Introduction
Early T-cell precursor-acute lymphoblastic leukemia
(ETP-ALL) has been identified as a distinct biological subtype of
T-cell lineage ALL (T-ALL), which accounts for 15% of T-ALL cases
(and ~2% of ALL cases). It is associated with poor clinical
outcomes even following the use of current treatment regimens.
Recently, the complete remission rate/complete remission with
incomplete platelet recovery rate in patients with ETP-ALL was
indicated to be significantly lower than that of non-ETP-ALL
patients (73 vs. 91%; P=0.03) in 2016 (1). Up to the last follow up, the median
overall survival for patients with ETP-ALL was 20 months vs. not
yet reached for the non-ETP-ALL/LBL patients (P=0.008) (1). This subtype is characterized by a lack
of expression of the T-cell surface markers cluster of
differentiation (CD) 1a and CD8, weak or absent expression of CD5,
and aberrant expression of at least one of the following myeloid or
hematopoietic stem cell markers: CD13, CD33, CD34, CD117, human
leukocyte antigen (HLA)-DR, CD11b and CD65 (2).
T-ALL is a genetically heterogeneous disease caused
by the accumulation of multiple genetic defects in developing
T-cells that affect critical cellular processes, including cell
differentiation, proliferation, survival and self-renewal capacity
(3). The presence of the nucleoporin
214-ABL proto-oncogene 1 (NUP214-ABL1) fusion gene has been
identified in 6% of all T-ALL cases (4). NUP214, also known as CAN, is a 214-kDa
FG-repeat-containing nucleoporin located at band 9q34.1 and
includes 36 exons. The encoded protein is located at the
cytoplasmic side of the nuclear pore complex (NPC) (5). The NUP214-ABL1 fusion protein is a
constitutively active protein tyrosine kinase. De Keersmaecker
et al (6) recently identified
that the sparse representation-based classifier (SRC) family kinase
lymphocyte-specific protein tyrosine kinase (LCK) is a protein
essential for the proliferation and survival of T-ALL cells
dependent on NUP214-ABL1 activity. These findings underscore the
potential of the dual ABL1/SRC kinase inhibitor dasatinib in the
treatment of NUP214-ABL1-positive T-ALL. Furthermore, NUP214 serves
a role in mRNA export and chromosome region maintenance 1
(CRM1)-mediated export of the 60S pre-ribosomal subunit, and may
serve a role in the transport of other proteins (7). Given that CRM1 inhibitors, such as
selinexor, suppress the export of proteins associated with NUP214
(7), there is potential for their
use to treat patients with T-ALL that also harbor
NUP214-ABL1.
The NUP214-ABL1 gene is highly specific for
T-ALL (8), however the prevalence of
NUP214-ABL1 gene expression in ETP-ALL has not been
verified. Zhang et al (9)
reported that NUP214-ABL1 was not detected in all the 64
ETP-ALL cases studied. The present case report describes a rare
case of a patient with ETP-ALL harboring the NUP214-ABL1
fusion gene. The present study also evaluated the therapeutic
efficiency of selinexor and dasatinib to treat
NUP214-ABL1-positive ETP-ALL cells in vitro. ETP-ALL
has higher rates of relapse and remission failure following
conventional chemotherapy and shorter patient survival times
(1), meaning that novel therapeutic
agents to treat ETP-ALL are urgently required.
Case report
A 29-year-old Han Chinese man was admitted to the
Zhongshan Hospital, Xiamen University (Xiamen, China) in September
2015 with a low-grade fever and stomachache, which he had
experienced for 5 days. The patient had no family history of
genetic or hematological disease. Ethical approval for the current
case report was granted by the Medical Ethics Committee of
Zhongshan Hospital, Xianmen University (Xiamen, China) and informed
written consent was obtained from the patient.
The patient presented with splenomegaly (spleen
size, 13.2×4.3 cm) determined via abdominal ultrasonography. The
patient's peripheral blood was analyzed with a hematology analyzer
(Beckman Coulter, Inc., Brea, CA, USA): White blood cell count was
5.86×109/l (neutrophils, 10%; lymphocytes, 66%;
monocytes, 20%; reference range, white blood cell count
3.5×109/l-9.5×109/l; neutrophils, 50–70%;
lymphocytes, 20–50%; monocytes, 3–10%), hemoglobin level (Hb) was
118 g/l (reference range, 130–175 g/l) and blood platelet count
(Plat) was 45×109/l (reference range, 125
×109/l-350 ×109/l). The proportion of
immature cells detected in the peripheral blood was low (4%). The
following blood coagulation characteristics were measured:
Prothrombin time, 15.6 sec (reference range, 11.0–15.0 sec);
activated partial thromboplastin time, 15.2 sec (reference range,
28.0–42.5 sec); fibrinogen level, 2.82 g/l (reference range,
2.00–4.00 g/l) and D-dimer quantification, 3,150 ng/ml (reference
range, 0.00–500.00 ng/ml. Lactate dehydrogenase levels were
elevated (605.1 U/l; reference range, 109.0–245.0 U/l), however
liver and renal functions were normal. The patient underwent bone
marrow (BM) harvesting under general anesthesia. BM was aspirated
from the posterior iliac crest in a sterile operating room, and BM
smears stained with Wright-Giemsa stains (Electron Microscopy
Sciences, Hatfield, PA, USA) were obtained and tested under an
optical microscope. The white blood cell nucleus and cytoplasm were
indicated by the characteristic blue or pink coloration. The
500-cell differential count from the BM aspirate on the first day
of hospital admission revealed that 80% of all nucleated cells were
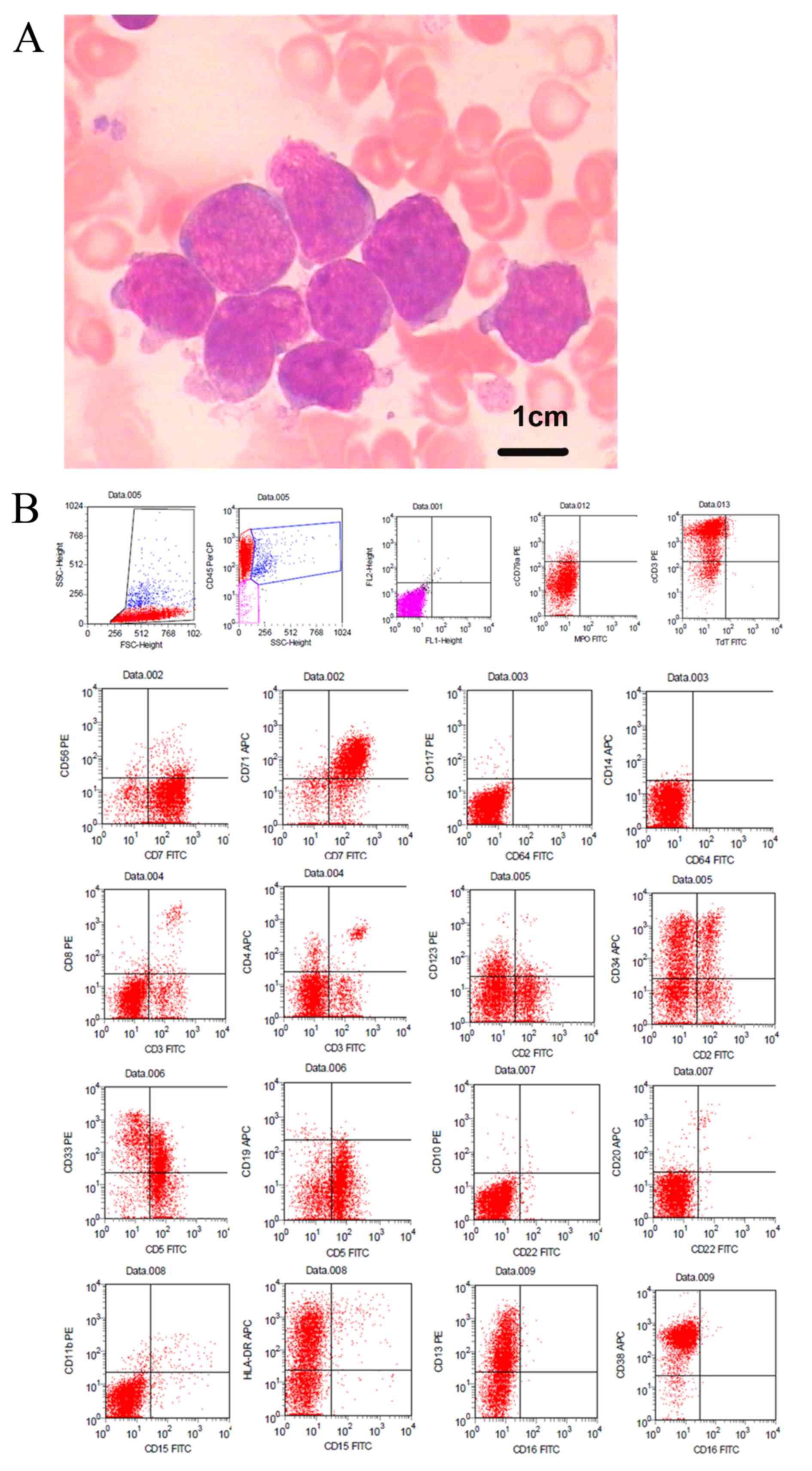
blasts. The majority of blasts exhibited large sizes, high
nuclear/cytoplasm ratios, irregularly shaped nuclei, dispersed
chromatin, indistinct nucleoli and basophilic cytoplasm that
occasionally formed pseudopods, but few exhibited moderate amounts
of granular cytoplasm, fine nuclear chromatin and 2–3 prominent
nucleoli (Fig. 1A). Cytochemical
staining (10) was negative for
myeloperoxidase (MPO) and ~3% positive for periodic acid-Schiff
staining.
Flow cytometry of the BM aspirate was performed
using a four-color Beckman Coulter Cytomics FC 500 flow cytometer
(Beckman Coulter, Inc.) as described previously (11). The results indicated that ~90% of the
blasts were positive for CD45, the T-cell markers cCD3, CD2 and
CD7, and the myeloid/stem cell markers CD34, CD38, CD13, CD33,
HLA-DR and CD123. However, the blasts were weak for CD5, negative
for CD1a, CD8, as well as CD19, CD10, CD79a, terminal
deoxynucleotidyl transferase, CD4, CD3, CD64, CD14, CD20, CD56,
CD16, CD15, CD11b and MPO (Fig. 1B),
suggesting a diagnosis of ETP-ALL. Bone marrow cells were directly
processed for chromosomal preparation and cytogenetic analyses were
performed using the standard method and/or the G-banding method
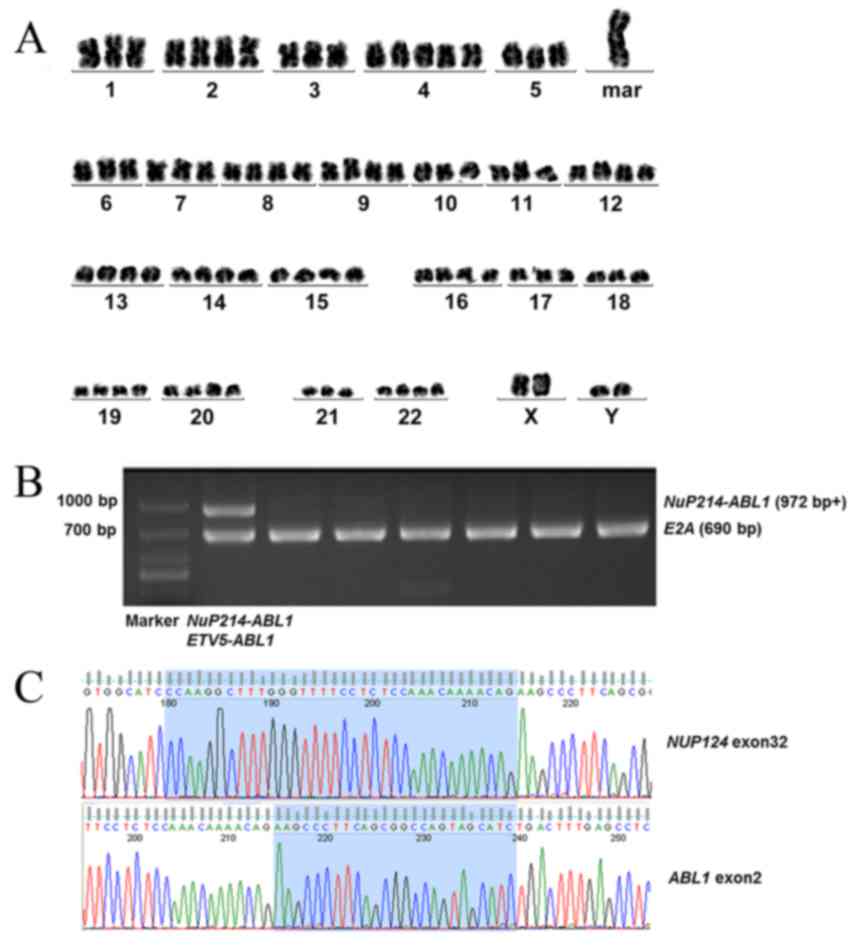
(12). The result of conventional
G-banding chromosomal analysis of the BM cells was
80–83<4n>,XXYY,+mar, inc[2]/46,XY[18] (Fig. 2A).
The results of multiplex nested reverse
transcription-polymerase chain reaction (RT-PCR), which was
performed as described previously (13), were negative for the fusion genes
listed in Table I. Multiplex RT-PCR
was subsequently used to detect Philadelphia chromosome-like fusion
genes that have a gene expression profile similar to that of
Ph+ ALL, but lack BCR ABL1 fusion. The results
demonstrated that the NUP214-ABL1 fusion gene was positive in this
case (Table I; Fig. 2B), which was further confirmed by DNA
sequencing (Fig. 2C). Also, gene
mutation analyses (Table I) were
performed. A TET2 missense mutation (c.86C>G; p.P29R), ASXL1
missense mutation (c.1954G>A; p.G652S) and ASXL1 same-sense
mutation (c.3759T>C; p.S1253S) were detected in this case, which
were single nucleotide polymorphism variants (SNP) of TET2 and
ASXL1 mutations. These SNP variants did not suggest any significant
prognostic impact on leukemia.
 | Table I.Genetic analysis of the early T-cell
precursor-acute lymphoblastic leukemia patient. |
Table I.
Genetic analysis of the early T-cell
precursor-acute lymphoblastic leukemia patient.
| Type of analysis | Gene | Method | Result |
|---|
| Gene mutation | FLT3-ITD | RT-PCR | – |
| Fusion gene | MLL/AF4 | Multiplex nested | – |
|
|
| RT-PCR |
|
|
| MLL/AF6 |
| – |
|
| MLL/AF9 |
| – |
|
| MLL/AF10 |
| – |
|
| MLL/AF17 |
| – |
|
| MLL/AF1P |
| – |
|
| MLL/AF1Q |
| – |
|
| MLL/AFX |
| – |
|
| MLL/ELL |
| – |
|
| MLL/ENL |
| – |
|
| dupMLL |
| – |
|
| CBFβ/MYH11 |
| – |
|
| AML1/ETO |
| – |
|
| AML1/MDS1 |
| – |
|
| SET/CAN |
| – |
|
| DEK/CAN |
| – |
|
| PML/RARα |
| – |
|
| PLZF/RARα |
| – |
|
| NPM/RARα |
| – |
|
| NPM/ALK |
| – |
|
| TEL/AML1 |
| – |
|
| E2A/PBX1 |
| – |
|
| BCR/ABL1 |
| – |
|
| NPM/MLF1 |
| – |
|
| TEL/ABL1 |
| – |
|
| E2A/HLF |
| – |
|
| TLS/ERG |
| – |
|
| SIL/TAL1 |
| – |
|
| TEL/PDGFR |
| – |
|
| EVI1 |
| – |
|
| HOX11 |
| – |
| Ph-like fusion
genes | RCSD1/ABL1 | Multiplex
RT-PCR | – |
|
| ZMIZ1/ABL1 |
| – |
|
| NUP214/ABL1 |
| + |
|
| PAG/ABL2 |
| – |
|
| TNIP1/PDGFRβ |
| – |
|
| RANBP2/ABL1 |
| – |
|
| SNX2/ABL1 |
| – |
|
| RCSD1/ABL2 |
| – |
|
| SSBP2/PDGFRβ |
| – |
|
| ETV6/ABL1 |
| – |
|
| ZC3HAV1/ABL2 |
| – |
|
| ZEB2/PDGFRβ |
| – |
|
| TEL/ABL1 |
| – |
|
| SSBP2/CSF1R |
| – |
|
| EBF1/PDGFRβ |
| – |
| Gene mutation | FLT3-ITD | RT-PCR | – |
|
|
IDH1R132 | Next-generation
sequencing | – |
|
| NPM1(exon12) |
| – |
|
| IDH2R140 |
| – |
|
| CEBPA |
| – |
|
| IDH2R172 |
| – |
|
| DNMT3A |
| – |
|
| C-kitD816 |
| – |
|
| PHF6 |
| – |
|
| TET2 |
| (c.86C>G;
p.P29R) |
|
| ASXL1 |
| (c.1954G>A;
p.G652S), (c.3759T>C; p.S1253S) |
The patient received pretreatment with an
intravenous drip of 10 mg/day dexamethasone (Cisen Pharmaceutical
Co., Ltd., Shandong, China). Following treatment for 7 days,
stomachache and fever were attenuated and the patient's lymphocyte
count decreased to <1.0×109/l. The patient
subsequently received induction chemotherapy with vincristine
(Hangzhou Minsheng Pharmaceutical Group Co., Ltd., Hangzhou,
China), idarubicin (Pfizer Inc., New York, NY, USA),
cyclophosphamide (Baxter Oncology GmbH, Shanghai, China) and
prednisone (Cisen Pharmaceutical Co., Ltd.) (VICP; 2 mg vincristine
on days 1, 8, 15 and 22; 10 mg idarubicinon days 1–3 and days
15–17; 1.2 g cyclophosphamide on days 1 and 15; and 60 mg
prednisone on days 1–28) and dasatinib (100 mg/day, on days 8–14).
On day 15, routine blood examination results were as follows:
Neutrophils, 4.76×109/l; lymphocytes,
1.85×109/l, Hb 65 g/l and Plat 74×109/l
(reference range, neutrophils,
1.8×109/l-6.3×109/l; lymphocytes,
1.10×109/l-3.2×109/l, Hb 130–175 g/l and Plat
125×109/l-350×109/l). BM aspiration revealed
<5% lymphoblasts, indicating that the patient had achieved a
complete hematological remission with incomplete blood count
recovery. On day 30, the patient had achieved complete
hematological remission (CR), which was defined as <5% marrow
blasts in the bone marrow aspirate and normalization of blood
counts. The patient then received consolidation chemotherapy [1.2 g
cyclophosphamide on day 1; 3 g cytarabine (Pfizer Inc.) every 12 h
on days 1–2; and 80 mg 6-mercaptopurine (Hangzhou Minsheng
Pharmaceutical Group Co., Ltd.) on days 1–7] and adequate central
nervous system prophylaxis with intrathecal therapy [10 mg
methotrexate (Pfizer Inc.), 50 mg cytarabine and 10 mg
dexamethasone] and high-dose systemic chemotherapy with 5
g/m2 methotrexate. The final date of follow-up was
January 11 2016, at which point the patient was alive and
healthy.
In vitro analysis of selinexor and
dasatinib treatment
Upon written informed consent, in vitro
analysis was conducted on cells from the patient. A total of
2×106 primary lymphoblast cells were obtained from BM
samples using Ficoll density gradient centrifugation (FicollPaque
PLUS solution; GE Healthcare Life Sciences, Little Chalfont, UK).
The protocol was as follows: 4 ml of Ficoll Histopaque was placed
in a 15 ml centrifuge tube, then the bone marrow sample was gently
layered on top. The tubes were centrifuged for 30 min at 100 × g at
4°C in a swing-out bucket. The buffy coat that formed in the
interphase between histopaque and medium was aspirated and washed
(centrifuged at 100 × g for 10 min) twice with 10 ml of sterile
PBS. Primary lymphoblast cells were maintained in RPMI 1640 medium
(Thermo Fisher Scientific, Inc.) and 15% fetal calf serum (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA), and incubated in an
atmosphere containing 5% CO2 at 37°C. The effect of
selinexor and dasatinib on apoptosis was evaluated in primary
lymphoblast cells. The primary cells were treated with 10 µM
selinexor (14) and/or 10 µM
dasatinib (15) for 48 h at 37°C.
Apoptosis was subsequently analyzed using the Annexin V/propidium
iodide staining (BD PharMingen, San Diego, CA, USA) and flow
cytometry was performed according to the manufacturer's protocol.
Poly ADP-ribose polymerase (PARP) cleavage was detected using
western blot analysis (16).
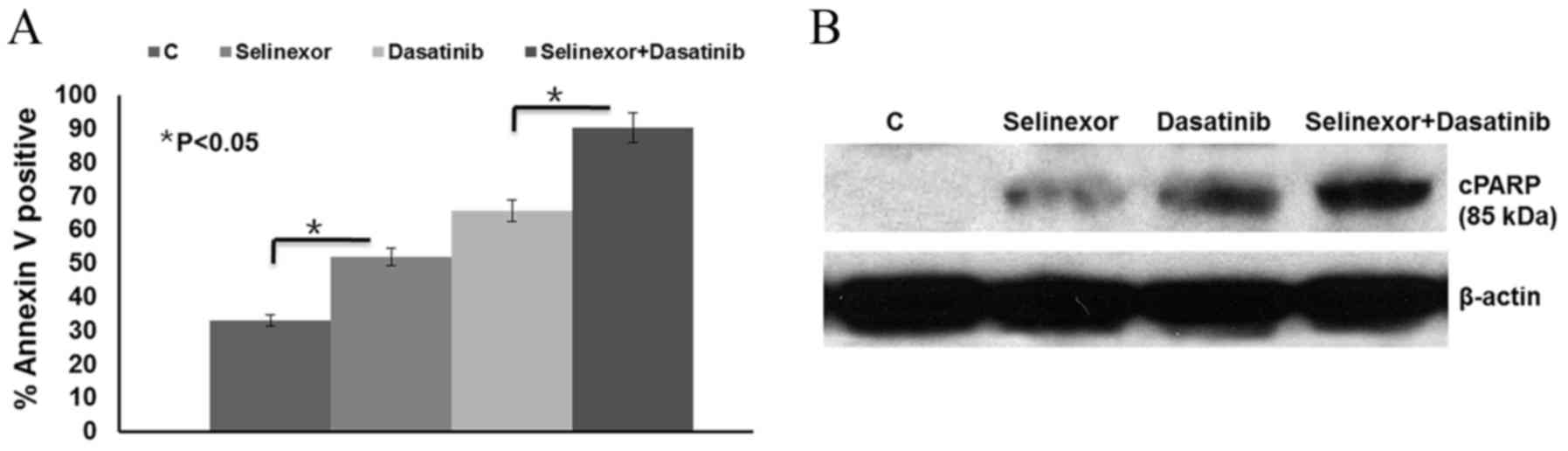
The results demonstrated that the apoptotic cell
population significantly increased following selinexor or dasatinib
treatment compared with the control (P<0.05) and combined
selinexor and dasatinib treatment led to a significant increase in
cell apoptosis compared with either treatment alone (P<0.05;
Fig. 3A). These results were further
confirmed by the detection of increased PARP cleavage by western
blot analysis following selinexor or dasatinib treatment, compared
with the control (Fig. 3B). Combined
selinexor and dasatinib treatment induced more PARP cleavage than
either treatment alone.
Discussion
Approximately 8% of T-ALL cases harbor fusions
involving the ABL1 tyrosine kinase gene (17). BCR-ABL1, the prototypic ABL1 fusion
kinase in chronic myeloid leukemia and subsets of B-cell ALL, is
rarely detected in T-ALL (18). By
contrast, 6% of T-ALL cases express the constitutively active
NUP214-ABL1 fusion kinase (5). To
the best of our knowledge, there have been no studies reporting
NUP214-ABL1 fusion gene expression in ETP-ALL to date. The
current case report describes a rare case of ETP-ALL with
NUP214-ABL1 fusion gene expression. ETP-ALL is recognized as
a form of T-ALL with high induction failure (1). However, CR was achieved in the current
case following 30 days induction therapy with VICP and dasatinib.
Further studies are required to confirm that dasatinib used in
combination with traditional induction chemotherapy is more
effective than chemotherapy alone.
The NUP214-ABL1 fusion protein is sensitive to
tyrosine kinase inhibitors including imatinib, dasatinib and
nilotinib (17); therefore, these
drugs may have potential in treating this subgroup of patients with
T-ALL. Although NUP214-ABL1 is sensitive to ABL1 kinase inhibitors,
the development of resistance to these compounds is a major
clinical problem, underlining the requirement for additional drug
targets in the sparsely studied NUP214-ABL1 signaling network. De
Keersmaecker et al (6)
identified LCK, mitotic arrest deficient-like 1, structural
maintenance of chromosomes protein 4 and nucleoporin 155 as
proteins that NUP214-ABL1-positive T-ALL tumor cells depend on for
their proliferation, indicating that these proteins are potential
drug targets for NUP214-ABL1-positive T-ALL. Targeting LCK in
NUP214-ABL1 may be possible to treat patients with T-ALL that are
NUP214-ABL1-positive, as dasatinib and bosutinib are able to
co-target ABL1 and LCK (6). The
effect of dasatinib on the apoptosis of primary isolated
lymphoblast cells from the patient in the present case study was
evaluated. Following treatment for 48 h, apoptosis was induced,
indicating that dasatinib induces a cytotoxic effect in
NUP214-ABL1-positive cells.
Furthermore, De Keersmaecker et al (8) reported that incorporating NUP214-ABL1
into the NPC may be critical for the activation of NUP214-ABL1
kinase. NUP214 is one component of NPC proteins. Evolutionarily
conserved NPC proteins mediate the transport of molecules between
the nucleoplasm and cytoplasm, which are CRM1 dependent. Mutations
in nucleoporins are often linked to specific developmental defects
and disease, and the resulting phenotypes are usually interpreted
as the consequences of perturbed nuclear transport activity
(8). The present study indicated
that CRM1 inhibition with selinexor was effective against a case of
NUP214-ABL1-positive T-ALL in vitro. Therefore, selinexor
may have a potential function as a salvage therapy at the time of
relapse.
In conclusion, expression of the NUP214-ABL1
fusion gene should be tested in cases of T-ALL, including ETP-ALL.
Dasatinib in combination with traditional induction chemotherapy
may reverse the high induction failure of ETP-ALL with
NUP214-ABL1 fusion gene, although further prospective
studies are required to confirm this. Therefore, selinexor with or
without dasatinib may serve as a potential salvage therapy
following relapse.
References
|
1
|
Jain N, Lamb AV, O'Brien S, Ravandi F,
Konopleva M, Jabbour E, Zuo Z, Jorgensen J, Lin P, Pierce S, et al:
Early T-cell precursor acute lymphoblastic leukemia/lymphoma
(ETP-ALL/LBL) in adolescents and adults: A high-risk subtype.
Blood. 127:1863–1869. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coustan-Smith E, Mullighan CG, Onciu M,
Behm FG, Raimondi SC, Pei D, Cheng C, Su X, Rubnitz JE, Basso G, et
al: Early T-cell precursor leukaemia: A subtype of very high-risk
acute lymphoblastic leukaemia. Lancet Oncol. 10:147–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Keersmaecker K, Marynen P and Cools J:
Genetic insights in the pathogenesis of T cell acute lymphoblastic
leukemia. Haematologica. 90:1116–1127. 2005.PubMed/NCBI
|
|
4
|
Graux C, Cools J, Melotte C, Quentmeier H,
Ferrando A, Levine R, Vermeesch JR, Stul M, Dutta B, Boeckx N, et
al: Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute
lymphoblastic leukemia. Nat Genet. 36:1084–1089. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pante N, Bastos R, McMorrow I, Burke B and
Aebi U: Interactions and three-dimensional localization of a group
of nuclear pore complex proteins. J Cell Biol. 126:603–617. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Keersmaecker K, Porcu M, Cox L, Girardi
T, Vandepoel R, de Beeck JO, Gielen O, Mentens N, Bennett KL and
Hantschel O: NUP214-ABL1-mediated cell proliferation in T-cell
acute lymphoblastic leukemia is dependent on the LCK kinase and
various interacting proteins. Haematologica. 99:85–93. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutten S and Kehlenbach RH: Nup214 is
required for CRM1-dependent nuclear protein export in vivo. Mol
Cell Biol. 26:6772–6785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Keersmaecker K, Rocnik JL, Bernad R,
Lee BH, Leeman D, Gielen O, Verachtert H, Folens C, Munck S,
Marynen P, et al: Kinase activation and transformation by
NUP214-ABL1 is dependent on the context of the nuclear pore. Mol
Cell. 31:134–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Ding L, Holmfeldt L, Wu G,
Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, et
al: The genetic basis of early T-cell precursor acute lymphoblastic
leukaemia. Nature. 481:157–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leinonen EA: Cytochemical studies of acute
leukemias. Acta Haematol. 43:219–227. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamul KR, Meyers DC, Bentley SA and Folds
JD: Two color flow cytometric analysis of concomitant acute myeloid
leukemia and chronic lymphocytic leukemia. Cytometry. 18:30–34.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Summer AT, Evans HJ and Buckland RA: New
technique for distinguishing human chromosomes. Nature. 232:31–32.
1971.
|
|
13
|
Pallisgaard N, Hokland P, Riishøj DC,
Pedersen B and Jørgensen P: Multiplex reverse
transcription-polymerase chain reaction for simultaneous screening
of 29 translocations and chromosomal aberrations in acute leukemia.
Blood. 92:574–588. 2014.
|
|
14
|
Etchin J, Sanda T, Mansour MR, Kentsis A,
Montero J, Le BT, Christie AL, McCauley D, Rodig SJ, Kauffman M, et
al: KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has
selective anti-leukaemic activity in preclinical models of T-cell
acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J
Haematol. 161:117–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quintás-Cardama A, Tong W, Manshouri T,
Vega F, Lennon PA, Cools J, Gilliland DG, Lee F, Cortes J,
Kantarjian H, et al: Activity of tyrosine kinase inhibitors against
human NUP214-ABL1-positive T cell malignancies. Leukemia.
22:1117–1124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YL, Guang MH, Zhuo HQ, Min XH, Yao
Q, Gu AQ, Wu SH, Zhang DB, Lu JY, Chen Y, et al: Carfilzomib
inhibits constitutive NF-κB activation in mantle cell lymphoma B
cells and leads to the induction of apoptosis. Acta Haematol.
137:106–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Keersmaecker K, Marynen P and Cools J:
Genetic insights in the pathogenesis of T-cell acute lymphoblastic
leukemia. Haematologica. 90:1116–1127. 2005.PubMed/NCBI
|
|
18
|
Westbrook CA, Hooberman AL, Spino C, Dodge
RK, Larson RA, Davey F, Wurster-Hill DH, Sobol RE, Schiffer C and
Bloomfield CD: Clinical significance of the BCR-ABL fusion gene in
adult acute lymphoblastic leukemia: A cancer and leukemia group B
study (8762). Blood. 80:2983–2990. 1992.PubMed/NCBI
|