Introduction
Cardiac arrest (CA) was one of the most significant
causes of global brain ischemia. Most patients had poor cerebral
function after discharge from the emergency room. According to
statistics, there were almost 326,200 patients died or disabled
from CA (1). However, only 10.6
percent of the emergency patients suffering from CA can survive,
and 8.3% of the patients can retain good neurological function
(1). The ischemia brain damage
causing by CA was mainly in the hippocampus (2). There were no therapies can treat the
behavioral and cognitive dysfunction deriving from the hippocampus
damage accompanied by memory loss, emotion change, or even coma,
persistent vegetative state and death. Therefore, cerebral
protection and cerebral resuscitation are significant after
cardiopulmonary resuscitation (CPR).
Because of the complex and diverse mechanisms of
brain damage, multi-mechanism treatment is necessary, for example,
mild hypothermia therapy. Current updated CPR guidelines
recommended that the patients who had out-of-hospital CA with
return of spontaneous circulation should have the body cooled to
32–36°C for maintenance of 24 h (3).
In general, preliminary study indicated the patients can benefit
from induced hypothermia, but the optimal level of hypothermia and
the best cooling methods at the beginning of treatment were still
under investigation. A number of cerebral protections should be
performed by comprehensive treatment, because of the complex
mechanisms of brain injury after CA (4). Therefore, the cerebral function may be
improved by developing more brain protection plans in cerebral
resuscitation study. Stem cell transplant has become important
research field of cerebral resuscitation, which can act on several
mechanisms of hypoxic-ischemic brain damage (HIBD). The purposes of
the study are to explore the protection of bone marrow mesenchymal
stem cells (BMSCs) against the global brain injury induced by
ischemic and hypoxic and to exam one of its mechanism.
MSCs do not derive from blood forming cells and bone
marrow is the most common sources of MSCs (5). Some research showed that BMSCs had the
following properties: (1) The BMSCs
derived from autologous bone marrow are not restricted by ethical
issues; (2) Colter and his
colleagues (6) discovered that the
culturing of 20 ml bone marrow can get 1013 BMSCs for 3
generations from 6-week culturing, almost three times more than the
original number. Chen et al (7) and Ding et al (8) found that MSCs can repair the nerve in
cerebral ischemic animal model; (3)
MSCs are able to pass the blood-brain barrier (BBB) to the brain
and survive, without the issue of organ rejection (9); (4) Chen
et al (10) established the
dose-response rat model of intracerebral injection, artery
injection and intravenous injection, whose results indicated that
neural function could benefit from thef continuous transplantation
of BMSCs a month after cerebral injury; (5) Wakabayashi and his colleagues (11) found that nerve injuries and cerebral
infarction areas were reduced by MSCs to the rat model of middle
cerebral artery occlusion (MCAO), and the transplantation
especially increased the insulin-like growth factor 1 (IGF-1)
secretion.
In this research, CA was induced by asphyxia. One
hour after ROSC, BMSCs were transplanted by injecting into the vein
of the tails. Observing the effects of BMSCs on nerve function of
rats with CA, this paper discussed the neuroprotection of global
cerebral ischemia and the mechanisms involved.
Materials and methods
Experimental animals
Primary cells were cultured from the specific
pathogen-free (SPF) male healthy Sprague-Dawley (SD) rats weighing
100–110 g, and the SPF male healthy SD rats weighing 250–350 g were
used to established rat model. The rats fasted except water the
night before experimental operation. All the rats were provided by
Center for Animal Testing of Beijing Vital River. Animal permit no.
SCXK (Jing) 2012–0001. All procedures were according to the
Guidance Suggestion of Caring Laboratory Animals (2006. 09.
30).
Isolation and culture of BMSCs
The young SD rats weighing 100–110 g were
sacrificed, and femurs and tibias were isolated from the muscle in
the laminar flow cabinet (Suzhou Antai Airtech Co., Ltd., Suzhou,
China). The bone cavity was repeatedly rinsed with Dulbecco's
modified Eagle's medium/Ham's F12 (DMEM/F12) without fetal bovine
serum (FBS) (both from Hyclone, Logan, UT, USA). The washing fluid
was put into centrifuge tube, and the supernatant was discarded
after centrifuging at 1,500 rpm for 5 min. The precipitation was
mixed by 8 ml DMEM/F12 with 10% FBS, divided into 25 cm2
culture flasks, and incubated in a Tri-Gas incubator (Sanyo, Osaka,
Japan). Half of the medium was changed after 24 h; the whole medium
was changed after 48 h; and the medium was changed every other
day.
The cells were trypsinized by 2 ml 0.25% pancreatin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 0.1 mM ethylenediaminetetraacetic acid (EDTA) for 2 min.
Cell passaged (1:2) when the cells fusion reach 90%, and subculture
was repeated when 90% of cells fused. The P3 generation cells were
used for further testing and transplantation. As the paper
described above (12,13), BMSCs were measured by flow cytometry
analysis of CD 29, CD 90, CD 45 and CD 11b (BioLegend, Inc., San
Diego, CA, USA), and the expressions of CD 29 and CD 90 were 97.15
and 99.02%. The expressions of CD 45 and CD 11b were 0.36 and
1.45%. It confirmed that BMSCs had been successfully isolated and
cultured, which can be used for further research.
Prior to transplantation, the BMSCs were collected
at P3 generation of growth, washed three times by
phosphate-buffered solution (PBS; Hyclone), and incubated with 10
µl of 1×108 TU/ml green fluorescent protein (GFP;
Shanghai GenePharma Co., Ltd., Shanghai, China). The medium was
changed after 24 h, and we observed the GFP under the fluorescence
microscope after 72 h. The GFP was transfected into the BMSCs
higher than 80% and can be used for further experiments.
Rat model of CPR
Adult male SD rats weighing 250–350 g were selected
and divided into three groups randomly: sham operation group
(n=15), CA group (n=15) and BMSCs treatment group (n=18), we made
same models of asphyxia-induced CA. The 1st day, the 3rd day and
the 7th day were chosen as the time-points to observe after ROSC.
One hour after ROSC, ~1×106 BMSCs were transplanted by
injecting into the vein of the tails. There were 6 rats on each
time-point in BMSCs treatment group, and 5 rats in the other
one.
The adult rats were anesthetized by intraperitoneal
injection 4.5 mg/100 g pentobarbital solution. The skin of the
neck, chest and groin was prepared before the rats' incisors were
fixed on the operation table in supine position. Endotracheal
intubation was performed by shining a flashlight penetrating
through the neck skin. The tracheal tube of 14 gauge cannula
(Becton Dickinson, Franklin Lakes, NJ, USA) was fixed to the jaw
with suture after the confirmation of successful intubation. In
anesthetic condition, right femoral artery was separated, and the
blood flow was occluded by ophthalmic forceps. The distal artery
was ligated; the proximal artery was cut a small slit by ophthalmic
scissors; and then a heparinized polyethylene 50 (PE-50) catheter
was advanced into the femoral artery for measurement of mean
arterial pressure (MAP) before the pipe was fixed. Map was measured
by BL-420 biological functional system (Chengdu Taimeng Technology
Co., Ltd., Chengdu, China). Electrocardiogram (ECG) was also
monitored by the system (lead II).
The physiological baseline parameters were recorded
when the rats was totally conscious. 0.05 mg/100 g vecuronium was
injected into three-way tube connected to the PE-50 catheter, and
the tracheal tube was blocked by a syringe. The standard of CA was
the MAP ≤25 mmHg, and the artery pulse wave without fluctuation
(14). After 5 min of CA, the
syringe was withdrawn from tracheal tube, and the freehand external
cardiac compression begun immediately with mechanical ventilation
with FiO2 of 100% by the rodent ventilator (Shanghai
Alcott Science and Technology Co., Ltd.). The tidal volume was 0.6
ml/100 g and the breathing rate was 100 bpm. The
compression/ventilation ratio was 2:1. The depth of compression was
1/3 of anteroposterior chest diameter, or adjusted by the change of
MAP. The 0.1 ml adrenal was injected into three-way tube after 2
min of compression, with the same dose every 2 min. The criterion
of ROSC was returned of supraventricular rhythm with the MAP ≥60
mmHg lasting for at least 10 min (15). The 5–15 µg/kg.min dopamine was
injected when the blood pressure dropped. If there was no ROSC
after 10 min of chest compression, the rat was to be considered as
recovery failure. Mechanical ventilation was continued for 30 min
after ROSC, and continuously recorded for ECG and MAP. The sham
operation group rats underwent the same operation except the CA.
All the operation was done by the same experimenter.
Transplantation of BMSCs
One hour after ROSC, in BMSCs treatment group,
~1×106 GFP-labeled BMSCs were prepared for
transplantation. The cells were resuspended in 0.5 ml PBS for
transplantation by injecting into the vein of the tails. After
injecting, the catheter and the cannula were removed with the
femoral artery ligated. 8 million IU of penicillin was injected by
intraperitoneal injection. The rats were totally awake and returned
to the animal cages. Incandescents were used for heating. Rats were
fed by 10% glucose injection on the first day after ROSC,
hereafter, fed by routine feed.
Nerve function defect grade in
rats
The neurological function of rats after ROSC at 1st
day, the 3rd day and the 7th day was assessed by the modified
neurological severity scores (mNSS; Table I), which can evaluate the extent of
neurological damage in motor, sensory, balance and reflex. The more
the scores, the more serious nerve injury (16).
 | Table I.Modified neurological severity
scores. |
Table I.
Modified neurological severity
scores.
| Items | Points |
|---|
| Motor tests |
| Raising
rat by tail | 3 |
|
Flexion of
forelimb | 1 |
|
Flexion of
hindlimb | 1 |
|
Head moved >10
to vertical axis within 30 | 1 |
| Placing
rat on the floor (normal, 0; maximum, 3) | 3 |
|
Normal walk | 0 |
|
Inability to walk
straight | 1 |
|
Circling toward
the paretic side | 2 |
|
Fall down to the
paretic side | 3 |
| Sensory tests | 2 |
|
Visual and tactile
placing | 1 |
|
Proprioceptive
test (deep sensory) | 1 |
| Beam balance
tests | 6 |
| Grasps
side of beam | 1 |
| Hugs
the beam and one limb falls down from the beam | 2 |
| Hugs
the beam and two limb fall down from the beam, or spin on beam
(>60 sec) | 3 |
| Attempt
to balance on the beam but fall off (>40 sec) | 4 |
| Attempt
to balance on the beam but fall off (>20 sec) | 5 |
| Fall
off with no attempt to balance or hand on to the beam | 6 |
| Reflexes (blunt or
sharp stimulation) absent of: | 4 |
| Pinna
reflex (a head shake when touching the auditory meatus) | 1 |
| Corneal
reflex (an eye blink when lightly touching the cornea with
cotton | 1 |
| Startle
reflex (a motor response to a brief loud paper noise) | 1 |
|
Seizures, myoclonus,
myodystony | 1 |
| Maximum points | 18 |
Serum levels of S100B
The rats at day 1, 3, 7 were anesthetized by
intraperitoneal injection 4.5 mg/100 g pentobarbital solution.
Thoracic cavity was opened to expose the heart, and the 5 ml blood
was taken from the right ventricle by a needle. The supernatant
kept under −80°C after centrifuging at 3,000 rpm for 15 min. Serum
levels of S100B were examined by enzyme-linked immunosorbent assay
(ELISA) kit (NeoBioscience, Shenzhen, China) following the
instructions.
Tissue sampling and slices
The skull was opened quickly after blood collection
by the steps. The left hippocampus was removed and placed in the 4%
paraformaldehyde rapidly, and immersed for 3 days, and then the
frozen sections were made 20 µm after dehydration. The right
hippocampus was placed in sterile tube, and stored in the liquid
nitrogen jar for real-time quantitative PCR.
Real-time quantitative PCR
analysis
Total RNA from 48 of right hippocampus samples were
extracted with TRIzol reagent (Qiagen GmbH, Hilden, Germany).
Primers were designed by Shanghai Sunny Biotechnology Co., Ltd.
(Shanghai, China). Reverse transcription was performed by
PrimeScript RT Master Mix (Takara Bio, Inc., Otsu, Japan).
Real-time quantitative PCR was performed by ECO fluorogenic
quantitative PCR (Illumina, Inc., San Diego, CA, USA) using
fluorescent quantitative reagent kit (Takara Bio, Inc.). Total two
primers were designed by Genergy Biotechnology (Shanghai) Co., Ltd.
(Shanghai, P.R. China). (Table
II).
 | Table II.The primers and GAPDH. |
Table II.
The primers and GAPDH.
| Primer name | Sequence
(5′-3′) | Length (bp) |
|---|
|
rat-GAPDH-forward |
AGTTCAACGGCACAGTCAAGG | 121 |
|
rat-GAPDH-reverse |
ACATACTCAGCACCAGCATCAC |
|
|
rat-IGF1-forward |
CTGGTGGACGCTCTTCAGTTC | 156 |
|
rat-IGF1-reverse |
ACAGTACATCTCCAGCCTCCTC |
|
The solution of reverse transcription was
centrifuged slightly, and the reverse transcription was reacted at
37°C for 15 min, and then reverse transcriptase was inactivated for
30 sec. The cDNA was stored at −20°C, and it was diluted 10 times
in this experiment. The parameters of thermal cycle of PCR were
showed in the Table III. The
expression of mRNA were calculated based on 2−ΔΔct
method (17).
 | Table III.Thermocycle features for polymerase
chain reaction. |
Table III.
Thermocycle features for polymerase
chain reaction.
| Temperature | Time (sec) | Cycles |
|---|
| 94°C
(pre-degeneration) | 30 | Stage 1 1
cycle |
| 95°C
(denature) | 5 | Stage 2 40
cycles |
| 61°C (primer
annealing) | 30 |
|
| 72°C
(extension) | 30 |
|
| 95°C | 15 | Melting curve 1
cycle |
| 55°C | 15 |
|
| 95°C | 15 |
|
Detection of the expression of IGF-1
in BMSCs by the double fluorescent labeling of GFP and IGF-1
Before the sections were covering with citric acid
buffer (pH 6.0), the frozen sections were washed in PBS for 5 min
three times, and the sections were washed in PBS again. The tissue
samples were blocked by normal serum (Abcam, Cambridge, UK) a 37°C
for 1 h. The IGF-1 antibody (Novus Biologicals, Littleton, CO, USA)
was added according to the manufacturer's directions, after the
normal serum was sop by filter. The tissue samples were washed in
PBS for 5 min three times, before covered by diluted secondary
antibody (Abcam), incubated at 37°C for 40 min, and then washed in
PBS for 5 min three times. After added the DAPI (1:1,000) for 10
min, the samples were washed in PBS for 5 min three times
immediately, and dehydrated and mounted with neutral gum.
Statistical analysis
Measurement data are showed as means ± standard
deviations, and the IBM SPSS 19.0 was used for statistical
analysis. One-way ANOVA was performed for the statistical method,
the homogeneity of variance and analysis of variance were compared
in every group. Measurement data were compared with S-N-K method,
and statistical significance was determined at P<0.05.
Results
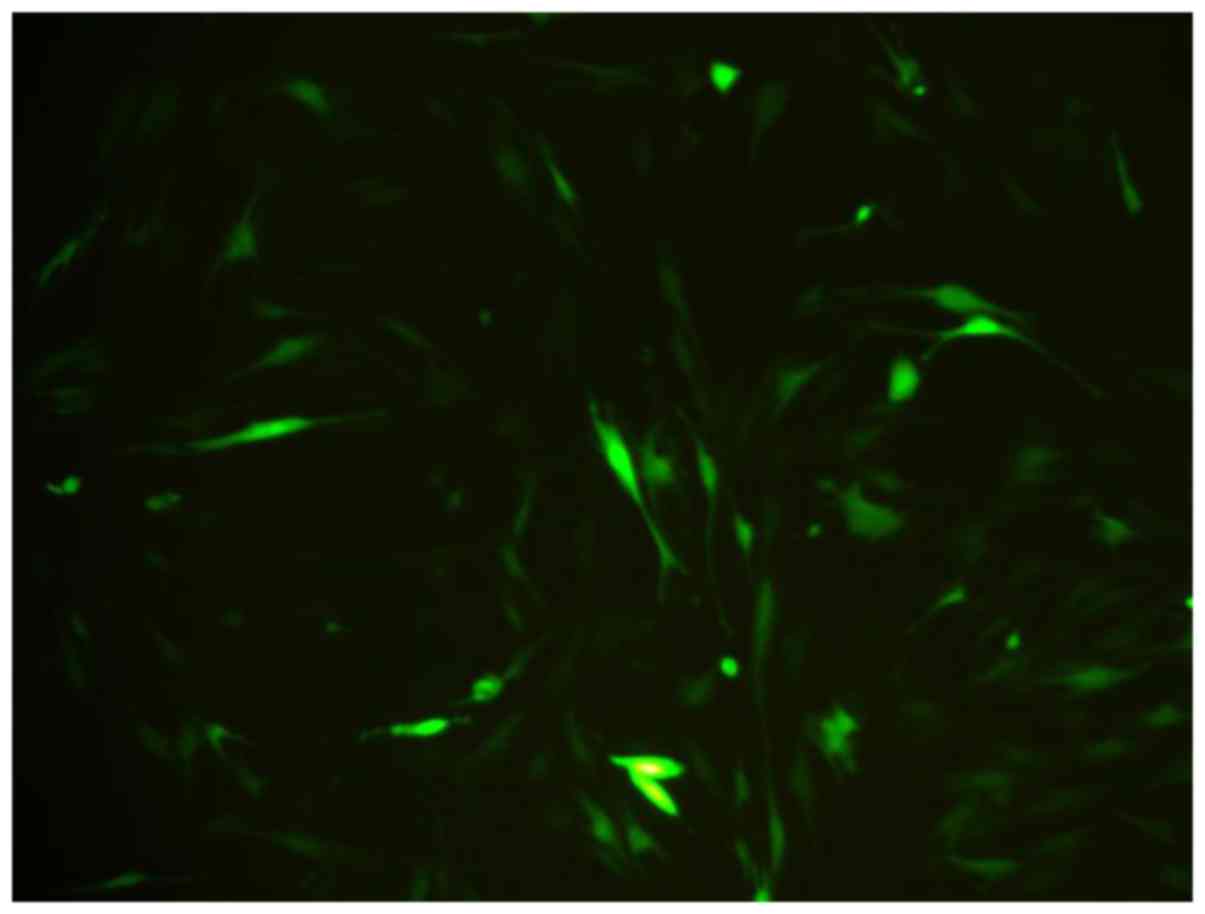
Observation of GFP-labeled BMSCs
BMSCs were spindle shaped as usual, after marked by
BMSCs. The growth, proliferation and activity of BMSCs were not
influenced by GFP. After 3 days, The BMSCs appeared fluorescent
green under a fluorescent microscope. The transfection efficiency
was greater than 80% of the BMSCs, and it showed GFP can perform as
the biomarker of BMSCs (Fig. 1).
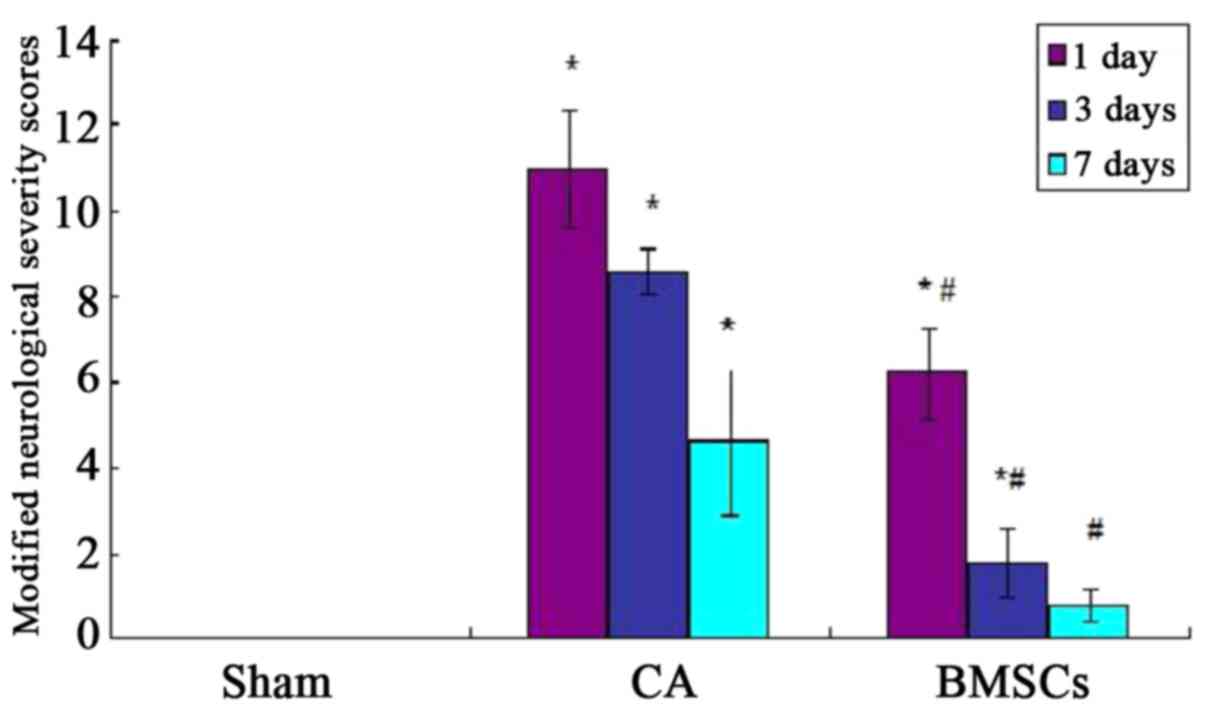
Nerve functional score in rats
Before the experimental operation, the score in rats
of all groups was zero, and there were no significant differences
(P>0.05). The mNSS score of CA group was higher than sham
operation group significantly after ROSC at 1st, 3rd and 7th day
(P<0.05); the mNSS score of BMSCs treatment group was reduced
significantly (P<0.05). However, compared with sham operation
group, the mNSS score of BMSCs treatment group was no statistical
differences at 7th day (P>0.05; Fig.
2).
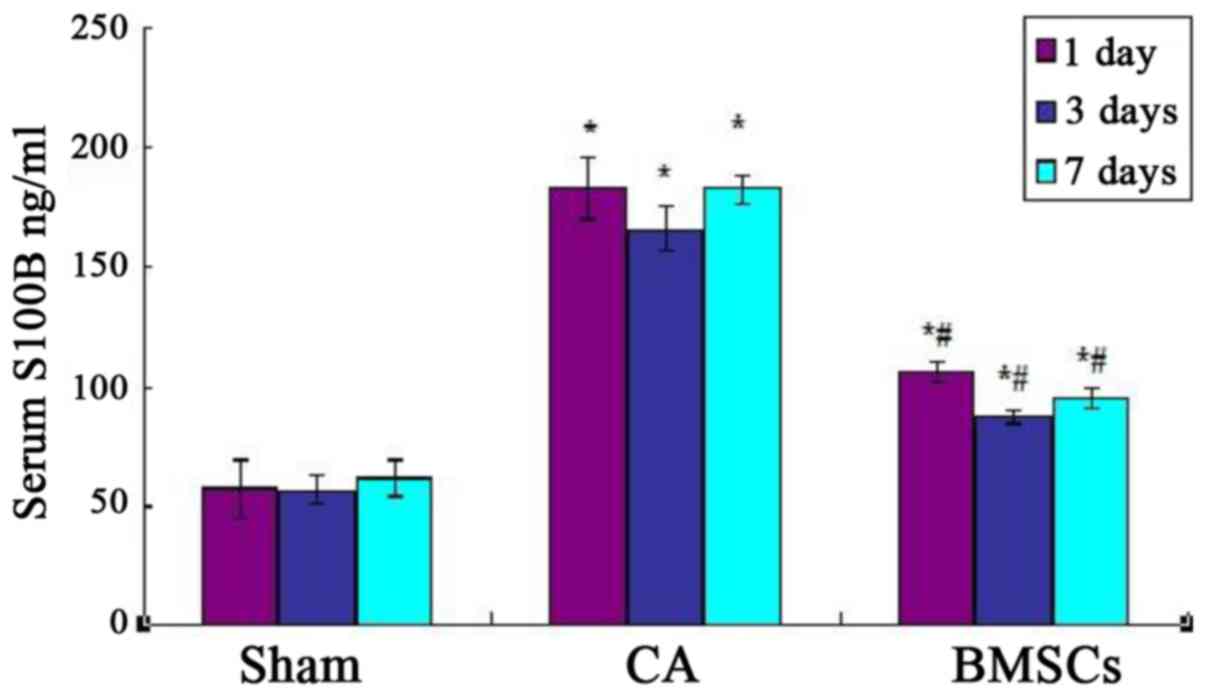
Serum levels of S100B
As shown in Fig. 3,
Serum levels of S100B of cell-transplanted group and CA group were
higher than sham operation group and CA group significantly after
ROSC at each time point. Cell-transplanted group decreased
significantly after ROSC at the 1st, 3rd, and 7th day than CA group
at the content of S100B. The standard curve of serum S100B levels
was showed in Fig. 4.
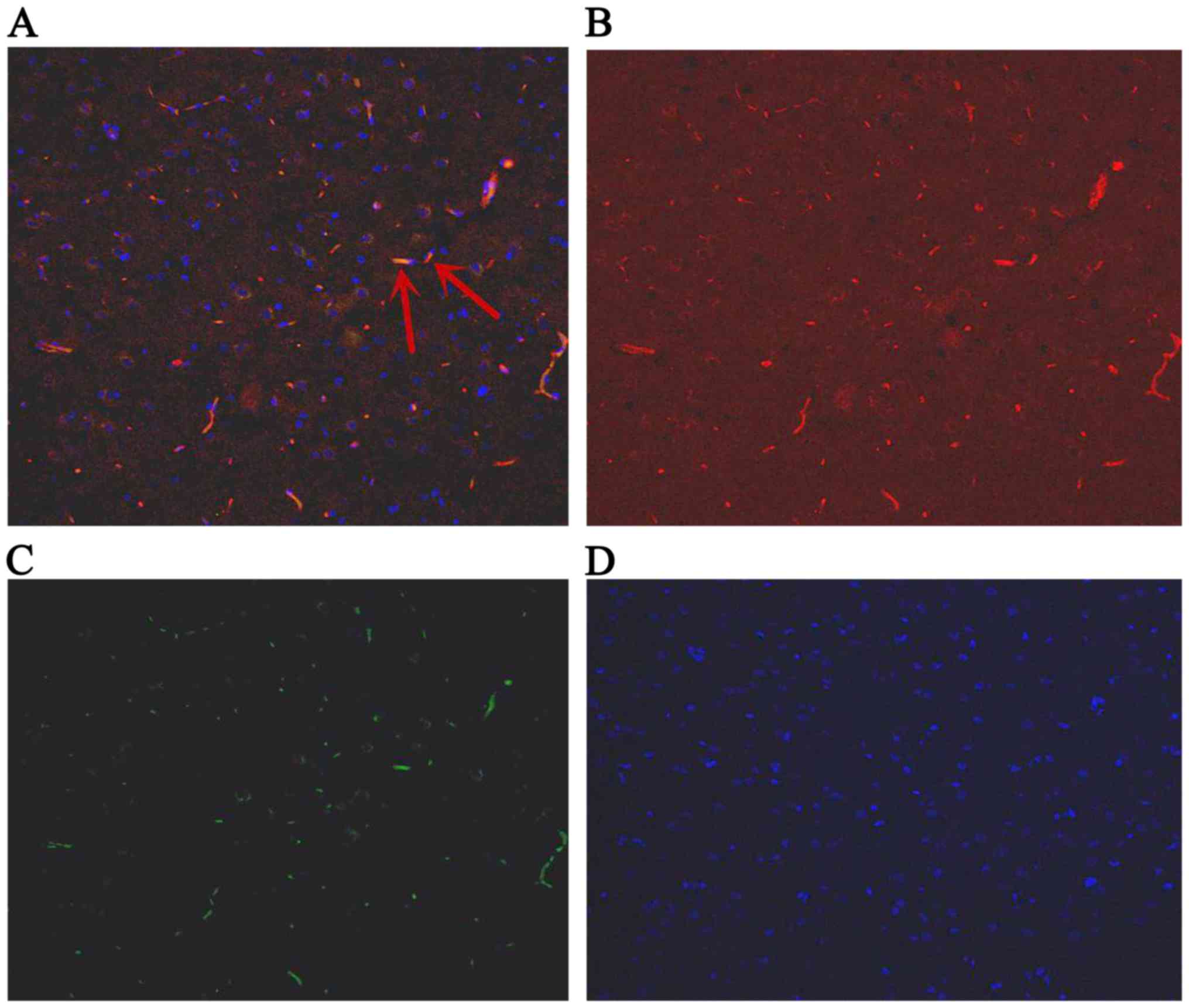
Detection of the expression of IGF-1
in BMSCs by the double fluorescent labeling of GFP and IGF-1
The GFP-labeled BMSCs can be detected in hippocampus
under a fluorescent microscope (Olympus, Tokyo, Japan) at day 1, 3
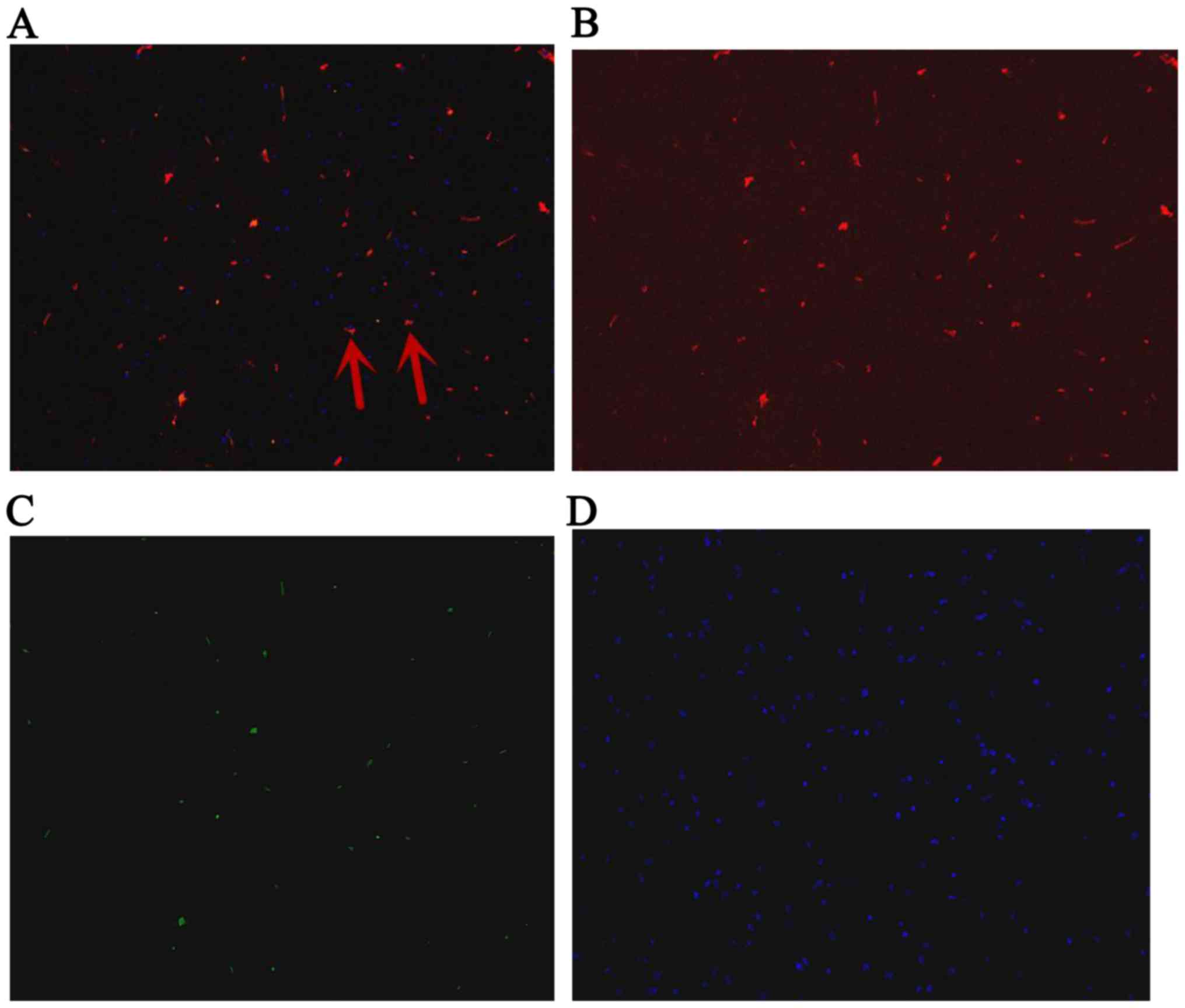
and 7. The red stain was the IGF-1; GFP was observed green; and the
cell nucleus was blue after DAPI dyeing. The cells located in
hippocampus were appeared green and blue. The BMSCs secreted IGF-1
were green dyeing and red dyeing. The proportion of positive cells
double stained by IGF-1 and GFP, and also labeled by DAPI were
6.89, 7.44, 7.26% at 1st day, 3rd and 7th day, respectively
(Figs. 4–6).
Quantitative PCR assays for IGF-1 in
the hippocampus
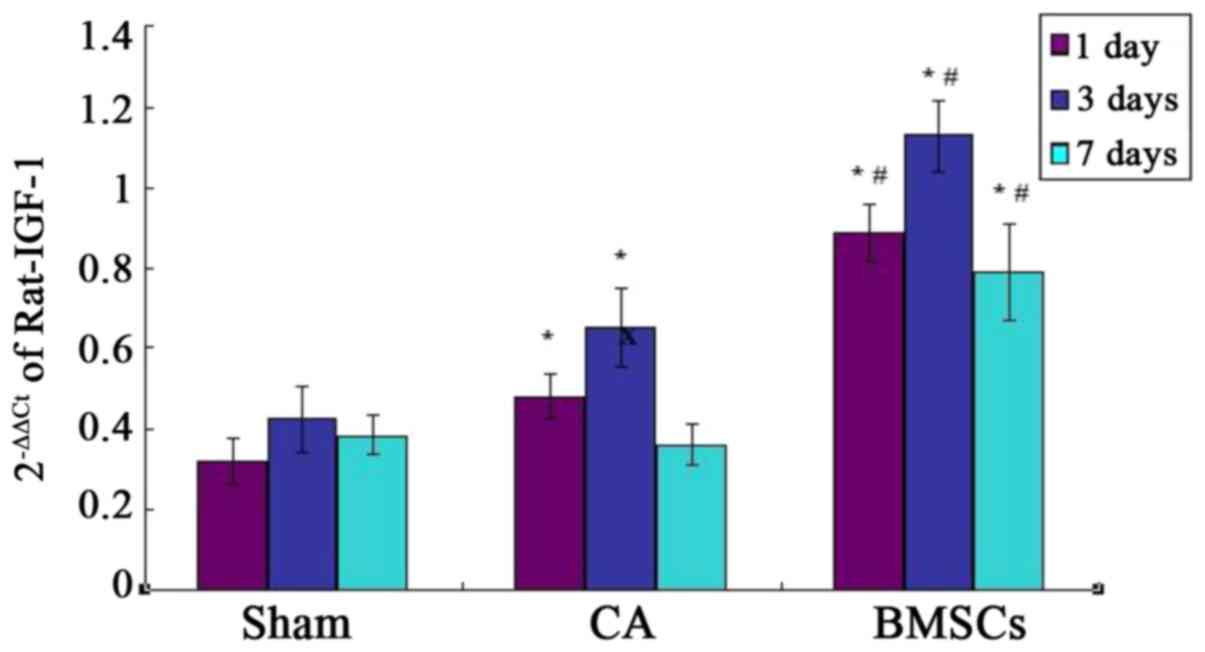
Real-time quantitative PCR analysis showed that
there was basic expression of IGF-1 in sham group. The results
confirmed IGF-1 mRNA relative amount of BMSCs treatment group was
significantly higher than that of sham operation group and CA group
at each time point. The expression of IGF-1 mRNA in CA group was
significantly increased compared with sham group at the 1st and 3rd
day. There was no statistical difference of the expression of IGF-1
mRNA between CA group and sham group at day 7, as shown in Fig. 7.
Observation of the expression of IGF-1
in hippocampus by immunohistochemistry
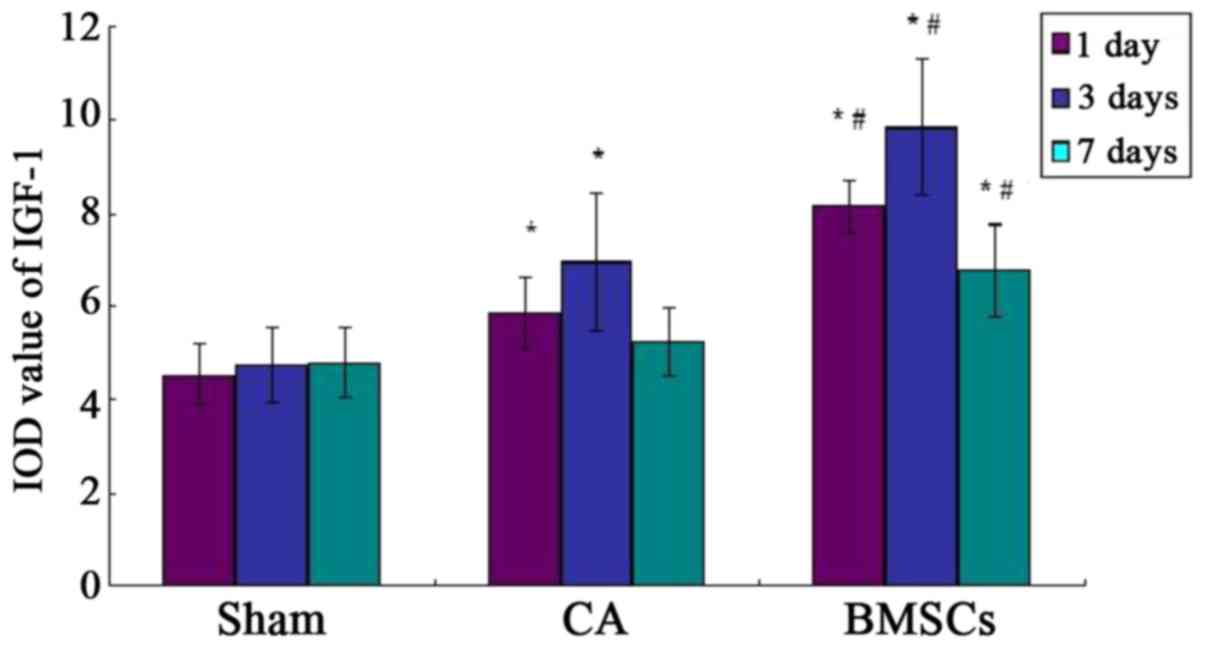
The IGF-1 positive-staining cells appeared light
brown dyeing. A few light brown dyeing can be detected in sham
group, which showed IGF-1 was weakly expressed in the hippocampus
of the rat. Immunohistochemistry results demonstrated the
hippocampus expressed IGF-1 positive, which were found in
cell-transplanted group. There was a little expression in CA group,
both groups were higher than that of sham operation group. But the
expression in CA group had no statistical difference with sham
operation group at 7th day, as shown in Figs. 8–11.
Discussion
BMSCs are pluripotent, low- immunogenic, can be
obtained easily and convenient (18). BMSCs which originate from bone
marrow, have the ability to self-renew, multi-differentiating,
nerve repair as well as to promote the nerve cells to
proliferation. This method is widely used in treating nervous
system diseases. The present studies on the effects of BMSCs
repairing the nerve damage after global ischemia induced by CA were
still comparatively little. Previous showed that the neuron loss
and the apoptosis of neurons in the hippocampus were improved in
the BMSCs-treated group compared to the CA group (19,20).
These researches proved that global ischemia can be improved by
BMSCs transplantation. In this study, the GFP-labeled BMSCs were
distributed in the hippocampus which was easily damaged after CA.
It illustrated BMSCs had the ability to pass the BBB and migrated
to the lesion. The rats' neurological status was assessed by mNSS
tests. The more the scores, the more serious the nerve injury was.
Our results showed that the abilities of motor, sensory, balance
and reflex were improved in the BMSCs treated group, compared to
the CA group. There was a number of behavior function damage in
rats after CA with the symptoms of twitching, convulsions, low
spirits and anorexia. The damaged neurological function had limited
recovery. Some researches shown that the Nogo-A secreted by
oligodendrocytes and glial scar formed by astrocytes can suppress
the growth of axon and myelination (21,22). The
expression of Nogo-A can be decreased by the transplantation of
BMSCs, and the time of reduction of scar was reduced (23).
The S100B were distributed in the central nevous
system and play a vital role in determining cell proliferation,
differentiation, nutrition and metabolism. If the brain tissue,
neurons or blood-brain barrier was damaged, the over-expressed
S100B were released into the blood. Researches found a positive
correlation between the levels of S100B and the damage degree of
injury brain, and serum levels of S100B can be an objective measure
of brain damage after CPR. The results of the study indicate that
BMSCs can effective reduce the levels of S100B and improve nerve
damage after cerebral resuscitation.
The mechanisms of BMSCs in brain resuscitation
included cell differentiation, immune regulation, promotion of
angiogenesis, anti-apoptosis and secretion of neurotrophic factors.
A study indicated that BMSCs can secrete brain-derived growth
factor (BDGF), nerve growth factor (NGF), basic fibroblast growth
factor (bFGF), hepatocyte growth factor (HGF), vascular endothelial
growth factor (VEGF) and IGF-1 (24). A study shown that the growth of axons
was promoted by hippocampal cells co-culturing with BMSCs, and the
cell apoptosis was suppressed; the results of proteome analysis and
ELISA demonstrated IGF-1 existed in the culture medium, and the DNA
sequencing analysis also indicated the high expression of IGF-1 in
neurons (25). Research shown that
the hippocampus and the learning ability were improved in the
neonatal rats of hypoxic-ischemic injury with the secretion of
IGF-1, after the adipose tissue-derived stem cells were
transplanted via jugular vein. Moreover, the nerve injuries and
cerebral infarction areas were reduced by the transplantation of
MSCs to the rat model of MCAO, and the secretion of IGF-1 was
increased. In this experiment, the result of the double fluorescent
labeling of GFP and IGF-1 showed that the IGF-1-secreting BMSCs
marked by DAPI were green dyed and red dyed, respectively, which
indicated that BMSCs can home to the lesion in the hippocampus and
survived to secrete IGF-1.
Insulin-like growth factor-1, a 70-amino-acid
polypeptide, named for the homology with insulin belongs to the
tyrosine kinase receptor E and regulated by IGF-1 binding proteins
(IGFBPs). IGF-1 mRNA expresses in the tissues of muscle, liver and
nerves, worked via the pathway of endocrine, autocrine and
paracrine (26). Study had found
that the expression of IGF-1 mRNA was increased on local cerebral
ischemia, and the expression quantity was proportional to the brain
injury extent (27). Lehtinen et
al (28) argued that IGF-1 was
good for the differentiation of the neural cells, axonal
connections, accumulation of contactin and the sodium channel open.
A study confirmed IGF-1 had the protective effect on the HIBD
(29). Previous studies showed that
IGF-1 can enhance the activity of BDNF and VEGF (30,31), and
there were no growth factors can enhance the activity of IGF-1.
Peripherally administered IGF-1 is not easily to pass BBB because
of its big molecular weight, and direct injection to brain can
cause twice trauma, so the clinical application of IGF-1 is limited
(32). However, BMSCs can secrete
the IGF-1 homing to the ischemia areas, and it provides an
appropriate administration route to avoid BBB.
In this study, the expression of IGF-1 mRNA in the
CA group was significantly increased compared with that of the sham
group, which confirmed the expression of IGF-1 in the model of CA
induced by asphyxia was similar to the model of focal cerebral
ischemia, and have a same tendency of IGF-1 mRNA. The results of
double fluorescent labeling confirmed BMSCs can definitely promote
the secretion of IGF-1 in the rats of CA induced by asphyxia. We
found that the expression in the CA group had no statistical
difference with the sham operation group at 7th day, but the
expression in the BMSCs-treated group increased significantly
compared to the other groups. Combining the results of the mNSS
test, the mNSS score of the BMSCs treatment group had no
statistical differences at 7th day, which illustrated BMSCs can
prolonge the time of expression result in the improvement of
neurological function.
However, this experiment also showed some
limitations. One limitation was that the study did not illustrate
the IGF-1 was a factor in improving cerebral resuscitation after
transplantation of BMSCs. The further research was planning to
block IGF-1 in rats performed by IGF-1 antibody or siRNA
technology. Another limitation was the mechanisms of brain
protection of IGF-1 were not explained. The mechanisms of IGF-1
were still in research. Possible mechanisms of IGF-1 included: i)
It can inhibit the apoptosis of the nerve cells (33–35); ii)
it can resist the function of excitatory amino acid (36); iii) It prevents the calcium overload
after cerebral ischemia reperfusion (35). (4) It
can decrease the cerebral vascular resistance and improve blood
circulation (37).
In summary, the hippocampal neuron in rats after CPR
can benefit from BMSCs treatment possibly via secreted IGF-1.
Acknowledgements
This study was funded by National Key Clinical
Specialty Construction Project of China (Emergency Medicine 2012)
and Fujian Provincial Natural Fund Subject (2014J01289).
References
|
1
|
Writing Group Members, . Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart Disease and Stroke
Statistics-2016 Update A Report From the American Heart
Association. Circulation. 133:E38–E360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Popp E, Vogel P, Teschendorf P and
Böttiger BW: Effects of the application of erythropoietin on
cerebral recovery after cardiac arrest in rats. Resuscitation.
74:344–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Callaway CW, Donnino MW, Fink EL, Geocadin
RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM
and Zimmerman JL: Part 8: Post-cardiac arrest care: 2015 American
heart association guidelines update for cardiopulmonary
resuscitation and emergency cardiovascular car. Circulation. 132 18
Suppl 2:S465–S482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teschendorf P, Vogel P, Wippel A, Krumnikl
JJ, Spöhr F, Böttiger BW and Popp E: The effect of
intracerebroventricular application of the caspase-3 inhibitor
zDEVD-FMK on neurological outcome and neuronal cell death after
global cerebral ischaemia due to cardiac arrest in rats.
Resuscitation. 78:85–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohal JS, Tailor HD and Khan WS: Sources
of adult mesenchymal stem cells and their applicability for
musculoskeletal applications. Curr Stem Cell Res Ther. 7:103–109.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colter DC, Class R, DiGirolamo CM and
Prockop DJ: Rapid expansion of recycling stem cells in cultures of
plastic-adherent cells from human bone marrow. Proc Natl Acad Sci
USA. 97:3213–3218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen A, Siow B, Blamire AM, Lako M and
Clowry GJ: Transplantation of magnetically labeled mesenchymal stem
cells in a model of perinatal brain injury. Stem Cell Res.
5:255–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen
X and Chopp M: The sonic hedgehog pathway mediates brain plasticity
and subsequent functional recovery after bone marrow stromal cell
treatment of stroke in mice. J Cereb Blood Flow Metab.
33:1015–1024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Moon GJ, Chang WH, Kim YH and Bang
OY: STARTING-2 (STem cell Application Researches and Trials In
NeuroloGy-2) collaborators: Intravenous transplantation of
mesenchymal stem cells preconditioned with early phase stroke
serum: Current evidence and study protocol for a randomized trial.
Trials. 14:3172013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JL, Li Y, Wang L, Lu M, Zhang XH and
Chopp M: Therapeutic benefit of intracerebral transplantation of
bone marrow stromal cells after cerebral ischemia in rats. J Neurol
Sci. 189:49–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wakabayashi K, Nagai A, Sheikh AM, Shiota
Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU and
Yamaguchi S: Transplantation of human mesenchymal stem cells
promotes functional improvement and increased expression of
neurotrophic factors in a rat focal cerebral ischemia model. J
Neurosci Res. 88:1017–1025. 2010.PubMed/NCBI
|
|
12
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dvorakova J, Hruba A, Velebny V and Kubala
L: Isolation and characterization of mesenchymal stem cell
population entrapped in bone marrow collection sets. Cell Biol Int.
32:1116–1125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashida K, Sano M, Kamimura N, Yokota T,
Suzuki M, Maekawa Y, Kawamura A, Abe T, Ohta S, Fukuda K and Hori
S: H2 gas improves functional outcome after cardiac arrest to an
extent comparable to therapeutic hypothermia in a rat model. J Am
Heart Assoc. 1:e0034592012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu K, Puchowicz MA, Lust WD and LaManna
JC: Adenosine treatment delays postischemic hippocampal CA1 loss
after cardiac arrest and resuscitation in rats. Brain Res.
1071:208–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen LH, Li Y, Chen J, Zhang J, Vanguri P,
Borneman J and Chopp M: Intracarotid transplantation of bone marrow
stromal cells increases axon-myelin remodeling after stroke.
Neuroscience. 137:393–399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lebouvier A, Poignard A, Cavet M, Amiaud
J, Leotot J, Hernigou P, Rahmouni A, Bierling P, Layrolle P, Rouard
H and Chevallier N: Development of a simple procedure for the
treatment of femoral head osteonecrosis with intra-osseous
injection of bone marrow mesenchymal stromal cells: Study of their
biodistribution in the early time points after injection. Stem Cell
Res Ther. 6:682015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng W, Honmou O, Miyata K, Harada K,
Suzuki J, Liu H, Houkin K, Hamada H and Kocsis JD: Therapeutic
benefits of human mesenchymal stem cells derived from bone marrow
after global cerebral ischemia. Brain Res. 1310:8–16. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohtaki H, Ylostalo JH, Foraker JE,
Robinson AP, Reger RL, Shioda S and Prockop DJ: Stem/progenitor
cells from bone marrow decrease neuronal death in global ischemia
by modulation of inflammatory/immune responses. Proc Natl Acad Sci
USA. 105:14638–14643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yiu G and He Z: Glial inhibition of CNS
axon regeneration. Nat Rev Neurosci. 7:617–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huber AB, Weinmann O, Brösamle C, Oertle T
and Schwab ME: Patterns of Nogo mRNA and protein expression in the
developing and adult rat and after CNS lesions. J Neurosci.
22:3553–3567. 2002.PubMed/NCBI
|
|
23
|
Shen LH, Li Y, Chen J, Cui Y, Zhang C,
Kapke A, Lu M, Savant-Bhonsale S and Chopp M: One-year follow-up
after bone marrow stromal cell treatment in middle-aged female rats
with stroke. Stroke. 38:2150–2156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shichinohe H, Ishihara T, Takahashi K,
Tanaka Y, Miyamoto M, Yamauchi T, Saito H, Takemoto H, Houkin K and
Kuroda S: Bone marrow stromal cells rescue ischemic brain by
trophic effects and phenotypic change toward neural cells.
Neurorehabil Neural Repair. 29:80–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakano N, Nakai Y, Seo TB, Yamada Y, Ohno
T, Yamanaka A, Nagai Y, Fukushima M, Suzuki Y, Nakatani T and Ide
C: Characterization of conditioned medium of cultured bone marrow
stromal cells. Neurosci Lett. 483:57–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skoff RP, Bessert D, Barks JD and
Silverstein FS: Plasticity of neurons and glia following neonatal
hypoxic-ischemic brain injury in rats. Neurochem Res. 32:331–342.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Deng J, Boyle DW, Zhong J and Lee
WH: Potential Role of IGF-I in hypoxia tolerance using a rat
hypoxic-ischemic model: Activation of hypoxia-inducible factor
1alpha. Pediatr Res. 55:385–394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lehtinen MK, Zappaterra MW, Chen X, Yang
YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, et al: The
cerebrospinal fluid provides a proliferative niche for neural
progenitor cells. Neuron. 69:893–905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Šerbedžija P, Madl JE and Ishii DN:
Insulin and IGF-I prevent brain atrophy and DNA loss in diabetes.
Brain Res. 1303:179–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Landi S, Ciucci F, Maffei L, Berardi N and
Cenni MC: Setting the pace for retinal development: Environmental
enrichment acts through insulin-like growth factor 1 and
brain-derived neurotrophic factor. J Neurosci. 29:10809–10819.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lopez-Lopez C, LeRoith D and Torres-Aleman
I: Insulin-like growth factor I is required for vessel remodeling
in the adult brain. Proc Natl Acad Sci USA. 101:9833–9838. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan J: Insulin-like growth factor-1
(IGF-1) derived neuropeptides, a novel strategy for the development
of pharmaceuticals for managing ischemic brain injury. CNS Neurosci
Ther. 17:250–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ueki K, Fruman DA, Brachmann SM, Tseng YH,
Cantley LC and Kahn CR: Molecular balance between the regulatory
and catalytic subunits of phosphoinositide 3-kinase regulates cell
signaling and survival. Mol Cell Biol. 22:965–977. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khokhlatchev AV, Canagarajah B, Wilsbacher
J, Robinson M, Atkinson M, Goldsmith E and Cobb MH: Phosphorylation
of the MAP kinase ERK2 promotes its homodimerization and nuclear
translocation. Cell. 93:605–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peruzzi F, Prisco M, Dews M, Salomoni P,
Grassilli E, Romano G, Calabretta B and Baserga R: Multiple
signaling pathways of the insulin-like growth factor 1 receptor in
protection from apoptosis. Mol Cell Biol. 19:7203–7215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scheid MP and Duronio V: Dissociation of
cytokine-induced phosphorylation of Bad and activation of PKB/akt:
Involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad
Sci USA. 95:7439–7444. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanaka R, Miyasaka Y, Yada K, Ohwada T and
Kameya T: Basic fibroblast growth factor increases regional
cerebral blood flow and reduces infarct size after experimental
ischemia in a rat model. Stroke. 26:2154–2159. 1995. View Article : Google Scholar : PubMed/NCBI
|