Introduction
Olfaction is an important function of the body. The
main functions of olfaction include odor discrimination,
environmental identification, appetite stimulation and emotional
regulation. Olfactory disorder is very common and a study on the
function of smell in 1.5 million people reports that 1.2% people
have a permanent loss and 62.4% have a temporary loss of the sense
of smell (1). Common causes of
olfactory dysfunction include infection of the upper respiratory
tract, nasal sinus disease, nasal operation, brain and nasal
tumors, endocrine disorders, head injury, age, radiation, toxic
chemicals, mental, drug and congenital factors and
neurodegenerative diseases amongst other causes (2).
Allergic rhinitis (AR) is the most common
otorhinolaryngological disease (3).
It is also one of the main causes of olfactory dysfunction
(4). The primary clinical
manifestations of AR include nasal blockage, running and itching
nose, sneezing, olfactory dysfunction amongst others (3). Moreover, the incidence rate of AR is
increasing each year (5,6). AR is currently a primary factor of
olfactory dysfunction (7,8); however, current epidemiological
investigations suggest that the incidence of AR is 10–30% of the
human population (9). It has also
been reported that the loss or impairment of olfaction is
associated with nasal allergy reactions (10). A survey by Cowart et al
(11) reported that 23.1% of
patients with AR have an impaired sense of smell, whereas, Rombaux
et al (12) report that the
incidence of smell disorder caused by AR is 15–20%. However, the
mechanism by which AR induces olfactory dysfunction remains
unclear. It is considered that nasal inflammation that blocks the
passage for odor molecules to reach olfactory receptors on the top
of the nasal cavity is the main reason that leads to olfactory
dysfunction, namely conductive olfactory dysfunction. However,
recent studies have demonstrated that pathological changes in
olfactory epithelium tissues caused by allergy, namely sensory
olfactory disorder, may be one of the direct causes of olfactory
dysfunction in AR patients (4,13–15).
Olfactory receptor neurons (ORNs) are the receptor
cells responsible for the olfactory sense. During breathing, odor
molecules arrive at the ORNs in the olfactory epithelium, cause
depolarization of the receptor cells and generate action potentials
(16,17). The action potentials are applied
along the axon to the olfactory bulb, then transferred onto the
olfactory center, resulting in the sense of smell (16,17).
Olfactory marker protein (OMP) is a type of protein of limited
solubility that is expressed in mature ORNs, and is considered to
be a sign for maturation of ORNs (18,19).
To date there has been no ideal treatment for
olfactory disorders induced by AR or other causes. In clinical
practice, glucocorticoid is often used for the treatment of
olfactory dysfunction. For example, the study by Faulcon et
al (20) indicated a good
therapeutic effect of glucocorticoid on 41 patients with olfactory
dysfunction. Moreover, the clinical study performed by Heilmann
et al (21) on 55 patients
with olfactory dysfunction demonstrates that oral administration of
prednisolone improves smell dysfunction caused by upper respiratory
tract infection, sinusitis, idiopathic anosmia amongst other
various reasons. Stevens (22)
observed that patients with nasal polyps still have olfactory
dysfunction following endoscopic sinus surgery performed to relieve
obstruction, and daily administration of 40 mg oral prednisone
(tapered) contributes to an improvement in olfaction. In addition,
local aerodynamic inhalation of glucocorticoid has achieved good
clinical results in the treatment of olfactory dysfunction
(23,24). However, there have been few clinical
studies performed on the effect of glucocorticoid in the treatment
of olfactory disorder caused by AR. In the present study, OMP
changes in the olfactory epithelium of mice are investigated.
Materials and methods
Animals and grouping
A total of 90 BALB/C mice of clean grade (male, 8
weeks old with a body weight of 25±1 g) were used in the present
study (Experimental Animal Center of Peking Union Medical College
Hospital, Chinese Academy of Medical Sciences, Beijing, China). The
mice were randomly divided into an AR model (80 mice) and control
(10 mice) groups. For sensitization, the AR model group of mice
were intraperitoneally injected with ovalbumin Al (OH)3
solution (300 µg/kg body weight; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) once every other day and 7 times in total.
Instead, ovalbumin solution was substituted with saline for the
control group. For excitation, the mice were anesthetized with an
intraperitoneal injection of 50 mg/kg 1% pentobarbital (Gene
Company Ltd., Hong Kong, China) on day 7 after the end of
sensitization. Next, ovalbumin solution (80 µg/kg body weight) was
slowly and steadily dripped into the bilateral anterior nostrils of
mice, and into the nasal cavity by breathing. Excitation was
performed one time a day for a consecutive 7 days. For the control
group, ovalbumin solution was replaced by saline. Moreover, the
symptom behavior superposition score method was used to evaluate
the model (25). In total, 30 min
after the last nasal excitation and secretion, sneezing frequency
and the nose-scratching times were observed and recorded. According
to the superposition quantization scoring (Table I), successful modeling was defined if
the total score was >5 points. All animal experiments were
conducted according to the ethical guidelines of Peking Union
Medical College Hospital.
 | Table I.Superposition quantization
scoring. |
Table I.
Superposition quantization
scoring.
| Score | Nose
scratching | Discharging | Sneezing |
|---|
| 0 | Never | None | Never |
| 1 | Occasionally | Reaching the
anterior nostril | 1–3 |
| 2 | Frequently | Over the anterior
nostril | 4–10 |
| 3 | Cannot stop | Flowing all the
face | >10 |
Animal model
In order to examine olfactory disorders, the buried
food test (BFT) was performed. Food pellets were randomly buried in
the litter at depths of 1–2 cm. Next, the mice were placed into the
location of the experiment and mice that were unable to find food
pellets within 300 sec (5 times on average) were defined to have
olfactory dysfunction. According to this standard, the AR model
group of mice was divided into a group with olfactory dysfunction
(55 mice) and a group without dysfunction (25 mice). The mice in
the control group were also assessed by BFT, and their results were
compared with those of the AR model group.
On day 3 after successful modeling, 9 mice in the
group with olfactory dysfunction and 9 mice in the group without
dysfunction were randomly selected. Moreover, all mice in the
control group were selected (8 mice as 2 mice died). The mice were
anesthetized with an intraperitoneal injection of pentobarbital (1%
pentobarbital 50 mg/kg; Gene Company Ltd.) and then sacrificed by
breaking marrow, removing the head fur and exposing the skulls. For
further treatment of pruning skull specimens, the upper part of the
nasal cavity was retained, including the nasal septum and lateral
wall as well as the ethmoid plate. The samples were then kept in 4%
polyformaldehyde solution (Beijing Dingguo Biotechnology Co., Ltd.,
Beijing, China) for 48 h, and soaked in 10% EDTA solution (Beijing
Sequoia Jinqiao Biological Technology Co., Ltd., Beijing, China)
for 14 days, with daily replacement of decalcifying fluid.
Following decalcification, the specimens were rinsed with tap water
for 24 h before being fixed in 4% polyformaldehyde solution again
for 24 h.
Medicine intervention was initiated on day 3 after
successful modeling. The remaining 46 mice in the group with
olfactory dysfunction were randomly divided into the following 5
groups: Budesonide group A (n=9), budesonide group B (n=10),
betamethasone group A (n=9), betamethasone group B (n=9) and
medicine-free group (n=9). The mice in the budesonide groups A and
B received nasal drips of 30 µl budesonide (Rhinocort; AstraZeneca
PLC, London, UK) into each nasal cavity once a day for consecutive
5 days. The mice in the betamethasone groups A and B were treated
with an intraperitoneal injection of 100 µl (3.5 mg/kg)
betamethasone solution (Shanghai Schering-Plough Pharmaceutical
Co., Ltd., Shanghai, China) once, and the mice in the medicine-free
group did not receive any medical intervention. On day 7 after the
start of intervention, the mice in the budesonide group A,
betamethasone group A and medicine-free group were sacrificed to
collect tissues. On day 14 after the start of intervention, the
mice in the budesonide group B and betamethasone group B were also
sacrificed to collect tissues.
Hematoxylin and eosin (H&E)
staining
In total 3 samples were randomly selected from the
control group, AR model group without olfactory dysfunction and AR
model group with olfactory dysfunction for gradient ethanol
dehydration, paraffin embedding and serial sections into 4–5 µm.
For conventional smear preparations, conventional smear glass
slides were fixed with 95% ethanol for at least 15 min, and then
treated with water for 1 min, hematoxylin for 10 min, running water
for 15 min, eosin for 30 sec, 95% ethanol for 1 min and 100%
ethanol for 2 min. Stained slides were cover-slipped with permount.
Finally, the entire H&E-stained cells were examined under a
light microscope using magnification of ×200-400.
Immunohistochemistry
A total of 3 samples were randomly selected from
each group. The samples were heated at 60°C for 1–2 h, dewaxed at
60°C for 10 min and dehydrated with ethanol for 2 min. The sections
were then washed with distilled water for 3 min, and soaked in 3%
hydrogen peroxide methanol solution at room temperature for 10 min.
Next, the sections were rinsed with 0.01 mol/l phosphate-buffered
saline (PBS) 3 times for 5 min each time. The sections were then
incubated with the primary antibody (1:8,000; goat anti-human OMP
monoclonal antibody; Wako Pure Chemical Industries, Ltd., Osaka,
Japan) overnight at 4°C, followed by rinsing with 0.01 mol/l PBS 3
times for 5 min each time. Thereafter, the sections were incubated
with horseradish peroxidase-labeled rabbit anti-goat antibody
(PV-6003; Beijing Sequoia Jinqiao Biological Technology Co., Ltd.,
Beijing, China) at 37°C in a humidified box for 30–60 min, followed
by rinsing with 0.01 mol/l PBS 3 times for 5 min each time. The
sections were stained with 3,3′-diaminobenzidine, counterstained
with hematoxylin, dehydrated in graded ethanol and made transparent
using xylene and sealed. Finally, the sections were observed under
an optical microscope (magnification, ×400), and all OMP-positive
cells were analyzed.
Statistical analysis
All the results were analyzed using SPSS 11.0
statistical analysis software (SPSS, Inc., Chicago, IL, USA). Each
group of data was compared using the paired t-test and numeral
materials were expressed as the mean ± standard deviation.
P<0.05 was used to indicate a statistically significant
difference.
Results
Establishment of the model of AR mice
is successful
In order to evaluate the establishment of models,
the symptom behavior superposition scoring method was adopted.
Following excitation, 7 mice in the AR model group and 2 mice in
the control group died. The mice that survived in both groups had a
shiny fur color, normal eating and drinking behavior, a sensitive
reaction and normal activities. Compared with the control group,
the mice in the AR model group demonstrated clear secretions in the
snout, and the number of times of nose-scratching occurred was
increased. Moreover, there was no significant difference in body
weight between the AR model (25.38±0.52 g) and control (25.52±0.70
g) groups (P>0.05). It was demonstrated that mice in the AR
model group were frequently scratching their nose using
fingernails, and they revealed an onset of sneezing, with nasal
secretions flowing out of the nose to form two wet marks of clear
liquid around the snout. However, no evident symptoms in the
control group of mice were observed (0 point). In addition, there
was a significant difference between the two groups (P<0.01;
Table II). The results suggested
that the establishment of the model of AR mice was successful.
 | Table II.Symptoms of mice in the AR model
group. |
Table II.
Symptoms of mice in the AR model
group.
| Score | Nose scratching
(mice) | Discharging
(mice) | Sneezing
(mice) |
|---|
| 0 | 0 | 0 | 0 |
| 1 | 4 | 17 | 14 |
| 2 | 36 | 46 | 21 |
| 3 | 33 | 10 | 38 |
Mice in the group with olfactory
dysfunction account for 75.34% of the total number of mice in the
AR model group
To determine the olfactory function of mice, BFT was
performed. The data demonstrated that all of the 8 mice in the
control group could find buried food pellets within 300 sec
(average time, 126±5 sec). In addition, 18 mice in the AR model
group could find food pellets within 300 sec (average time, 144±7
sec), and were classified into groups without olfactory
dysfunction. The remaining 55 mice in the AR model group could not
find buried food pellets in 300 sec, and were classified into the
group with olfactory dysfunction. The results indicate that mice in
the group with olfactory dysfunction account for 75.34% of the
total number of mice in the AR model group.
AR induces morphological changes in
the nasal mucosa even if olfactory dysfunction occurs
In order to observe morphological changes in the
nasal mucosa, H&E staining was used. Mouse olfactory mucosa was
located on the roof of the bilateral nasal cavity, inferior to the
middle part of the nasal septum and bilateral lateral wall, and
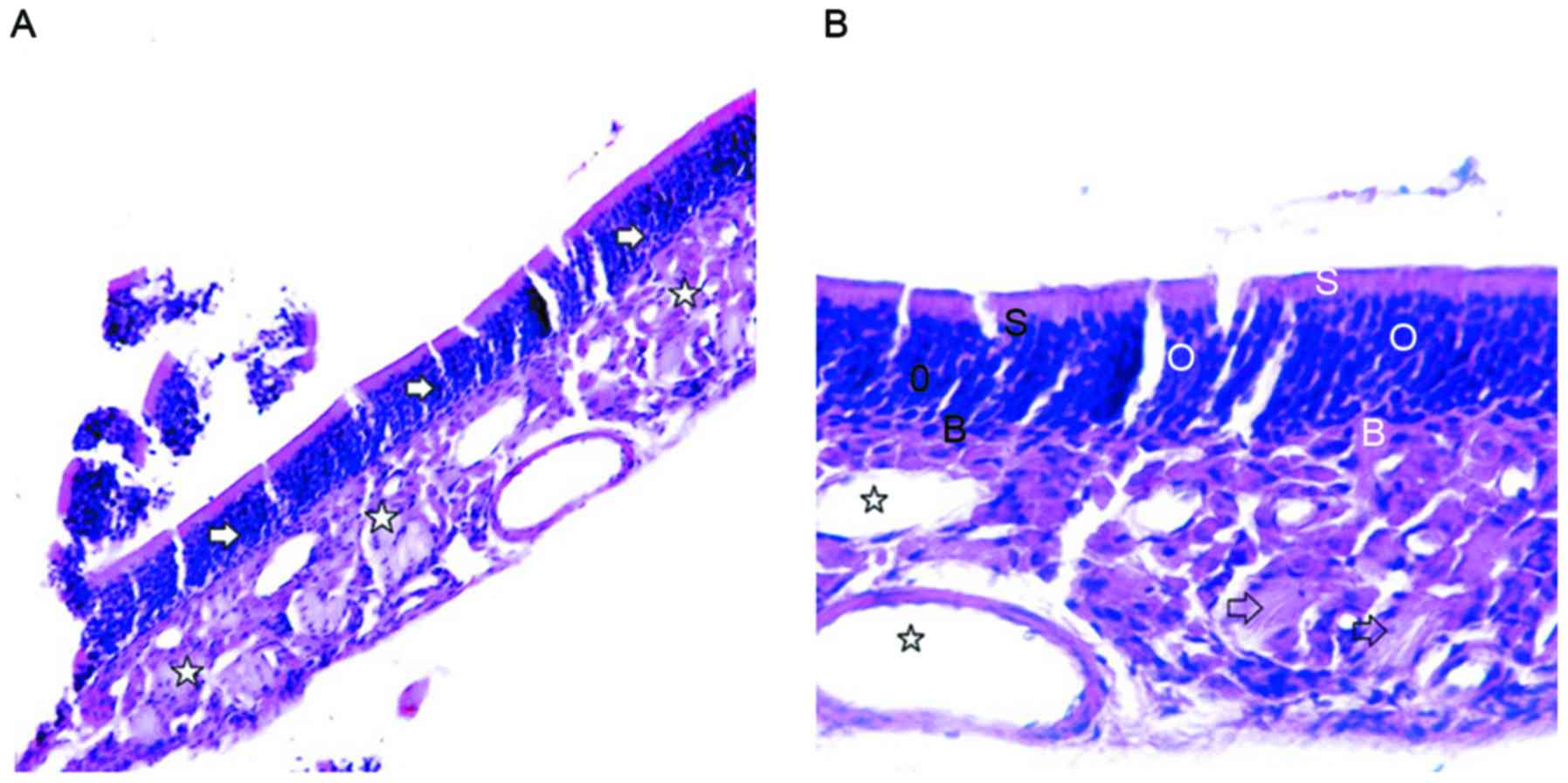
composed of epithelium and lamina propria (Fig. 1A). H&E staining revealed that the
olfactory mucosa epithelial layer in the control group contained
ORNs, supporting cells (SCs) and basal cells (BCs). Moreover, ORNs
were located in the middle part of the olfactory epithelium, with 7
or 8 layers, and with round, dark blue nuclei. SCs were located
near the surface of the olfactory epithelium, with elliptical and
light blue nuclei. BCs were located on the bottom of the olfactory
epithelium, close to the basement membrane, and with small and
oblate nuclei. Moreover, the lamina propria contained olfactory
nerves and blood vessels (Fig. 1B).
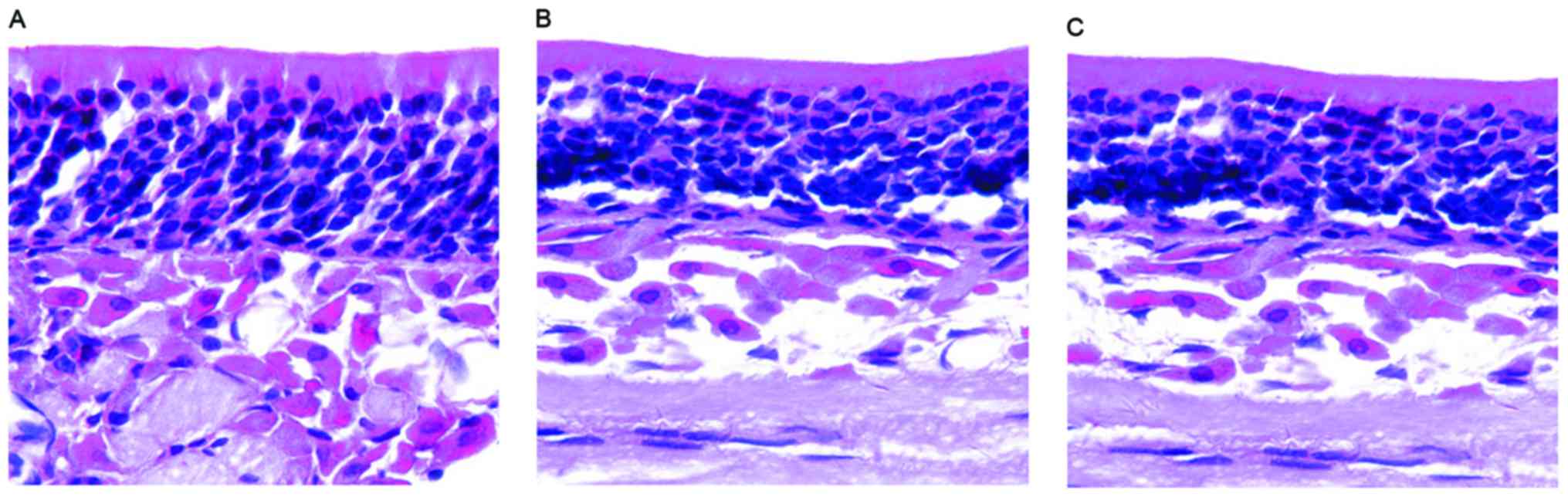
Each layer of epithelial cells was arranged in neat rows, and with
an apparent polarity and the epithelium became thinner in mice in
either group (with or without olfactory dysfunction). Furthermore,
the layers of ORNs were reduced and arranged in disorder. In
addition, there was no evident morphological difference between the
two groups, but the morphology of the nasal mucosa in the AR model
group was different from that of the control group (Fig. 2). These results indicate that AR
induces morphological changes in the nasal mucosa even if olfactory
dysfunction occurs.
Treatment with budesonide or
betamethasone restores a reduced number of OMP-positive cells in AR
mice with olfactory dysfunction to levels similar to that in
healthy mice
In order to observe histological changes in the
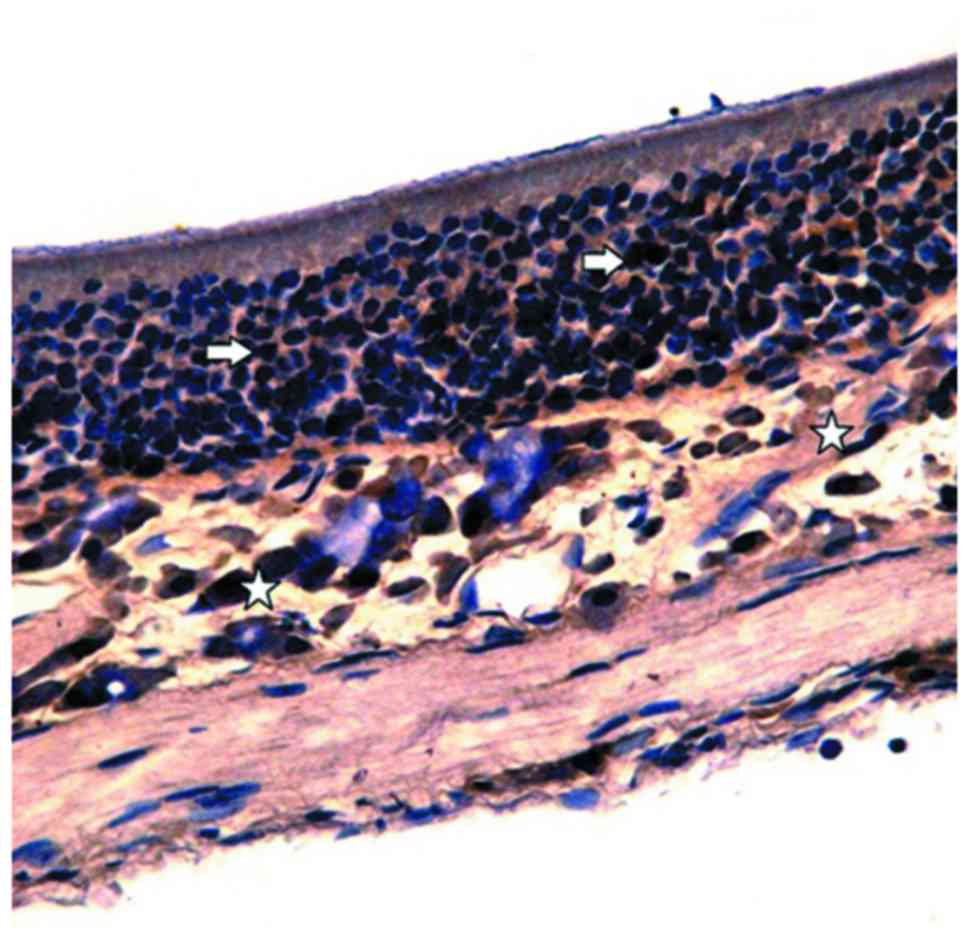
nasal mucosa, immunohistochemistry was performed. OMP-positive
cells in the control group were stained brown under an optical
microscope and were then distributed in the olfactory mucosal
epithelium and lamina propria. In addition, the OMP reaction was
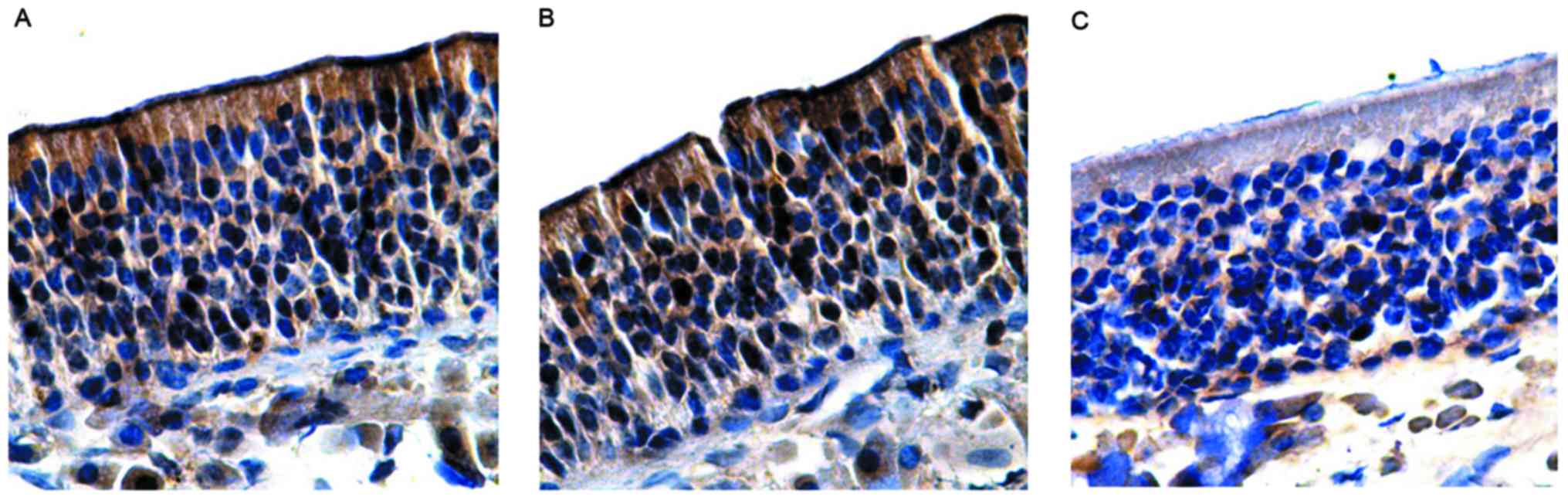
also observed in the olfactory mucosal surface cilia (Fig. 3). A large number of OMP-positive
cells were observed in the epithelial layer of the olfactory
mucosa.
In the lamina propria, brown olfactory nerve fibers
were observed in the network, while no OMP-positive reaction was
observed in the vascular wall (Fig.
4A). The number of OMP-positive cells in the control group was
66.38±1.52 (Table III). Moreover,
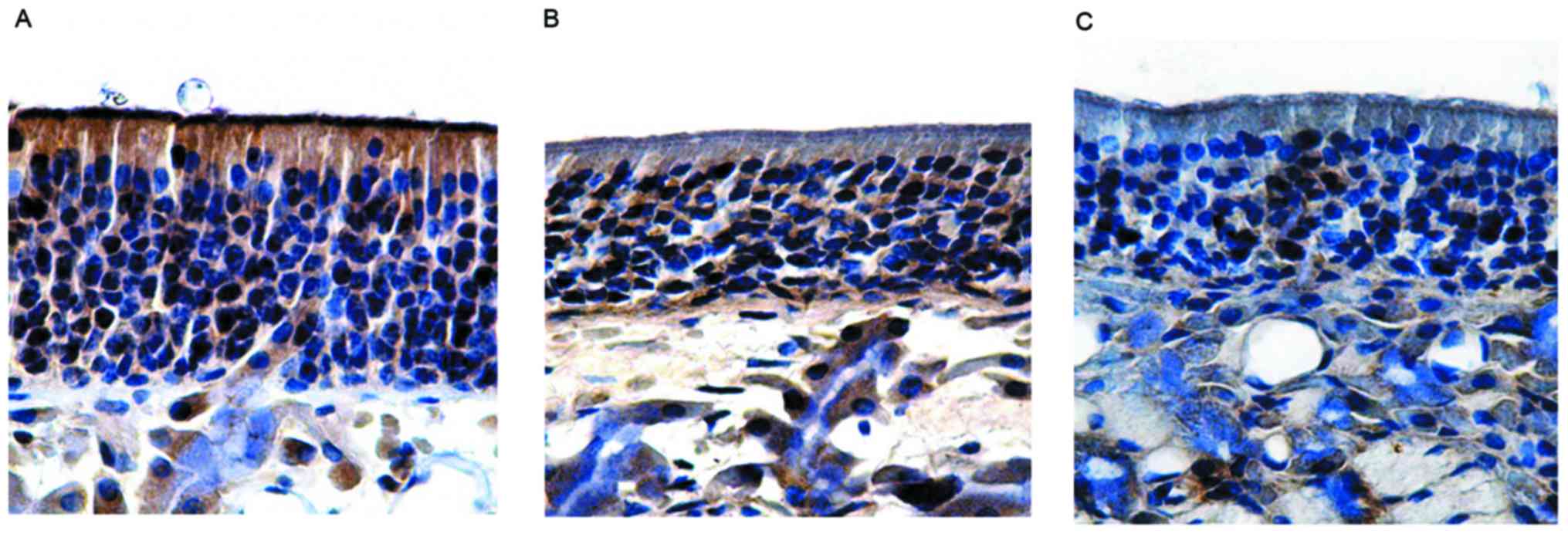
in the group without olfactory dysfunction, the olfactory mucosa
epithelial layer was thin and pale under a microscope, with the
expression of OMP being lower than that in the control group. The
number of OMP-positive cells in the group without olfactory
dysfunction was 59.50±0.56, which was not significantly different
to the control (P>0.05; Fig. 4B
and Table III). Similarly, in the
group with olfactory dysfunction, the olfactory epithelium layer
was also thin and pale, and the expression of OMP was significantly
lower than the control, with no positive brown staining in the
cilia of the surface layer. The number of OMP-positive cells in the
group with olfactory dysfunction was 39.77±2.01, which was
significantly different from those in the control group and the
group without olfactory dysfunction (P<0.05; Fig. 4C and Table III).
 | Table III.Number of OMP-positive cells in each
group. |
Table III.
Number of OMP-positive cells in each
group.
| Groups | OMP-positive cells
(n) |
|---|
| Control |
66.38±1.52 |
| Group without
olfactory dysfunction |
59.50±0.56 |
| Group with
olfactory dysfunction |
39.77±2.01a |
| Budesonide group
A |
61.51±1.62b |
| Betamethasone group
A |
62.04±1.23 |
| Medicine-free |
47.34±1.81c |
| Budesonide group
B |
60.19±1.32 |
| Betamethasone group
B |
63.82±1.26 |
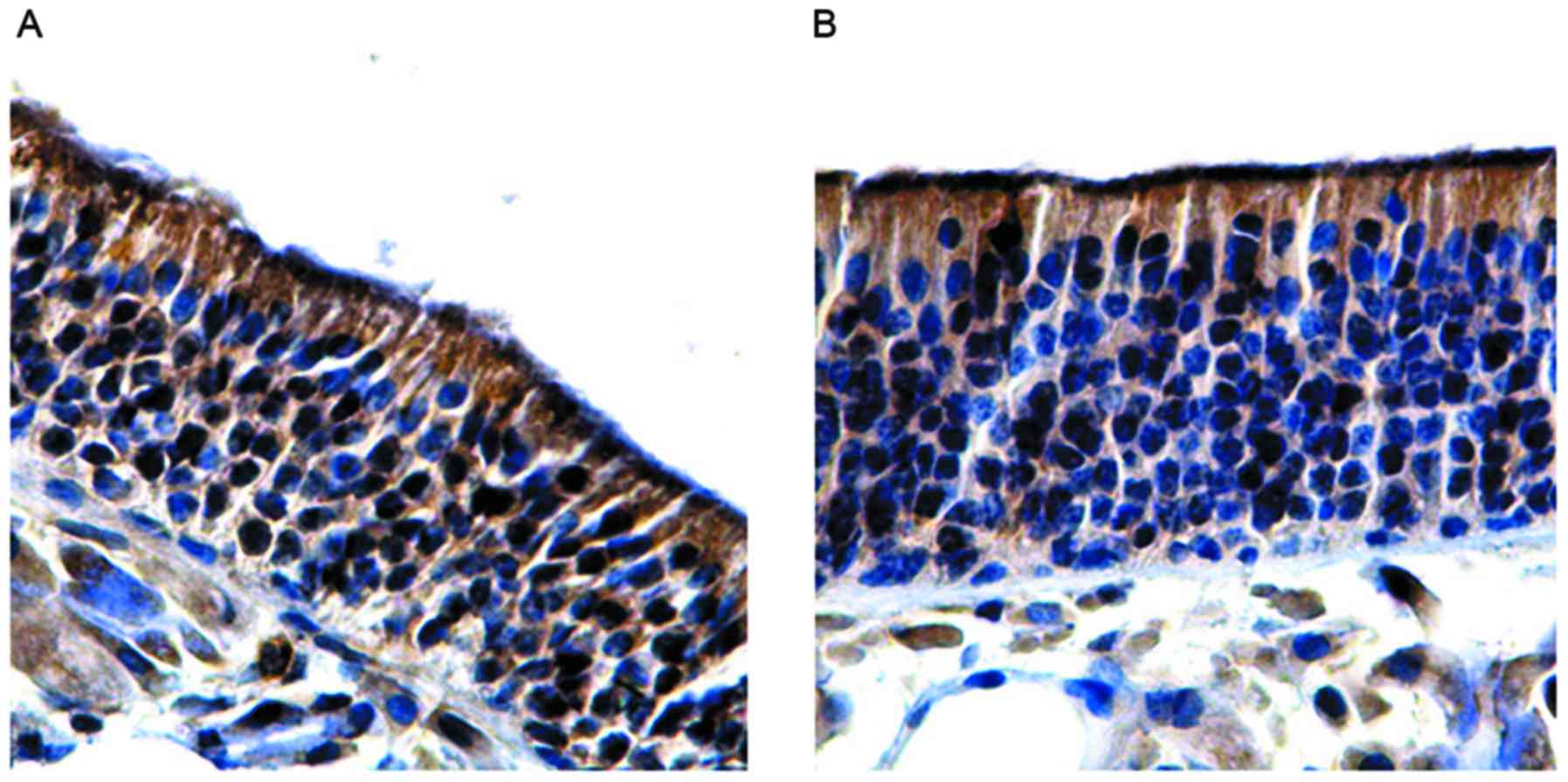
In the budesonide group A, the olfactory mucosa
epithelial layer was thickened, the expression of OMP was increased
and the number of OMP-positive cells (61.51±1.62) was significantly
higher compared with the group with olfactory dysfunction
(P<0.05). However, no significant differences were observed
between the budesonide group A and control group (P>0.05;
Fig. 5A and Table III). In the betamethasone group A,
the olfactory mucosa epithelial layer was thickened, the expression
of OMP was increased and the number of OMP-positive cells
(62.04±1.23) was significantly higher compared with the group with
olfactory dysfunction (P<0.05). However, no significant
differences were observed between the betamethasone group A,
control or budesonide group A (P>0.05; Fig. 5B and Table III). In the medicine-free group,
the thickness of the olfactory epithelial layer was increased and
cell arrangement was disordered. Moreover, the expression of OMP
was increased slightly, and the number OMP-positive cells
(47.34±1.81) was not significantly different from the group with
olfactory dysfunction (P>0.05), but was significantly less than
the number of OMP-positive cells in either the budesonide group A
or betamethasone group A (P<0.05; Fig. 5C and Table III). Finally, in the budesonide
group B, the expression of OMP was similar to that of the
budesonide group A. Moreover, the number of OMP-positive cells
(60.19±1.32) was slightly less than the budesonide group A
(P>0.05; Fig. 6A and Table III). In the betamethasone group B,
the expression of OMP was similar to the betamethasone group A, and
the number of OMP-positive cells (63.82±1.254) was not
significantly different from the betamethasone group A (P>0.05)
or budesonide group B (P>0.05; Fig.
6B and Table III). These
results indicate that treatment with budesonide or betamethasone
restores a reduced number of OMP-positive cells in AR mice with
olfactory dysfunction to levels similar to that in healthy
mice.
Discussion
The establishment of an animal model is an effective
means of studying the pathogenesis and pathophysiological basis of
diseases. The principle of establishing a model is to reliably and
specifically reflect the clinical features and pathological
changes, with good repeatability. Toluene diisocyanate (TDI) and
OVA are mainly used as allergens in the establishment of AR animal
models (26–28). Since TDI has a stimulation effect on
mucosa and inflammatory injury, it cannot be widely used. By
contrast, OVA is more widely used because its stimulation effect is
small and sensitization is better (29,30).
When selecting animal models, mice have congenital favorable
factors for allergic diseases, including mild temperament, low
aggressivity, short growth period, high fecundity, a developed
lymph system and sensitivity to outside stimuli. In addition, the
mouse gene map has nearly been completed, resulting in a deeper
understanding of the immune system of mice. Therefore, BALB/C mice
were selected as model animals in the present study. Moreover, OVA
and adjuvant Al(OH)3 were used to sensitize animal
bodies and to maintain nasal excitation. This model provides the
basis for the study of OMP expression in the olfactory epithelium
in AR and the effect of glucocorticoid invention.
Evaluation of the olfactory function trough
behavioral changes is currently a widely used experimental method
(31,32). BFT is the olfactory evaluation method
that has been applied to evaluate mouse olfactory disorder
behavior, and this method has also improved (33,34), and
it is easy to operate with a low cost, good feasibility and
reproducibility. The present study performed preliminary assessment
with BFT on all mice before grouping, and all mice could find food
pellets within 300 sec, demonstrating that 300 sec is and feasible
as a grouping criterion in olfactory behavioral experiments. In
total, ~74.55% of AR model mice demonstrated olfactory dysfunction
in the present study, and the results confirm that olfactory
dysfunction is a common symptom of AR.
The mechanism by which AR causes olfactory
dysfunction is not entirely clear yet. It is thought that the loss
of smell is conductive, due to the blocked channels for odor
molecules to reach to the top of the nasal cavity olfactory
receptor, and the intact olfactory epithelium (35). Recent studies tend to think that the
degree of nasal obstruction is not directly associated with
olfactory dysfunction caused by AR (4,36).
Moreover, the use of epinephrine nasal decongestants cannot return
the olfactory function of AR patients to normal (37). The study by Cowart et al
(11) also demonstrated that even if
the lower passage of the nasal cavity is completely blocked, it is
still not enough to cause a significant decrease in the olfactory
sensitivity. This indicates that pathological changes of the
olfactory epithelium itself may be the main reason for olfactory
dysfunction caused by AR. In the present study, the olfactory
epithelium of AR model mice is thinner, the layer of cells is
arranged in irregular order, the plies of cells are significantly
reduced and the polarity is lost. The results show that the
olfactory mucosa has evident pathological changes and that the ORNs
have pathological injury.
In the studies related to olfactory sense, OMP has
recently attracted a lot of attention. OMP is a type of protein
closely associated with the sense of smell, and it is specifically
expressed in mature ORNs. Moreover, ORNs are the only neurons in
the olfactory mucosa. Their function is to sense odor molecules in
the air, to change chemical signals into electrical signals and to
transmit olfactory information to olfactory bulb and olfactory
senior center (16,17,38). The
results in the present study demonstrated that expression of OMP in
the AR model group was lower than the control group, and the OMP
expression level in the group with olfactory dysfunction was
significantly lower than that in the control. This indicates that
the olfactory mucosa has pathological changes.
There is no ideal and standard treatment for
olfactory dysfunction to date. However, previous results
demonstrate a method to enhance olfactory sensitivity by short-term
systemic contact with multiple specific odors (39), and attempts have been made to improve
the olfactory sensitivity by repeated magnetic stimulation of the
frontal cortex (40). However, these
new efforts have not been clinically applied yet. The treatment for
olfactory dysfunction at present still uses corticosteroids as a
first choice. There are numerous reports on glucocorticoid
treatment for olfactory dysfunction. Hotchkiss (41) reported that oral glucocorticoid
improves olfactory dysfunction induced by nasal polyps. Moreover,
Fukazawa et al (42) gave
nasal septum mucosal local injection of dexamethasone to 102
patients with a different etiology of olfactory disorder, and 63.7%
patients demonstrated an improved sense of smell. Finally, Guan
et al (24) used intranasal
pneumatic jet atomization inhalation of budesonide suspension for
the treatment of patients with olfactory dysfunction with different
causes, and achieved better therapeutic effects. Furthermore, other
studies revealed that glucocorticoid combined with extracts of
Ginkgo biloba may have an improved treatment effect
(43,44).
The mechanism of action of glucocorticoid in the
treatment for olfactory dysfunction is not fully understood yet. It
is known that glucocorticoid improves the sense of smell by
inhibiting inflammatory cytokine production, induces the
anti-inflammatory factor to reduce the inflammation of olfactory
mucosa, alleviates the congestion and edema to increase the contact
area of olfactory mucosa with odor molecules in the air and
promotes odor molecules to combine with ORNs. In addition,
glucocorticoid has a direct effect on the olfactory mucosa itself.
A previous study found that there were glucocorticoid receptors in
the olfactory mucosa (45).
Glucocorticoid directly induces basal cell proliferation and
formation of new ORNs that replace the aged and deactivated ORNs
(46). Glucocorticoid also
upregulates the expression of cyclic nucleotide-gated channel
protein mRNA, strengthens the adenylate cyclase and cyclic
adenosine monophosphate pathway in the process of olfactory signal
transduction and promotes the olfactory signal transduction,
thereby improving the olfactory function (47).
The present study selects budesonide and the
betamethasone compound as two different routes of administration of
corticosteroids. The results reveal that after 1 week of
glucocorticoid intervention by budesonide or betamethasone compound
in the AR model of mice with olfactory dysfunction, the number of
ORNs in the olfactory mucosa had increased, reaching levels that
were comparable to the control group. However, observation of mice
in the medicine-free group demonstrated that the number of ORNs in
the olfactory mucosa is not significantly increased. These results
demonstrated that the application of glucocorticoid has an explicit
intervention effect for olfactory disorder in AR. Glucocorticoid
effectively improves the number of ORNs in the olfactory mucosa and
demonstrates protective effects on it. After 2 weeks of medicine
intervention, the number of ORNs is not significantly changed in
the two groups of different routes of medicine administration. This
result reveals that the treatment effects of the two different
routes of administration of glucocorticoid on olfactory dysfunction
in AR can be maintained for a considerable amount of time.
Intranasal local glucocorticoid, including budesonide, is not
easily absorbed into the blood of the nasal mucosa; therefore, the
incidence of systemic adverse reactions is low. Thus, intranasal
application of local glucocorticoid is expected to be an ideal
treatment method for olfactory dysfunction in AR. In conclusion, AR
is an important factor causing olfactory dysfunction. Moreover,
glucocorticoid alleviates olfactory dysfunction by acting on ORNs
in the olfactory mucosa. In the future, further studies should be
focused on how glucocorticoid activates the glucocorticoid
receptors as well as the targeting mechanism in the olfactory
system.
Acknowledgements
The present study was supported by the Chinese
Academy of Medical Science and Peking Union Medical College.
References
|
1
|
Ni DF: Olfactory disorders and olfactory
function test. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 17:571–575.
2003.(In Chinese).
|
|
2
|
Stenner M, Vent J, Huttenbrink KB, Hummel
T and Damm M: Topical therapy in anosmia: Relevance of
steroid-responsiveness. Laryngoscope. 18:1681–1686. 2008.
View Article : Google Scholar
|
|
3
|
Bousquet J, Khaltaev N, Cruz AA, Denburg
J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica
GW, van Weel C, et al: Allergic rhinitis and its impact on asthma
(ARIA) 2008 update (in collaboration with the world health
organization, GA(2)LEN and AllerGen). Allergy. 86 Suppl 63:86–160.
2008.
|
|
4
|
Guilemany JM, García-Piñero A, Alobid I,
Cardelús S, Centellas S, Bartra J, Valero A, Picado C and Mullol J:
Persistent allergic rhinitis has a moderate impact on the sense of
smell, depending on both nasal congestion and inflammation.
Laryngoscope. 119:233–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu ZY: Respiratory tract inflammation.
Zhonghua Er Bi Yan Hou Ke Za Zhi. 36:397–399. 2001.(In
Chinese).
|
|
6
|
Amit A, Saxena VS, Pratibha N, D'Souza P,
Bagchi M, Bagchi D and Stohs SJ: Mast cell stabilization,
lipoxygenase inhibition, hyaluronidase inhibition, antihistaminic
and antispasmodic activities of Aller-7, a novel botanical
formulation for allergic rhinitis. Drugs Exp Clin Res. 29:107–115.
2003.PubMed/NCBI
|
|
7
|
Mott AE, Cain WS, Lafreniere D, Leonard G,
Gent JF and Frank ME: Topical corticosteroid treatment of anosmia
associated with nasal and sinus disease. Arch Otolaryngol Head Neck
Surg. 123:367–372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simola M and Malmberg H: Sense of smell in
allergic and nonallergic rhinitis. Allergy. 53:190–194. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kirtsreesakul V and Naclerio RM: Role of
allergy in rhinosinusitis. Curr Opin Allergy Clin Immunol. 4:17–23.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moll B, Klimek L, Eggers G and Mann W:
Comparison of olfactory function in patients with seasonal and
perennial allergic rhinitis. Allergy. 53:297–301. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cowart BJ, Flynn-Rodden K, McGeady SJ and
Lowry LD: Hyposmia in allergic rhinitis. J Allergy Clin Immunol.
91:747–751. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rombaux P, Collet S, Eloy P, Ledeghen S
and Bertrand B: Smell disorders in ENT clinic. B-ENT. 1 Suppl
1:97–109. 2005.PubMed/NCBI
|
|
13
|
Doty RL and Mishra A: Olfactory and its
alteration by nasal obstruction, rhinitis, and rhinosinusitis.
Laryngoscope. 111:409–423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guss J, Doghramji L, Reger C and Chiu AG:
Olfactory dysfunction in allergic rhinitis. ORL J Otorhinolaryngol
Relat Spec. 71:268–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Beek TA and Montoro P: Chemical
analysis and quality control of Ginkgo biloba leaves,
extracts, and phytopharmaceuticals. J Chromatogr A. 1216:2002–2032.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berghard A, Buck LB and Liman ER: Evidence
for distinct signaling mechanisms in two mammalian olfactory sense
organ. Proc Natl Acad Sci USA. 93:2365–2369. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buck LB: The molecular architecture of
odor and pheromone sensing in mammals. Cell. 100:611–618. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kass MD, Moberly AH, Rosenthal MC, Guang
SA and McGann JP: Odor-specific, olfactory marker protein-mediated
sparsening of primary olfactory input to the brain after odor
exposure. J Neurosci. 33:6594–6602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee AC, He J and Ma M: Olfactory marker
protein is critical for functional maturation of olfactory sensory
neurons and development of mother preference. J Neurosci.
31:2974–2982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faulcon P, Biacabe B and Bonfils P:
Contribution of corticosteroid treatment in neurosensorial
anosomia: A series of 62 patients. Ann Otolaryngol Chir Cervicofac.
117:374–377. 2000.(In French). PubMed/NCBI
|
|
21
|
Heilmann S, Huettenbrink KB and Hummel T:
Local and systemic administration of corticosteroid in the
treatment of olfactory loss. Am J Rhinol. 18:29–33. 2004.PubMed/NCBI
|
|
22
|
Stevens MH: Steroid-dependent anosmia.
Laryngoscope. 111:200–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hilberg O: Effect of terfenadine and
budesonide on nasal symptoms, olfaction, and nasal airway patency
following allergen challenge. Allergy. 50:683–688. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guan J, Ni DF, Wang J, Zhu Y, Xu C, Chen X
and Liu J: Therapy for olfactory disorder associated with URTI
along with nasal and accessory nasal diseases. Lin Chung Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 24:484–488. 2010.(In Chinese).
PubMed/NCBI
|
|
25
|
Miescher SM and Vogel M: Molecular aspects
of allergy. Mol Aspects Med. 23:413–462. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahashi N, Aramaki Y and Tsuchiy S:
Allergic rhinitis model with Brown Norway rat and evaluation of
antiallergic drugs. J Pharmacobiodyn. 13:414–420. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakaya M, Dohi M, Okunishi K, Nakagome K,
Tanaka R, Imamura M, Baba S, Takeuchi N, Yamamoto K and Kaga K:
Noninvasive system for evaluating allergen-induced nasal
hypersensitivity in murine allergic rhinitis. Lab Invest.
86:917–926. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka K, Okamoto Y, Nagaya Y, Nishimura
F, Takeoka A, Hanada S, Kohno S and Kawai M: A nasal allergy model
developed in the guinea pig by application of 2,4-toluene
diisocyanate. Int Arch Allergy Appl Immunol. 85:392–397. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sehmi R, Wood LJ, Watson R, Foley R, Hamid
Q, O'Byrne PM and Denburg JA: Allergen-induced increases in IL-5
receptor alpha-subunit expression on bone marrow-derived CD34+
cells from asthmatic subjects. A novel marker of progenitor cell
commitment towards eosinophilic differentiation. J Clin Invest.
100:2466–2475. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Wei YX, Wang XD and Yang L:
Observation of the olfactory mucosa in mice with allergic rhinitis
olfactory dysfunction. Zhongguo Er Bi Yan Hou Tou Jing Wai Ke.
15:465–468. 2008.(In Chinese).
|
|
31
|
Carr VM, Robinson AM and Kern RC:
Tissue-specific effects of allergic rhinitis in mouse nasal
epithelia. Chem Senses. 37:655–668. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wirth S, Stemmelin J, Will B, Christen
YVES and Di Scala G: Facilitative effects of EGb 761 on olfactory
recognition in young and aged rats. Pharmacol Biochem Behav.
65:321–326. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nathan BP, Yost J, Litherland MT, Struble
RG and Switzer PV: Olfactory function in apoE knockout mice. Behav
Brain Res. 150:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liebenauer LL and Slotnick BM: Social
organization and aggression in a group of olfactory bulbectomized
male mice. Physiol Behav. 60:403–409. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seiden AM and Duncan HJ: The diagnosis of
a conductive olfactory loss. Laryngoscope. 111:9–14. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klimek L and Eggers G: Olfactory
dysfunction in allergic rhinitis is related to nasal eosinophilic
inflammation. J Allergy Clin Immunol. 100:158–164. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doty RL and Mishra A: Olfaction and its
alternation by nasal obstruction, rhinitis, and rhinosinusitis.
Laryngoscope. 111:409–423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen ZH and Ni DF: Research progress of
odorant receptor. Guo Ji Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
15:465–468. 2008.(In Chinese).
|
|
39
|
Hummel T, Rissom K, Reden J, Hähner A,
Weidenbecher M and Hüttenbrink KB: Effects of olfactory training in
patients with olfactory loss. Laryngoscope. 119:496–499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Henkin RI, Potolicchio SJ Jr and Levy LM:
Improvement in smell and taste dysfunction after repetitive
transcranial magnetic stimulation. Am J Otolarnygol. 32:38–46.
2011. View Article : Google Scholar
|
|
41
|
Hotchkiss WT: Influence of prednisone on
nasal polyposis with anosmia; preliminary report. AMA Arch
Otolaryngol. 64:478–479. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fukazawa K, Fujii M, Tomofuji S, Ogasawara
H, Seo W and Sakagami M: Local injection of dexamethasone acetate
suspension into the nasal mucosa in cases of olfactory disturbance.
Nippon Jibinkoka Gakkai Kaiho. 102:1175–1183. 1999.(In Japanese).
View Article : Google Scholar
|
|
43
|
Seo BS, Lee HJ, Mo JH, Lee CH, Rhee CS and
Kim JW: Treatment of postviral olfactory loss with glucocorticoids,
Ginkgo biloba, and mometasone nasal spray. Arch Otolaryngol
Head Neck Surg. 135:1000–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee GS, Cho JH, Park CS, Jung SH, Lee DH,
Jun BC, Song CE and Cho KJ: The effect of Ginkgo biloba on
the expression of intermediate-early antigen (c-fos) in the
experimentally induced anosmic mouse. Auris Nasus Larynx.
36:287–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Robinson AM, Kern RC, Foster JD, Fong KJ
and Pitovski DZ: Expression of glucocorticoid receptor mRNA and
protein in the olfactory mucosa: Physiologic and pathophysiologic
implications. Laryngoscope. 108:1238–1242. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takanosawa M, Nishino H, Ohta Y and
Ichimura K: Glucocorticoids enhance regeneration of murine
olfactory epithelium. Acta Otolaryngol. 129:1002–1009. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei Y, Zhang C, Miao X, Xing F, Liu X,
Zhao H, Zhan X and Han D: Effects of glucocorticoid on cyclic
nucleotide-gated channels of olfactory receptor neurons. J
Otolaryngol Head Neck Surg. 38:90–95. 2009.PubMed/NCBI
|