Smith AN and Varma
JS: Rectal hypersensitivity in the irritable bowel syndrome. Int J
Colorectal Dis. 12:29–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haub S, Ritze Y, Bergheim I, Pabst O,
Gershon MD and Bischoff SC: Enhancement of intestinal inflammation
in mice lacking interleukin 10 by deletion of the serotonin
reuptake transporter. Neurogastroenterol Motil. 22:826–834, e229.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller T, Dürk T, Blumenthal B, Grimm M,
Cicko S, Panther E, Sorichter S, Herouy Y, Di Virgilio F, Ferrari
D, et al: 5-hydroxytryptamine modulates migration, cytokine and
chemokine release and T-cell priming capacity of dendritic cells in
vitro and in vivo. PLoS One. 4:e64532009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elsebai MF, Mocan A and Atanasov AG:
Cynaropicrin: A comprehensive research review and therapeutic
potential as an anti-hepatitis C virus agent. Front Pharmacol.
7:4722016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho JY, Baik KU, Jung JH and Park MH: In
vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene
lactone, from saussurea lappa. Eur J Pharmacol. 398:399–407. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emendörfer F, Emendörfer F, Bellato F,
Noldin VF, Cechinel-Filho V, Yunes RA, Delle Monache F and Cardozo
AM: Antispasmodic activity of fractions and cynaropicrin from
Cynara scolymus on guinea-pig ileum. Biol Pharm Bull. 28:902–904.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishida K, Kojima R, Tsuboi M, Tsuda Y and
Ito M: Effects of artichoke leaf extract on acute gastric mucosal
injury in rats. Biol Pharm Bull. 33:223–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liebregts T, Adam B, Bertel A, Jones S,
Schulze J, Enders C, Sonnenborn U, Lackner K and Holtmann G: Effect
of E. coli Nissle 1917 on post-inflammatory visceral sensory
function in a rat model. Neurogastroenterol Motil. 17:410–414.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Chaer ED, Kawasaki M and Pasricha PJ: A
new model of chronic visceral hypersensitivity in adult rats
induced by colon irritation during postnatal development.
Gastroenterology. 119:1276–1285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tammpere A, Brusberg M, Axenborg J, Hirsch
I, Larsson H and Lindström E: Evaluation of pseudo-affective
responses to noxious colorectal distension in rats by manometric
recordings. Pain. 116:220–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Zhang XJ, Xu HX, Sung JJ and Bian
ZX: Intracolonical administration of protease-activated receptor-2
agonists produced visceral hyperalgesia by up-regulating serotonin
in the colon of rats. Eur J Pharmacol. 606:199–204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu X, Liu Z, Niu W, Wang Y, Zhang A, Qu
H, Zhou J, Bai L, Yang Y and Li J: Effects of electroacupuncture at
ST25 and BL25 in a Sennae-induced rat model of
diarrhoea-predominant irritable bowel syndrome. Acupunct Med.
35:216–223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu X, Shinohara H, Miyatake R and Hohsaka
T: Novel biosensor system model based on fluorescence quenching by
a fluorescent streptavidin and carbazole-labeled biotin. J Mol
Recognit. 29:485–491. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Liu Z, Qu H, Niu W, Gao L, Wang Y,
Zhang A and Bai L: The effect and mechanism of electroacupuncture
at LI11 and ST37 on constipation in a rat model. Acupunct Med.
34:194–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertrand PP and Bertrand RL: Serotonin
release and uptake in the gastrointestinal tract. Auton Neurosci.
153:47–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bischoff SC, Mailer R, Pabst O, Weier G,
Sedlik W, Li Z, Chen JJ, Murphy DL and Gershon MD: Role of
serotonin in intestinal inflammation: Knockout of serotonin
reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic
acid colitis in mice. Am J Physiol Gastrointest Liver Physiol.
296:G685–G695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghia JE, Li N, Wang H, Collins M, Deng Y,
El-Sharkawy RT, Côté F, Mallet J and Khan WI: Serotonin has a key
role in pathogenesis of experimental colitis. Gastroenterology.
137:1649–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linden DR, Chen JX, Gershon MD, Sharkey KA
and Mawe GM: Serotonin availability is increased in mucosa of
guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest
Liver Physiol. 285:G207–G216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Motomura Y, Ghia JE, Wang H, Akiho H,
El-Sharkawy RT, Collins M, Wan Y, McLaughlin JT and Khan WI:
Enterochromaffin cell and 5-hydroxytryptamine responses to the same
infectious agent differ in Th1 and Th2 dominant environments. Gut.
57:475–481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oshima S, Fujimura M and Fukimiya M:
Changes in number of serotonin-containing cells and serotonin
levels in the intestinal mucosa of rats with colitis induced by
dextran sodium sulfate. Histochem Cell Biol. 112:257–263. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin HY, Luo JL, Qi SD, Xu HX, Sung JJ and
Bian ZX: Visceral hypersensitivity induced by activation of
transient receptor potential vanilloid type 1 is mediated through
the serotonin pathway in rat colon. Eur J Pharmacol. 647:75–83.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spiller R: Serotonin, inflammation, and
IBS: Fitting the jigsaw together? J Pediatr Gastroenterol Nutr. 45
(Suppl 2):S115–S119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cloez-Tayarani I, Petit-Bertron AF,
Venters HD and Cavaillon JM: Differential effect of serotonin on
cytokine production in lipopolysaccharide-stimulated human
peripheral blood mononuclear cells: Involvement of
5-hydroxytryptamine2A receptors. Int Immunol. 15:233–240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dürk T, Panther E, Müller T, Sorichter S,
Ferrari D, Pizzirani C, Di Virgilio F, Myrtek D, Norgauer J and
Idzko M: 5-Hydroxytryptamine modulates cytokine and chemokine
production in LPS-primed human monocytes via stimulation of
different 5-HTR subtypes. Int Immunol. 17:599–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Idzko M, Panther E, Stratz C, Müller T,
Bayer H, Zissel G, Dürk T, Sorichter S, Di Virgilio F, Geissler M,
et al: The serotoninergic receptors of human dendritic cells:
Identification and coupling to cytokine release. J Immunol.
172:6011–6019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iken K, Chheng S, Fargin A, Goulet AC and
Kouassi E: Serotonin upregulates mitogen-stimulated B lymphocyte
proliferation through 5-HT1A receptors. Cell Immunol. 163:1–9.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laberge S, Cruikshank WW, Beer DJ and
Center DM: Secretion of IL-16 (lymphocyte chemoattractant factor)
from serotonin-stimulated CD8+ T cells in vitro. J Immunol.
156:310–315. 1996.PubMed/NCBI
|
|
34
|
Moser B and Loetscher P: Lymphocyte
traffic control by chemokines. Nat Immunol. 2:123–128. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Segal DM, Taurog JD and Metzger H: Dimeric
immunoglobulin E serves as a unit signal for mast cell
degranulation. Proc Natl Acad Sci USA. 74:2993–2997. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Young MR, Kut JL, Coogan MP, Wright MA,
Young ME and Matthews J: Stimulation of splenic T-lymphocyte
function by endogenous serotonin and by low-dose exogenous
serotonin. Immunology. 80:395–400. 1993.PubMed/NCBI
|