|
1
|
Lee VW, Schwander B and Lee VH:
Effectiveness and cost-effectiveness of erlotinib versus gefitinib
in first-line treatment of epidermal growth factor
receptor-activating mutation-positive non-small-cell lung cancer
patients in Hong Kong. Hong Kong Med J. 20:178–186. 2014.PubMed/NCBI
|
|
2
|
Chen WQ, Zheng RS, Zeng HM, Zou XN, Zhang
SW and He J: Report of cancer incidence and mortality in China,
2011. China Cancer. 24:1–10. 2015. View Article : Google Scholar
|
|
3
|
Li S, Zhang X, Yan Y, Wang K, Rui D, Pang
L and Li F: High cancer burden in elderly chinese, 2005–2011. Int J
Environ Res Public Health. 12:12196–12211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim
SW, Jung SH, Park YH, Ahn JS, Park K and Ahn MJ: Randomized phase
II study of gefitinib versus erlotinib in patients with advanced
non-small cell lung cancer who failed previous chemotherapy. Lung
Cancer. 75:82–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ellis PM, Coakley N, Feld R, Kuruvilla S
and Ung YC: Use of the epidermal growth factor receptor inhibitors
gefitinib, erlotinib, afatinib, dacomitinib and icotinib in the
treatment of non-small-cell lung cancer: A systematic review. Curr
Oncol. 22:e183–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Sheng Z, Zhang Y and Li G: The
Efficacy of epidermal growth factor receptor tyrosine kinase
inhibitors for molecularly selected patients with non-small cell
lung cancer: A meta-analysis of 30 randomized controlled trials.
Targ Oncol. 11:49–58. 2016. View Article : Google Scholar
|
|
7
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, et al: Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced
non-small-cell lung cancer. J Clin Oncol. 21:2237–2246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thatcher N, Chang A, Parikh P, Pereira
Rodrigues J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH,
Pemberton K, Archer V and Carroll K: Gefitinib plus best supportive
care in previously treated patients with refractory advanced
non-small-cell lung cancer: Results from a randomised,
placebo-controlled, multicentre study (iressa survival evaluation
in lung cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shepherd FA, Pereira Rodrigues J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Togashi Y, Masago K, Fujita S, Hatachi Y,
Fukuhara A, Nagai H, Sakamori Y, Kim YH, Mio T and Mishima M:
Differences in adverse events between 250 mg daily gefitinib and
150 mg daily erlotinib in Japanese patients with non-small cell
lung cancer. Lung Cancer. 74:98–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan WC, Yu CJ, Tsai CM, Huang MS, Lai CL,
Hsia TC, Tien YJ, Huang SF, Wu CH, Chou KT, et al: Different
efficacies of erlotinib and gefitinib in taiwanese patients with
advanced non-small cell lung cancer: A retrospective multicenter
study. J Thorac Oncol. 6:148–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urata Y, Katakami N, Morita S, Kaji R,
Yoshioka H, Seto T, Satouchi M, Iwamoto Y, Kanehara M, Fujimoto D,
et al: Randomized phase III study comparing gefitinib with
erlotinib in patients with previously treated advanced lung
adenocarcinoma: WJOG 5108 L. J Clin Oncol. 34:3248–3257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
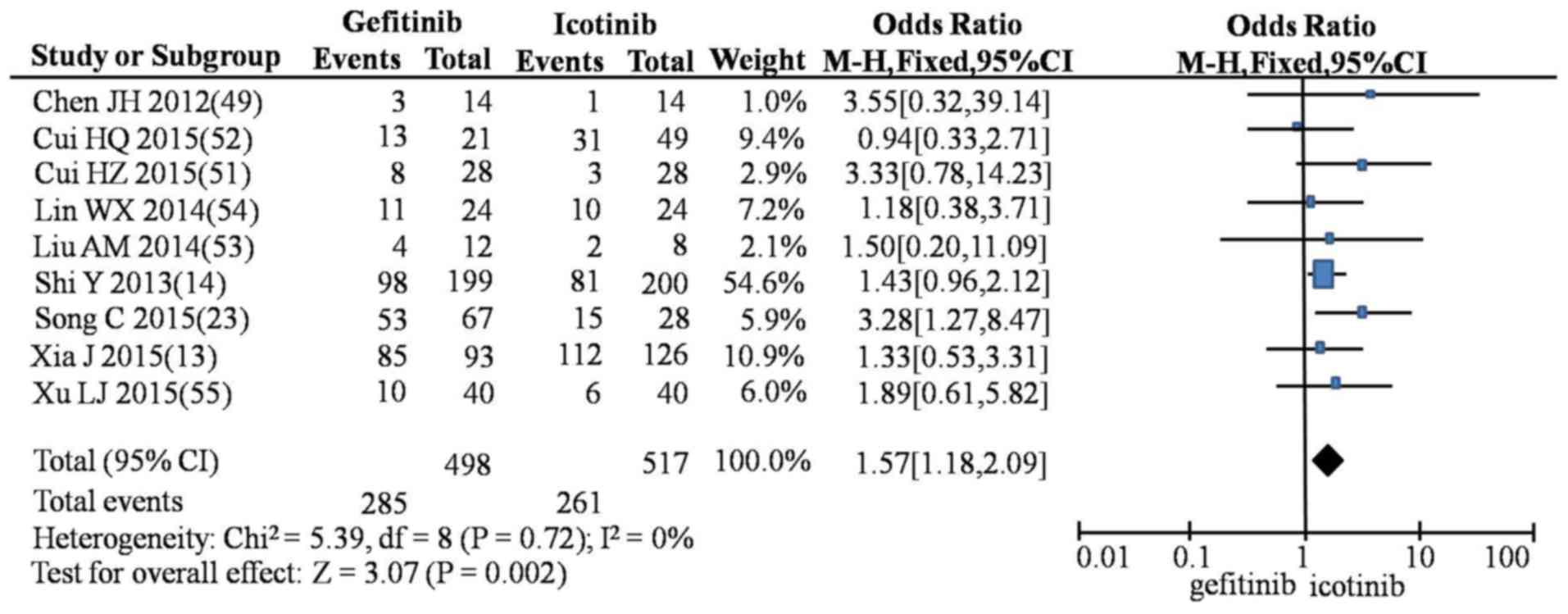
Xia J, Si RR and Wu YF: Clinical research
on icotinib hydrochloride in the treatment of advanced non-small
cell lung cancer. J Basic Clin Oncol. 28:210–212. 2015.
|
|
14
|
Shi Y, Zhang L, Liu X, Zhou C, Zhang L,
Zhang S, Wang D, Li Q, Qin S, Hu C, et al: Icotinib versus
gefitinib in previously treated advanced non-small-cell lung cancer
(ICOGEN): A randomised, double-blind phase 3 non-inferiority trial.
Lancet Oncol. 14:953–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YP: Evidence-based Medicine. People's
Medical Publishing House; Beijing: 2014, View Article : Google Scholar
|
|
16
|
Zeng XT, Bao CP, Cao SY and Liu JY: The
quality assessments for randomized controlled trials. Chin J Evid
Based Cardiovasc Med. 4:183–184. 2012.
|
|
17
|
Zeng XT, Liu H, Chen X and Leng WD: The
quality assessments for observational study. Chin J Evid Based
Cardiovasc Med. 4:297–299. 2012.
|
|
18
|
Yuhara H, Steinmaus C, Cohen SE, Corley
DA, Tei Y and Buffler PA: Is diabetes mellitus an independent risk
factor for colon cancer and rectal cancer? Am J Gastroenterol.
106:1911–1921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the United States, national cancer institute of
Canada. J Nati Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
20
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi YX, Zhang W, Liu XY, Zhang J, Zhu DJ
and Lv QY: Result interpretation of network meta-analysis. Chin J
Evidence Based Med. 15:103–109. 2015.
|
|
22
|
Wang HY and Zhang DF: Comparison of the
efficacy of gefitinib and erlotinib as a second line treatment for
advanced non-small cell lung cancer. J Practical Med. 28:3444–3446.
2012.
|
|
23
|
Song C, Xu LY, Qiao JJ, Li M, Zhao JB and
Sun LM: Efficacy and safety of EGFR-TKIs as first-line treatment in
112 elder patients with advanced non-small cell lung cancer. J
Dalian Med Univ. 37:282–285. 2015.
|
|
24
|
Wu X, Zhang HY, Lv WZ and Lin Z:
Comparation of clinical effects and safety between gefitinib and
erlotinib in treatment of patients with NSCLC. Chin J of
Misdiagnostics. 11:3534–3536. 2011.
|
|
25
|
Yuan HF: Comparison of clinical
effectiveness of gefitinib and erlotinib on non-small cell lung
cancer. Sci and Tech of West China. 14:108–109. 2015.
|
|
26
|
Qu WF: Clinical effect of erlotinib on non
- small cell lung cancer and its impact on immunoglobulin levels
and T-lymphocyte subsets. Practical J of Cardiac Cerebral Pneumal
and Vascular Disease. 23:73–75. 2015.
|
|
27
|
Wang YX: Comparation of the efficacy and
care between gefitinib and erlotinib for the patients with NSCLC.
Medical Inform. 27:174–175. 2014.
|
|
28
|
Xie Y, Liang J and Su N: Gefitinib versus
erlotinib as first-line treatment for patients with advanced EGFR
mutation-positive non-small-cell lung cancer. Nan Fang Yi Ke Da Xue
Xue Bao. 35:446–449. 2015.(In Chinese). PubMed/NCBI
|
|
29
|
Xie YK: The clinical effects and safety of
gefitinib for patients with non-small cell lung cancer. Yiayao
Qianyan. 4:192. 2014.
|
|
30
|
Weng XL: Clinical effect and
pharmacoeconomics of gefitinib and erlotinib in advanced
non-small-cell lung cancer. Chin Med Pharm. 5:100–102. 2015.
|
|
31
|
Zhang J, Liu SQ, Zhang J, Ban LY and Zhou
T: Effect and cost-efficacy analysis of the second-line treatment
of advanced non-small cell lung cancer. Chin Clin Oncol.
17:908–911. 2012.
|
|
32
|
Zhang CW: The observation of toxicities
for targeted therapy in advanced non-small cell lung cancer. Hainan
Med J. 25:2273–2274. 2014.
|
|
33
|
Chen XP, Hang XS, Gao X, Xu WH, Li C and
Zhao J: Adverse drug reaction of gefitinib in therapy for patients
with advanced non-small cell lung cancer. Chin J of Hemorheology.
19:579–582. 2009.
|
|
34
|
Zhang YQ, Li YP, Ni J and Liu GL: Clinical
effect and pharmacoeconomics of gefitinib and erlotinib in advanced
non-small-cell lung cancer. Chin J New Drugs Clin Rem. 28:837–840.
2009.
|
|
35
|
Zhang YJ, Li HB, Li XD, Liu XC and Han JC:
Clinical effect and safety of gefitinib and erlotinib second line
treatment of lung adenocarcinoma. Chin J Clin Pharmacol.
31:899–901. 2015.
|
|
36
|
Li YY, Li L and Lv EJ: The comparison of
efficacy between gefitinib and erlotinib for patients with brain
metastases from non-small cell lung cancer. Chin J Clin Res.
28:1308–1311. 2015.
|
|
37
|
Bai H, Xiong LW, Han BH and Jiang LY:
Clinical observation of gefitinib and erlotinib for brain
metastases of non-small cell lung cancer. Chin Clin Oncol.
20:1028–1031. 2015.
|
|
38
|
Li L: Clinical observation of 70 patients
with brain metastases. Dalian Medical Univ. 2013.
|
|
39
|
Zhang JX, Cai D, Li SY, Zhou CZ, Qin YY
and Ouyang M: Clinical comparison of erlotinib and gefitinib in
non-small cell lung cancer with brain metastases. Chin J Cancer
Prevent Treat. 22:285–288. 2015.
|
|
40
|
Ma Y, Huang Y, Zhao H, Liu J, Chen L, Wu H
and Zhou N: The cost-effectiveness analysis of gefitinib or
erlotinib in the treatment of advanced EGFR mutant non-small cell
lung cancer patients. Zhongguo Fei Ai Za Zhi. 16:203–210. 2013.(In
Chinese). PubMed/NCBI
|
|
41
|
Shao YY, Shau WY, Lin ZZ, Chen HM, Kuo R,
Yang JC and Lai MS: Comparison of gefitinib and erlotinib
efficacies as third-line therapy for advanced non-small-cell lung
cancer. Eur J Cancer. 49:106–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lim SH, Lee JY, Sun JM, Ahn JS, Park K and
Ahn MJ: Comparison of clinical outcomes following gefitinib and
erlotinib treatment in non-small-cell lung cancer patients
harboring an epidermal growth factor receptor mutation in either
exon 19 or 21. J Thorac Oncol. 9:506–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida T, Yamada K, Azuma K, Kawahara A,
Abe H, Hattori S, Yamashita F, Zaizen Y, Kage M and Hoshino T:
Comparison of adverse events and efficacy between gefitinib and
erlotinib in patients with non-small-cell lung cancer: A
retrospective analysis. Med Oncol. 30:3492013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hong J, Kyung SY, Lee SP, Park JW, Jung
SH, Lee JI, Park SH, Sym SJ, Park J, Cho EK, et al: Pemetrexed
versus gefitinib versus erlotinib in previously treated patients
with non-small cell lung cancer. Korean J Intern Med. 25:294–300.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Emery IF, Battelli C, Auclair PL, Carrier
K and Hayes DM: Response to gefitinib and erlotinib in non-small
cell lung cancer: A retrospective study. BMC Cancer. 9:3332009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu WS, Chen YM, Tsai CM, Shih JF, Chiu CH,
Chou KT, Lai SL, Wu CH, Luo YH, Huang CY, et al: Erlotinib has
better efficacy than gefitinib in adenocarcinoma patients without
EGFR-activating mutations, but similar efficacy in patients with
EGFR-activating mutations. Exp Ther Med. 3:207–213. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu JY, Wu SG, Yang CH, Chang YL, Chang YC,
Hsu YC, Shih JY and Yang PC: Comparison of gefitinib and erlotinib
in advanced NSCLC and the effect of EGFR mutations. Lung Cancer.
72:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim ST, Lee J, Kim JH, Won YW, Sun JM, Yun
J, Park YH, Ahn JS, Park K and Ahn MJ: Comparison of gefitinib
versus erlotinib in patients with nonsmall cell lung cancer who
failed previous chemotherapy. Cancer. 116:3025–3033. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen JH, Luo YZ, Wang W, Zhou WW and Wen
XP: Efficacy and toxicity of ecotinib and gefitinib in the
treatment for 28 patients with non-small cell lung cancer who have
failed previous chemotherapy. Chin J New Drugs. 21:2056–2059.
2012.
|
|
50
|
Chen JH, Luo YZ, Wang W, Zhou WW and Wen
XP: A phase III study on icotinib hydrochloride for non-small cell
lung cancer. Anti-Tumor Pharmacy. 1:441–443. 2011.
|
|
51
|
Cui HZ, Guan JZ, Liao GQ, Liu PH, Li LL
and Shao Y: Efficacy of icotinib and gefitinib in advanced lung
adenocarcinoma with EGFR mutation. Acad J Chin Pla Med School.
36:326–328, 341. 2015.
|
|
52
|
Cui HQ, Liu HF and Lv JR: Icotinib in
treatment for 49 patients with non small cell lung cancer in
epidermal growth factor receptor mutant. Chin J New Drugs Clin Rem.
34:556–559. 2015.
|
|
53
|
Liu AM and Liu HQ: The observation and
care of EGFR-TKIs therapy for patients with advanced non-small cell
lung cancer. Med Inf. 27:175–176. 2014.
|
|
54
|
Lin WX and Zhang LJ: Clinical observation
of icotinib for non-small cell lung cancer. Chin J Prim Med Pharm.
21:106–107. 2014. View Article : Google Scholar
|
|
55
|
Xu LJ, Liu H, Li J and Gao Y: Clinical
observation of icotinib and gefitinib in first-line therapy for
advanced non-small cell lung cancer. J Hunan Normal Univ (Med Sci).
12:94–97. 2015.
|
|
56
|
Huang Y: Clinical observation of icotinib
hydrochloride and erlotinib in the treatment of patients with
advanced lung cancer who failed previous chemotherapy. Chin Foreign
Med Treat. 33:8–9. 2014.
|
|
57
|
Sun Y, Song C, Li M, Zhao JB and Sun LM:
The comparison of efficacy between icotinib and erlotinib for
patients with advanced non-small cell lung cancer. Shandong Med J.
55:34–36. 2015.
|
|
58
|
Zhang JX, Yu X, Zhang BB, Guan Q, Chen X,
Zhang Z, Yang BJ and Zhao MF: Comparison of clinical effects and
safety between icotinib and erlotinib in treatment of advanced
non-small cell lung cancers. J of Chin Phy. 17:1032–1035. 2015.
|
|
59
|
Hu X, Zhang L, Shi Y, Zhou C, Liu X, Wang
D, Song Y, Li Q, Feng J, Qin S, et al: The efficacy and safety of
icotinib in patients with advanced non-small cell lung cancer
previously treated with chemotherapy: A single-arm, multi-center,
prospective study. PLoS One. 10:e01425002015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shao YY, Lin CC and Yang CH: Gefitinib or
erlotinib in the treatment of advanced non-small cell lung cancer.
Discov Med. 9:538–545. 2010.PubMed/NCBI
|
|
61
|
Niu M, Hu J, Wu S, Xiaoe Z, Xu H, Zhang Y,
Zhang J and Yang Y: Structural bioinformatics-based identification
of EGFR inhibitor gefitinib as a putative lead compound for BACE.
Chem Biol Orug Des. 83:81–88. 2014.
|
|
62
|
Takeda M, Okamoto I, Fukuoka M and
Nakagawa K: Successful treatment with erlotinib after
gefitinib-related severe hepatotoxicity. J Clin Oncol. 28:e273–274.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Q and Zhou W: The new choice for
non-small cell lung cancer patients - iootinib hydrochloride. J
Pharmaceuti Res. 32:121–124. 2013.
|
|
64
|
Peters S, Zimmermann S and Adjei AA: Oral
epidermal growth factor receptor tyrosine kinase inhibitors for the
treatment of non-small cell lung cancer: Comparative
pharmacokinetics and drug-drug interactions. Cancer Treat Rev.
40:917–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan
DM and Song Y: Skin rash could predict the response to EGFR
tyrosine kinase inhibitor and the prognosis for patients with
non-small cell lung cancer: A systematic review and meta-analysis.
PLoS one. 8:e551282013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li HR and Sun YF: The severe adverse
events and prevention measures for erlotinib. Chin J
Pharmacoepidemiol. 19:232–233. 2010.
|