Introduction
Lung cancer is the one of the leading causes of
cancer-associated mortality worldwide (1). The majority (~80–85%) of patients with
lung cancer patients have non-small cell lung cancer (NSCLC) and
70% of patients with NSCLC are at an advanced stage by the time of
diagnosis (1). In China, lung cancer
was a common type of cancer (48.32/100,000) and cause of
cancer-associated mortality (39.27/100,000) in 2011 (2). The burden created by elderly Chinese
patients with lung cancer is high (3). For patients with NSCLC who cannot
undergo surgery due to having an advanced stage of the disease,
platinum-based combination chemotherapy has become the primary
treatment (4,5). However, in the majority of patients
with NSCLC, the disease will eventually progress despite
combination therapy (4). Therefore,
drugs with better efficacies for the treatment of advanced NSCLC
are required.
During the last decade, the development of molecular
targeted drugs has increased the effectiveness of NSCLC therapy
(5). Epidermal growth factor
receptor tyrosine kinase inhibitors (EGFR-TKIs), including
gefitinib, erlotinib and icotinib, have been demonstrated to be
effective for the treatment of advanced NSCLC with few adverse
effects, particularly in patients with NSCLC harboring EGFR
mutations (5,6). A multi-institutional randomized phase
II trial demonstrated that gefitinib had effective clinical
antitumor activity and provided symptomatic relief in patients with
NSCLC (7). However, the ISEL study
reported that treatment with gefitinib did not significantly
improve the survival of patients with NSCLC (8). The BR.21 clinical trial reported that
erlotinib, another EGFR-TKI, could prolong survival in patients
with NSCLC (9). Although the
gefitinib and erlotinib have similar molecular and chemical
structures, studies have reported different effects of the two
drugs on the survival of patients with NSCLC (8,9).
Furthermore, other studies have reported that, compared with
gefitinib, erlotinib possesses an improved disease control rate and
prolongs progression-free survival with increased median survival
time, but has more adverse effects, in patients with NSCLC
(1,10,11). The
WJOG5108L clinical trial reported that gefitinib and erlotinib have
similar efficacies (12). Xia et
al (13) reported that icotinib
exhibited a similar effectiveness and toxicity compared with
gefitinib for the treatment of advanced NSCLC, but icotinib
exhibited better disease control rate (DCR) and improved rate of
diet and sleep period (13). In
addition, the ICOGEN clinical trial demonstrated that icotinib and
gefitinib achieved similar clinical cure rates in patients with
NSCLC (14).
As demonstrated by the findings discussed above, the
effectiveness of gefitinib, erlotinib and icotinib for the
treatment of patients with advanced NSCLC remains controversial. To
the best of our knowledge, no clinical trials comparing the success
rate of gefitinib, erlotinib and icotinib have been reported. In
the current study, a network meta-analysis was performed to compare
the effectiveness and adverse effects of gefitinib, erlotinib and
icotinib for the treatment of patients with advanced NSCLC.
Materials and methods
Search strategy
According to the patient, intervention, control,
outcome (PICO) principle (15), the
Cochrane (http://www.cochranelibrary.com), PubMed (http://www.ncbi.nlm.nih.gov/pmc), Embase
(http://www.embase.com), ScienceDirect (http://www.sciencedirect.com/), China National
Knowledge Infrastructure (http://www.cnki.net), VIP Database for Chinese
Technical Periodicals (http://qikan.cqvip.com/) and Wanfang (http://g.wanfangdata.com.cn/) databases were searched
using the following key words gefitinib, erlotinib, icotinib and
non-small cell lung cancer. Search strategies were as follows:
‘gefitinib’ AND ‘erlotinib’ AND ‘non-small cell lung cancer OR
non-small cell lung carcinoma OR NSCLC’; ‘gefitinib’ AND ‘icotinib’
AND ‘non-small cell lung cancer OR non-small cell lung carcinoma OR
NSCLC’; ‘erlotinib’ AND ‘icotinib’ AND ‘non-small cell lung cancer
OR non-small cell lung carcinoma OR NSCLC’.
Eligibility criteria
Studies were selected based on primary screening of
the identified abstracts or titles, followed by a secondary
screening of the full text. According to the PICO principle, the
following eligibility criteria were established: i) Research
includes a randomized controlled trial, case-control study or
cohort study; ii) subjects are patients with advanced NSCLC
confirmed by pathological investigation; iii) intervention measures
were gefitinib, erlotinib or icotinib; and iv) end-points included
complete response (CR), partial response (PR), stable disease (SD),
progressive disease (PD), overall response rate (ORR), DCR,
progression-free survival (PFS), median survival time (MST) or
adverse effects. Exclusion criteria were as follows: i) Patients
having tumors other than NSCLC; ii) initial treatment contained
drugs that function via the same molecular mechanism as EGFR-TKIs;
and iii) study design devoid of control group. Reviews and case
reports were also excluded.
Data extraction and quality
assessment
Two reviewers independently evaluated the quality of
the studies to be included and then extracted the data. The
following information was extracted from eligible studies: First
author, date of publication, country of affiliations, type of
study, number of patients analyzed, interventions, CR, PR, SD, PD,
ORR, DCR, PFS, MST and adverse events.
The quality of randomized controlled trials from the
Cochrane network was evaluated in terms of the presence or absence
of a randomized patient grouping method, concealed assignment,
blinding method, incomplete data reporting, selective reporting and
other bias (16). Incomplete data
reporting (16) was defined as
describing the completeness of outcome data for each main outcome.
This includes the numbers in each intervention group (compared with
total randomized participants), reasons for attrition/exclusions
where reported and any re-inclusions in analyses performed by the
review authors. Selective reporting (16) was defined as stating how the
possibility of selective outcome reporting was examined by the
review authors, and what was found. This includes the study
protocol is available and all of the study's pre-specified (primary
and secondary) outcomes that are of interest in the review have
been reported in the pre-specified way; or the study protocol is
not available but it is clear that the published reports include
all expected outcomes, including those that were pre-specified. All
included studies met the following criteria: Randomized, blinding,
incomplete data and selective outcome reporting. Allocation
concealment and other sources of bias were unclear in all
randomized controlled trials.
The Newcastle-Ottawa Scale questionnaire was used to
evaluate the quality of case-control and cohort studies (17). The methods of assessment primarily
focused on the following three areas: Choice of subjects,
comparability and exposure (outcome). The results of the quality
assessment are shown in Tables I and
II. The included studies were
indicated to be of a good quality (18).
 | Table I.Quality assessment of the case
control studies. |
Table I.
Quality assessment of the case
control studies.
|
| Selection |
| Exposure |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Study (author,
year) | Case
definition | Representative
cases | Selection of
controls | Definition of
controls | Comparability | Ascertainment of
exposure | Consistency of
exposure | Non-response
rate | Total score | (Refs.) |
|---|
| Song et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (23) |
| Wu et al,
2011 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (24) |
| Wang, 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (27) |
| Weng, 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (30) |
| Zhang et al,
2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (31) |
| Zhang, 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (32) |
| Zhang et al,
2009 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (34) |
| Zhang et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (35) |
| Li et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (36) |
| Bai et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (37) |
| Li, 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (38) |
| Zhang et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (39) |
| Ma et al,
2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (40) |
| Lim et al,
2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (42) |
| Fan et al,
2011 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (11) |
| Yoshida et
al, 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (43) |
| Hong et al,
2010 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (44) |
| Emery et al,
2009 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (45) |
| Togashi et
al, 2011 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (10) |
| Wu et al,
2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (46) |
| Wu et al,
2011 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (47) |
| Kim et al,
2010 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (48) |
| Cui et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (52) |
| Liu and Liu,
2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (53) |
| Xia et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (13) |
| Sun et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (57) |
| Zhang et al,
2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | (58) |
 | Table II.Quality assessment of the cohort
study. |
Table II.
Quality assessment of the cohort
study.
|
| Selection |
| Outcome |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Study (author,
year) | Exposed cohort | Non-exposed
cohort | Ascertainment of
exposure | Outcome of
interest | Comparability | Assessment of
outcome | Length of
follow-up | Adequacy of
follow-up | Total score | (Refs.) |
|---|
| Shao et al,
2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | (41) |
Response assessment
Patient response was assessed according to the
Response Evaluation Criteria in Solid Tumors (19), which included CR, PR, SD, PD, ORR,
DCR, PFS and MST. CR and PR were added together to calculate the
ORR, while disease control rate was defined by CR, PR and SD.
Toxicities were determined according to the Common Terminology
Criteria for Adverse Events version 3.0 (20).
Statistical analysis
Statistical analyses were conducted using RevMan
(version 5.2; Cochrane Collaboration, Copenhagen, Denmark), SPSS
(version 20.0; IBM Corp., Armonk, NY, USA), R (version 3.3.0;
http://mirror.bjtu.edu.cn/cran/) and
Stata (version 13.0; StataCorp LLC, College Station, TX, USA)
software. R and Stata software were used to perform node-splitting
analysis of inconsistencies, network meta-analysis and ranking for
drug efficacy. The model for R software is considered a good
indicator when potential scale reduction factor (PSRF) is close to
1 (21). A pooled analysis was
performed using RevMan software in order to evaluate indicators
(CR, PR, SD, PD, ORR, DCR, rash, diarrhea, nausea and vomiting,
fatigue and abnormal liver function) among drugs. The adjusted odds
ratio (OR) and 95% confidence interval (CI) or 95% credible
interval (CrI) were used as measures of response for enumeration
data. The fixed-effects model (Mantel-Haenszel method) was used for
cases with no significant heterogeneity (P>0.1 and
I2<50%). Otherwise, the random-effects model was
used. PFS and MST were calculated by the weighted average method
using SPSS. The non-parametric Kruskal-Wallis test was used to
compare differences in the PFS and MST between groups. Funnel plots
were used to assess possible publication bias amongst the included
studies. Stata software was used to analyze publication bias with
the ‘metabias’ command and to evaluate sensitivity with the
‘metaninf’ command. All tests were two-tailed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Literature search
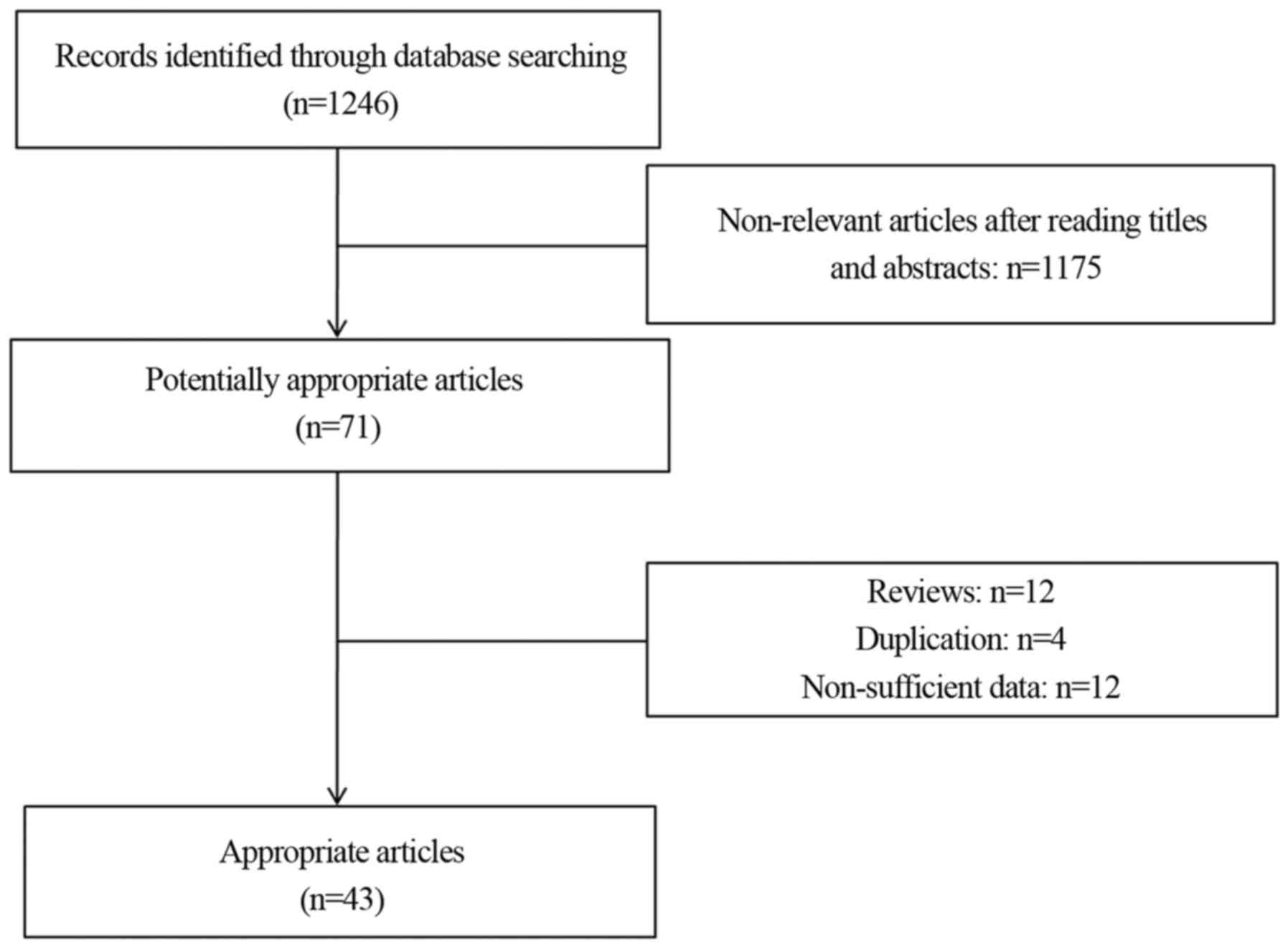
As illustrated in Fig.
1, 1,246 potentially relevant articles were identified through
database searching. According to the aforementioned selection
criteria, 43 articles (4,10–14,22–58)
containing data on 7,168 patients were selected for the network
meta-analysis. The characteristics of the eligible studies are
described in Table III.
 | Table III.Characteristics of the studies
included in the network meta-analysis. |
Table III.
Characteristics of the studies
included in the network meta-analysis.
| Study (author,
year) | Country | Type of study | Intervention | n | CR (n) | PR (n) | SD (n) | PD (n) | ORR (%) | DCR (%) | PFS (months) | MST (months) | Rash (n) | Diarrhea (n) | Nausea and vomiting
(n) | Abnormal liver
function (n) | Fatigue (n) | (Refs.) |
|---|
| Wang and | China | Randomized | Gefitinib | 41 | 2 | 13 | 17 | 9 | 36.69 | 78.05 | – | 13 | – | – | – | – | – | (22) |
| Zhang, 2012 |
|
| Erlotinib | 42 | 1 | 13 | 23 | 5 | 33.33 | 88.10 | – | 12 | – | – | – | – | – |
|
| Song et al,
2015 | China | Case-control | Gefitinib | 67 | 0 | 18 | 27 | 22 | 26.8 | 67.2 | 6 | – | 9 | – | – | 5 | – | (23) |
|
|
|
| Erlotinib | 14 | 0 | 3 | 3 | 8 | 21.4 | 42.9 | 2 | – | 9 | – | – | 2 | – |
|
|
|
|
| Icotinib | 28 | 0 | 5 | 12 | 11 | 17.9 | 60.7 | 3 | – | 15 | – | – | 3 | – |
|
| Wu et al,
2011 | China | Case-control | Gefitinib | 24 | 0 | 7 | 13 | 4 | 29.2 | 83.3 | 7.6 | – | 10 | 3 | – | 2 | – | (24) |
|
|
|
| Erlotinib | 20 | 0 | 5 | 8 | 7 | 25 | 75 | 6.2 | – | 12 | 4 | – | 3 | – |
|
| Yuan, 2015 | China | Randomized | Gefitinib | 9 | 6 |
| 2 | 1 | 66.67 | 88.89 | – | – | – | – | – | – | – | (25) |
|
|
|
| Erlotinib | 9 | 5 |
| 3 | 1 | 55.56 | 88.89 | – | – | – | – | – | – | – |
|
| Qu, 2015 | China | Randomized | Gefitinib | 50 | 2 | 11 | 25 | 12 | 26 | 76 | – | – | – | – | – | – | – | (26) |
|
|
|
| Erlotinib | 50 | 8 | 21 | 18 | 3 | 58 | 94 | – | – | – | – | – | – | – |
|
| Wang, 2014 | China | Case-control | Gefitinib | 30 | 4 | 12 | 4 | 10 | 53.33 | 66.67 | – | – | 7 | 8 | 4 | 5 | – | (27) |
|
|
|
| Erlotinib | 30 | 3 | 15 | 4 | 8 | 60 | 73.33 | – | – | 12 | 5 | 7 | 8 | – |
|
| Xie et al,
2015 | China | Randomized | Gefitinib | 27 | 1 | 14 | 8 | 4 | 55.6 | 85.2 | 8 | – | 17 | 4 | – | – | – | (28) |
|
|
|
| Erlotinib | 23 | 2 | 12 | 6 | 3 | 60.9 | 87.0 | 8.5 | – | 16 | 3 | – | – | – |
|
| Xie, 2014 | China | Randomized | Gefitinib | 34 | 5 | 13 | 10 | 6 | 52.94 | 82.35 | – | – | – | – | – | – | – | (29) |
|
|
|
| Erlotinib | 34 | 2 | 10 | 10 | 12 | 35.29 | 64.7 | – | – | – | – | – | – | – |
|
| Weng, 2015 | China | Case-control | Gefitinib | 38 | 2 | 13 | 11 | 12 | 39.47 | 68.42 | 6.12 | – | 29 | – | – | – | – | (30) |
|
|
|
| Erlotinib | 34 | 2 | 10 | 8 | 14 | 35.29 | 58.82 | 6.14 | – | 26 | – | – | – | – |
|
| Zhang et al,
2012 | China | Case-control | Gefitinib | 40 | 0 | 9 | 12 | 19 | 22.5 | 52.5 | 3.5 | – | 29 | 18 | 2 | 4 | – | (31) |
|
|
|
| Erlotinib | 40 | 0 | 8 | 18 | 14 | 20.0 | 65.0 | 3.5 | – | 32 | 17 | 0 | 5 | – |
|
| Zhang, 2014 | China | Case-control | Gefitinib | 71 | 3 | 12 | 36 | 20 | 21.13 | 71.83 | – | – | – | – | – | 5 | – | (32) |
|
|
|
| Erlotinib | 54 | 2 | 9 | 29 | 14 | 20.37 | 74.07 | – | – | – | – | – | 4 | – |
|
| Chen et al,
2009 | China | Case-control | Gefitinib | 25 | 0 | 13 | 5 | 7 | 52 | 72 | 6.8 | 9.8 | 15 | – | – | 1 | – | (33) |
|
|
|
| Erlotinib | 24 | 0 | 16 | 4 | 4 | 66.67 | 83.33 | 7.3 | 10.3 | 20 | – | – | 2 | – |
|
| Zhang et al,
2009 | China | Case-control | Gefitinib | 50 | 2 | 17 | 15 | 16 | 38 | 68 | – | – | 38 | 33 | – | – | – | (34) |
|
|
|
| Erlotinib | 50 | 3 | 15 | 12 | 20 | 36 | 60 | – | – | 38 | 35 | – | – | – |
|
| Zhang et al,
2015 | China | Case-control | Gefitinib | 41 | 1 | 9 | 13 | 18 | 24.3 | 56.1 | – | 13.5 | 5 | 3 | 1 | 2 | 5 | (35) |
|
|
|
| Erlotinib | 45 | 0 | 12 | 15 | 18 | 26.7 | 60 | – | 13.2 | 6 | 5 | 0 | 2 | 5 |
|
| Li et al,
2015 | China | Case-control | Gefitinib | 37 | 3 | 13 | 18 | 3 | 42.11 | 91.89 | – | – | 20 | 16 | 15 | 2 | – | (36) |
|
|
|
| Erlotinib | 36 | 3 | 13 | 17 | 3 | 44.44 | 91.67 | – | – | 32 | 28 | 26 | 11 | – |
|
| Bai et al,
2015 | China | Case-control | Gefitinib | 38 | 0 | 16 | 19 | 3 | 42.1 | 92.1 | 10.6 | 14.8 | 24 | 13 | 3 | 4 | – | (37) |
|
|
|
| Erlotinib | 29 | 0 | 14 | 13 | 2 | 48.3 | 93.1 | 11.7 | 15.7 | 22 | 9 | 2 | 3 | – |
|
| Li, 2013 | China | Case-control | Gefitinib | 20 | 0 | 6 | 8 | 6 | 30.0 | 70.0 | 6.2 | – | 11 | – | 3 | – | – | (38) |
|
|
|
| Erlotinib | 11 | 0 | 5 | 3 | 3 | 45.5 | 72.7 | 6.5 | – | 6 | – | 1 | – | – |
|
| Zhang et
al, | China | Case-control | Gefitinib | 39 | 6 | 12 | 17 | 4 | 46.15 | 89.74 | 9.5 | – | – | – | – | – | – | (39) |
| 2015 |
|
| Erlotinib | 42 | 4 | 15 | 19 | 4 | 45.24 | 90.48 | 9.0 | – | – | – | – | – | – |
|
| Ma et al,
2013 | China | Case-control | Gefitinib | 49 | 0 | 25 | 20 | 4 | 51.0 | 91.1 | 17.5 | – | 31 | 12 | – | 13 | – | (40) |
|
|
|
| Erlotinib | 17 | 0 | 7 | 8 | 2 | 41.2 | 86.7 | 13.0 | – | 16 | 12 | – | 2 | – |
|
| Shao et al,
2013 | China | Cohort | Gefitinib | 655 | − | – | – | – | – | – | 5.5 | 10.2 | – | – | – | – | – | (41) |
|
|
|
| Erlotinib | 329 | − | – | – | – | – | – | 3.4 | 9.9 | – | – | – | – | – |
|
| Lim et al,
2014 | Korea | Case-control | Gefitinib | 121 | 0 | 93 | 16 | 12 | 76.9 | 90.1 | 11.7 | – | – | – | – | – | – | (42) |
|
|
|
| Erlotinib | 121 | 0 | 90 | 15 | 16 | 74.4 | 86.8 | 9.6 | – | – | – | – | – | – |
|
| Kim et al,
2012 | Korea | Randomized | Gefitinib | 48 | 1 | 22 | 12 | 12 | 47.9 | 72.9 | 4.9 | – | 30 | 16 | 4 | – | 0 | (4) |
|
|
|
| Erlotinib | 48 | 1 | 18 | 13 | 15 | 39.6 | 66.7 | 3.1 | – | 35 | 17 | 2 | – | 8 |
|
| Fan et al,
2011 | China | Case-control | Gefitinib | 715 | 246 | 175 | 294 | 34.4 | 58.9 | 3.6 | 9.6 | – | – | – | – | – |
| (11) |
|
|
|
| Erlotinib | 407 | 145 | 123 | 139 | 35.6 | 65.8 | 4.6 | 10.7 | – | – | – | – | – |
|
|
| Yoshida et
al, | Japan | Case-control | Gefitinib | 107 | − | – | – | – | – | – | – | – | 67 | 39 | 8 | 14 | 32 | (43) |
| 2013 |
|
| Erlotinib | 35 | − | – | – | – | – | – | – | – | 33 | 6 | 5 | 2 | 21 |
|
| Hong et
al, | Korea | Case-control | Gefitinib | 20 | 5 | 3 | 12 | 25.0 | 40.0 | 3.5 | 21.8 | 7 | 3 | 2 | 0 | 0 |
| (44) |
| 2010 |
|
| Erlotinib | 17 | 2 | 7 | 8 | 12.5 | 50.0 | 4.4 | 21.5 | 10 | 5 | 0 | 3 | 2 |
|
|
| Emery et
al, | USA | Case-control | Gefitinib | 115 | 3 | 3 | 30 | 75 | 5.2 | 31.3 | 2.4 | – | 46 | 43 | 8 | – | 7 | (45) |
| 2009 |
|
| Erlotinib | 45 | 2 | 2 | 21 | 18 | 8.9 | 55.6 | 2.8 | – | 26 | 17 | 8 | – | 5 |
|
| Togashi et
al, | Japan | Case-control | Gefitinib | 85 | 44 | 17 | 21 | 51.7 | 71.8 | – | – | 53 | 28 | 10 | 22 | – |
| (10) |
| 2011 |
|
| Erlotinib | 69 | 25 | 17 | 19 | 36.2 | 60.9 | – | – | 54 | 36 | 36 | 24 | – |
|
|
| Urata et al,
2016 | Japan | Randomized | Gefitinib | 244 | 1 | 111 | 61 | 61 | 45.9 | 70.9 | 6.5 | 22.8 | 207 | 118 | 82 | – | – | (12) |
|
|
|
| Erlotinib | 227 | 3 | 97 | 71 | 46 | 44.1 | 75.3 | 7.5 | 24.5 | 255 | 141 | 96 | – | – |
|
| Wu et al,
2012 | China | Case-control | Gefitinib | 124 | 0 | 52 | 46 | 26 | 41.9 | 79.0 | 7.6 | – | – | – | – | – | – | (46) |
|
|
|
| Erlotinib | 100 | 0 | 42 | 27 | 31 | 42.0 | 69.0 | 7.9 | – | – | – | – | – | – |
|
| Wu et al,
2011 | China | Case-control | Gefitinib | 440 | − | – | – | – | – | – | 5.0 | 18.0 | – | – | – | – | – | (47) |
|
|
|
| Erlotinib | 276 | − | – | – | – | – | – | 2.9 | 11.6 | – | – | – | – | – |
|
| Kim et al,
2010 | Korea | Case-control | Gefitinib | 171 | 0 | 65 | 43 | 61 | 38.0 | 63.2 | 4.6 | 12.6 | – | – | – | – | – | (48) |
|
|
|
| Erlotinib | 171 | 1 | 54 | 56 | 57 | 32.2 | 64.9 | 2.7 | 12.1 | – | – | – | – | – |
|
| Chen et al,
2012 | China | Randomized | Gefitinib | 14 | 0 | 1 | 7 | 2 | 7.1 | 57.1 | – | 3.8 | 3 | 1 | – | – | – | (49) |
|
|
|
| Icotinib | 14 | 0 | 3 | 5 | 4 | 21.4 | 57.1 | – | 11 | 1 | 0 | – | – | – |
|
| Chen et al,
2011 | China | Randomized | Gefitinib | 6 | 0 | 1 | 3 | 2 | 16.7 | 66.6 | 3.2 | 4.8 | – | – | – | – | – | (50) |
|
|
|
| Icotinib | 6 | 0 | 2 | 2 | 2 | 33.3 | 66.6 | 3.6 | 6 | – | – | – | – | – |
|
| Cui et al,
2015 | China | Randomized | Gefitinib | 28 | 0 | 7 | 8 | 13 | 25.0 | 53.6 | – | – | 8 | 5 | – | – | – | (51) |
|
|
|
| Icotinib | 28 | 0 | 11 | 7 | 10 | 39.3 | 64.3 | – | – | 3 | 1 | – | – | – |
|
| Cui et al,
2015 | China | Case-control | Gefitinib | 21 | 0 | 12 | 5 | 4 | 57 | 81 | – | – | 13 | 7 | 4 | 2 | – | (52) |
|
|
|
| Icotinib | 49 | 1 | 28 | 12 | 8 | 59 | 84 | – | – | 31 | 16 | 10 | 6 | – |
|
| Liu and Liu,
2014 | China | Case-control | Gefitinib | 12 | 3 | 5 | 2 | 2 | 66.7 | 83.3 | – | – | 4 | 5 | 4 | 3 | 1 | (53) |
|
|
|
| Erlotinib | 10 | 2 | 5 | 1 | 2 | 70.0 | 80.0 | – | – | 6 | 4 | 6 | 4 | 2 |
|
|
|
|
| Icotinib | 8 | 1 | 4 | 1 | 2 | 62.5 | 75.0 | – | – | 2 | 3 | 5 | 3 | 2 |
|
| Lin and Zhang, | China | Randomized | Gefitinib | 24 | 0 | 2 | 12 | 3 | 8.3 | 58.3 | 6.5 | 8.8 | 11 | 9 | – | – | – | (54) |
| 2014 |
|
| Icotinib | 24 | 0 | 5 | 9 | 7 | 20.8 | 58.3 | 6.8 | 9.2 | 10 | 7 | – | – | – |
|
| Xia et al,
2015 | China | Case-control | Gefitinib | 93 | 11 | 31 | 27 | 17 | 45.2 | 74.2 | – | – | 85 | 7 | – | 1 | – | (13) |
|
|
|
| Icotinib | 126 | 14 | 44 | 51 | 17 | 46.0 | 86.5 | – | – | 112 | 11 | – | 3 | – |
|
| Xu et al,
2015 | China | Randomized | Gefitinib | 40 | 0 | 9 | 14 | 13 | 22.2 | 55.6 | 9 | – | 10 | 4 | – | 4 | – | (55) |
|
|
|
| Icotinib | 40 | 0 | 10 | 13 | 12 | 27.7 | 61.1 | 11 | – | 6 | 6 | – | 1 | – |
|
| Shi et al,
2013 | China | Randomized | Gefitinib | 196 | 0 | 53 | 93 | 40 | 27.2 | 74.9 | 3.4 | 13.9 | 98 | 58 | 14 | 25 | – | (14) |
|
|
|
| Icotinib | 199 | 1 | 54 | 95 | 42 | 27.6 | 75.4 | 4.6 | 13.3 | 81 | 43 | 11 | 16 | – |
|
| Huang, 2014 | China | Randomized | Erlotinib | 13 | 0 | 2 | 7 | 4 | 15.4 | 69.2 | – | – | 7 | 1 | 1 | – | – | (56) |
|
|
|
| Icotinib | 13 | 0 | 3 | 5 | 5 | 23.1 | 61.6 | – | – | 5 | 2 | 1 | – | – |
|
| Sun et al,
2015 | China | Case-control | Erlotinib | 41 | 8 | 13 | 20 | 19.5 | 48.8 | 4 | – | 13 | 3 | – | 2 | – |
| (57) |
|
|
|
| Icotinib | 43 | 12 | 15 | 16 | 27.9 | 62.8 | 6 | – | 17 | 1 | – | 0 | – |
|
|
| Zhang et al,
2015 | China | Case-control | Erlotinib | 34 | 1 | 6 | 19 | 8 | 20.6 | 76.5 | 6.86 | – | 17 | 5 | – | 3 | – | (58) |
|
|
|
| Icotinib | 34 | 0 | 12 | 17 | 5 | 35.3 | 85.3 | 8.53 | – | 12 | 3 | – | 2 | – |
|
Network meta-analysis for the clinical
effectiveness and adverse effects of gefitinib, erlotinib and
icotinib
Network maps (Fig. 2)
and network forest plots (Fig. 3)
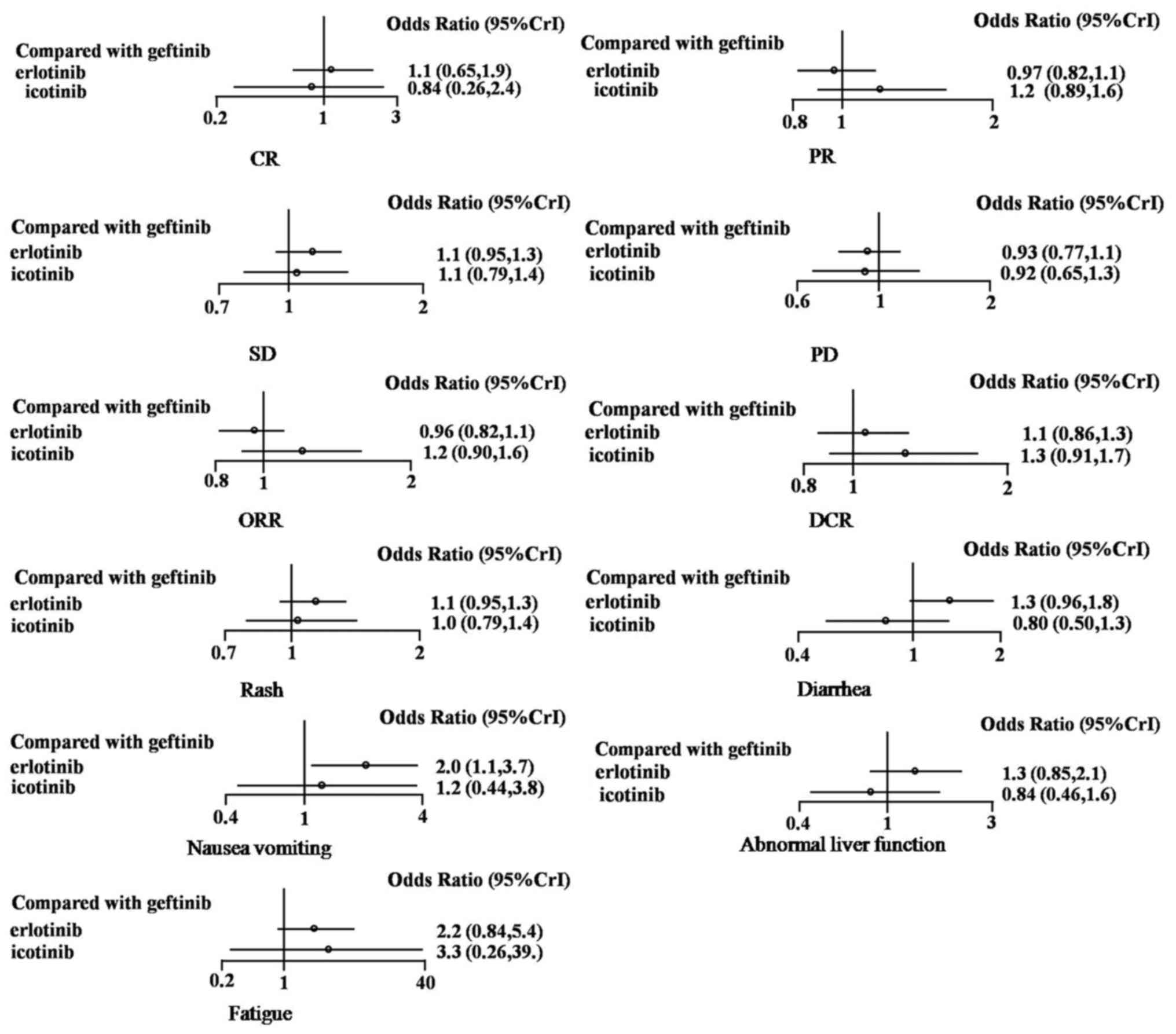
were produced for all indicators. Compared with gefitinib, the
network meta-analysis indicated that erlotinib had no significant
differences in OR. The ORs for erlotinib were as follows: CR, 1.1
(95% CrI, 0.65–1.9); PR, 0.97 (95% CrI, 0.82–1.1); SD, 1.1 (95%
CrI, 0.95–1.3); PD, 0.93 (95% CrI, 0.77–1.1); ORR, 0.96 (95% CrI,
0.82–1.1); and DCR, 1.1 (95% CrI, 0.86–1.3). Icotinib also had no
significant differences in OR compared with gefitinib. The ORs for
icotinib were as follows: CR, 0.84 (95% CrI, 0.26–2.4); PR, 1.2
(95% CrI, 0.89–1.6); SD, 1.1 (95% CrI, 0.79–1.4); PD, 0.92 (95%
CrI, 0.65–1.3); ORR, 1.2 (95% CrI, 0.90–1.6); and DCR, 1.3 (95%
CrI, 0.91–1.7).
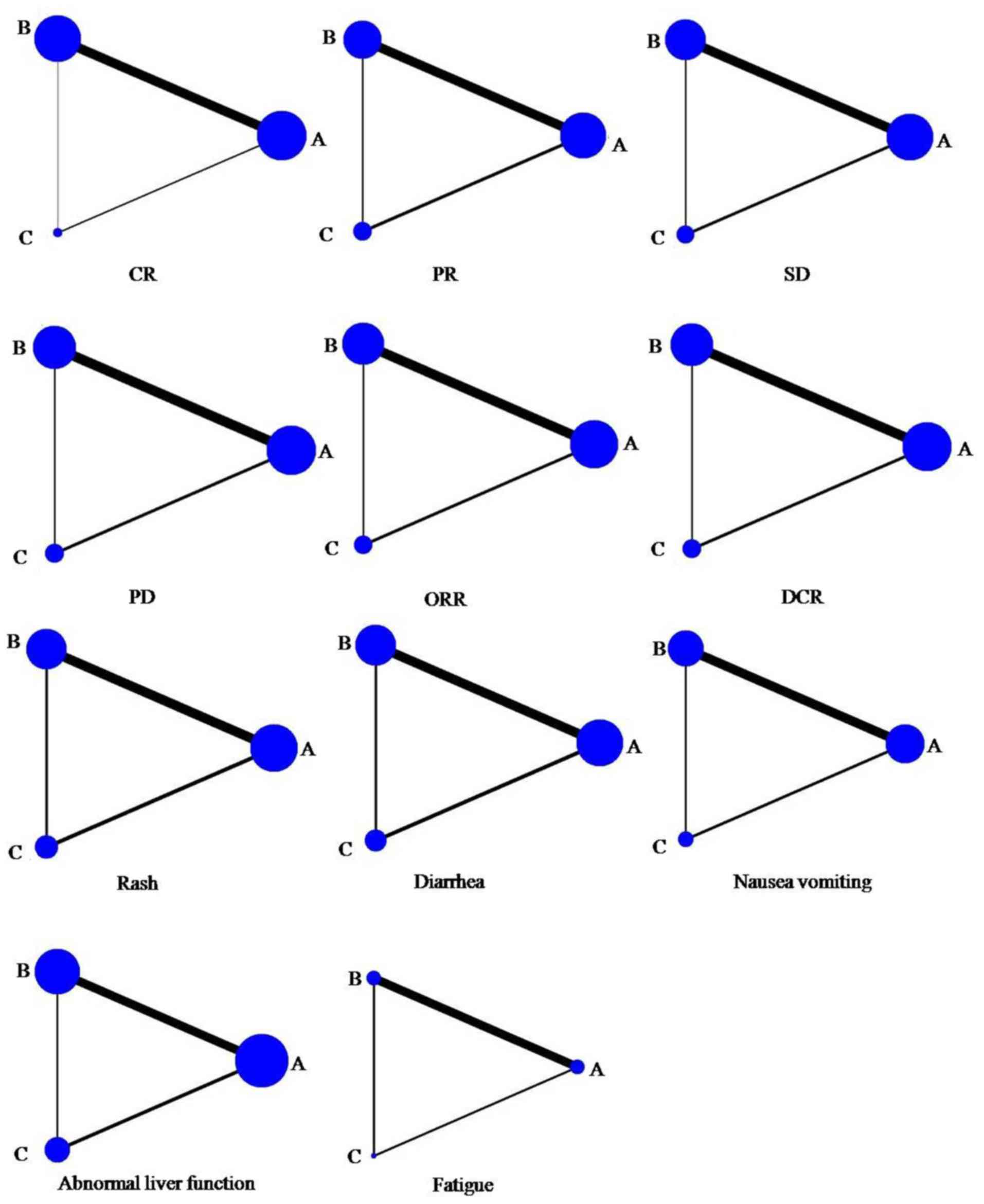 | Figure 2.Network map of the clinical
efficacies and adverse events of A, B and C. Node size and line
width are based on the number of intervention studies included in
the meta-analysis. Larger nodes and thicker lines indicate a higher
frequency of intervention with the indicated drug. A, gefitinib; B,
erlotinib; C, icotinib; CR, complete response; PR, partial
response; SD, stable disease; PD, progressive disease; ORR, overall
response rate; DCR, disease control rate. |
In terms of adverse events, compared with gefitinib,
erlotinib resulted in higher rates of nausea and vomiting (OR=2.0;
95% CrI, 1.1–3.7) during network meta-analysis. However, there were
no significant differences in OR for rash (OR=1.1; 95% CrI,
0.95–1.3), diarrhea (OR=1.3; 95% CrI, 0.96–1.8), fatigue (OR=2.2;
95% CrI, 0.84–5.4) or abnormal liver function (OR=1.3; 95% CrI,
0.85–2.1). Compared with gefitinib, icotinib also had no
significant differences in OR for rash (OR=1.0; 95% CrI, 0.79–1.4),
diarrhea (OR=0.80; 95% CrI, 0.50–1.3), nausea and vomiting (OR=1.2;
95% CrI, 0.44–3.8), fatigue (OR=3.3; 95% CrI, 0.26–39.0) or
abnormal liver function (OR=0.84; 95% CrI, 0.46–1.6).
Meta-analysis of two congruent
drugs
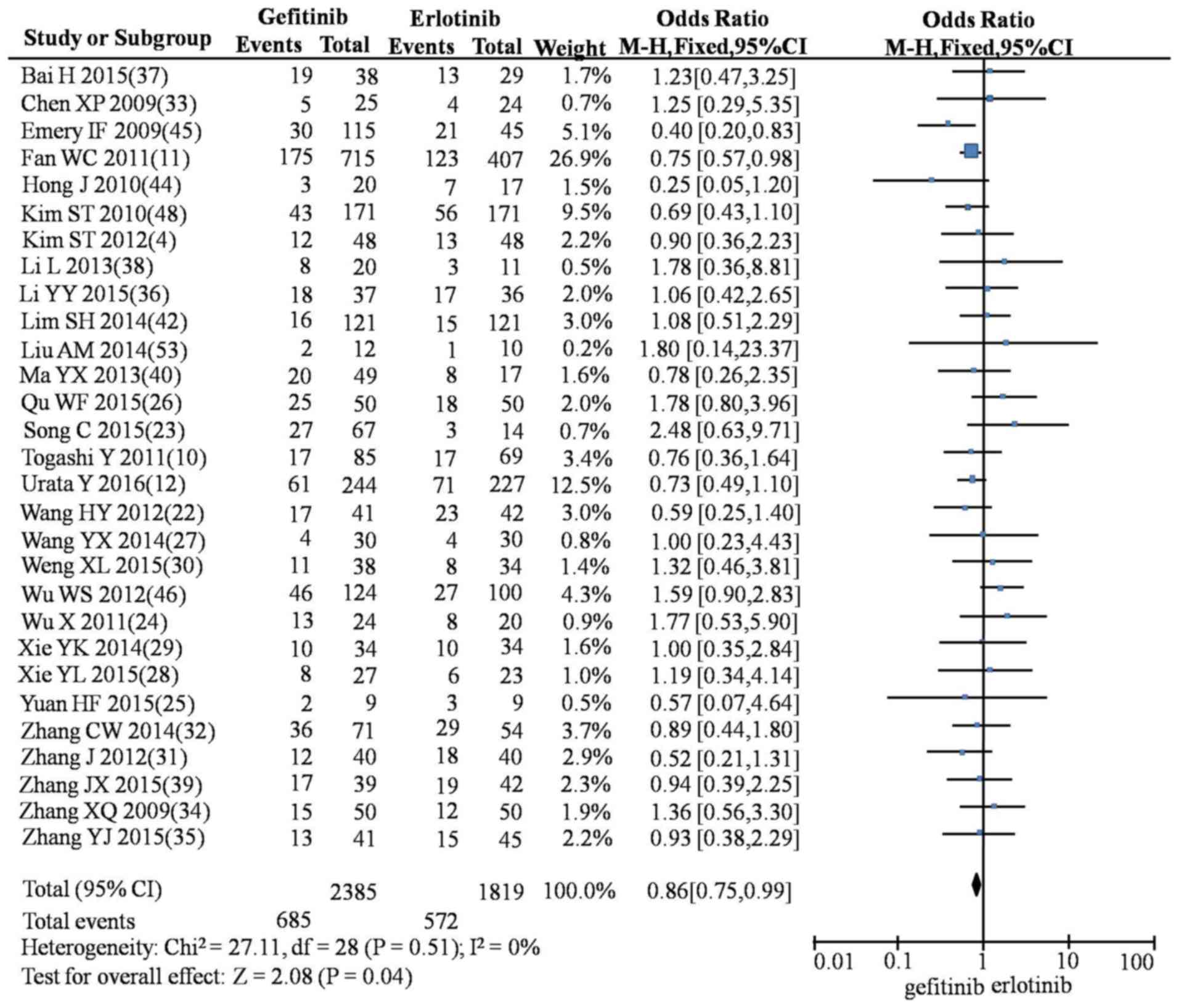
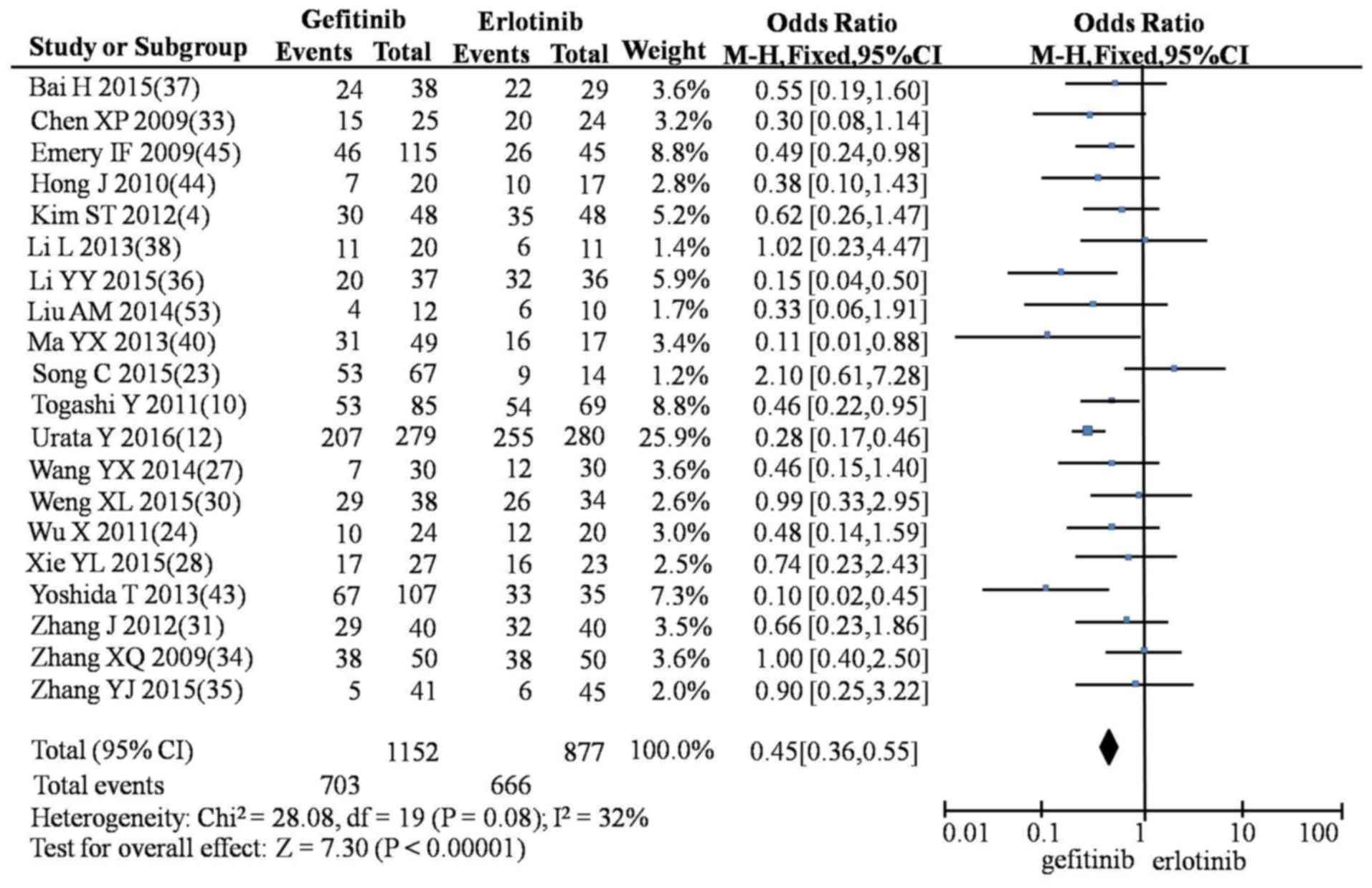
In the meta-analysis, compared with erlotinib,
gefitinib produced lower rates of SD (OR=0.86; 95% CI, 0.75–0.99;
P=0.04; Fig. 4), rash (OR=0.45; 95%
CI, 0.36–0.55; P<0.00001; Fig.
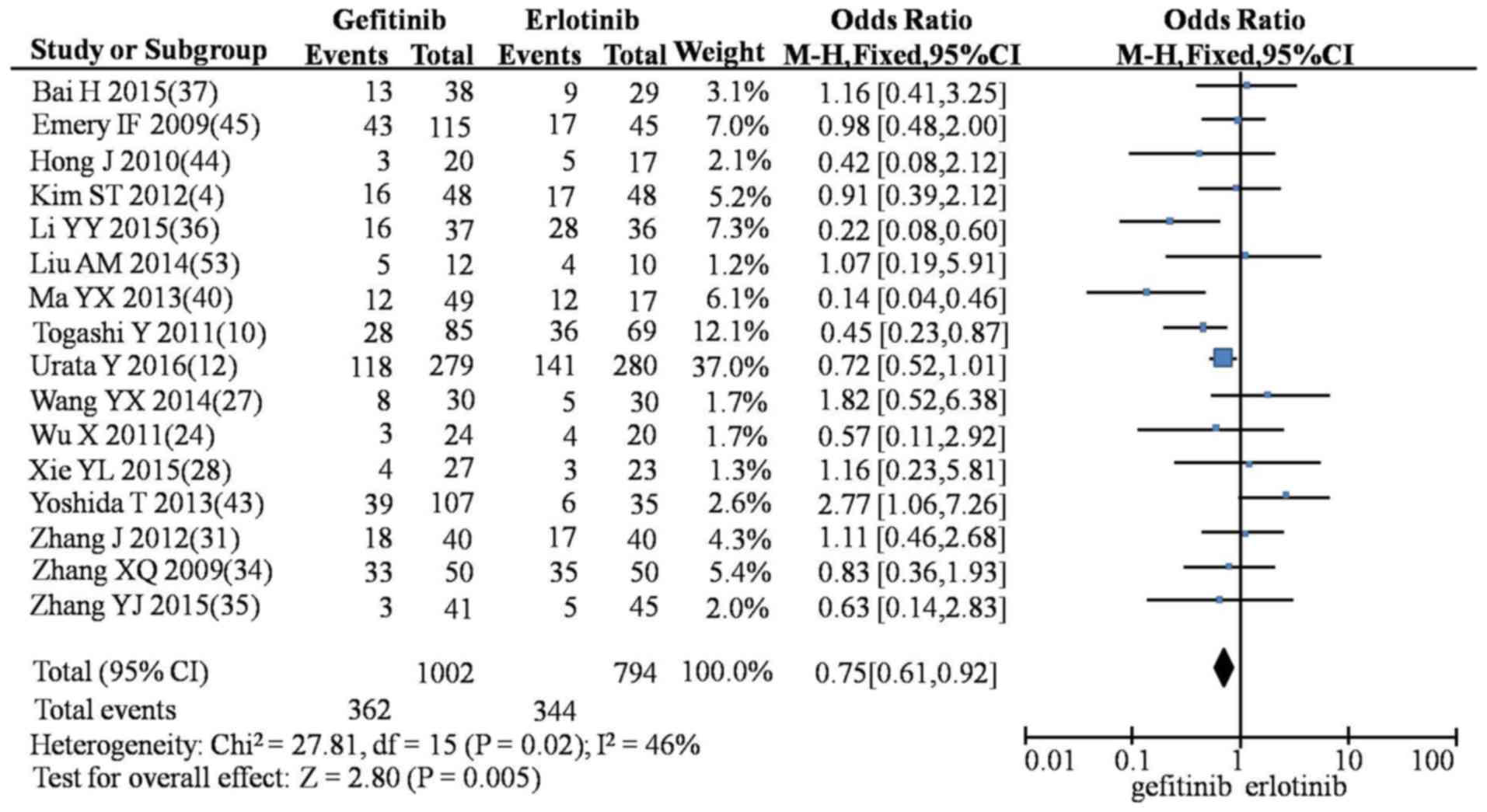
5), diarrhea (OR=0.75; 95% CI, 0.61–0.92; P=0.005; Fig. 6), nausea and vomiting (OR=0.47; 95%
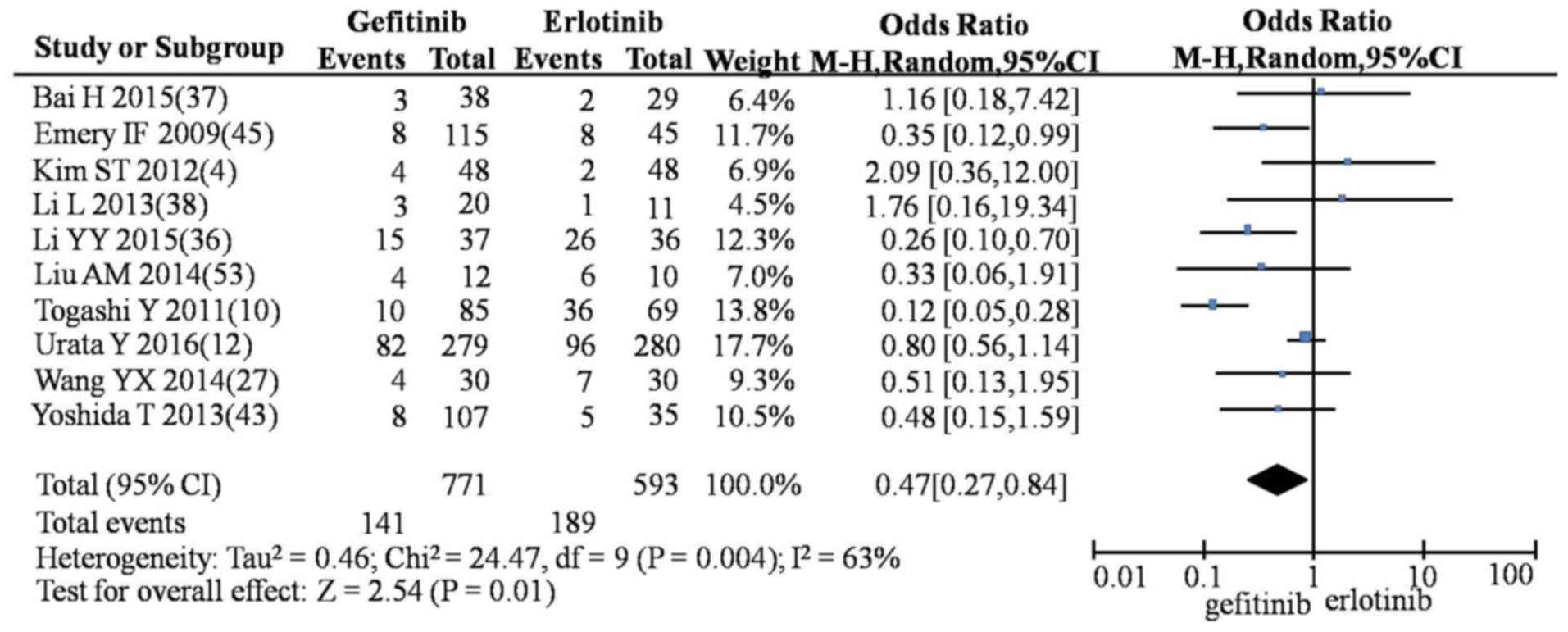
CI, 0.0.27–0.84; P=0.01; Fig. 7) and
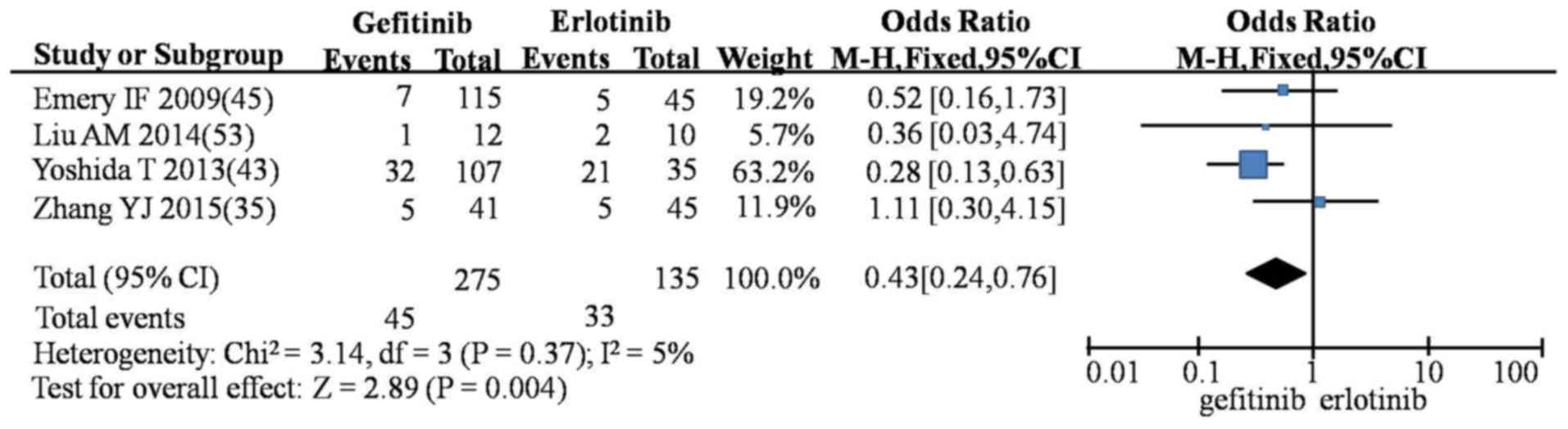
fatigue (OR=0.43; 95% CI, 0.24–0.76; P=0.004; Fig. 8). However, gefitinib produced a
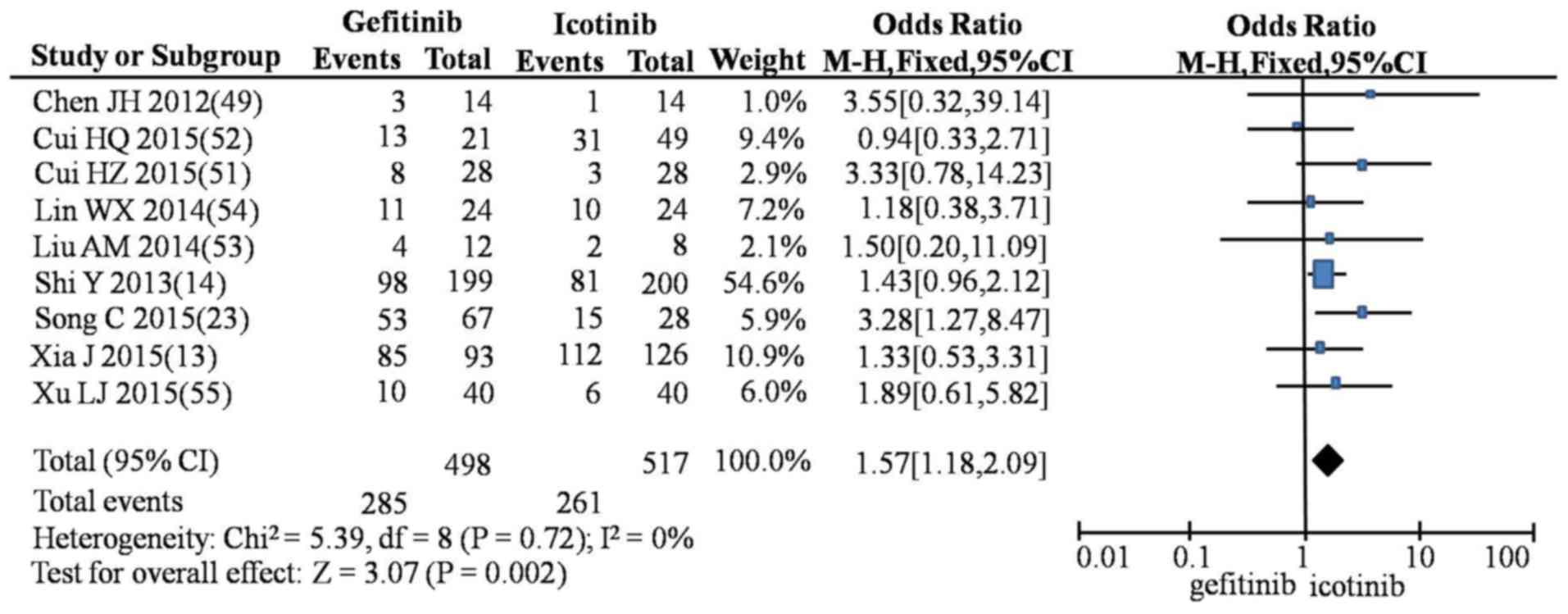
higher incidence of rash compared with icotinib (OR=1.57; 95% CI,
1.18–2.09; P=0.002; Fig. 9). No
significant differences were observed between gefitinib and
icotinib for CR, PR, PD, ORR, DCR, diarrhea or abnormal liver
function (Table IV).
 | Table IV.Meta-analysis of the clinical
efficacies and adverse events associated with G, E and I. |
Table IV.
Meta-analysis of the clinical
efficacies and adverse events associated with G, E and I.
|
|
|
|
| Heterogeneity
testing |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Indicator | Intervention | n | OR (95% CI) | χ2 | P-value | I2 | Model | Z-value | P-value |
|---|
| CR | G vs. E | 14 | 0.90
(0.56–1.44) |
8.11 | 0.84 | 0 | Fixed | 0.43 | 0.67 |
|
| G vs. I | 2 | 1.17
(0.53–2.58) |
0.34 | 0.56 | 0 | Fixed | 0.40 | 0.69 |
|
| E vs. I | 1 | 1.75
(0.13–23.70) | – | – | – | – | 0.42 | 0.67 |
| PR | G vs. E | 25 | 1.03
(0.88–1.21) | 12.57 | 0.97 | 0 | Fixed | 0.41 | 0.68 |
|
| G vs. I | 10 | 0.90
(0.68–1.19) |
4.86 | 0.85 | 0 | Fixed | 0.72 | 0.47 |
|
| E vs. I | 4 | 0.63
(0.30–1.35) |
1.62 | 0.65 | 0 | Fixed | 1.19 | 0.23 |
| SD | G vs. E | 29 | 0.86
(0.75–0.99) | 27.11 | 0.51 | 0 | Fixed | 2.08 | 0.04 |
|
| G vs. I | 10 | 0.95
(0.74–1.23) |
4.85 | 0.85 | 0 | Fixed | 0.37 | 0.71 |
|
| E vs. I | 5 | 0.94
(0.55–1.62) |
2.74 | 0.60 | 0 | Fixed | 0.21 | 0.83 |
| PD | G vs. E | 29 | 1.14
(0.99–1.31) | 35.44 | 0.16 | 21 | Fixed | 1.87 | 0.06 |
|
| G vs. I | 10 | 0.99
(0.73–1.34) |
5.07 | 0.83 | 0 | Fixed | 0.06 | 0.95 |
|
| E vs. I | 5 | 1.48
(0.85–2.59) |
1.51 | 0.82 | 0 | Fixed | 1.38 | 0.17 |
| ORR | G vs. E | 29 | 1.03
(0.91–1.18) | 23.76 | 0.69 | 0 | Fixed | 0.51 | 0.61 |
|
| G vs. I | 10 | 0.91
(0.69–1.20) |
4.81 | 0.85 | 0 | Fixed | 0.68 | 0.49 |
|
| E vs. I | 5 | 0.68
(0.37–1.25) |
1.53 | 0.82 | 0 | Fixed | 1.24 | 0.21 |
| DCR | G vs. E | 29 | 0.90
(0.78–1.03) | 37.53 | 0.11 | 25 | Fixed | 1.56 | 0.12 |
|
| G vs. I | 10 | 0.85
(0.64–1.13) |
5.34 | 0.80 | 0 | Fixed | 1.11 | 0.27 |
|
| E vs. I | 5 | 0.67
(0.39–1.18) |
1.51 | 0.82 | 0 | Fixed | 1.38 | 0.17 |
| Rash | G vs. E | 20 | 0.45
(0.36–0.55) | 28.08 | 0.08 | 32 | Fixed | 7.30 | <0.01 |
|
| G vs. I | 9 | 1.57
(1.18–2.09) |
5.39 | 0.72 | 0 | Fixed | 3.07 | <0.01 |
|
| E vs. I | 5 | 1.37
(0.81–2.30) |
3.89 | 0.42 | 0 | Fixed | 1.17 | 0.24 |
| Diarrhea | G vs. E | 16 | 0.75
(0.61–0.92) | 27.81 | 0.02 | 46 | Fixed | 2.80 | <0.01 |
|
| G vs. I | 7 | 1.32
(0.94–1.85) |
4.20 | 0.65 | 0 | Fixed | 1.60 | 0.11 |
|
| E vs. I | 4 | 1.45
(0.57–3.72) |
1.43 | 0.70 | 0 | Fixed | 0.78 | 0.44 |
| Nausea and | G vs. E | 10 | 0.47
(0.27–0.84) | 24.47 | 0.00 | 63 | Random | 2.54 | 0.01 |
| vomiting | G vs. I | 3 | 0.99
(0.53–1.88) |
2.01 | 0.37 | 1 | Fixed | 0.02 | 0.98 |
|
| E vs. I | 2 | 0.93
(0.19–4.57) |
0.00 | 0.95 | 0 | Fixed | 0.09 | 0.93 |
| Abnormal liver | G vs. E | 13 | 0.73
(0.51–1.05) | 10.94 | 0.53 | 0 | Fixed | 1.70 | 0.09 |
| function | G vs. I | 6 | 1.27
(0.77–2.10) |
4.33 | 0.50 | 0 | Fixed | 0.94 | 0.35 |
|
| E vs. I | 3 | 1.34
(0.45–4.00) |
0.06 | 0.97 | 0 | Fixed | 0.53 | 0.60 |
| Fatigue | G vs. E | 4 | 0.43
(0.24–0.76) |
3.14 | 0.37 | 5 | Fixed | 2.89 | <0.01 |
|
| G vs. I | 1 | 0.27
(0.02–3.67) | – | – | – | – | 0.98 | 0.33 |
|
| E vs. I | 1 | 0.75
(0.08–6.96) | – | – | – | – | 0.25 | 0.80 |
Ranking of interventions
Interventions were ranked by how often they caused
certain adverse effects (Table V).
Erlotinib was observed to produce the highest rate of SD, followed
by icotinib. However, erlotinib was also associated with highest
risk of rash, followed by gefitinib (data not shown). Icotinib was
associated with the highest risk of diarrhea, followed by
gefitinib. Erlotinib was associated with the highest risk of nausea
and vomiting, followed by icotinib. Icotinib was associated with
the highest risk of fatigue, followed by erlotinib.
 | Table V.Ranking of interventions. |
Table V.
Ranking of interventions.
| A, Stable
disease |
|---|
|
|---|
| Ranking | Gefitinib (%) | Erlotinib (%) | Icotinib (%) |
|---|
| 1 |
2.4 | 71.2 | 26.5 |
| 2 | 34.7 | 25.6 | 39.7 |
| 3 | 62.9 |
3.2 | 33.8 |
|
| B, Diarrhea |
|
| Ranking | Gefitinib (%) | Erlotinib (%) | Icotinib (%) |
|
| 1 |
1.7 | 95.0 |
3.2 |
| 2 | 77.5 |
4.6 | 18.0 |
| 3 | 20.8 |
0.4 | 78.8 |
|
| C, Nausea and
vomiting |
|
| Ranking | Gefitinib (%) | Erlotinib (%) | Icotinib (%) |
|
| 1 | 65.8 |
0.2 | 34.0 |
| 2 | 34.0 | 15.7 | 50.2 |
| 3 |
0.1 | 84.1 | 15.8 |
|
| D, Fatigue |
|
| Ranking | Gefitinib (%) | Erlotinib (%) | Icotinib (%) |
|
| 1 | 83.6 |
1.4 | 15.0 |
| 2 | 15.8 | 62.0 | 22.2 |
| 3 |
0.6 | 36.6 | 62.8 |
Analysis of inconsistency and
convergence
Using the consistency model, the PSRF for all
indicators of clinical efficacies and adverse events was close to 1
(data not shown). Following node-splitting analysis of
inconsistencies, no significant differences were observed for CR,
PR, SD, PD, ORR, DCR, diarrhea, nausea and vomiting, fatigue or
abnormal liver function (data not shown).
PFS and MST
The PFS rate for gefitinib, erlotinib and icotinib
was 5.48, 5.15 and 5.81 months, respectively (Table VI). The MST was 13.26, 13.52 and
12.58 months for gefitinib, erlotinib and icotinib, respectively
(Table VI). Gefitinib and icotinib
had a significantly higher PFS rate compared with erlotinib
(P<0.01); however, no significant difference in PFS was observed
between gefitinib and icotinib (Table
VI). Erlotinib had a significantly longer MST compared with
gefitinib and icotinib (P<0.05), and gefitinib had a
significantly longer MST compared with icotinib (P<0.05)
(Table VI).
 | Table VI.Comparison of PFS and MST. |
Table VI.
Comparison of PFS and MST.
| Intervention | PFS (months) | MST (months) |
|---|
| Gefitinib | 5.48a | 13.26a,b |
| Erlotinib | 5.15 | 13.52a,c |
| Icotinib | 5.81c | 12.58b,c |
Publication bias
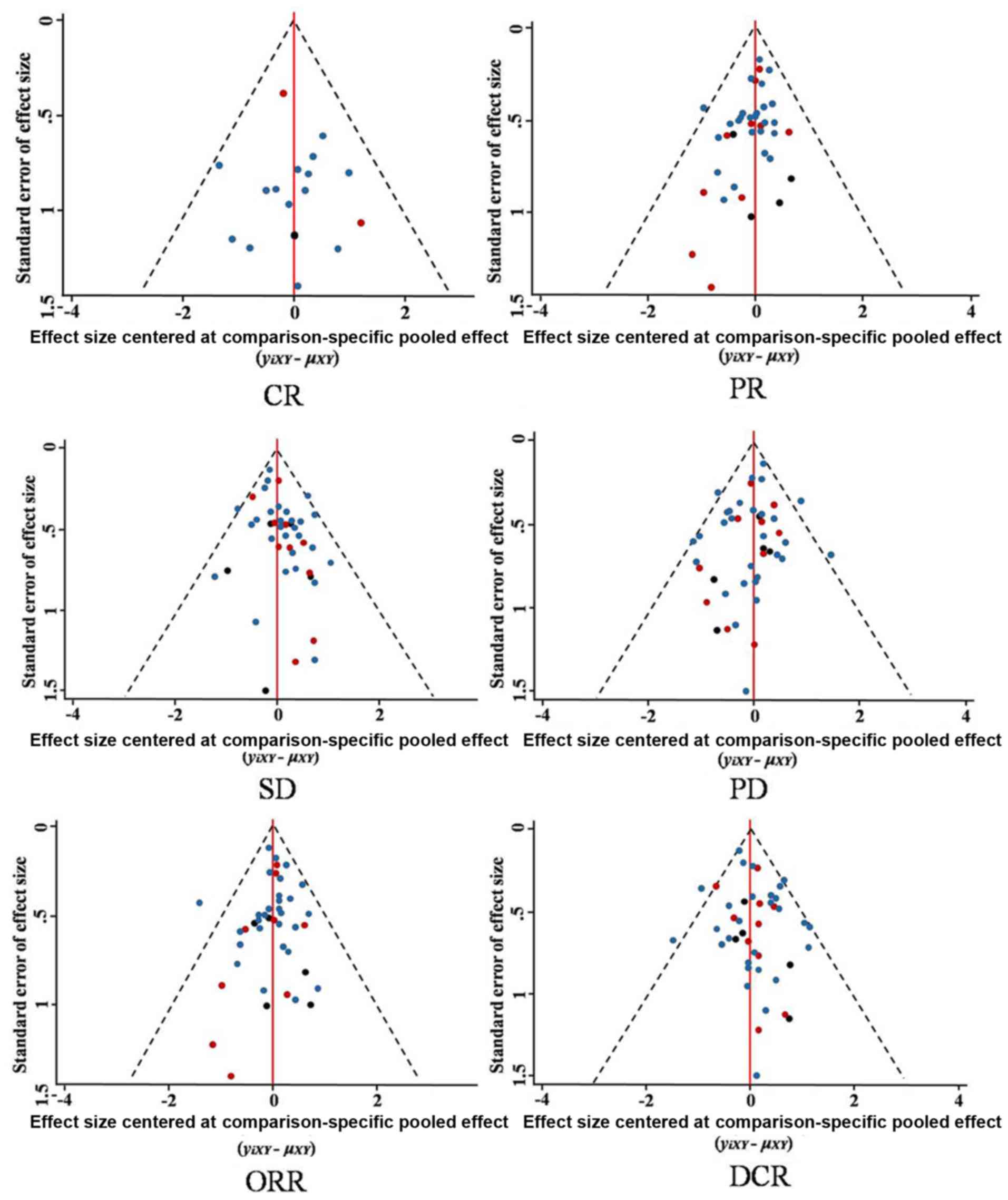
Funnel plots (Fig.
10) revealed that all included studies were symmetrical in
terms of standard error of the effect size and the effect size
centered at the comparison-specific pooled effect for CR, PR, SD,
PD, ORR and DCR. This indicates that there was minimal publication
bias.
Sensitivity analysis
According to sensitivity analysis, there was little
difference for the pooled effect among each study for the indexes
ORR and DCR (data not shown).
Discussion
Lung cancer has the highest morbidity and mortality
rate of any malignant tumor type, and is most commonly NSCLC
(1,2). In total >50% of patients diagnosed
with NSCLC are at an advanced stage of the disease (40). Platinum-based combination
chemotherapy is the most common course of treatment for patients
with NSCLC, but its clinical efficacy is limited (4). Targeted therapy, including gefitinib,
erlotinib and icotinib, has improved the treatment of advanced
NSCLC (7,9,59).
However, there remains controversy surrounding the effectiveness
and adverse effects of these three drugs (1,10–14,60).
In the current meta-analysis, no significant
differences for CR, PR, SD, PD, ORR, DCR, rash, diarrhea, fatigue
or abnormal liver function were observed between gefitinib,
erlotinib and icotinib by using network meta analysis. The
frequency of fatigue, and nausea and vomiting, was lower for
gefitinib compared with icotinib or erlotinib. In addition, the MST
was longer for erlotinib compared with the other two drugs.
Furthermore, the frequency of rash for icotinib was lower compared
with the other two drugs. The present study revealed that the three
drugs had similar efficacies for the treatment of patients with
advanced NSCLC. Overall, gefitinib had a lower frequency of nausea
and vomiting and fatigue, while erlotinib has a longer MST, but a
higher frequency for rash and nausea and vomiting. A lower
frequency of rash may occur with icotinib.
The results of the network meta-analysis conducted
in the present study revealed that there were no significant
differences between the efficacies of gefitinib, erlotinib or
icotinib for the treatment of NSCLC. All three targeted drugs act
as EGFR-TKIs and bind to the Mg-ATP binding site of the EGFR
tyrosine kinase catalytic domain competitively (45,58).
This inhibits EGFR phosphorylation and subsequent signal
transduction. Thus, these drugs have antitumor activity (10,59,61).
Since these drugs function via the same molecular mechanism, they
may have similar efficacies in the treatment of patients with
NSCLC. In the current study, no significant statistical differences
were observed in CR, PR, PD, ORR or DCR by performing meta-analysis
of two congruent drugs.
Rash and diarrhea were the most frequent adverse
effects in patients with advanced NSCLC treated with EGFR-TKIs. The
network meta-analysis conducted in the current study demonstrated
that erlotinib resulted in a higher frequency of nausea and
vomiting compared with gefitinib and icotinib. In addition,
erlotinib resulted in a higher frequency of rash, nausea and
vomiting and fatigue in patients with advanced NSCLC compared with
gefitnib and icotinib in the meta-analysis for two congruent drugs
and ranking interventions. The adverse events caused by erlotinib
treatment can be dose-dependent (10). The approved daily dose of erlotinib
(150 mg) is equal to the maximum tolerated dose (10). Therefore, the increase in the
toxicity of erlotinib was associated with the dose (10). Furthermore, the adverse effects of
erlotinib, icotinib and gefitinib on the liver occur through
different mechanisms (62). The
different toxicity profiles of these drugs are due to differences
in their chemical structure and pharmacokinetics (63,64).
The results of the current study revealed that
erlotinib had a longer MST compared with gefitinib and icotinib. Wu
et al (46) reported that
erlotinib had a higher efficacy compared with gefitinib for the
treatment of patient with NSCLC patients without activating
mutations of EGFR. The longer MST following erlotinib treatment
might be associated with different characteristics of the
population such as the mutation of EGFR in the population. Previous
studies have revealed that the efficacy of EGFR-TKIs can be
associated with the appearance of rash; the more frequent the rash,
the more effective the drug was for the treatment of NSCLC
(65,66). Therefore, the increased frequency of
rash observed following erlotinib treatment may be associated with
its longer MST. In the present study, the network meta-analysis
also indicated that erlotinib may have a higher SD rate compared
with erlotinib and icotinib.
The efficacy of EGFR-TKIs in the treatment of
patients with advanced NSCLC may be associated with gender,
smoking, ethnicity and tumor pathology (47). However, these clinical factors could
not be accounted for in the current analysis due to the limited
number of studies evaluated. The present study was also limited by
its retrospective nature and the heterogeneity of the treatment
regimens.
In the present study, the most common adverse events
of EGFR-TKIs in patients with advanced NSCLC were rash and
diarrhea. The efficacy of these drugs may be associated with the
frequency of rash (65,66); however, the underlying molecular
mechanism by which this occurs remains unknown. EGFR-TKI efficacy
may also be associated with gender, smoking, ethnicity, tumor
pathology, pharmacokinetics and population characteristics
(47,63,64).
In conclusion, the results of the present study
indicate that gefitinib, erlotinib and icotinib exhibit similar
efficacy in the treatment of patients with advanced NSCLC.
Erlotinib may increase survival rates compared with gefitinib or
icotinib, but more frequently results in side effects. Further
clinical trials evaluating the efficacy of erlotinib, icotinib and
gefitinib are required.
Acknowledgements
The present study was supported by Xinjiang
Production and Construction Corps (grant nos. 2014BA039 and
2015AG014), and Shihezi University (grant no. GJHZ201602).
References
|
1
|
Lee VW, Schwander B and Lee VH:
Effectiveness and cost-effectiveness of erlotinib versus gefitinib
in first-line treatment of epidermal growth factor
receptor-activating mutation-positive non-small-cell lung cancer
patients in Hong Kong. Hong Kong Med J. 20:178–186. 2014.PubMed/NCBI
|
|
2
|
Chen WQ, Zheng RS, Zeng HM, Zou XN, Zhang
SW and He J: Report of cancer incidence and mortality in China,
2011. China Cancer. 24:1–10. 2015. View Article : Google Scholar
|
|
3
|
Li S, Zhang X, Yan Y, Wang K, Rui D, Pang
L and Li F: High cancer burden in elderly chinese, 2005–2011. Int J
Environ Res Public Health. 12:12196–12211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim
SW, Jung SH, Park YH, Ahn JS, Park K and Ahn MJ: Randomized phase
II study of gefitinib versus erlotinib in patients with advanced
non-small cell lung cancer who failed previous chemotherapy. Lung
Cancer. 75:82–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ellis PM, Coakley N, Feld R, Kuruvilla S
and Ung YC: Use of the epidermal growth factor receptor inhibitors
gefitinib, erlotinib, afatinib, dacomitinib and icotinib in the
treatment of non-small-cell lung cancer: A systematic review. Curr
Oncol. 22:e183–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Sheng Z, Zhang Y and Li G: The
Efficacy of epidermal growth factor receptor tyrosine kinase
inhibitors for molecularly selected patients with non-small cell
lung cancer: A meta-analysis of 30 randomized controlled trials.
Targ Oncol. 11:49–58. 2016. View Article : Google Scholar
|
|
7
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, et al: Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced
non-small-cell lung cancer. J Clin Oncol. 21:2237–2246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thatcher N, Chang A, Parikh P, Pereira
Rodrigues J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH,
Pemberton K, Archer V and Carroll K: Gefitinib plus best supportive
care in previously treated patients with refractory advanced
non-small-cell lung cancer: Results from a randomised,
placebo-controlled, multicentre study (iressa survival evaluation
in lung cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shepherd FA, Pereira Rodrigues J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Togashi Y, Masago K, Fujita S, Hatachi Y,
Fukuhara A, Nagai H, Sakamori Y, Kim YH, Mio T and Mishima M:
Differences in adverse events between 250 mg daily gefitinib and
150 mg daily erlotinib in Japanese patients with non-small cell
lung cancer. Lung Cancer. 74:98–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan WC, Yu CJ, Tsai CM, Huang MS, Lai CL,
Hsia TC, Tien YJ, Huang SF, Wu CH, Chou KT, et al: Different
efficacies of erlotinib and gefitinib in taiwanese patients with
advanced non-small cell lung cancer: A retrospective multicenter
study. J Thorac Oncol. 6:148–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urata Y, Katakami N, Morita S, Kaji R,
Yoshioka H, Seto T, Satouchi M, Iwamoto Y, Kanehara M, Fujimoto D,
et al: Randomized phase III study comparing gefitinib with
erlotinib in patients with previously treated advanced lung
adenocarcinoma: WJOG 5108 L. J Clin Oncol. 34:3248–3257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia J, Si RR and Wu YF: Clinical research
on icotinib hydrochloride in the treatment of advanced non-small
cell lung cancer. J Basic Clin Oncol. 28:210–212. 2015.
|
|
14
|
Shi Y, Zhang L, Liu X, Zhou C, Zhang L,
Zhang S, Wang D, Li Q, Qin S, Hu C, et al: Icotinib versus
gefitinib in previously treated advanced non-small-cell lung cancer
(ICOGEN): A randomised, double-blind phase 3 non-inferiority trial.
Lancet Oncol. 14:953–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YP: Evidence-based Medicine. People's
Medical Publishing House; Beijing: 2014, View Article : Google Scholar
|
|
16
|
Zeng XT, Bao CP, Cao SY and Liu JY: The
quality assessments for randomized controlled trials. Chin J Evid
Based Cardiovasc Med. 4:183–184. 2012.
|
|
17
|
Zeng XT, Liu H, Chen X and Leng WD: The
quality assessments for observational study. Chin J Evid Based
Cardiovasc Med. 4:297–299. 2012.
|
|
18
|
Yuhara H, Steinmaus C, Cohen SE, Corley
DA, Tei Y and Buffler PA: Is diabetes mellitus an independent risk
factor for colon cancer and rectal cancer? Am J Gastroenterol.
106:1911–1921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the United States, national cancer institute of
Canada. J Nati Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
20
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi YX, Zhang W, Liu XY, Zhang J, Zhu DJ
and Lv QY: Result interpretation of network meta-analysis. Chin J
Evidence Based Med. 15:103–109. 2015.
|
|
22
|
Wang HY and Zhang DF: Comparison of the
efficacy of gefitinib and erlotinib as a second line treatment for
advanced non-small cell lung cancer. J Practical Med. 28:3444–3446.
2012.
|
|
23
|
Song C, Xu LY, Qiao JJ, Li M, Zhao JB and
Sun LM: Efficacy and safety of EGFR-TKIs as first-line treatment in
112 elder patients with advanced non-small cell lung cancer. J
Dalian Med Univ. 37:282–285. 2015.
|
|
24
|
Wu X, Zhang HY, Lv WZ and Lin Z:
Comparation of clinical effects and safety between gefitinib and
erlotinib in treatment of patients with NSCLC. Chin J of
Misdiagnostics. 11:3534–3536. 2011.
|
|
25
|
Yuan HF: Comparison of clinical
effectiveness of gefitinib and erlotinib on non-small cell lung
cancer. Sci and Tech of West China. 14:108–109. 2015.
|
|
26
|
Qu WF: Clinical effect of erlotinib on non
- small cell lung cancer and its impact on immunoglobulin levels
and T-lymphocyte subsets. Practical J of Cardiac Cerebral Pneumal
and Vascular Disease. 23:73–75. 2015.
|
|
27
|
Wang YX: Comparation of the efficacy and
care between gefitinib and erlotinib for the patients with NSCLC.
Medical Inform. 27:174–175. 2014.
|
|
28
|
Xie Y, Liang J and Su N: Gefitinib versus
erlotinib as first-line treatment for patients with advanced EGFR
mutation-positive non-small-cell lung cancer. Nan Fang Yi Ke Da Xue
Xue Bao. 35:446–449. 2015.(In Chinese). PubMed/NCBI
|
|
29
|
Xie YK: The clinical effects and safety of
gefitinib for patients with non-small cell lung cancer. Yiayao
Qianyan. 4:192. 2014.
|
|
30
|
Weng XL: Clinical effect and
pharmacoeconomics of gefitinib and erlotinib in advanced
non-small-cell lung cancer. Chin Med Pharm. 5:100–102. 2015.
|
|
31
|
Zhang J, Liu SQ, Zhang J, Ban LY and Zhou
T: Effect and cost-efficacy analysis of the second-line treatment
of advanced non-small cell lung cancer. Chin Clin Oncol.
17:908–911. 2012.
|
|
32
|
Zhang CW: The observation of toxicities
for targeted therapy in advanced non-small cell lung cancer. Hainan
Med J. 25:2273–2274. 2014.
|
|
33
|
Chen XP, Hang XS, Gao X, Xu WH, Li C and
Zhao J: Adverse drug reaction of gefitinib in therapy for patients
with advanced non-small cell lung cancer. Chin J of Hemorheology.
19:579–582. 2009.
|
|
34
|
Zhang YQ, Li YP, Ni J and Liu GL: Clinical
effect and pharmacoeconomics of gefitinib and erlotinib in advanced
non-small-cell lung cancer. Chin J New Drugs Clin Rem. 28:837–840.
2009.
|
|
35
|
Zhang YJ, Li HB, Li XD, Liu XC and Han JC:
Clinical effect and safety of gefitinib and erlotinib second line
treatment of lung adenocarcinoma. Chin J Clin Pharmacol.
31:899–901. 2015.
|
|
36
|
Li YY, Li L and Lv EJ: The comparison of
efficacy between gefitinib and erlotinib for patients with brain
metastases from non-small cell lung cancer. Chin J Clin Res.
28:1308–1311. 2015.
|
|
37
|
Bai H, Xiong LW, Han BH and Jiang LY:
Clinical observation of gefitinib and erlotinib for brain
metastases of non-small cell lung cancer. Chin Clin Oncol.
20:1028–1031. 2015.
|
|
38
|
Li L: Clinical observation of 70 patients
with brain metastases. Dalian Medical Univ. 2013.
|
|
39
|
Zhang JX, Cai D, Li SY, Zhou CZ, Qin YY
and Ouyang M: Clinical comparison of erlotinib and gefitinib in
non-small cell lung cancer with brain metastases. Chin J Cancer
Prevent Treat. 22:285–288. 2015.
|
|
40
|
Ma Y, Huang Y, Zhao H, Liu J, Chen L, Wu H
and Zhou N: The cost-effectiveness analysis of gefitinib or
erlotinib in the treatment of advanced EGFR mutant non-small cell
lung cancer patients. Zhongguo Fei Ai Za Zhi. 16:203–210. 2013.(In
Chinese). PubMed/NCBI
|
|
41
|
Shao YY, Shau WY, Lin ZZ, Chen HM, Kuo R,
Yang JC and Lai MS: Comparison of gefitinib and erlotinib
efficacies as third-line therapy for advanced non-small-cell lung
cancer. Eur J Cancer. 49:106–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lim SH, Lee JY, Sun JM, Ahn JS, Park K and
Ahn MJ: Comparison of clinical outcomes following gefitinib and
erlotinib treatment in non-small-cell lung cancer patients
harboring an epidermal growth factor receptor mutation in either
exon 19 or 21. J Thorac Oncol. 9:506–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida T, Yamada K, Azuma K, Kawahara A,
Abe H, Hattori S, Yamashita F, Zaizen Y, Kage M and Hoshino T:
Comparison of adverse events and efficacy between gefitinib and
erlotinib in patients with non-small-cell lung cancer: A
retrospective analysis. Med Oncol. 30:3492013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hong J, Kyung SY, Lee SP, Park JW, Jung
SH, Lee JI, Park SH, Sym SJ, Park J, Cho EK, et al: Pemetrexed
versus gefitinib versus erlotinib in previously treated patients
with non-small cell lung cancer. Korean J Intern Med. 25:294–300.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Emery IF, Battelli C, Auclair PL, Carrier
K and Hayes DM: Response to gefitinib and erlotinib in non-small
cell lung cancer: A retrospective study. BMC Cancer. 9:3332009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu WS, Chen YM, Tsai CM, Shih JF, Chiu CH,
Chou KT, Lai SL, Wu CH, Luo YH, Huang CY, et al: Erlotinib has
better efficacy than gefitinib in adenocarcinoma patients without
EGFR-activating mutations, but similar efficacy in patients with
EGFR-activating mutations. Exp Ther Med. 3:207–213. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu JY, Wu SG, Yang CH, Chang YL, Chang YC,
Hsu YC, Shih JY and Yang PC: Comparison of gefitinib and erlotinib
in advanced NSCLC and the effect of EGFR mutations. Lung Cancer.
72:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim ST, Lee J, Kim JH, Won YW, Sun JM, Yun
J, Park YH, Ahn JS, Park K and Ahn MJ: Comparison of gefitinib
versus erlotinib in patients with nonsmall cell lung cancer who
failed previous chemotherapy. Cancer. 116:3025–3033. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen JH, Luo YZ, Wang W, Zhou WW and Wen
XP: Efficacy and toxicity of ecotinib and gefitinib in the
treatment for 28 patients with non-small cell lung cancer who have
failed previous chemotherapy. Chin J New Drugs. 21:2056–2059.
2012.
|
|
50
|
Chen JH, Luo YZ, Wang W, Zhou WW and Wen
XP: A phase III study on icotinib hydrochloride for non-small cell
lung cancer. Anti-Tumor Pharmacy. 1:441–443. 2011.
|
|
51
|
Cui HZ, Guan JZ, Liao GQ, Liu PH, Li LL
and Shao Y: Efficacy of icotinib and gefitinib in advanced lung
adenocarcinoma with EGFR mutation. Acad J Chin Pla Med School.
36:326–328, 341. 2015.
|
|
52
|
Cui HQ, Liu HF and Lv JR: Icotinib in
treatment for 49 patients with non small cell lung cancer in
epidermal growth factor receptor mutant. Chin J New Drugs Clin Rem.
34:556–559. 2015.
|
|
53
|
Liu AM and Liu HQ: The observation and
care of EGFR-TKIs therapy for patients with advanced non-small cell
lung cancer. Med Inf. 27:175–176. 2014.
|
|
54
|
Lin WX and Zhang LJ: Clinical observation
of icotinib for non-small cell lung cancer. Chin J Prim Med Pharm.
21:106–107. 2014. View Article : Google Scholar
|
|
55
|
Xu LJ, Liu H, Li J and Gao Y: Clinical
observation of icotinib and gefitinib in first-line therapy for
advanced non-small cell lung cancer. J Hunan Normal Univ (Med Sci).
12:94–97. 2015.
|
|
56
|
Huang Y: Clinical observation of icotinib
hydrochloride and erlotinib in the treatment of patients with
advanced lung cancer who failed previous chemotherapy. Chin Foreign
Med Treat. 33:8–9. 2014.
|
|
57
|
Sun Y, Song C, Li M, Zhao JB and Sun LM:
The comparison of efficacy between icotinib and erlotinib for
patients with advanced non-small cell lung cancer. Shandong Med J.
55:34–36. 2015.
|
|
58
|
Zhang JX, Yu X, Zhang BB, Guan Q, Chen X,
Zhang Z, Yang BJ and Zhao MF: Comparison of clinical effects and
safety between icotinib and erlotinib in treatment of advanced
non-small cell lung cancers. J of Chin Phy. 17:1032–1035. 2015.
|
|
59
|
Hu X, Zhang L, Shi Y, Zhou C, Liu X, Wang
D, Song Y, Li Q, Feng J, Qin S, et al: The efficacy and safety of
icotinib in patients with advanced non-small cell lung cancer
previously treated with chemotherapy: A single-arm, multi-center,
prospective study. PLoS One. 10:e01425002015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shao YY, Lin CC and Yang CH: Gefitinib or
erlotinib in the treatment of advanced non-small cell lung cancer.
Discov Med. 9:538–545. 2010.PubMed/NCBI
|
|
61
|
Niu M, Hu J, Wu S, Xiaoe Z, Xu H, Zhang Y,
Zhang J and Yang Y: Structural bioinformatics-based identification
of EGFR inhibitor gefitinib as a putative lead compound for BACE.
Chem Biol Orug Des. 83:81–88. 2014.
|
|
62
|
Takeda M, Okamoto I, Fukuoka M and
Nakagawa K: Successful treatment with erlotinib after
gefitinib-related severe hepatotoxicity. J Clin Oncol. 28:e273–274.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Q and Zhou W: The new choice for
non-small cell lung cancer patients - iootinib hydrochloride. J
Pharmaceuti Res. 32:121–124. 2013.
|
|
64
|
Peters S, Zimmermann S and Adjei AA: Oral
epidermal growth factor receptor tyrosine kinase inhibitors for the
treatment of non-small cell lung cancer: Comparative
pharmacokinetics and drug-drug interactions. Cancer Treat Rev.
40:917–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan
DM and Song Y: Skin rash could predict the response to EGFR
tyrosine kinase inhibitor and the prognosis for patients with
non-small cell lung cancer: A systematic review and meta-analysis.
PLoS one. 8:e551282013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li HR and Sun YF: The severe adverse
events and prevention measures for erlotinib. Chin J
Pharmacoepidemiol. 19:232–233. 2010.
|