Introduction
The conjunctival epithelium and the corneal
epithelium form the outer surface of the eye, and injury to one
part may result in system-wide secondary dysfunction (1). Normal function of the conjunctiva is
critical for supporting the normal function of the ocular surface
and ensuring clarity of the corneal epithelium, as it provides the
mucin (MUC) component of the tear film (2,3).
Therefore, reconstruction of the conjunctiva is the prerequisite
for successful ocular surface reconstruction. Upon injury,
conjunctival epithelium spontaneously re-epithelializes. However,
this is usually accompanied with a range of complications,
particularly if an extensive area is affected, such as in patients
with chemical or thermal burns, Stevens-Johnson syndrome or ocular
cicatricial pemphigoid (4,5). In these situations, an appropriate
substitute needs to be applied for repairing the coloboma
tissue.
The ideal conjunctival scaffolds should be a stable,
biocompatible and easy to manipulate, and importantly, mimic the
structure and biological function of the natural extracellular
matrix (ECM) (6). Although various
therapies have been used in clinical studies or in animal models,
such as autologous conjunctiva, human amniotic membrane (hAM)
(7) and artificial membranes
(8,9), they are limited due to numerous
reasons. For instance, hAM is widely used as a substitute for
conjunctival reconstruction due to the ability to reduce scarring,
anti-microbial, anti-angiogenic and anti-inflammatory properties,
but concerns regarding the possible transmission of infectious
diseases are the main drawbacks (10). Thus, there is a need for artificial
scaffolds with well-defined composition and mimicking the ECM for
ocular surface application.
Electrospinning has recently attracted increased
attention as a method of fabricating nanofibrous scaffolds with
high porosity and high surface area to resemble the topographic
features of the ECM. The unique nanofibrous structure facilitates
cell growth and differentiation, and allows for efficient exchange
of nutrients and metabolic wastes between the scaffolds and their
environment (11,12).
Type I collagen, the most abundant stromal protein
conjunctiva, is biocompatible and biodegradable and possesses low
immunogenicity (13). Therefore,
collagen, as the main component of ECM, is appropriate for use in
the formation of a tissue-engineered conjunctival scaffold, but its
poor mechanical properties have hampered its applications (14). Poly(L-lactic acid-co-ε-caprolactone)
(PLCL), a co-polymer of poly(L-lactic acid) and
poly-ε-caprolactone, is one of the most common biodegradable
polyesters for tissue engineering due to its favorable mechanical
properties. However, the drawbacks of PLCL, such as its strong
hydrophobicity and lack of natural cell recognition sites, have
greatly limited its application as scaffolds in tissue engineering
(15). Therefore, the present study
hypothesized that blending the bioactive functions of collagen with
the good mechanical properties of PLCL may generate a novel
material with the desired cell adhesion, degradation rate and
mechanical properties for conjunctival reconstruction.
The present study attempted to employ collagen/PLCL
scaffolds to engineer a conjunctival equivalent containing
proliferative cells and goblet cells that is an essential
indication of a functional conjunctival epithelium. Characteristics
of collagen/PLCL scaffolds, such as the diameter of the nanofibers,
wettability, mechanical properties and cell viability were
determined. To study the cyto-compatibility of the nanofibrous
structure for applications in conjunctival tissue engineering,
conjunctival epithelial cells were seeded onto the scaffolds. Cell
morphology, phenotypes, viability and proliferation were evaluated.
Furthermore, histological analysis of the cell-scaffold complexes
was performed by hematoxylin and eosin (H&E) staining.
Materials and methods
Fabrication of collagen/PLCL scaffolds
by electrospinning
1,1,1,3,3,3,-hexafluoro-2-propanol (HFIP) and a
copolymer of PLCL with a composition of 50% w/w L-lactide and 50%
w/w ε-caprolactone monomers were obtained from Donghua University
(Shanghai, China) (12). Type I
collagen (Mingrang Biotechnology Co., Chengdu, China) and PLCL were
dissolved separately in HFIP at a concentration of 8% w/w and
stirred vigorously at room temperature for 24 h. Prior to
electrospinning, Type I collagen solution and the PLCL solution
were mixed at a 25:75 volume ratio, followed by stirring at room
temperature for 1 h. The electrospinning conditions were as
follows: Injection rate, 1.0 ml/h; voltage, 16 kV and distance, 12
cm. Collagen/PLCL scaffolds were dried in a vacuum oven for 1 week
at room temperature to remove residual solvent and stored in
desiccators until use.
Characterization of collagen/PLCL
scaffolds
The morphology of collagen/PLCL scaffolds was
observed by scanning electron microscopy (SEM; JSM-6701; JEOL,
Tokyo, Japan). Prior to imaging by SEM, the samples were
sputter-coated with gold for 50 sec to increase conductivity. The
mean diameter of the nanofibers was measured by Image Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA). At least 50
nanofibers from the SEM image were analyzed.
The hydrophilicity of collagen/PLCL scaffolds was
determined by water contact angle measurement as described
previously (16). The contact angle
was measured by a video contact angle instrument (Attension Theta;
Attension, Espoo, Finland). Droplets of 1.0 µl were dropped onto
the scaffolds. The contact angle indicating the wetting ability of
the scaffolds was automatically calculated.
The mechanical properties of collagen/PLCL scaffolds
were determined using an uniaxial material testing machine
(Instron-3343; Instron, Norwood, MA, USA) equipped with a 10-N load
cell (17). Rectangular-shaped
specimens (30×10 mm) were stretched at a constant crosshead speed
of 10 mm/min. For each specimen, the greatest slope in the linear
region of the stress-strain curve corresponding to a strain of
0–20% was used to calculate the Young's modulus. At least five
samples were tested.
To evaluate whether collagen/PLCL scaffolds altered
the cell cycle, the B4G12 human corneal endothelial cell line
(Creative Bioarray, Shirley, NY, USA) was seeded onto the scaffolds
in a 24-well plate and cultured in a humidified environment at 37°C
with 5% CO2 for 3 days (18). The cells were then harvested using
0.25% trypsin (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
followed by washing with pre-cooled (4°C) PBS, and then fixed in
pre-cooled (4°C) 70% ethanol for 1 h. Fixed cells were treated
using propidium iodide (PI, Abcam, Cambridge, MA, USA). The total
number of cells in different phases of the cell cycle was detected
using flow cytometry (Cytomics™ FC500 flow cytometer; Beckman
Coulter Ltd., Brea, CA, USA).
Cell isolation and culture
Conjunctival epithelium was isolated and cultured as
previously described (19). All
animal experiments were approved by the Medical Ethics Committee of
Ninth People's Hospital, Shanghai Jiao Tong University School of
Medicine. In brief, after sacrification, the conjunctiva was
carefully dissected from BALB/c mice (age, 8 weeks; weight, 20±2 g;
10 male and 10 female; purchased from the Animal Centre of Ninth
People's Hospital, Shanghai Jiao Tong University School of
Medicine) with the underlying connective tissue removed. The sheet
was rinsed three times with PBS (1X; 130 mM NaCl, 3 mM KCl, 10 mM
Na2HPO4, 2 mM KH2PO4,
pH 7.4) containing 100 U/ml penicillin and was then incubated with
dispase II (2.4 units/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 4°C for 10 h. The detached epithelial layer was then
scattered into single cells with 0.05% trypsin/EDTA for 10 min at
37°C. Then cells were seeded on a cell culture dish in Dulbecco's
modified Eagle's medium/Ham's nutrient mixture F12 (1:1 DMEM/F12;
Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare, Little Chalfont, UK), 5
µg/ml insulin (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), 5
µg/ml transferrin (Santa Cruz Biotechnology, Inc.), 5 ng/ml
selenium (Santa Cruz Biotechnology, Inc.), 1%
penicillin/streptomycin, 10 ng/ml human epidermal growth factor
(R&D Systems, Inc., Minneapolis, MN, USA) and 100 ng/ml nerve
growth factor (R&D Systems). After 2 days of culture, the
non-adherent cells were removed by washing with PBS. When reaching
confluence, conjunctival epithelial cells were passaged with 0.05%
trypsin/EDTA and subcultured for further experiments.
Cell morphology and phenotype on
collagen/PLCL scaffolds
The morphology of conjunctival epithelial cells on
collagen/PLCL scaffolds was observed by SEM. Conjunctival
epithelial cells were seeded on collagen/PLCL scaffolds at a
density of 2×106 cells/well in 24-well plates. Two days
after cell seeding, the samples were fixed with 0.25%
glutaraldehyde (Merck KGaA) overnight at 4°C. Samples were rinsed
and dehydrated with graded concentrations of ethanol (30, 50, 70,
80, 90 and 100% v/v) for 10 min each. Subsequently, the samples
were critical-point dried. After drying, the samples were coated
with gold sputter and examined by SEM.
Immunofluorescence staining was performed using
standard procedures as described previously (19). Briefly, two days after cell seeding
the cell-scaffold complexes were fixed with 4% (w/v)
paraformaldehyde for 30 min at room temperature. The samples were
incubated at 4°C overnight in the primary antibodies [rabbit
monoclonal anti-cytokeratin (CK) 19 (ab52625, Abcam, 1:200), rabbit
monoclonal anti-CK4 (ab183329, Abcam, 1:200) and mouse monoclonal
anti-MUC 5, subtypes A and C (MUC5AC) (ab24071, Abcam, 1:200)].
After washing with PBS, Alexa Fluor488 goat
anti-mouse/rabbit (BD Biosciences, San Jose, CA, USA, BD5002) was
diluted 1:500 in PBS and applied for 1 h at room temperature. A
control sample was prepared by omitting the primary antibody.
Nuclei were stained with Hoechst 33342 (Invitrogen; Thermo Fisher
Scientific, Inc.). Images were captured under a confocal laser
scanning microscope.
Cell proliferation and viability on
collagen/PLCL scaffolds
To detect the effect of collagen/PLCL scaffolds on
cell proliferation, a Cell Counting Kit-8 assay (CCK-8; Dojindo,
Kumamoto, Japan) was performed (20). In brief, conjunctival epithelial
cells were seeded onto collagen/PLCL scaffolds at a density of
2×104 cells/well in 24-well plates. After 1, 3, 5 or 7
days of cell seeding, the cells were washed with PBS and incubated
with 10% CCK-8 in DMEM/F12. After incubation for 3 h, the
absorbance of each well was measured at 450 nm with a microplate
reader (Bio-Tek ELx800; Bio-Tek, Winooski, VT, USA). At least six
samples were measured at each time-point. For the cell viability
study, viability staining was performed using a
calcein-acetoxymethylester (CAM)/ethidium homodimer 2 (EthD-2)
(Invitrogen; Thermo Fisher Scientific, Inc.) assay, which is based
on differential permeability of live and dead cells. When the cells
reached confluence, live cells were stained with green-fluorescent
CAM, and dead cells were stained with red-fluorescent EthD-2. A
fluorescent microscope (Olympus BX51; Olympus, Tokyo, Japan) was
used to capture images of the cell staining.
Gene expression detection of the
cell-scaffolds complexes
The cell-scaffold complexes were taken from
coverslips by using microscope forceps and immersed in TRIzol
reagent (Thermo Fisher Scientific, Inc.). The complexes were ground
using a Bio-gen pro200 Homogenizer (PRO Scientific Inc., Oxford,
CT, USA). Total RNA was extracted using TRIzol reagent according to
the manufacturer's instructions. The concentration and purity of
the total RNA were determined spectrophotometrically at optical
density at 260 and 280 nm. DNase I was used to eliminate genomic
DNA contamination. The complementary (c)DNA was synthesized from 1
mg total RNA using a PrimeScript™ RT reagent kit (Takara Bio Inc.,
Otsu, Japan). Real-time polymerase chain reaction (PCR) was
conducted using a 7500 Real-Time PCR Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and activated at 95°C
for 10 min and 40 cycles of amplification (15 sec at 95°C and 1 min
at 60°C). The efficiency of the reaction was measured with primers
using serial dilutions of the cDNA (1:1, 1:5, 1:25, 1:125, 1:625
and 1:3125). The primer sequences used for Real-time PCR were as
follows: CK4 (192 bp) forward, 5′-TTGAGCAATGACAAAGGTCGCC-3′ and
reverse, 5′-AAGGCTTTCCATCTTGGCCTCT-3′; CK19 (143 bp) forward,
5′-CAGGTCAGTGTGGAGGTGGATT-3′ and reverse,
5′-TTCAGCTCCTCAATCCGAGCAA-3′; MUC5AC (150 bp) forward,
5′-ACCACTTTCTCCTTCTCCACAC-3′ and reverse,
5′-AACAGGGCTCTTCACAGACAATA-3′; interleukin 4 (IL-4; 160 bp)
forward, 5′-CGTCCTCACAGCAACGAAGAAC-3′ and reverse,
5′-GCATCGAAAAGCCCGAAAGAGT-3′; IL-5 (128 bp) forward,
5′-ATACTCCCTCCCCCTCATCCTC-3′ and reverse,
5′-GTATGTGATCCTCCTGCGTCCA-3′; IL-6 (103 bp) forward,
5′-TTGCCTTCTTGGGACTGATGCT-3′ and reverse,
5′-TAGACAGGTCTGTTGGGAGTGG-3′. The relative mRNA levels were
expressed as the fold change relative to the control sample [cells
cultured on a tricalcium phosphate scaffold (TCPS; Corning Life
Sciences, Amsterdam, The Netherlands)] after being normalized to
the expression of GAPDH. Relative gene expression was analyzed
using the Pfaffl method (21).
Histological findings
After culture for 1 or 2 weeks in vitro, the
complexes were fixed in 4% (w/v) paraformaldehyde, embedded in
Tissue-Tek Optimal Cutting Temperature compound (Sakura Seiki,
Tokyo, Japan) and then cut into 10-µm sections. H&E staining
was performed to assess epithelial cell stratification.
Statistical analysis
SPSS 18.0 software was used for statistical analysis
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. The statistical analysis was performed using
the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of collagen/PLCL
scaffolds
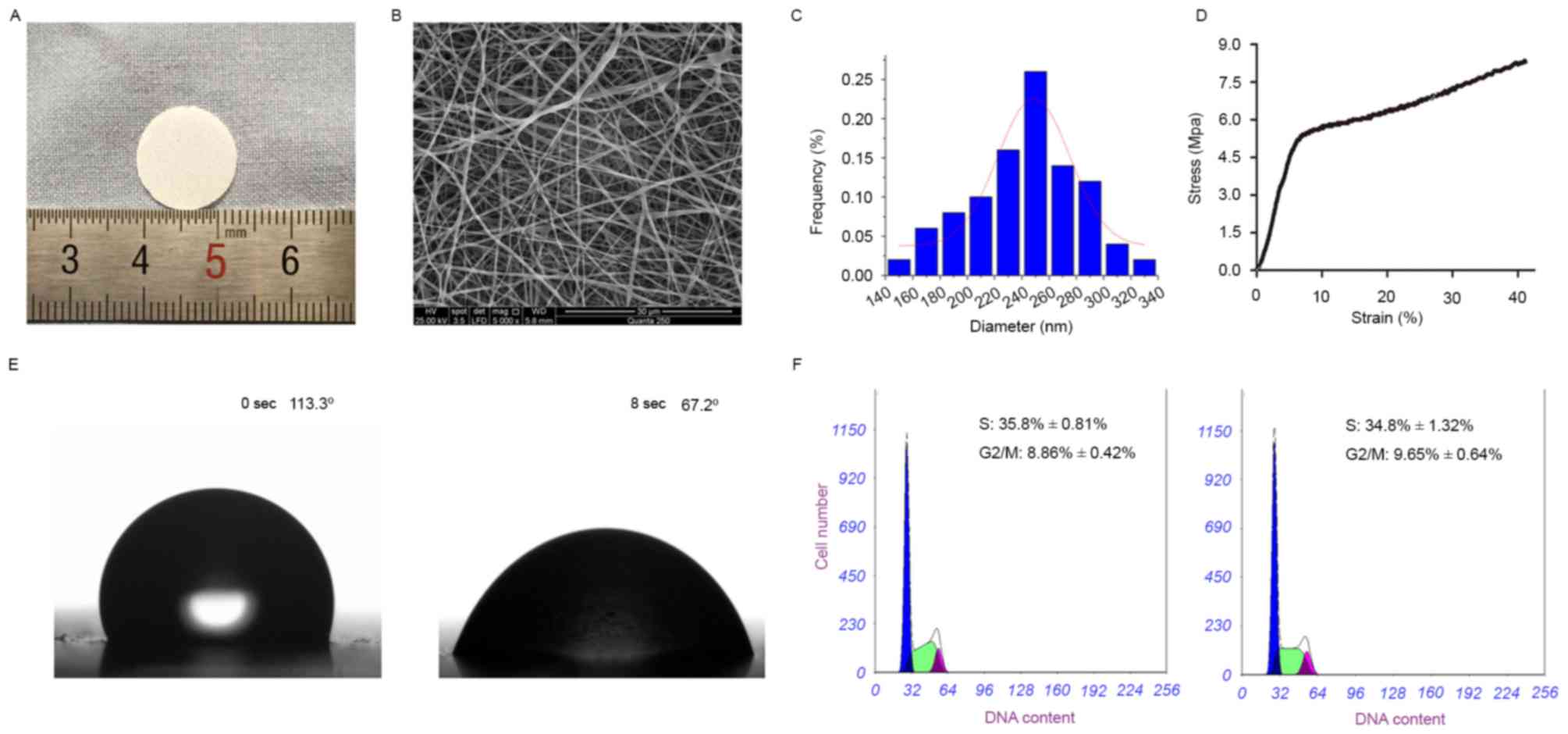
Under light, ultrathin scaffolds composed of
collagen and PLCL were successfully produced by electrospinning
(Fig. 1A). As displayed in Fig. 1B and C, the scaffolds were composed
of randomly oriented, defect-free fibers and thoroughly
interconnected pore structures, and the average fiber diameter was
248.83±26.44 nm.
The tensile strength was measured to confirm the
operability of the scaffolds in tissue engineering. A typical
tensile stress-strain curve of collagen/PLCL scaffolds is presented
in Fig. 1D, exhibiting considerable
tensile strength.
Surface wettability is an important parameter
affecting the attachment, proliferation, migration and viability of
cells. To determine the wettability of the scaffolds, the water
contact angle was measured. It was observed that the water drop was
immediately absorbed into the scaffolds, indicating that
collagen/PLCL scaffolds were hydrophilic (Fig. 1E).
As shown in Fig. 1F,
there was no marked difference in the amount of B4G12 cells in the
active cell cycle (G2/M+S) between those on collagen/PLCL scaffolds
and those on TCPS, indicating that collagen/PLCL scaffolds did not
have any adverse effect on cell proliferation.
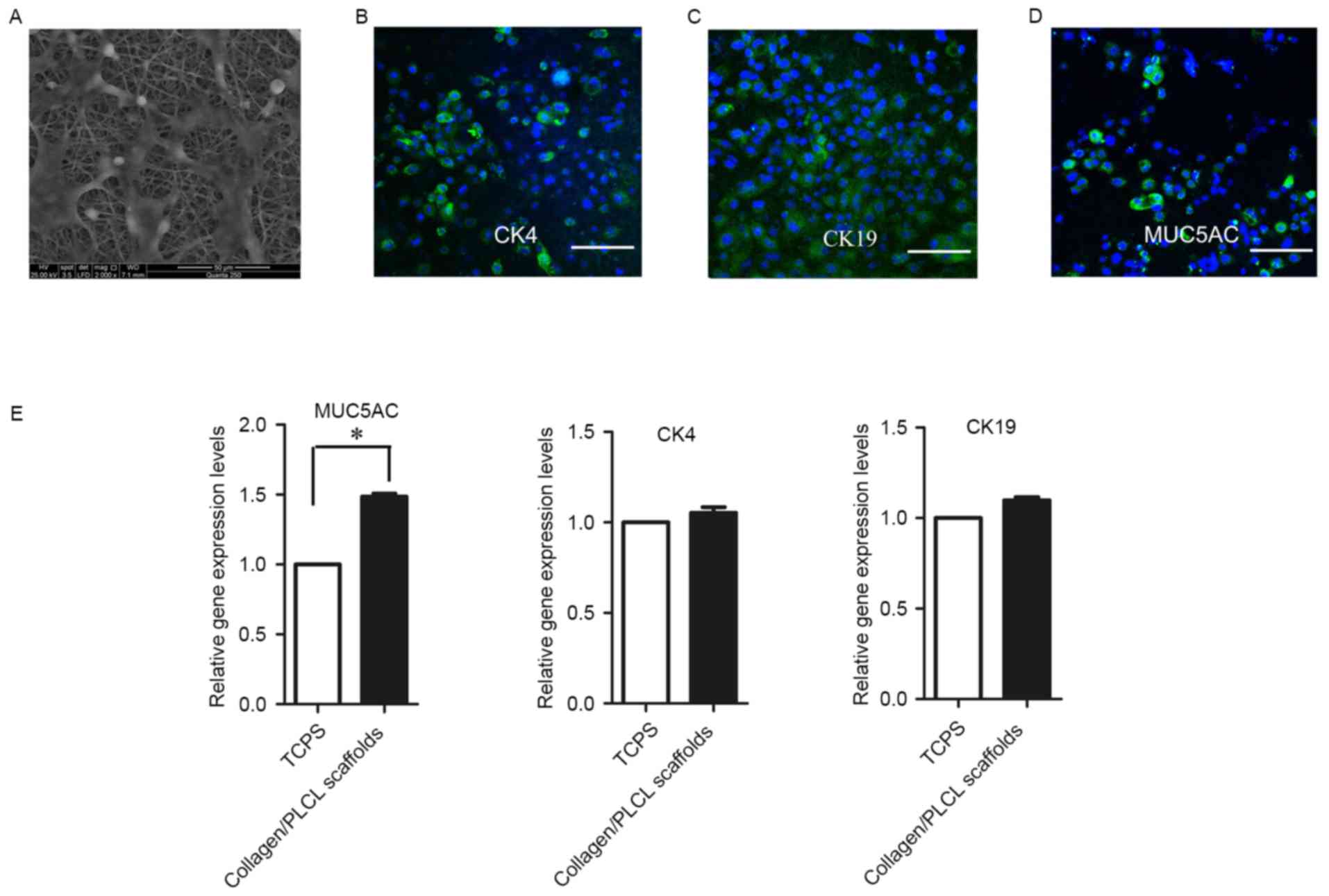
Morphology and phenotypes of
conjunctival epithelial cells on collagen/PLCL scaffolds
Ideal scaffolds for tissue engineering should
maintain a normal morphology and differentiation of the cells. At
two days after conjunctival epithelial cells were seeded onto
collagen/PLCL scaffolds, cells with polygonal shape adhered and
spread on the scaffolds (Fig.
2A).
To study the cell phenotype, the putative
differentiation marker proteins of conjunctival epithelial cells
were identified (Fig. 2B-D). The
cells were positive for CK4 and CK19, specific markers of
conjunctival epithelial cells. Furthermore, staining for specific
secretory conjunctival MUC5AC was positive, indicating the presence
of goblet cells.
Reverse-transcription quantitative (RT-q)PCR was
also performed to characterize gene expression of conjunctival
epithelial cells cultured on the collagen/PLCL scaffolds and TCPS
(Fig. 2E). There was no significant
difference in CK4 and CK19 expression between cells grown on the
collagen/PLCL scaffolds and TCPS; however, MUC5AC transcripts
exhibited a significant, 1.5-fold increase in cells grown on
collagen/PLCL scaffolds in comparison with those in cells grown on
TCPS.
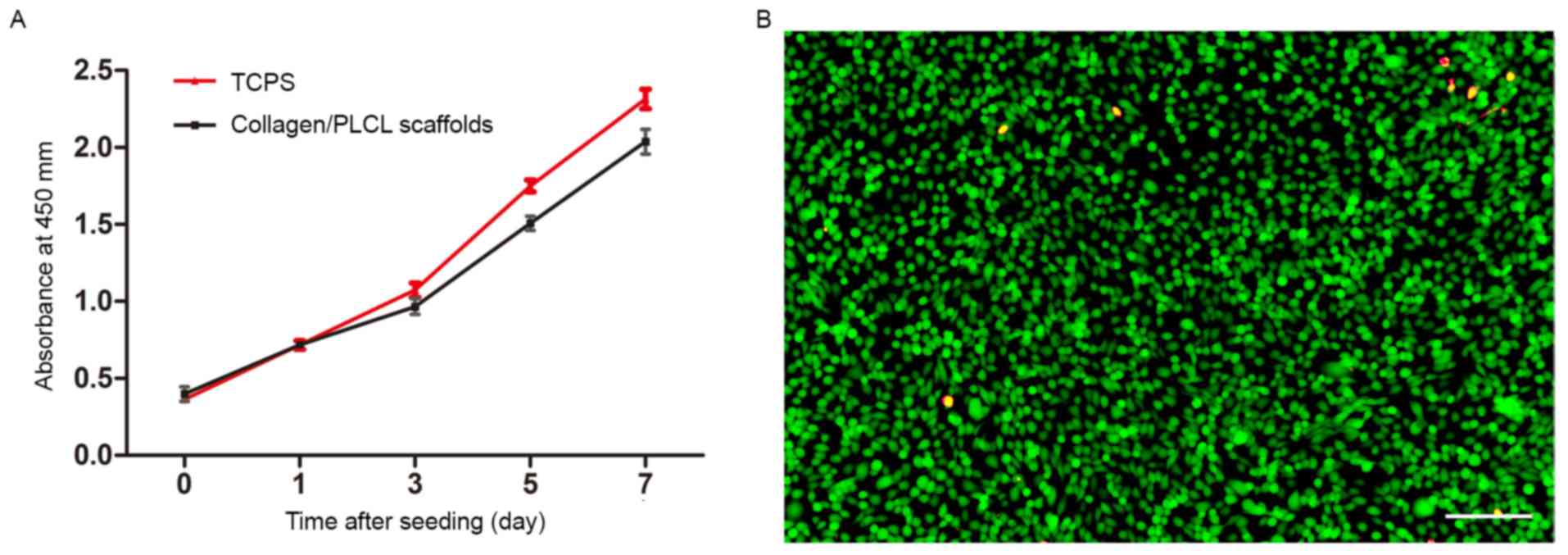
Proliferation and viability of
conjunctival epithelial cells on collagen/PLCL scaffolds
A CCK-8 assay was used to quantify cell
proliferation on collagen/PLCL scaffolds and TCPS (Fig. 3A). Starting from the same seeding
density, one day after cell seeding, cell proliferation was not
significantly different between the scaffolds and TCPS. Although
the proliferation was lower than that of cells on the TCPS,
conjunctival epithelial cells proliferated well and the number of
cells increased with culture time, indicating that the scaffolds
were non-toxic.
The live/dead staining was performed, with live
cells staining green and red color indicating cell death, revealing
only a minor proportion of dead cells on the scaffolds (Fig. 3B). This result further confirmed that
the scaffolds were non-toxic.
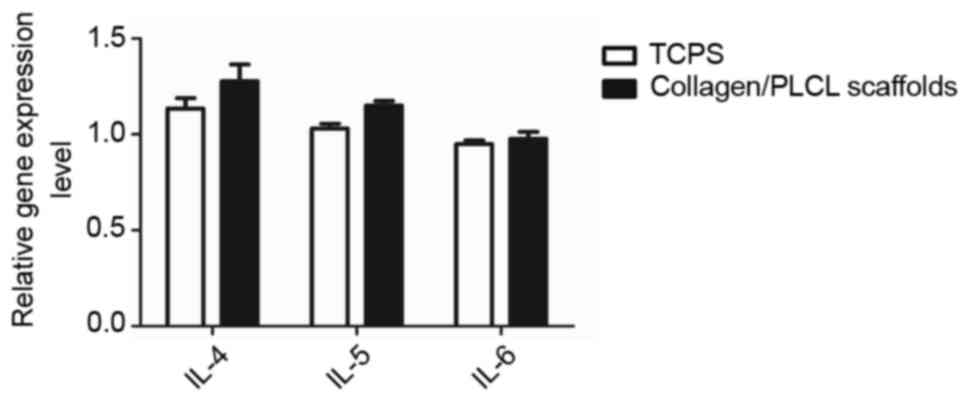
Expression of inflammatory cytokines
by conjunctival epithelial cells on collagen/PLCL scaffolds
It is known that numerous scaffolds induce elevated
expression of inflammatory genes. Using RT-qPCR analysis, is was
demonstrated that IL-4, IL-5 and IL-6 expression between
conjunctival epithelial cells cultured on collagen/PLCL scaffolds
and those cultured on TCPS was not obviously different (Fig. 4), suggesting that the scaffolds may
not elicit any obvious inflammatory responses.
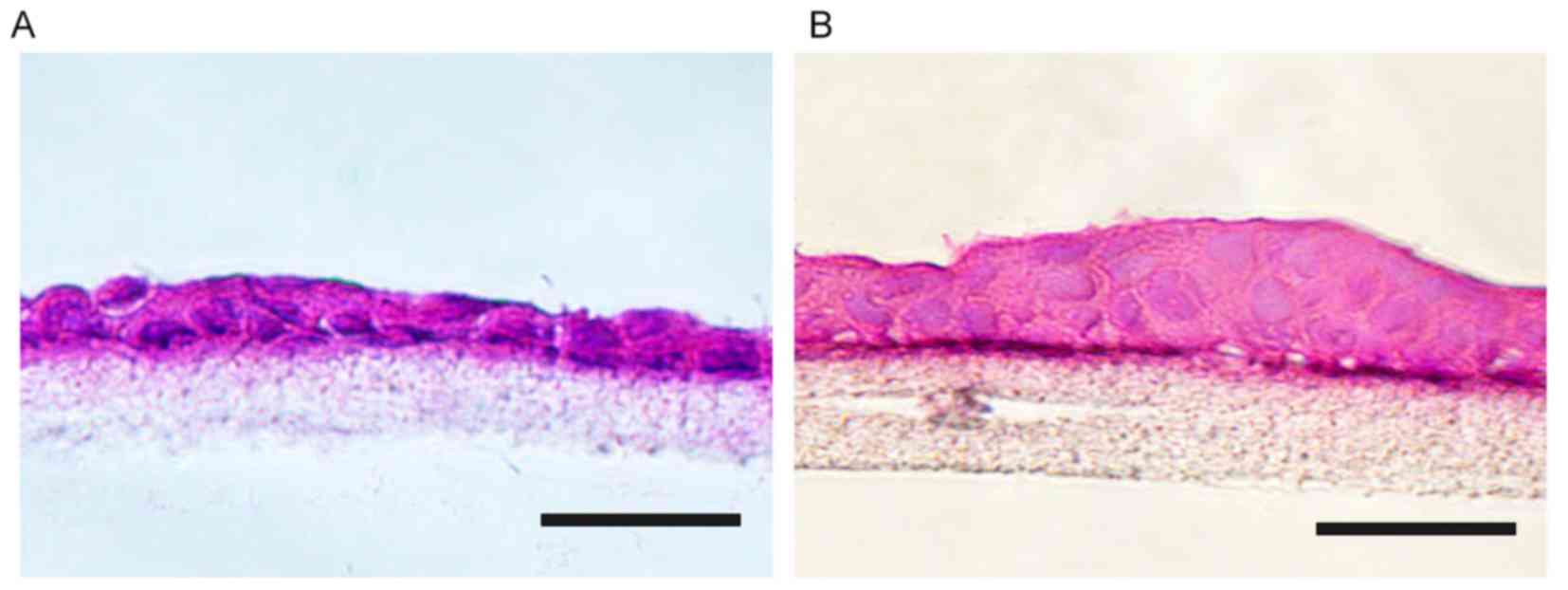
Histological analysis
After 1 or 2 weeks of culture in vitro, cell
stratification of the cell-scaffold complexes was examined by
H&E staining (Fig. 5). The cells
adhered tightly to the upper surface of the scaffolds, and 1–2
stratified epithelial layers were present on the surface of the
scaffolds after 1 week of culture. With the extension of culture
time (2 weeks), cell stratification of the cell-scaffold complexes
(3–4 layers) became more similar to the native conjunctiva.
Discussion
Conjunctiva-associated injuries compromise the
homeostasis and functionality of the ocular surface (22). Therefore, conjunctival tissue
engineering is the prerequisite for successful ocular surface
reconstruction. In the present study, collagen/PLCL scaffolds
fabricated by electrospinning were used for conjunctival
reconstruction, and it was demonstrated that conjunctival
epithelial cells cultured on the scaffolds formed a stratified
epithelium containing proliferative cells and goblet cells.
Cell differentiation largely depends on the
surrounding microenvironment (23).
Therefore, to achieve optimal outcomes in tissue engineering, the
applied biomaterials should imitate the structure and biological
functions of the natural ECM, which is most favorable for tissue
engineering. Recently, electrospinning has attracted increasing
interest for application in fabricating biomimetic nanofibrous
scaffolds due to their structural resemblance to topographic
features of the ECM (24). In the
present study, collagen/PLCL scaffolds were successfully prepared
by electrospinning. SEM analysis revealed that the collagen/PLCL
scaffolds were composed of defect-free nanofibers with a high
porosity to mimic the topographic features of the ECM.
Goblet cells, one of the hallmarks of conjunctival
epithelium, are responsible for the secretion of large gel-forming
mucins in the tear film (25). Mucin
component alterations or goblet cell loss are always found in
patients with conjunctival disorders (26). Therefore, functional restoration of
goblet cells may be a critical procedure for the reconstruction of
the ocular surface. It is commonly accepted that if conjunctival
epithelium is cultured in vitro, is difficult to maintain
the differentiation of goblet cells (5). However, the results of the present
study revealed that collagen/PLCL scaffolds successfully supported
the growth and differentiation of goblet cells. The epithelium
formed on top of the scaffolds expressed markers of goblet cells
detected by gene expression and immunocytochemical analysis. It was
speculated that the beneficial effect of collagen/PLCL scaffolds in
maintaining the differentiation of cells may be attributed to their
structure, which closely mimics the ECM.
Previous studies have examined whether scaffolds
altered the secretion of inflammatory factors by conjunctival
epithelial cells. IL-4, IL-5 and IL-6 are important molecules in
conjunctival inflammation (3,27,28).
The present study found that conjunctival epithelial cells on
collagen/PLCL scaffolds did not increase the expression of IL-4,
IL-5 and IL-6 compared with those obtained under TCPS culture
conditions.
The present study generated collagen/PLCL scaffolds
by electrospinning. The scaffolds showed desirable mechanical
properties, wettability and the ability to promote cell
proliferation. The cultured stratified epithelium displayed a cell
stratification similar to that of native conjunctival epithelium.
The present study suggested that collagen/PLCL may be a desirable
scaffold for the regeneration of conjunctival epithelium.
Acknowledgements
The authors are grateful for the assistance of Mo
Xiumei of Donghua University (Shanghai, China). The study was
supported by the National Natural Science Foundation of China (nos.
81370992, 81570812, 81500765), Shanghai Municipal Education
Commission: Gaofeng Clinical Medicine Grant Support (grant no.
20161421) and Shanghai Municipal Science and Technology Commission:
the Commercialization and Industrialization of Research Findings
Project (grant no. 17411963800).
References
|
1
|
Eidet JR, Fostad IG, Shatos MA, Utheim TP,
Utheim ØA, Raeder S and Dartt DA: Effect of biopsy location and
size on proliferative capacity of ex vivo expanded conjunctival
tissue. Invest Ophthalmol Vis Sci. 53:2897–2903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mason SL, Stewart RM, Kearns VR, Williams
RL and Sheridan CM: Ocular epithelial transplantation: Current uses
and future potential. Regen Med. 6:767–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
García-Posadas L, Arranz-Valsero I,
López-García A, Soriano-Romaní L and Diebold Y: A new human primary
epithelial cell culture model to study conjunctival inflammation.
Invest Ophthalmol Vis Sci. 54:7143–7152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schrader S, Notara M, Beaconsfield M, Tuft
S, Geerling G and Daniels JT: Conjunctival epithelial cells
maintain stem cell properties after long-term culture and
cryopreservation. Regen Med. 4:677–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou H, Lu Q, Guo Q, Chae J, Fan X,
Elisseeff JH and Grant MP: Vitrified collagen-based conjunctival
equivalent for ocular surface reconstruction. Biomaterials.
35:7398–7406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schrader S, Notara M, Beaconsfield M, Tuft
SJ, Daniels JT and Geerling G: Tissue engineering for conjunctival
reconstruction: Established methods and future outlooks. Curr Eye
Res. 34:913–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Selvam S, Thomas PB and Yiu SC: Tissue
engineering: Current and future approaches to ocular surface
reconstruction. Ocul Surf. 4:120–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rama P, Bonini S, Lambiase A, Golisano O,
Paterna P, De Luca M and Pellegrini G: Autologous fibrin-cultured
limbal stem cells permanently restore the corneal surface of
patients with total limbal stem cell deficiency. Transplantation.
72:1478–1485. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu WC, Spilker MH, Yannas IV and Rubin
PA: Inhibition of conjunctival scarring and contraction by a porous
collagen-glycosaminoglycan implant. Invest Ophthalmol Vis Sci.
41:2404–2411. 2000.PubMed/NCBI
|
|
10
|
Ye J, Shi X, Chen X, Xie J, Wang C, Yao K,
Gao C and Gou Z: Chitosan-modified, collagen-based biomimetic
nanofibrous membranes as selective cell adhering wound dressings in
the treatment of chemically burned corneas. J Mater Chem B.
2:4226–4236. 2014. View Article : Google Scholar
|
|
11
|
Chen H, Fan X, Xia J, Chen P, Zhou X,
Huang J, Yu J and Gu P: Electrospun chitosan-graft-poly
(ε-caprolactone)/poly (ε-caprolactone) nanofibrous scaffolds for
retinal tissue engineering. Int J Nanomedicine. 6:453–461.
2011.PubMed/NCBI
|
|
12
|
Zhang K, Wang H, Huang C, Su Y, Mo X and
Ikada Y: Fabrication of silk fibroin blended P(LLA-CL) nanofibrous
scaffolds for tissue engineering. J Biomed Mater Res A. 93:984–993.
2010.PubMed/NCBI
|
|
13
|
Freyria AM, Ronzière MC, Cortial D, Galois
L, Hartmann D, Herbage D and Mallein-Gerin F: Comparative
phenotypic analysis of articular chondrocytes cultured within type
I or type II collagen scaffolds. Tissue Eng Part A. 15:1233–1245.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Fu W, Feng B, Wang H, Liu Z, Yin M,
Wang W and Zheng J: Electrospun collagen-poly(L-lactic
acid-co-ε-caprolactone) membranes for cartilage tissue engineering.
Regen Med. 8:425–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim BS and Mooney DJ: Development of
biocompatible synthetic extracellular matrices for tissue
engineering. Trends Biotechnol. 16:224–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu W, Liu Z, Feng B, Hu R, He X, Wang H,
Yin M, Huang H, Zhang H and Wang W: Electrospun gelatin/PCL and
collagen/PLCL scaffolds for vascular tissue engineering. Int J
Nanomedicine. 9:2335–2344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin A, Zhang K, McClure MJ, Huang C, Wu J,
Fang J, Mo X, Bowlin GL, Al-Deyab SS and El-Newehy M:
Electrospinning collagen/chitosan/poly(L-lactic
acid-co-epsilon-caprolactone) to form a vascular graft: Mechanical
and biological characterization. J Biomed Mater Res A.
101:1292–1301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Yan C, Zhu M, Yao Q, Shao C, Lu W,
Wang J, Mo X, Gu P, Fu Y and Fan X: Electrospun nanofibrous
SF/P(LLA-CL) membrane: A potential substratum for endothelial
keratoplasty. Int J Nanomedicine. 10:3337–3350. 2015.PubMed/NCBI
|
|
19
|
Yao Q, Zhu M, Chen J, Shao C, Yan C, Wang
Z, Fan X, Gu P and Fu Y: Reconstruction of conjunctival
epithelium-like tissue using a temperature-responsive culture dish.
Mol Vis. 21:1113–1121. 2015.PubMed/NCBI
|
|
20
|
Zhang D, Ni N, Chen J, Yao Q, Shen B,
Zhang Y, Zhu M, Wang Z, Ruan J, Wang J, et al: Electrospun SF/PLCL
nanofibrous membrane: A potential scaffold for retinal progenitor
cell proliferation and differentiation. Sci Rep. 5:143262015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gipson IK: The ocular surface: The
challenge to enable and protect vision: The Friedenwald lecture.
Invest Ophthalmol Vis Sci. 48:4390–4398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elisseeff J, Ferran A, Hwang S, Varghese S
and Zhang Z: The role of biomaterials in stem cell differentiation:
Applications in the musculoskeletal system. Stem Cells Dev.
15:295–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vasita R and Katti DS: Nanofibers and
their applications in tissue engineering. Int J Nanomedicine.
1:15–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian L, Qu M, Wang Y, Duan H, Di G, Xie L
and Zhou Q: Inductive differentiation of conjunctival goblet cells
by γ-secretase inhibitor and construction of recombinant
conjunctival epithelium. Exp Eye Res. 123:37–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dogru M, Okada N, Asano-Kato N, Tanaka M,
Igarashi A, Takano Y, Fukagawa K, Shimazaki J, Tsubota K and
Fujishima H: Atopic ocular surface disease: Implications on tear
function and ocular surface mucins. Cornea. 24 (8 Suppl):S18–S23.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng X, Ma P, de Paiva CS, Cunningham MA,
Hwang CS, Pflugfelder SC and Li DQ: TSLP and downstream molecules
in experimental mouse allergic conjunctivitis. Invest Ophthalmol
Vis Sci. 51:3076–3082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barisani-Asenbauer T, Inic-Kanada A, Belij
S, Marinkovic E, Stojicevic I, Montanaro J, Stein E, Bintner N and
Stojanovic M: The ocular conjunctiva as a mucosal immunization
route: A profile of the immune response to the model antigen
tetanus toxoid. PLoS One. 8:e606822013. View Article : Google Scholar : PubMed/NCBI
|