Introduction
Hypertrophic scars are a common type of
post-traumatic tissue repair abnormality (1). Hypertrophic scars formed during wound
healing are mainly characterized by the overexpression of multiple
fibrogenic factors, as well as excessive synthesis and reduced
degradation of extracellular matrix, such as collagen (2). However, their pathogenesis has remained
to be fully elucidated.
Chymase secreted by mast cells is a type of serine
protease and its release activates transforming growth factor
(TGF)-β1 via paracellular secretion stimulation and fibrinolysin
(3–5). A previous study demonstrated that
chymase has a potential role in tissue fibrosis (6). In addition, chymase secreted by mast
cells was found to promote the release of TGF-β1 in the
extracellular matrix of human epithelial and endothelial cells
(7). It was also reported that
chymase facilitates cell proliferation via the TGF-β1 signaling
pathway (8).
The TGF-β1/Smads signaling pathway has been
demonstrated to have important roles in the formation of
hypertrophic scars (9). TGF-β1
stimulates the proliferation of fibroblasts and the production of
collagen, and inhibits matrix degradation, finally leading to the
formation of hypertrophic scars (10). Inhibition of the TGF-β1/Smads
signaling pathway was reported to suppress the formation of
hypertrophic scars (11). TGF-β is a
cytokine that activates the intracellular Smad signaling pathway
and has a key role in promoting wound healing and tissue remodeling
after repair (10). Smad proteins
are among the most important intracellular signal transduction
proteins downstream of the TGF-β superfamily (12–14).
Mast cells exist in hypertrophic scars and have a role in their
formation (15). However, it has
remained elusive whether chymase secreted by mast cells activates
the Smad signaling pathway via TGF-β1 in hypertrophic scars. In the
present study, the role of mast cell chymase in the in hypertrophic
scar fibroblast formation via the TGF-β1/Smads signaling pathway
was investigated.
Materials and methods
Subjects
A total of 5 patients with hypertrophic scars and an
additional 5 patients subjected to repair and reconstruction of
other tissue defects at the Department of Burns and Plastic
Surgery, the First Affiliated Hospital of Xinjiang Medical
University between May 2014 and May 2015 were included in the
present study. The subjects were otherwise healthy and none of them
had received any anti-scar treatment within two years or had any
systemic disease. A piece of tissue was cut from each hypertrophic
scar for histochemical examination in order to further confirm the
validity of the clinical diagnosis (Table I). All procedures were approved by
the Ethics Committee of Xinjiang Medical University (Urumqi,
China). Written informed consent was obtained from all subjects or
their families.
 | Table I.Sources of hypertrophic scars. |
Table I.
Sources of hypertrophic scars.
| Group | Age (years) | Gender | Scar location | Family history | Course of
disease |
|---|
| HSFs | 24 | Female | Chest | − | 9 months |
|
| 31 | Female | Neck | + | 25 months |
|
| 26 | Female | Upper arm | − | 10 months |
|
| 29 | Female | Chest | + | 8 months |
|
| 21 | Female | Ear | − | 14 months |
| NFs | 20 | Male | Abdomen | − | 1 year |
|
| 25 | Female | Abdomen | − | 2 years |
|
| 27 | Female | Shank | − | 6 months |
|
| 41 | Female | Forearm | − | 3 years |
|
| 23 | Female | Forearm | − | 8 months |
Cells
Hypertrophic scar samples were washed with PBS
containing ampicillin and streptomycin (HyClone; GE Healthcare,
Little Chalfont, UK), and epithelial and subcutaneous adipose
tissues were removed. The samples were cut into 1-mm3
pieces and cultured in low-glucose Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum (both from HyClone;
GE Healthcare) at 37°C under 5% CO2. The medium was
refreshed twice a week and finally, hypertrophic scar fibroblasts
were obtained. When the fibroblasts reached 70–80% confluence,
0.25% trypsin (HyClone; GE Healthcare, Logan, UT, USA) was used to
digest the cells, which were then collected split for further
culture. The medium was refreshed every two days, and the growth
speed and morphology of the fibroblasts were observed under an
inverted phase contrast microscope. The cells at passage 3–6 were
used for the experiments.
Immunohistochemical staining
Sample tissues from each group (hypertrophic scar
and normal skin) were enclosed with pathological filter paper,
fixed immediately with 10% buffered formalin at room temperature
overnight, processed through graded alcohols and xylene, embedded
in paraffin, and sectioned at 5-µm thickness (3 replicates for each
specimen). Mast cells and mast cell chymase were
immunohistochemically stained using polyclonal rabbit anti-human
CD117 (1:500; cat no. LS-C20514; c-Kit; LifeSpan BioSciences, Inc.,
Seattle, WA, USA) antibody and polyclonal mouse anti-chymase
antibody (1:500; cat no. kl086Bo01; Gene Company Ltd., Shanghai,
China), respectively, and incubated at 4°C overnight. Samples were
subsequently incubated with horseradish peroxidase-conjugated
anti-rabbit secondary antibody (1:1,000; cat no. GK500705; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) at 37°C for 30
min. Positive expression of CD117 and chymase was indicated by
brown staining of cell membrane and cytoplasm. As a negative
control, PBS was used instead of primary antibodies.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Hypertrophic scar fibroblasts were treated with
different concentrations (0, 15, 30, 60 and 120 ng/ml) of chymase
(cat no. C8118; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
6, 12 and 24 h prior to analysis. Total RNA was extracted from the
cells at the first passage using TRIzol reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Complementary DNA was obtained
by RT using the TIANScript RT kit (Tiangen, Beijing, China) at 37°C
for 15 min, followed by an enzyme inactivation reaction at 98°C for
5 min. The primers were as follows: TGF-β1 (158 bp),
5′-ACACCAACTATTGCTTCAG-3′ (forward) and 5′-TGTCCAGGCTCCAAATG-3′
(reverse); collagen type I, α1 (COL1A1; 147 bp),
5′-CCCGGGTTTCAGAGAGACAACTTC-3′ (forward) and
5′-TCCACATGCTTTATTCCAGCAATC-3′ (reverse); COL3A1 (244 bp),
5′-CTTCTCTCCAGCCGAGCTTC-3′ (forward) and 5′-GTAGTCTCACAGCCTTGCGT-3′
(reverse); angiotensin (197 bp), 5′-CAAGGTGGAGGGTCTCAC-3′ (forward)
and 5′-CTGATGCGGTCATTGCTC-3′ (reverse); β-actin (187 bp),
5′-TGGCACCCACAATGAA-3′ (forward) and
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse). PCR was performed on a
thermo cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using
a QuantiFast SYBR-Green PCR kit (Qiagen, Hilden, Germany) according
to the manufacturer's manual. The PCR reaction protocol was as
follows: Initial denaturation at 95°C for 5 min, followed by 39
cycles of 95°C for 10 sec and 60°C for 30 sec. Quantitative
measurements were performed using the 2−∆∆Cq method
(16), and β-actin was used as an
internal reference. Each sample was measured in triplicate.
Western blot analysis
After washing with ice-cold PBS twice, the 4th
passage of the cells were lysed using pre-cooled
radioimmunoprecipitation assay lysis buffer and
phenylmethanesulfonyl fluoride (both from Thermo Fisher Scientific,
Inc.) for 50 min on ice. The mixture was then centrifuged at 12,000
× g and 4°C for 5 min. The supernatant was used to determine the
protein concentration by using a bicinchoninic acid protein
concentration determination kit (BioTeke Corp., Beijing, China).
After denaturation, the samples were subjected to 12% SDS-PAGE. The
resolved proteins were transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA) on ice and blocked
with 5% skimmed milk at room temperature for 1 h. The membranes
were then incubated with TGF-β1 antibody (1:300 dilution; cat no.
sc-146), Smad7 (1:300 dilution; cat no. sc-11392), Smad4 (1:300
dilution; cat no. sc-7966), GAPDH (1:300 dilution; cat no.
sc-25778) (all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) or p-Smad2/3 (1:1,000 dilution; cat no. 8828s; Cell Signaling
Technology, Inc., Beverly, MA, USA) at 4°C overnight. After washing
with Tris-buffered saline with Tween-20 for 2 h, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:1,000 dilution; cat no. 77054s; Cell Signaling Technology, Inc.)
at room temperature for 2 h. The membrane was then developed using
an enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck
KGaA) for imaging on X-ray film (ChemiDoc MP; Bio-Rad Laboratories,
Inc.). Band intensity was acquired and analyzed using Quantity One
software (version 4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
The results were analyzed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). Values are
expressed as the mean ± standard deviation. Differences between
groups were analyzed using general linear single-factor analysis of
variance and two-tailed t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
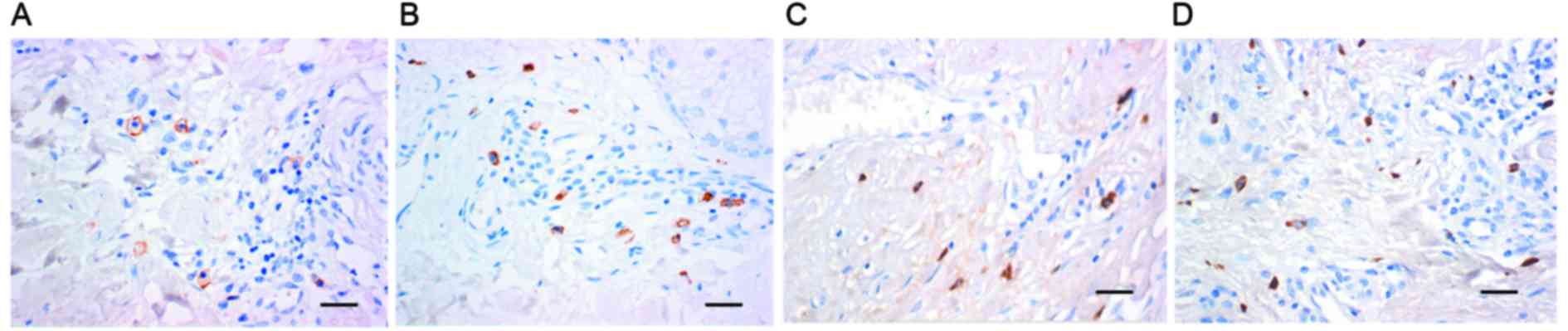
Mast cells and active expression of
chymase are present in hypertrophic scar tissues
To detect the existence of mast cells and mast cell
chymase in hypertrophic scars, immunohistochemistry was employed.
The results demonstrated that in hypertrophic scar tissues, the
number of mast cells, identified by brown staining of membranes,
was greater than that in normal skin tissues (Fig. 1A and B). In addition, chymase
expression in hypertrophic scar tissues was higher than that in
normal skin, as indicated by the higher number of brown particles
in the cell cytoplasm (Fig. 1C and
D). These results suggested that mast cells and active
expression of chymase were present in hypertrophic scar
tissues.
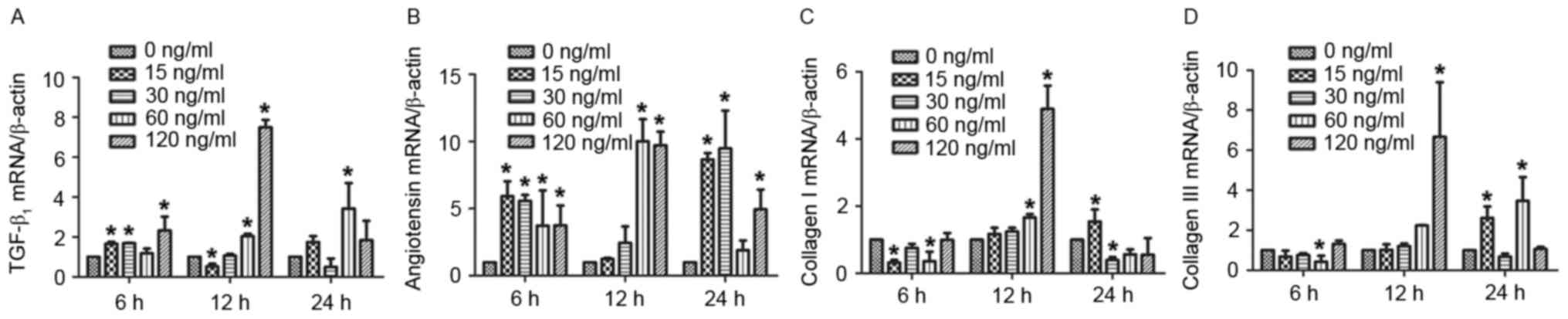
Mast cell chymase promotes the mRNA
expression of TGF-β1, angiotensin and type I and III collagen in
hypertrophic scar fibroblasts in a time- and dose-dependent
manner
To assess the effect of chymase on TGF-β1,
angiotensin, and type I and III collagen mRNA expression in
hypertrophic scar fibroblasts, RT-qPCR was performed. The results
demonstrated that treatment with various concentrations of chymase
for 6, 12 and 24 h increased TGF-β1 mRNA expression compared with
that in the control group, particularly treatment with 120 ng/ml
chymase for 12 h (Fig. 2A). Similar
results were obtained for angiotensin mRNA expression, particularly
after treatment with 60 and 120 ng/ml chymase for 12 h, and 15 and
30 ng/ml chymase for 24 h (Fig. 2B).
Particularly after treatment with 60 and 120 ng/ml chymase for 12
h, type I collagen mRNA expression was enhanced compared with that
in the control cells (Fig. 2C). Type
III collagen mRNA expression was increased after incubation with
various chymase concentrations for 12 or 24 h (Fig. 2D). These results indicated that mast
cell chymase promotes the mRNA expression of TGF-β1, angiotensin,
and type I and III collagen in hypertrophic scar fibroblasts in a
time- and dose-dependent manner.
Treatment with 60 ng/ml mast cell
chymase for 12 h upregulates TGF-β1, P-Smad2/3, Smad4 and Smad7
protein expression in hypertrophic scar fibroblasts
To investigate how chymase affects TGF-β1, Smad4 and
Smad7 protein expression as well as P-Smad2/3 levels, western blot
analysis was performed. The results revealed that treatment with 60
or 120 ng/ml chymase for 12 h significantly enhanced TGF-β1 protein
expression compared with that in the control cells (0 ng/ml
chymase) (P<0.05; Fig. 3A). In
addition, treatment with 60 ng/ml chymase for 12 h significantly
increased P-Smad2/3 levels compared with those in the control cells
(P<0.05; Fig. 3B). Similarly,
treatment with 60 ng/ml chymase for 12 h significantly increased
Smad4 protein expression compared with that in the control
(P<0.05; Fig. 3C). Furthermore,
treatment with 60 or 120 ng/ml chymase for 6 or 12 h significantly
elevated Smad 4 and Smad7 protein expression compared with that in
the control (P<0.05; Fig. 3D and
E). The results suggested that treatment with 60 ng/ml mast
cell chymase for 12 h led to an upregulation of TGF-β1, P-Smad2/3,
Smad4 and Smad7 in hypertrophic scar fibroblasts.
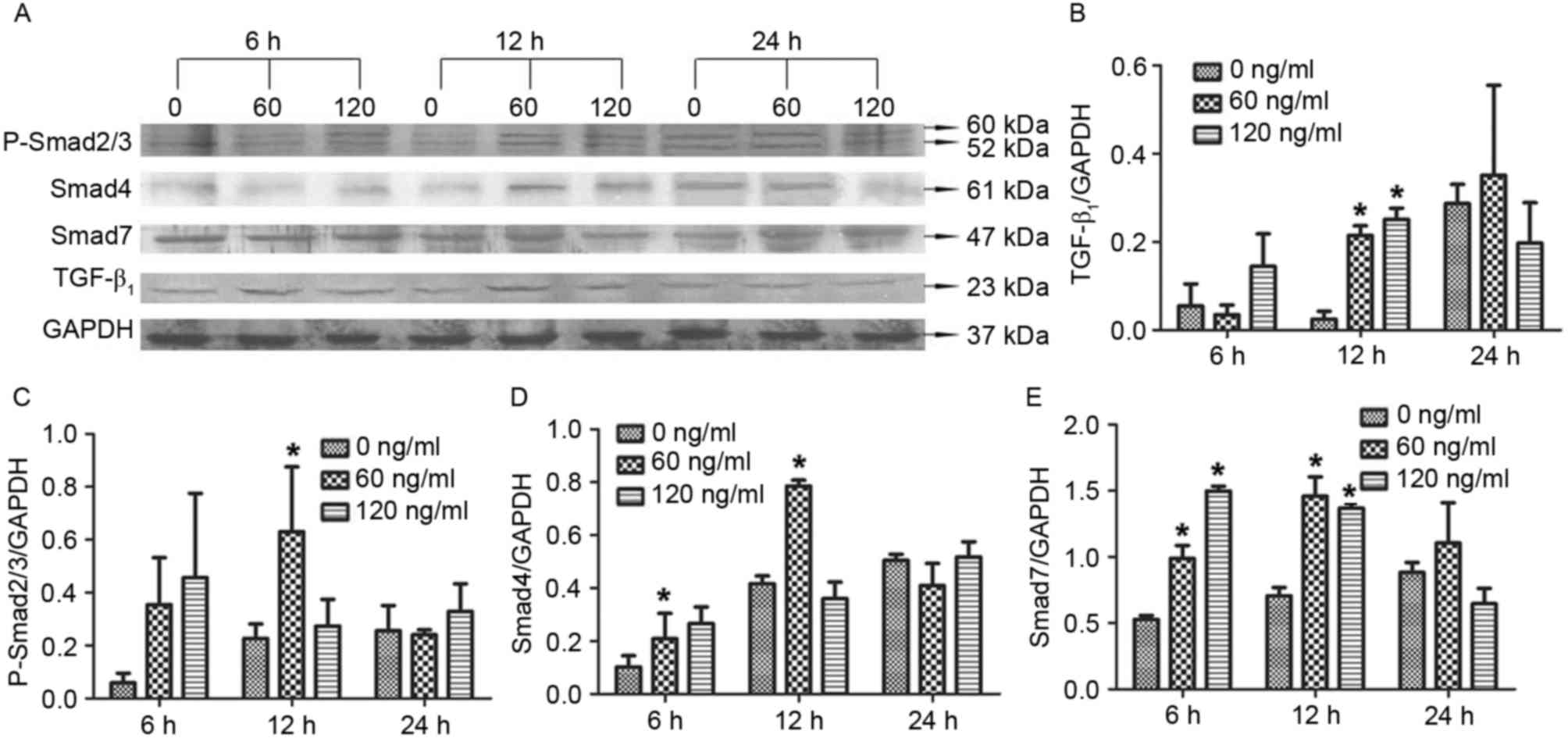 | Figure 3.Effect of chymase on TGF-β1,
P-Smad2/3, Smad4 and Smad7 protein expression in hypertrophic scar
fibroblasts. (A) Western blot image displaying TGF-β1, P-Smad2/3,
Smad4 and Smad7 protein levels after treatment with different
concentrations of chymase (0, 60 or 120 ng/ml) for 6, 12 or 24 h.
Protein expression of (B) TGF-β1, (C) P-Smad2/3, (D) Smad4 and (E)
Smad7 normalized to GAPDH. All experiments were performed in
triplicate. Values are expressed as the mean ± standard deviation.
*P<0.05 compared with control (0 ng/ml chymase). TGF,
transforming growth factor; P-SMAD2/3, phosphorylated SMAD2/3. |
Discussion
Hypertrophic scars are a type of skin tissue
fibrosis mediated by inflammatory cells. In the formation stage of
hypertrophic scars, an increase in the mast cell number is
positively correlated with the severity of fibrosis (17). Compared with normal skin, active
transmitters associated with mast cells are significantly
increased, suggesting that mast cells and their transmitters are
key factors for promoting hypertrophic scar formation (17). Chymase secreted by mast cells
participates in the formation of skin matrix (18,19).
Castagnoli et al (20)
demonstrated that serine proteinase secreted by mast cells
facilitates the proliferation of hypertrophic scar fibroblasts by
eliminating contact inhibition, and promotes collagen synthesis,
secretion and deposition in extracellular matrix. Mature mast cells
are only present around blood vessels, or in the skin and mucus
membranes, with specific phenotypes. CD117 (c-Kit) is an important
receptor on the mast cell surface that regulates its function. In
the present study, immunohistochemical staining demonstrated that
the number of mast cells and the level of chymase activity in
hypertrophic scars are significantly higher than those in normal
skin, suggesting that mast cell chymase is present and active in
hypertrophic scars.
Chymase has been demonstrated to induce the
proliferation of cardiac fibroblasts, and to have important roles
in the development of fibrosis in the lungs and heart via
angiotensin II (21,22). Angiotensin II is an important growth
factor in the renin-angiotensin system. The renin-angiotensin
system exists in the skin, and hypertrophic scar fibroblasts
express angiotensin II receptors. The present study revealed that
chymase upregulated angiotensin expression in fibroblasts isolated
from hypertrophic scars. TGF-β stimulates the differentiation and
proliferation of fibroblasts by regulating the synthesis and
deposition of extracellular matrix. It inhibits the production of
collagenase and increases the production of collagenase inhibitors,
finally leading to the sustained growth of hypertrophic scars.
Overexpression of TGF-β1 is a reason for the formation of
hypertrophic scars (23,24). Ghahary et al (25) reported that the level of TGF-β1 mRNA
in hypertrophic scars is 61% higher than that in normal skin.
Tredget et al (26)
demonstrated that the mRNA and protein expression of TGF-β1 in
hypertrophic scars and their fibroblasts is significantly higher
than that in normal skin and its cells. Similarly, the results of
the present study demonstrated that mast cell chymase enhances the
expression of TGF-β1 in hypertrophic fibroblasts. Type I and III
collagen is the main interstitial collagen that has important roles
in the structural composition of extracellular matrix (26,27).
Verhaegen et al (27)
demonstrated that excessive deposition of collagen promotes the
formation of hypertrophic scars. The results of the present study
revealed that mast cell chymase (60 and 120 ng/ml) increases the
expression of type I and III collagen in hypertrophic scar
fibroblasts. The present study also demonstrated that mast cell
chymase enhances P-Smad2/3 as well as the protein expression of
Smad4 and Smad7. The phosphorylation of Smad2/3 is a key step and
marker in the Smad signaling pathway (28–30).
Enhanced expression of Smad4 positively regulates the signaling
pathway and activates downstream effector molecules (29,30).
Smad7 is activated by TGF-β and exerts its biological effects
together with TGF-β (29,30).
In conclusion, the present study demonstrated that
mast cell chymase is present in hypertrophic scars with active
expression. Mast cell chymase activates TGF-β1 and facilitated the
synthesis of collagen in hypertrophic scar fibroblasts in
vitro. In addition, chymase activated the Smads signaling
pathway and enhanced Smad protein expression. Therefore, it is
concluded that mast cell chymase has important roles in the
formation of hypertrophic scars through the TGF-β1/Smads signaling
pathway. The present study provided a basis for research into the
treatment of hypertrophic scars by inhibiting the activation and
release of mast cell chymase in the future.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant no. 81260291) and the Youth
Science Foundation of the First Affiliated Hospital of Xinjiang
Medical University (grant no. 2012QN02).
References
|
1
|
Beldon P: Abnormal scar formation in wound
healing. Nurs Times. 96:44–45. 2000.PubMed/NCBI
|
|
2
|
Shi Y and Massagé J: Mechanisms of
TGF-beta signaling from cell membrance to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douaiher J, Succar J, Lancerotto L, Gurish
MF, Orgill DP, Hamilton MJ, Krilis SA and Stevens RL: Development
of mast cells and importance of their tryptase and chymase serine
proteases in inflammation and wound healing. Adv Immunol.
122:211–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao XY, Zhao LY, Zheng QS, Su JL, Guan H,
Shang FJ, Niu XL, He YP and Lu XL: Chymase induces profibrotic
response via transforming growth factor-beta 1/Smad activation in
rat cardiac fibroblasts. Mol Cell Biochem. 310:159–166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lindstedt KA, Wang Y, Shiota N, Saarinen
J, Hyytiäinen M, Kokkonen JO, Keski-Oja J and Kovanen PT:
Activation of paracrine TGF-beta1 signaling upon stimulation and
degranulation of rat serosal mast cells: A novel function for
chymase. FASEB J. 15:1377–1388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muto T and Fukami H: Recent chymase
inhibitors and their effects in in vivo models. IDrugs.
5:1141–1150. 2002.PubMed/NCBI
|
|
7
|
Taipale J, Lohi J, Saarinen J, Kovanen PT
and Keski-Oja J: Human mast cell chymase and leukocyte elastase
release latent transforming growth factor-beta 1 from the
extracellular matrix of cultured human epithelial and endothelial
cells. J Biol Chem. 270:4689–4696. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Algermissen B, Hermes B,
Feldmann-Boeddeker I, Bauer F and Henz BM: Mast cell chymase and
tryptase during tissue turnover: Analysis on in vitro mitogenesis
of fibroblasts and keratinocytes and alterations in cutaneous
scars. Exp Dermatol. 8:193–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan SQ, Cai JL, Qin LY, Wang ZH, Liu ZZ
and Sun ML: Effect of heparin on production of transforming growth
factor (TGF)-beta1 and TGF-beta1 mRNA expression by human normal
skin and hyperplastic scar fibroblasts. Ann Plast Surg. 60:299–305.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cowin AJ, Holmes TM, Brosnan P and
Ferguson MW: Expression of TGF-beta and its receptors in murine
fetal and adult dermal wounds. Eur J Dermatol. 11:424–431.
2001.PubMed/NCBI
|
|
11
|
Walraven M, Gouverneur M, Middelkoop E,
Beelen RH and Ulrich MM: Altered TGF-β signaling in fetal
fibroblasts: What is known about the underlying mechanisms? Wound
Repair Regen. 22:3–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor beta in human disease. N Engl J Med.
342:1350–1358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto T, Hartmann K, Eckes B and Krieg
T: Role of stem cell factor and monocyte chemoattrctant protein in
the interaction between fibroblasts and mast cells in fibrosis. J
Dermatol Sci. 26:106–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu H, Bock O, Bayat A, Ferguson MW and
Mrowietz U: Decreased expression of inhibitory SMAD6 and SMAD7 in
keloid scarring. J Plast Reconstr Aesthet Surg. 59:221–229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hermes B, Feldmann-Böddeker I, Welker P,
Algermissen B, Steckelings MU, Grabbe J and Henz BM: Altered
expression of mast cell chymase and tryptase and of c-Kit in human
cutaneous scar tissue. J Invest Dermatol. 114:51–55. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller HR, Wright SH, Knight PA and
Thornton EM: A novel function for transforming growth factor-beta1:
Upregulation of the expression and the IgE-independent
extracellular release of a mucosal mast cell granule-specific
beta-chymase, mouse mast cell protease-1. Blood. 93:3473–3486.
1999.PubMed/NCBI
|
|
19
|
Moyer KE, Saggers GC and Ehrlich HP: Mast
cells promote fibroblast populated collagen lattice contraction
through gap junction intercellular communication. Wound Repair
Regen. 12:269–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castagnoli C, Stella M, Berthod C,
Magliacani G and Richiardi PM: TNF production and hypertrophic
scarring. Cell Immunol. 147:51–63. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakaguchi H, Takai S, Sakaguchi M,
Sugiyama T, Ishihara T, Yao Y, Miyazaki M and Ikeda T: Chymase and
angiotensin converting enzyme activities in a hamster model of
glaucoma filtering surgery. Curr Eye Res. 24:325–331. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Younan G, Suber F, Xing W, Shi T, Kunori
Y, Abrink M, Pejler G, Schlenner SM, Rodewald HR, Moore FD Jr, et
al: The inflammatory response following an epidermal burn depends
on the activities of mouse mast cell proteases 4 and 5. J Immunol.
185:7681–7690. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan JM, Ng YY, Hill PA, Nikolic-Paterson
DJ, Mu W, Atkins RC and Lan HY: Transforming growth factor-beta
regulates tubular epithelial-myofibroblast transdiffetrntiation in
vitro. Kideny Int. 56:1455–1467. 1999. View Article : Google Scholar
|
|
24
|
Willis BC and Borok Z: TGF-beta-induced
EMT: Mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghahary A, Shen YJ, Scott PG, Gong Y and
Tredget EE: Enhanced expression of mRNA for transforming growth
factor-beta, type I and type III procollagen in human postburn
hypertrophic scar tissues. J Lab Clin Med. 122:465–473.
1993.PubMed/NCBI
|
|
26
|
Tredget EE, Wang R, Shen Q, Scott PG and
Ghahary A: Transforming growth factor-beta mRNA and protein in
hypertrophic scar tissues and fibroblasts: Antagonism by IFN-alpha
and IFN-gamma in vitro and in vivo. J Interferon Cytokine Res.
20:143–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verhaegen PD, van Zuijlen PP, Pennings NM,
van Marle J, Niessen FB, van der Horst CM and Middelkoop E:
Differences in collagen architecture between keloid, hypertrophic
scar, normotrophic scar, and normal skin: An objective
histopathological analysis. Wound Repair Regen. 17:649–656. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tao SJ and Sampath K: Alternative splicing
of SMADs in differentiation and tissue homeostasis. Dev Growth
Differ. 52:335–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dabiri G, Campaner A, Morgan JR and Van De
Water L: A TGF-beta1-dependent autocrine loop regulates the
structure of focal adhesions in hypertrophic scar fibroblasts. J
Invest Dermatol. 126:963–970. 2006. View Article : Google Scholar : PubMed/NCBI
|