Introduction
As one of the major causes of lower back pain,
intervertebral disc degeneration (IDD) remains an important global
problem, severely affecting the quality of life and leading to a
serious socioeconomic burden (1–3).
Multiple risk factors can cause IDD, including genetic
predisposition, lifestyle and aging among others (4,5). The
incidence of IDD is higher in developing countries, particularly in
China. It has been indicated that various cellular events, ranging
from matrix synthesis to cytokine expression, are involved in the
progression of human IDD (6).
Increasing evidence supports the observation that nucleus pulposus
(NP) cells, which produce type II collagen, aggrecan and other
components of the extracellular matrix (ECM), serve a critical role
in maintaining the integrity of intervertebral discs (IVDs)
(7,8). In addition, excessive apoptosis of IVD
cells and of the components of ECM occurs during the IDD progress.
Loss of the proteoglycan (PG) content of IVDs is one of the main
features of IDD; thus, as a strong promoter of inflammation,
lipopolysaccharide (LPS) can reduce the PG content and cause the
occurrence of IDD (9,10). Furthermore, proinflammatory factors,
including interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α
(TNF-α), also serve important roles in IDD. However, the underlying
molecular and cellular mechanisms of IDD remain largely
unknown.
MicroRNAs (miRNAs or miRs) are a class of small
non-coding RNA molecules with a length of 20–22 nucleotides that
serve key roles in post-transcriptional regulation of gene
expression (11,12). miRNAs have a central role in the
development of cancer, as well as in inflammatory,
neurodegenerative and the majority of degenerative disorders
(13,14). Recently, multiple studies have
demonstrated that miRNAs serve an important role in degenerative
disc diseases, such as IDD (15–19). As
a multifunctional miRNA, miR-27a is expressed in various tissues,
and abnormal expression of miR-27a has been detected in various
diseases (20–22). Previously, miR-27a has been reported
to be upregulated in human degenerative NP cells when compared with
the control NP cells (19); however,
the underlying mechanisms remain unknown.
Thus, the present study aimed to verify the
expression and to investigate the role of miR-27a in IDD, as well
as to examine the underlying mechanisms involved.
Materials and methods
Materials
ELISA kits for the detection of IL-1β (Cat no.
E-EL-H0149c), IL-6 (Cat no. E-EL-H0102c) and TNF-α (Cat no.
E-EL-H0109c) protein levels were obtained from Elabscience
Biotechnology Co., Ltd (Wuhan, China). Antibodies against p-p38
(Cat no. 1170), NF-κB (Cat no. 8214) and β-actin (Cat no. 12620),
as well as the secondary antibodies, were supplied by Cell
Signaling Technology, Inc. (Danvers, MA, USA). LPS was purchased
from Sigma-Aldrich (Merck, Darmstadt, Germany). The miR-27a
inhibitor (lentiviral antigomiR-27a) and the cell transfection kit
(Cat no. sc-36868) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). All other chemicals and reagents were
purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,
China).
Specimens
The study was approved by the Human Ethics Committee
Review Board at the First Affiliated Hospital of Soochow University
(Suzhou, China), and written informed consent was obtained from
each patient. In total, 20 patients with degenerative disc disease,
presenting with lumbar intervertebral disc herniation (LIDH), a
medical condition that is representative of IDD (IDD group) and 20
spinal cord injury patients (control group) were enrolled into the
present study. The inclusion and exclusion criteria of the patients
were as previously described by Zhao et al (23). The degenerative condition of IDD and
IVDs (control patients who were suffering from a lower grade of
IDD) was assessed by magnetic resonance imaging (MRI) according to
the Pfirrmann's grading system (24). Samples graded as 4 from patients were
identified as the IDD group, samples graded 1 from cadaveric donors
were classified as the control group. The NP tissues were carefully
dissected during the disc excision surgery following a protocol
approved by the Institutional Review Board at the First Affiliated
Hospital of Soochow University, and then subjected to various
methods of analysis, according to the corresponding procedures.
Briefly, NP tissues were separated from the annulus using a
stereotaxic microscope; herniation tissues and granulation tissues
were excluded. Subsequently, as described previously (23), the whole tissue specimens were washed
with phosphate-buffered saline (pH 7.2) and then divided into two
sections of equal size. One of the sections was fixed with 4%
paraformaldehyde at 4°C for 30 min and used in subsequent studies,
while the remaining section was snap-frozen and stored in liquid
nitrogen within 30 min of removal from the patients, with
subsequent storage at −80°C, and used in RT-qPCR analysis.
Cell culture
Primary human NP cells (Cat no. #4800) were
purchased from the ScienCell Research Laboratories, Inc. (Carlsbad,
CA, USA). Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 1% L-glutamine (Gibco; Thermo Fisher Scientific,
Inc.), 100 mg/ml streptomycin and 100 U/ml penicillin were added,
and cells were incubated in a 5% CO2 incubator at 37°C.
Cell culture medium was changed every 2 days and the cells were
passaged until they reached 90% confluence.
Cell transfection and LPS
treatment
Human NP cells were plated in a 6-well plate
(5×104 cells/well) the day prior to transfection. NP
cells were transiently transfected with an miR-27a inhibitor
(lentiviral antigomiR-27a; GenScript, Piscataway, NJ, USA) or its
negative control (lentiviral vector control; GenScript) with 30 µl
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. At 24
h after the transfection, cells were stimulated with LPS (10 ng/ml)
in serum-free medium for 24 h at 37°C under 5% CO2.
Next, the supernatants were collected by centrifugation (1,000 × g
at 4°C for 10 min) 24 h after the initiation of treatment for each
group. The treatment for each groups is as follows: Con group,
cells without any treatment; LPS group, cells treated with LPS;
NC/LPS group, cells transfected with negative control and then
treated with LPS; 27a/LPS group, cells transfected with miR-27a
inhibitor and then treated with LPS. Then cells were harvested for
use in subsequent experiments. The NP cells in the control group
did not undergo any treatment.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from NP tissues or NP cells was isolated
using TRIzol reagent (Takara Bio, Inc., Shiga, Japan) and a mirVana
PARIS kit (Ambion; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. The RNA concentration was then
quantified using a Nanodrop spectrophotometer (Thermo Fisher
Scientific, Inc.) at 260 nm. Next, qPCR was performed for miR-27a
expression detection. Briefly, total RNA was reverse transcribed
into cDNA using the TaqMan microRNA Reverse Transcription kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
instructions provided by the manufacturer. Subsequently, qPCR was
performed to analyze the synthesized cDNA. The 20 µl master mix
contained 2 µl 10× reverse transcription buffer, 1 µl dNTPs (100
mM; with dTTP), 3.75 µl nuclease-free water, 0.25 µl 2 µM forward
and reverse primer, 3.5 µl distilled H2O, and 5 µl 1
ng/µl cDNA. The conditions used for amplification were as follows:
40 cycles of denaturation at 95°C for 10 sec, followed by 60°C for
60 sec to allow annealing and extension. The primers used are
listed in Table I. The levels of
miR-27a were normalized to the level of the GAPDH, which served as
an internal control, by using the 2−ΔΔCq method
(25). The experiment was performed
three times in triplicate.
 | Table I.Primer sequences for polymerase chain
reaction. |
Table I.
Primer sequences for polymerase chain
reaction.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| IL-1β |
CTGTGACTCGTGGGATGATG |
AGGGATTTTGTCGTTGCTTG |
| IL-6 |
GTGCTCCTGGTATTGCTGGT |
GGCTCCTCGTTTTCCTTCTT |
| TNF-α |
CCTGTCTCTTCCTACCCAACC |
GCAGGAGTGTCCGTGTCTTC |
| miR-27a |
ACAGGCTAGCGCCGCCTAAC |
CCTTAAGGCCCAAGATTACG |
| NF-kB |
ACACCTCTGCATATAGCGGC |
GGTACCCCCAGAGACCTCAT |
| GAPDH |
CTTTGGTATCGTGGAAGGACTC |
GTAGAGGCAGGGATGATGTTCT |
Western blot analysis
Western blot analysis was performed using the
transfected cell samples using standard methods. Total cellular
protein was extracted using radio immunoprecipitation assay buffer
(RIPA buffer, Cell Signaling Technology, Danvers, MA, USA).
Briefly, cells were washed with cold PBS 3 times for 5 min, then
200 µl RIPA buffer was added and incubated for 40 min (on ice), the
supernatants were collected by centrifugation (1,000 × g at 4°C for
15 min). A BCA protein assay kit (Thermo Fisher Scientific, Inc.)
was used to detect the protein concentration of samples according
to the instructions provided by the manufacturer. Next, proteins
(25 µg each sample) were resolved by 10% SDS-PAGE and then
transferred to a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). Subsequent to blocking in 5% nonfat dried milk
in Tris-buffered saline-Tween 20 for 2 h, the blots were incubated
overnight at 4°C with a primary antibody against p-p38 (1:1,000),
NF-κB (1:1,000) or β-actin (1:2,000), and then incubated with a
HRP-conjugated secondary antibody (Anti-rabbit IgG; 1:5,000; Cat
no. 7074; Cell signaling Technology, Inc., Danvers, MA, USA) at
room temperature for 1 h. Protein bands were observed using
enhanced chemiluminescence using Super Signal West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.), and
the ImageTool version 3.0 gray-scale scanning software (Microsoft
Corporation, Redmond, WA, USA) was applied to quantify the band
density. The mean normalized optical density (OD) of the p38
phosphorylation band relative to the OD of the β-actin band from
the same sample was calculated using ImageTool version 3.0
gray-scale scanning software. The expression levels of p38
phosphorylation were expressed as fold changes compared with the
control group.
ELISA for IL-1β, IL-6 and TNF-α level
determination
Cells were stimulated with LPS (10 ng/ml) in
serum-free medium 24 h after transfection and incubated for a
further 24 h at 37°C under 5% CO2. Then, the
supernatants were collected by centrifugation (1,000 × g at 4°C for
10 min). To investigate the expression levels of proinflammatory
factors IL-1β, IL-6 and TNF-α in the cellular supernatant of the
transfected cells following 24 h of treatment, ELISA was performed,
following the manufacturer's instructions of each kit. Each
experiment was independently performed three times.
Statistical analysis
Data are displayed as the mean ± standard deviation.
Statistical comparisons between two groups were analyzed with the
Student's t-test and between multiple groups with one-way analysis
of variance. A difference with a value of P<0.05 was considered
as statistically significant.
Results
Basic patient information
A total of 20 patients with IDD were selected as the
IDD group, including 12 males and 8 females, with an age range of
39–69 years and a mean age of 54.9±7.5 years. The degenerative IVDs
of these patients were the L3-L5 segments, and according to the
magnetic resonance imaging (MRI) results, all patients with IDD
were classified as grade IV. In addition, 20 patients with spinal
cord injury were selected as the control group, including 13 males
and 7 females, with an age of 25–56 years and an average age of
42.2±8.9 years. The degenerative IVDs of all the control patients
(who were suffering from a lower grade of IDD) were the L2-L5
segment, and were classified as grade I based on MRI examination
(Table II).
 | Table II.Basic patient information (n=20 in
each group). |
Table II.
Basic patient information (n=20 in
each group).
| Parameter | IDD patients | Spinal cord injury
(control) |
|---|
| Mean age (years) | 54.9±7.5 | 42.2±8.9 |
| Gender | 12 M, 8 F | 13 M, 7 F |
| Segment | L3-L5 | L2-L5 |
| MRI grade | IV | I |
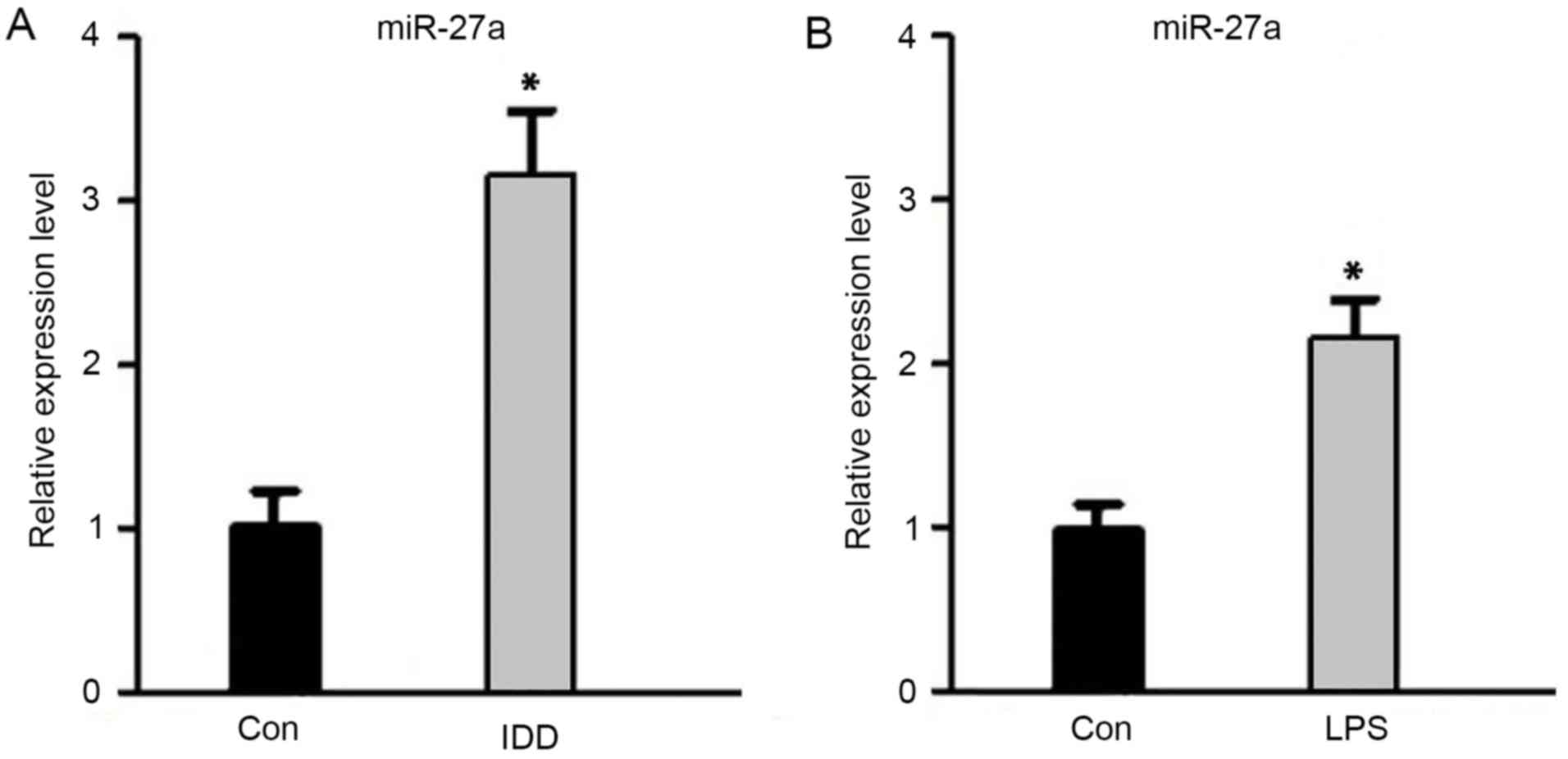
Upregulation of miR-27a expression in
IDD
To verify the expression of miR-27a in IDD, RT-qPCR
was performed. As shown in Fig. 1,
the miR-27a expression level was significantly higher in IDD
patients when compared with the control subjects (P<0.05).
Simultaneously, the miR-27a expression level in NP cells after
stimulation with LPS was evidently increased compared with the
untreated control cells (P<0.05). These data indicated that
miR-27a was upregulated in IDD patients, as well as in
LPS-stimulated NP cells, suggesting that miR-27a may be involved in
the development of IDD.
Downregulation of miR-27a decreases
proinflammatory cytokine levels in LPS-stimulated NP cells
In order to investigate the role of miR-27a in IDD,
a stable NP cell line exhibiting reduced miR-27a expression was
generated by transfection of NP cells with an miR-27a inhibitor,
while negative control oligonucleotides was used as the negative
control group. Subsequently, the cells were stimulated with LPS. At
24 h after the stimulation, miR-27a expression was analyzed by
RT-qPCR, while the mRNA and protein levels of proinflammatory
cytokines were detected by RT-qPCR and ELISA, respectively. The
results suggested that, following transfection with the miR-27a
inhibitor, miR-27a expression was efficiently downregulated
(Fig. 2A; P<0.05). Compared with
the LPS-stimulated only group, the downregulation of miR-27a
resulted in a significant decrease in the mRNA (Fig. 2B-D) and protein (Fig. 3) expression levels of proinflammatory
cytokines IL-1β, IL-6 and TNF-α (P<0.05). By contrast, negative
control oligonucleotides presented no significant effect on the
production of IL-1β, TNF-α and IL-6 (Figs. 2 and 3). The data indicated that downregulation
of miR-27a significantly decreased the proinflammatory cytokine
expression in LPS-stimulated NP cells.
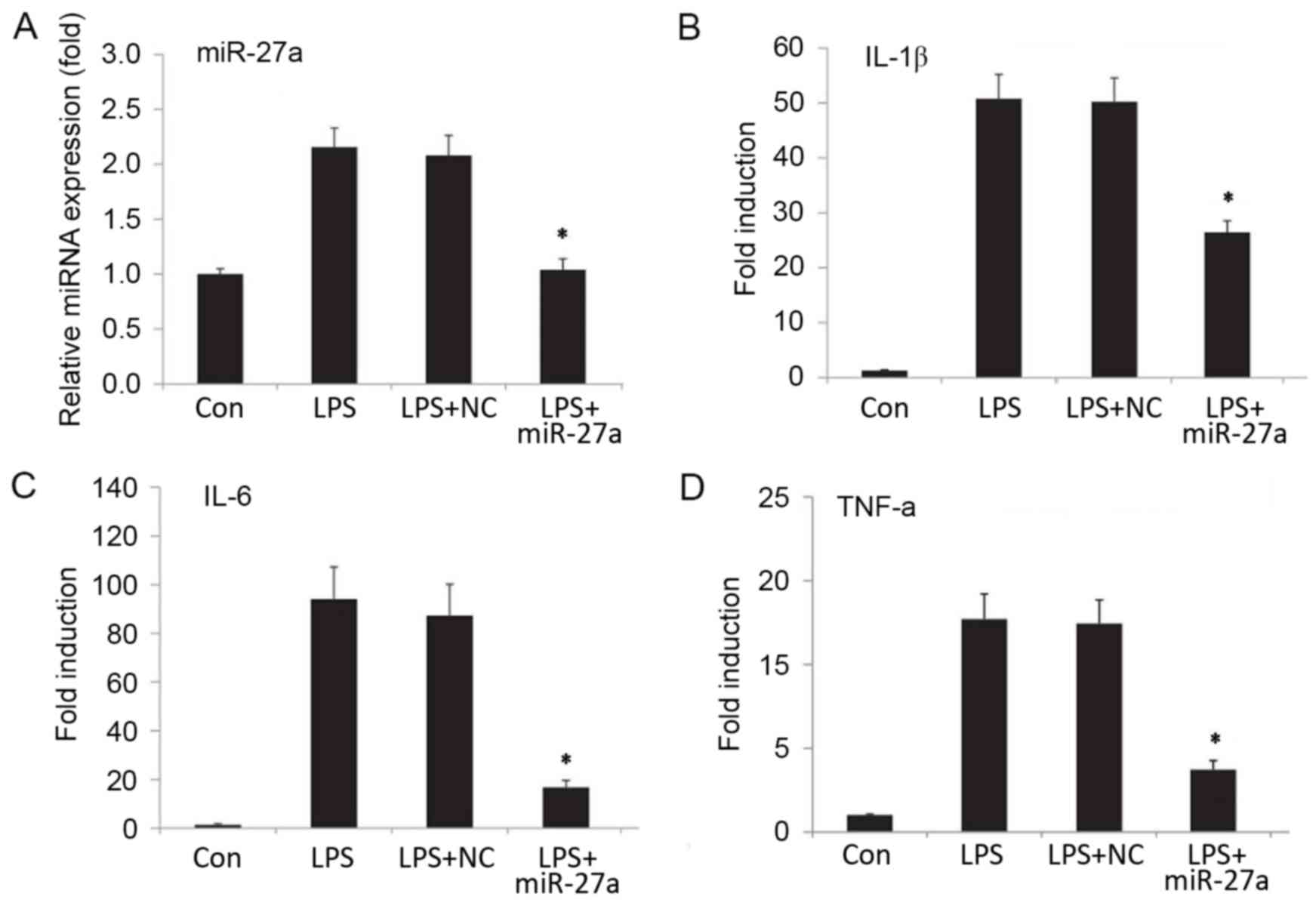 | Figure 2.(A) Relative miR-27a expression, and
mRNA expression levels of the proinflammatory factors (B) IL-1β,
(C) IL-6 and (D) TNF-α. Human nucleus pulposus cells were
transfected with miR-27a mimic or NC (sham transfection without
miR-27a, serving as the control group). Next, cells were treated
with or without LPS (10 ng/ml) for 24 h, and expression levels were
detected by reverse transcription-quantitative polymerase chain
reaction. The relative expression of miRNA/mRNA was normalized to
GAPDH. *P<0.05 vs. Con group. All results presented as the mean
± standard deviation of three independent experiments. miR,
microRNA; LPS, lipopolysaccharide; Con, control; NC, negative
control vector; IL, interleukin; TNF, tumor necrosis factor; Con,
control group, cells without any treatment; LPS group, cells
treated with LPS; NC+LPS, cells transfected with negative control
and then treated with LPS; LPS+miR-27a, cells transfected with
miR-27a inhibitor and then treated with LPS. |
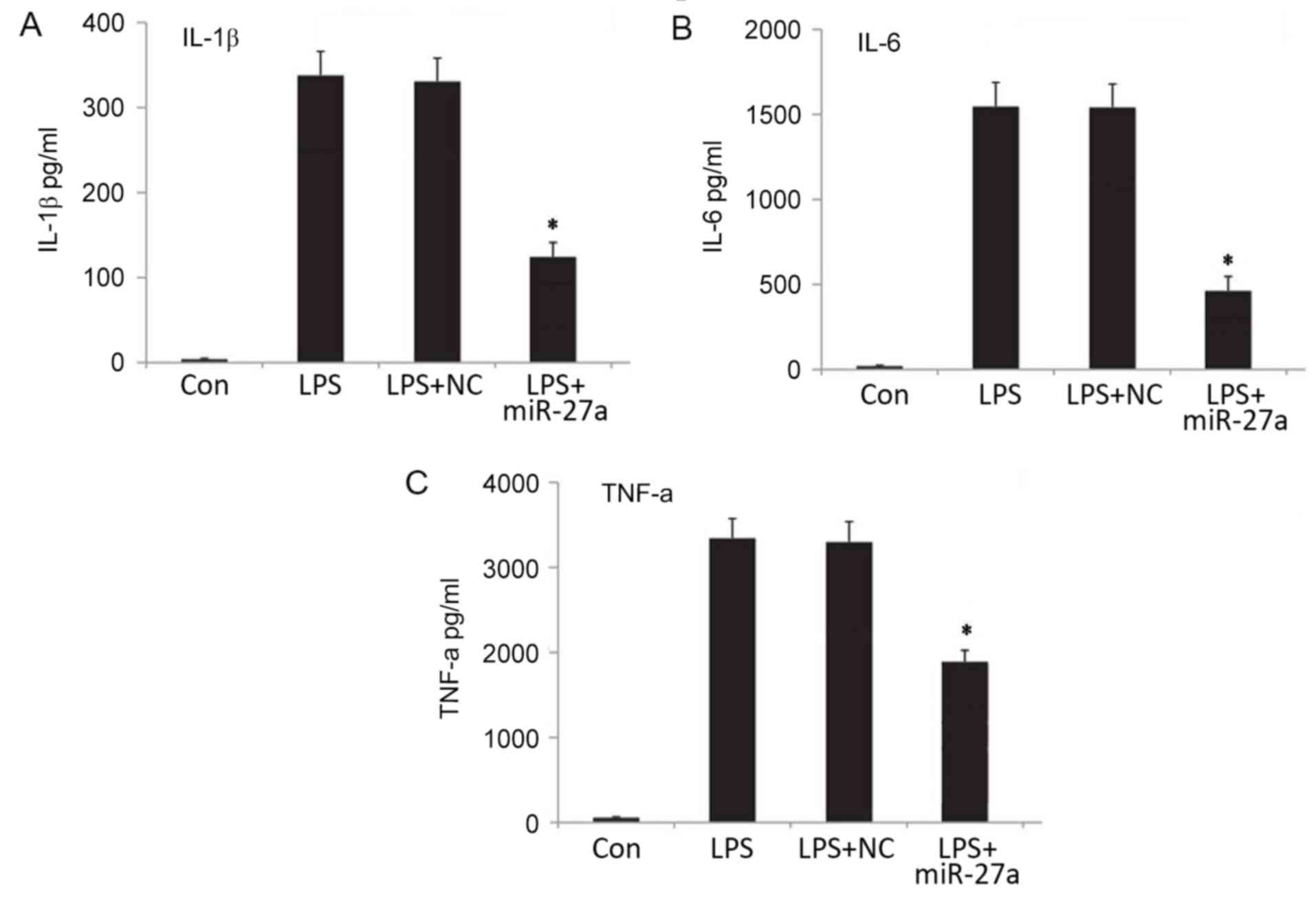 | Figure 3.Expression levels of proinflammatory
factors (A) IL-1β, (B) IL-6 and (C) TNF-α in the cellular
supernatant, as determined by ELISA. *P<0.05 vs. Con group. All
results presented as the mean ± standard deviation of three
independent experiments. miR, microRNA; LPS, lipopolysaccharide;
Con, control; NC, negative control vector; IL, interleukin; TNF,
tumor necrosis factor; Con, control group, cells without any
treatment; LPS group, cells treated with LPS; NC+LPS, cells
transfected with negative control and then treated with LPS;
LPS+miR-27a, cells transfected with miR-27a inhibitor and then
treated with LPS. |
Downregulation of miR-27a suppresses
the activation of p38/mitogen activated protein kinases (MAPK) in
LPS-stimulated NP cells
The present study further investigated whether
miR-27a was able to regulate inflammation via the MAPK signaling
pathway using western blot analysis. Following stimulation with LPS
for 24 h, p38 phosphorylation and NF-κB protein expression were
detected using western blot analysis (Fig. 4A). As presented in Fig. 4B and C, downregulation of the miR-27a
expression in LPS-stimulated NP cells significantly reduced the
protein expression levels of p-p38 and NF-κB when compared with the
LPS-simulated NP cells without miR-27a inhibitor (P<0.05).
However, negative control oligonucleotides exhibited no significant
effect on the expression levels of p-p38 and NF-κB in
LPS-stimulated NP cells. In addition, RT-qPCR was further performed
to measure the mRNA expression level of NF-κB, which is presented
in Fig. 4D (P<0.05). Changes in
the NF-κB mRNA level were consistent with those in the protein
levels. These results suggested that downregulation of miR-27a was
able to suppress the activation of the p38/MAPK in LPS-stimulated
NP cells. All these data suggest that miR-27a may function as a
promoter in IDD development, and that inhibition of miR-27a may
ameliorate inflammation via the p38/MAPK-signaling pathway in IVD
cells.
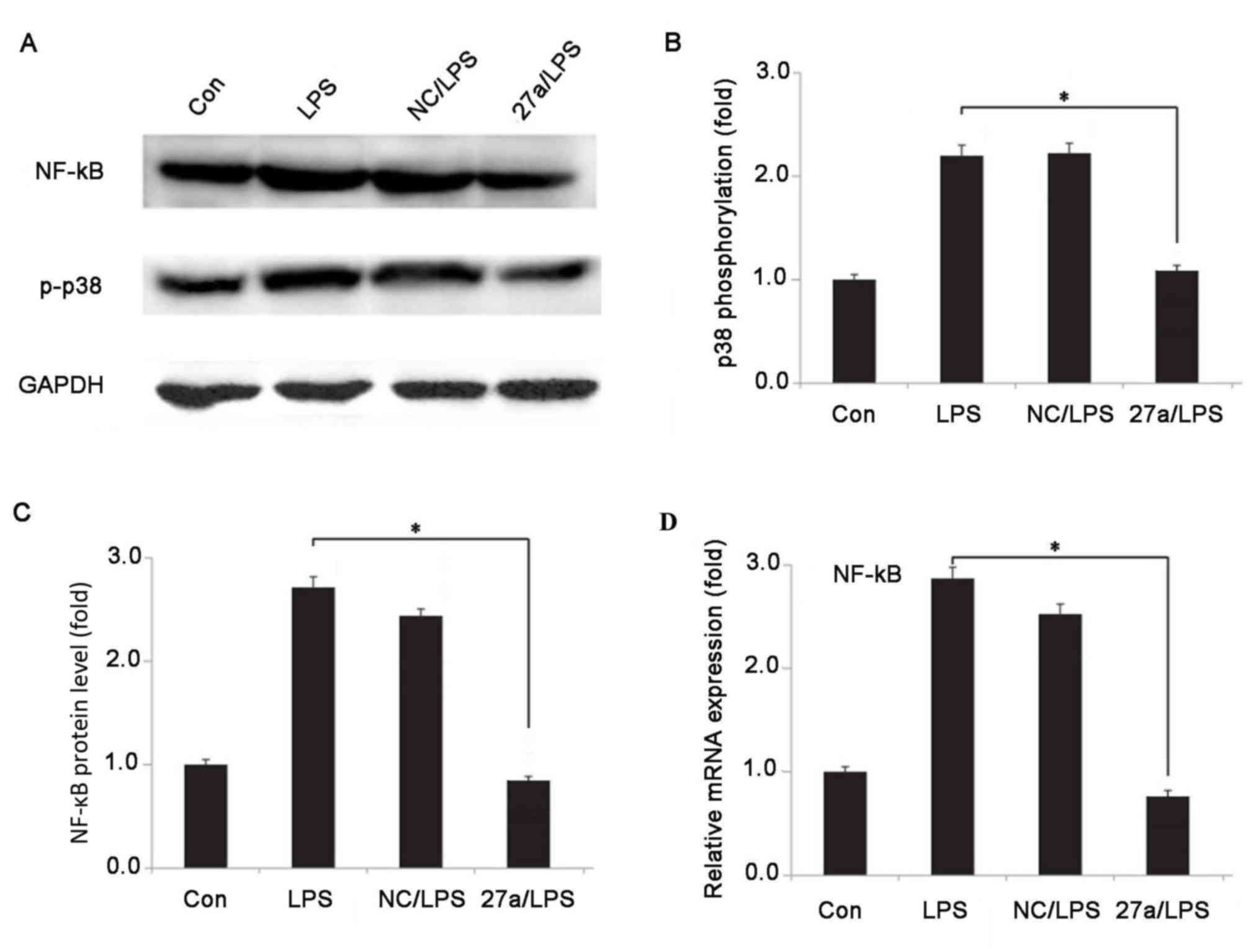 | Figure 4.Effect of miR-27a on p38/MAPK pathway
in LPS-stimulated human nucleus pulposus cells. (A) Western blots
of NF-κB and p-p38 protein expression levels. (B) p38
phosphorylation detected using western blot analysis. (C)
Quantified NF-κB protein level. (D) Relative mRNA expression of
NF-κB, measured using reverse transcription-quantitative polymerase
chain reaction. GAPDH was used as an internal control. *P<0.05
vs. Con group. All results presented as the mean ± standard
deviation of three independent experiments. miR, microRNA; LPS,
lipopolysaccharide; Con, control; NC, negative control vector; NF,
nuclear factor; MAPK, mitogen-activated protein kinase; Con,
control group, cells without any treatment; LPS group, cells
treated with LPS; NC+LPS, cells transfected with negative control
and then treated with LPS; LPS+miR-27a, cells transfected with
miR-27a inhibitor and then treated with LPS. |
Discussion
Increasing evidence has indicated that miRNAs serve
critical roles in a number of normal biological and pathological
processes, including embryogenesis, lineage determination, as well
as in the regulation of cell differentiation, proliferation and
apoptosis (26). However, knowledge
of the aberrant expression and roles of miRNAs in IDD remain
largely uncharacterized. Therefore, identification of
IDD-associated miRNAs and exploration of their roles in IDD may be
important for developing novel targets for IDD therapy. In the
present study, the role of miR-27a in IDD, as well as the
pathological links between miR-27a, IDD and inflammatory pathways
associated with IDD, were investigated.
Studies have been suggested that an abnormal
expression of miR-27a was associated with various diseases, and
that the expression of miR-27a was upregulated in degenerative NP
cells (19,27–29).
However, the role of miR-27a in IDD remains to be investigated. In
present study, the expression level of miR-27a in IDD was first
verified using RT-qPCR, and the results confirmed that upregulation
of miR-27a was observed in IDD.
Inflammation serves a critical role in disc
degeneration. Endogenous factors, including crystal deposits in the
annulus of human intervertebral discs and ECM breakdown products,
can trigger the IVD inflammatory response (30,31).
Various proinflammatory cytokines, including IL-1β, IL-6, IL-12 and
TNF-α, were significantly increased due to immunoreactivity in
herniated and generative IVD tissues (32,33). To
investigate the role of miR-27a in IDD, a stable miR-27a-knockdown
NP cell line was generated by transfection with an miR-27a
inhibitor, and an IDD cell model was established by LPS stimulation
of cells. RT-qPCR and ELISA assay were then performed for the
detection of proinflammatory cytokine expression levels in the
cells. The results demonstrated that downregulation of miR-27a
significantly reduced the levels of proinflammatory cytokines,
including IL-1β, TNF-α and IL-6. This indicated that inhibition of
miR-27a was able to suppress inflammatory response in IVD
cells.
MAPK signaling pathways are responsible for
regulating a variety of cellular activities, including
proliferation, differentiation, and apoptosis, in response to
certain environmental stimuli. Three major MAPK pathways have been
identified in mammals: MAPK/ERK, SAPK/JNK and p38 MAPK. p38 MAPK is
activated by external stress, inflammatorycytokines or UV
radiation. A previous study reported that the p38 MAPK pathway is
involved in development of IDD (34). Therefore, the present study aimed to
further investigate the underlying mechanism of the regulation of
cell immunoreactivity by miR-27a and the p38/MAPK pathway was also
assessed in the current study. As a result of miR-27a
downregulation, the p38 phosphorylation was notably decreased.
Furthermore, the mRNA and protein expression levels of NF-κB were
significantly reduced in the LPS-stimulated NP cells. These results
suggested that downregulation of miR-27a was able to suppress the
activation of the p38/MAPK pathway in LPS-stimulated NP cells.
In conclusion, the present study revealed that
miR-27a may function as a promoter in the development of IDD.
Inhibition of miR-27a may suppress inflammatory factors released by
IVD cells by regulating the MAPK signaling pathway. To the best of
our knowledge, this is the first study addressing the underlying
mechanisms of miR-27a in IDD, and these findings also highlight
miR-27a as a novel potential therapeutic target for IDD.
References
|
1
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gore M, Sadosky A, Stacey BR, Tai KS and
Leslie D: The burden of chronic low back pain: Clinical
comorbidities, treatment patterns, and health care costs in usual
care settings. Spine (Phila Pa 1976). 37:E668–E677. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Millecamps M, Tajerian M, Naso L, Sage EH
and Stone LS: Lumbar intervertebral disc degeneration associated
with axial and radiating low back pain in ageing SPARC-null mice.
Pain. 153:1167–1179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine (Phila
Pa 1976). 31:2151–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayer JE, Iatridis JC, Chan D, Qureshi SA,
Gottesman O and Hecht AC: Genetic polymorphisms associated with
intervertebral disc degeneration. Spine J. 13:299–317. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Liang J, Wu WK, Yu X, Yu J, Weng X
and Shen J: Leptin activates RhoA/ROCK pathway to induce
cytoskeleton remodeling in nucleus pulposus cells. Int J Mol Sci.
15:1176–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G
and Liu J: The role of leptin on the organization and expression of
cytoskeleton elements in nucleus pulposus cells. J Orthop Res.
31:847–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellman MB, Kim JS, An HS, Chen D, KC R, An
J, Dittakavi T, van Wijnen AJ, Cs-Szabo G, Li X, et al: Toll-like
receptor adaptor signaling molecule MyD88 on intervertebral disk
homeostasis: In vitro, ex vivo studies. Gene. 505:283–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwata M, Ochi H, Asou Y, Haro H, Aikawa T,
Harada Y, Nezu Y, Yogo T, Tagawa M and Hara Y: Variations in gene
and protein expression in canine chondrodystrophic nucleus pulposus
cells following long-term three-dimensional culture. PLoS One.
8:e631202013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee RC, Feinbaum RL and Ambros V: The C.
Elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teague EM, Print CG and Hull ML: The role
of microRNAs in endometriosis and associated reproductive
conditions. Hum Reprod Update. 16:142–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HQ, Yu XD, Liu ZH, Cheng X, Samartzis
D, Jia LT, Wu SX, Huang J, Chen J and Luo ZJ: Deregulated miR-155
promotes Fas-mediated apoptosis in human intervertebral disc
degeneration by targeting FADD and caspase-3. J Pathol.
225:232–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X
and Qiu G: MicroRNA-10b promotes nucleus pulposus cell
proliferation through RhoC-Akt pathway by targeting HOXD10 in
intervetebral disc degeneration. PLoS One. 8:e830802013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohrt-Nissen S, Dөssing KB, Rossing M,
Lajer C, Vikeså J, Nielsen FC, Friis-Hansen L and Dahl B:
Characterization of miRNA expression in human degenerative lumbar
disks. Connect Tissue Res. 54:197–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Huang X, Liu X, Xiao S, Zhang Y,
Xiang T, Shen X, Wang G and Sheng B: miR-21 promotes human nucleus
pulposus cell proliferation through PTEN/AKT signaling. Int J Mol
Sci. 15:4007–4018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu G, Cao P, Chen H, Yuan W, Wang J and
Tang X: MiR-27a regulates apoptosis in nucleus pulposus cells by
targeting PI3K. PLoS One. 8:e752512013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji J, Zhang J, Huang G, Qian J, Wang X and
Mei S: Over-expressed microRNA-27a and 27b influence fat
accumulation and cell proliferation during rat hepatic stellate
cell activation. FEBS Lett. 583:759–766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao B, Yu Q, Li H, Guo X and He X:
Characterization of microRNA expression profiles in patients with
intervertebral disc degeneration. Int J Mol Med. 33:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Lu X and Cao Y: MicroRNA and
signal transduction pathways in tumor radiation response. Cell
Signal. 25:1625–1634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao X, Yang L and Hu J: Down-regulation
of miR-27a might inhibit proliferation and drug resistance of
gastric cancer cells. J Exp Clin Cancer Res. 30:552011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Drayton RM, Dudziec E, Peter S, Bertz S,
Hartmann A, Bryant HE and Catto JW: Reduced expression of miRNA-27a
modulates cisplatin resistance in bladder cancer by targeting the
cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 20:1990–2000.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hezova R, Kovarikova A, Bienertova-Vasku
J, Sachlova M, Redova M, Vasku A, Svoboda M, Radova L, Kiss I,
Vyzula R and Slaby O: Evaluation of SNPs in miR-196-a2, miR-27a and
miR-146a as risk factors of colorectal cancer. World J
Gastroenterol. 18:2827–2831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gruber HE, Norton HJ, Sun Y and Hanley EN
Jr: Crystal deposits in the human intervertebral disc: Implications
for disc degeneration. Spine J. 7:444–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wuertz K, Vo N, Kletsas D and Boos N:
Inflammatory and catabolic signalling in intervertebral discs: The
roles of NF-kB and MAP kinases. Eur Cell Mater. 23:103–120.
2012.PubMed/NCBI
|
|
32
|
Kokubo Y, Uchida K, Kobayashi S, Yayama T,
Sato R, Nakajima H, Takamura T, Mwaka E, Orwotho N, Bangirana A and
Baba H: Herniated and spondylotic intervertebral discs of the human
cervical spine: Histological and immunohistological findings in 500
en bloc surgical samples. Laboratory investigation. J Neurosurg
Spine. 9:285–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shamji MF, Setton LA, Jarvis W, So S, Chen
J, Jing L, Bullock R, Isaacs RE, Brown C and Richardson WJ:
Proinflammatory cytokine expression profile in degenerated and
herniated human intervertebral disc tissues. Arthritis Rheum.
62:1974–1982. 2010.PubMed/NCBI
|
|
34
|
Liu C, Yang H, Gao F, Li X, An Y, Wang J
and Jin A: Resistin promotes intervertebral disc degeneration by
upregulation of ADAMTS-5 through p38MAPK signaling pathway. Spine
(Phila Pa 1976). 41:1414–1420. 2016. View Article : Google Scholar : PubMed/NCBI
|