Introduction
Cryptococcosis is a potentially life-threatening
fungal disease affecting humans and animals worldwide. The disease
has increased in prevalence in recent years, accounting for 1
million infections and 700,000 deaths annually (1,2). In
China, cryptococcosis has also gradually increased over the past 20
years (3). The majority of these
infection cases are due to the inhalation of Cryptococcus
spores existing in pigeon excreta and decayed wood. Among >38
species of the genus Cryptococcus, Cryptococcus
neoformans and Cryptococcus gattii are the major
causative agents of human cryptococcosis (2). These two species are genetically
associated with each other; however, they differ genotypically,
epidemiologically, ecologically, geographically and phenotypically
(2,3). Cryptococcus neoformans occurs
most frequently in immunocompromised individuals, particularly
those with acquired immunodeficiency syndrome (AIDS) or other
causes of impaired T cell-mediated immunity, and less frequently
infects immunocompetent patients (4). By contrast, Cryptococcus gattii
is more likely to infect healthy individuals, and is associated
with tropical and subtropical regions (5). In addition, Cryptococcus gattii
infections cause more frequent neurological complications as
compared with infections associated with Cryptococcus
neoformans. Infections with Cryptococcus gattii are also
more difficult to treat due to its reduced sensitivity to
antifungal therapy in comparison with Cryptococcus
neoformans; therefore, more aggressive therapeutic strategies
are usually required for these infections (6,7).
Pulmonary cryptococcosis may be the first
manifestation of cryptococcosis since the respiratory tract is
considered to be the entry site and is most frequently involved
when cryptococcal infection develops (8). Depending on the host immune status, the
infection may remain dormant in the lung or may undergo
hematogenous spread to any organ system, including the central
nervous system (CNS), bones and skin (7,9).
However, pulmonary cryptococcosis does not present specific
clinical manifestations and radiological findings. The clinical
expression is variable and may present as coughing, expectoration,
chest tightness, fever or asymptomatic. The most common
radiological characteristics are known to be solitary or multiple
pulmonary nodules, and segmental or lobar consolidation (10,11). For
these reasons, pulmonary cryptococcosis is frequently misdiagnosed
as other lung diseases, such as cancer and pneumonia. Thus, the
main diagnostic methods include culture of clinical samples,
detection of cryptococcal antigen in body fluids and/or
histopathological examinations in tissue sections (12). However, certain of these methods are
associated with invasive acquisition of biopsies from the patients.
In addition, the sensitivities and accuracies of these techniques
are limited due to sampling errors or pollution, and heterogeneity
of Cryptococcus spores in biopsy samples (13,14).
Computed tomography (CT) scanning allows a quantitative repeated
noninvasive evaluation of the overall course of pulmonary disease
in patients. Therefore, a chest CT scan is helpful for
identification of pulmonary disease, patient stratification,
prediction of the response to therapy and evaluation of drug
efficacy. Although there are certain studies in the literature
reporting plain chest CT imaging data of pulmonary cryptococcosis
(4,10,11),
poorly specific manifestations in plain chest CT scans often cause
misdiagnosis. Contrast-enhanced chest CT scans can provide more
valuable information for the diagnosis of pulmonary nodules in
comparison with plain chest CT scans (15). Thus, the aim of the present study was
to retrospectively review the finding of plain and
contrast-enhanced chest CT scans in immunocompetent patients with
pulmonary cryptococcosis, in order to improve the diagnosis of this
type of pulmonary disease.
Materials and methods
Patients
The current study retrospectively reviewed the
characteristics and imaging findings of 27 immunocompetent patients
diagnosed with pulmonary cryptococcosis who were admitted to
Xinglin Branch, the First Affiliated Hospital of Xiamen University
(Xiamen, China) and underwent chest CT examination (plain and
contrast-enhanced, or only plain) between January 2005 and October
2014. The patient's medical records were collected, including
demographics, respiratory symptoms, occupational environment
exposure history, physical examination results, laboratory tests,
imaging data, pathology data, treatment and outcomes. The diagnosis
of pulmonary cryptococcosis was confirmed by histological or
cytological presence of the organism in lung biopsy specimens, by
positive findings in respiratory specimen cultures or in the serum
cryptococcal antigen test The protocol was approval by the Ethics
Committee of Xinglin Branch Hospital and all patients or legally
authorized representatives provided written informed consent upon
enrolment into the present study.
Chest CT scanning
Of the 27 patients, 14 patients underwent plain and
contrast-enhanced chest CT scans, and 13 patients only underwent
plain chest CT scans using a Philips Brilliance 16 CT scanner
(Philips Medical Systems, Inc., Cleveland, OH, USA) or a Toshiba
Aquilion 16 CT scanner (Toshiba Medical Systems Corp., Tokyo,
Japan). Patients in the supine position were asked to hold their
breath during scanning. Scanning was performed from the lung apices
to the level of the middle portion of the two kidneys. The CT
images were obtained using the following parameters: Detector
collimation, 5 mm; tube voltage, 120 kV; tube current, 100–200 mA
(using an automatic exposure control system); and reconstruction
interval, 5 mm. Contrast-enhanced CT scans were conducted by
administration of a nonionic iodine contrast agent (Iopromide 300;
Bayer Schering Pharma AG, Leverkusen, Germany). Patients were
initially fast injected with Iopromide 300 intravenously at the
dose of 1.5 ml/kg and speed of 3–4 ml/sec with a high-pressure
syringe, and then artery and vein double-phase scans were performed
with automatically triggered technology. All images were processed
in a picture archiving and communication system (PACS; version
UnSight 4.2; DJ HealthUnion Systems Corp., Shanghai, China) for
coronal, sagittal and oblique reconstructions.
Image analysis
CT images were reviewed retrospectively by two
independent experienced radiologists who were unaware of any
clinical data. A final conclusion was reached by consensus. When CT
images were reviewed, the size, number, location, border and
internal characteristics of any pulmonary nodules or masses were
recorded. The presence of an air bubble, air bronchogram, halo
sign, cavity, calcification and pleural effusions was also noted.
The CT attenuation values of the nodule or mass were subsequently
measured in the largest parts of the lesions, which were away from
the air density area. The mean attenuation values were calculated
from three randomly selected nodule locations.
Results
Patient characteristics
The demographics and clinical features of the 27
immunocompetent patients with pulmonary cryptococcosis included
into the present study are summarized in Table I. These patients consisted of 16
(59.26%) men and 11 (40.74%) women, with an age range between 26
and 65 years (median, 45.6 years). The most common clinical
manifestations were coughing (9 cases; 33.33%), expectoration (6
cases; 22.22%), fever (5 cases; 18.52%) and chest pain (4 cases;
14.81%), while 3 (11.11%) patients were asymptomatic. In term of
disease history, 8 (29.63%) patients were healthy, whereas 7
(25.93%) patients had a history of chronic pulmonary disease, 6
(22.22%) diabetes, 5 (18.52%) hypertension and 1 (3.70%)
malignancy. Regarding environmental factors, 8 (29.63%) patients
had no exposure history, while 9 (33.33%) patients had a history of
direct exposure to pigeon droppings. The remaining 10 (37.04%)
patients have a history of keeping cats, dogs or poultry.
 | Table I.Demographics and clinical features of
27 immunocompetent patients with pulmonary cryptococcosis. |
Table I.
Demographics and clinical features of
27 immunocompetent patients with pulmonary cryptococcosis.
|
Characteristics | Subjects |
|---|
| Age, years |
|
|
Range | 26–65 |
|
Mean | 45.6 |
| Gender, n (%) |
|
|
Male | 16 (59.26) |
|
Female | 11 (40.74) |
| Symptoms, n
(%) |
|
|
Asymptomatic | 3
(11.11) |
|
Coughing | 9
(33.33) |
|
Expectoration | 6
(22.22) |
|
Fever | 5
(18.52) |
| Chest
pain | 4
(14.81) |
| Disease history, n
(%) |
|
|
Healthy | 8
(29.63) |
| Chronic
pulmonary disease | 7
(25.93) |
|
Diabetes | 6
(22.22) |
|
Hypertension | 5
(18.52) |
|
Malignancy | 1
(3.70) |
| Occupational
environment exposure history, n (%) |
|
| History
of exposure to pigeon droppings | 9
(33.33) |
| History
of keeping cats, dogs or poultry | 10 (37.04) |
| No
exposure history | 8
(29.63) |
| Treatment and
follow-up, n (%) |
|
| No
treatment | 4
(14.81) |
|
Antifungal drugs | 23 (85.19) |
| Diagnostic method,
n (%) |
|
| Lung
biopsy specimens | 12 (44.44) |
|
Positive findings in
respiratory specimen culture | 10 (37.04) |
|
Positive results of serum
cryptococcal antigen test | 5
(18.52) |
According to the results of laboratory examination
(Table II), elevation of the
peripheral white blood cell count was detected in 8 (29.63%)
patients (normal range, 3.5–9.5×10−9 cells/l). A
lymphocyte cell count of <1.1×10−9/l (normal range,
1.1–3.2×10−9 cells/l) was present in 4 (14.81%) patients
(3 with chronic pulmonary disease receiving corticosteroids and 1
with malignancy), and a hemoglobin level of <115 g/l (normal
range, 115–150 g/l) was observed in 5 (18.52%) patients (2 with
diabetes, 1 with malignancy and 2 with chronic pulmonary disease
receiving corticosteroids). In addition, an erythrocyte
sedimentation rate of >15 mm/h (normal range, 0–15 mm/h) was
reported in 2 (7.41%) male patients and >20 mm/h (normal range,
0–20 mm/h) in 4 (22.22%) female patients (4 with chronic pulmonary
disease and 2 with hypertension), while a c-reactive protein level
of >8 mg/l (normal range, 0–8 mg/l) was observed in 5 (18.52%)
patients (3 with chronic pulmonary disease and 2 with diabetes).
None of the patients presented CNS involvement of the disease.
 | Table II.Laboratory test of 27 immunocompetent
patients with pulmonary cryptococcosis. |
Table II.
Laboratory test of 27 immunocompetent
patients with pulmonary cryptococcosis.
| Parameters | Unit | Normal range | Abnormal, n
(%) |
|---|
| White blood
cells |
x10−9/l | 3.5–9.5 | 8 (29.63) |
| Lymphocytes |
x10−9/l | 1.1–3.2 | 4 (14.81) |
| Hemoglobin | g/l | 115–150 | 5 (18.52) |
| Platelet |
x10−9/l | 125–350 | 0 |
| ESR of males | mm/H |
0–15 | 2 (7.41) |
| ESR of females | mm/H |
0–20 | 4 (22.22) |
| C-reactive
protein | mg/l |
0–8 | 5 (18.52) |
The diagnosis of these pulmonary cryptococcosis
cases was confirmed by histological or cytological presence of the
organism in lung biopsy specimens (12 cases; 44.44%), by positive
findings in respiratory specimen culture (10 cases; 37.04%) or by
positive results of the serum cryptococcal antigen test (5 cases;
18.52%). Treatment included antifungal drugs alone in 23 (85.19%)
patients, and no treatment was administered in 4 (14.81%) patients,
one of whom suffered from pancreatic cancer that progressed
rapidly, and thus antifungal treatment was impossible. Of the 23
patients who received antifungal therapy, 22 patients improved and
1 succumbed due to acute myocardial infarction during the follow-up
period.
Radiological features
The pulmonary abnormalities observed on the CT scans
are summarized in Table III. The
most common radiologic feature of pulmonary crptococcosis was the
presence of one or more nodules (11 out of 27 patients; 40.74%). A
solitary nodule was identified in 36.36% (4 out of 11) of patients
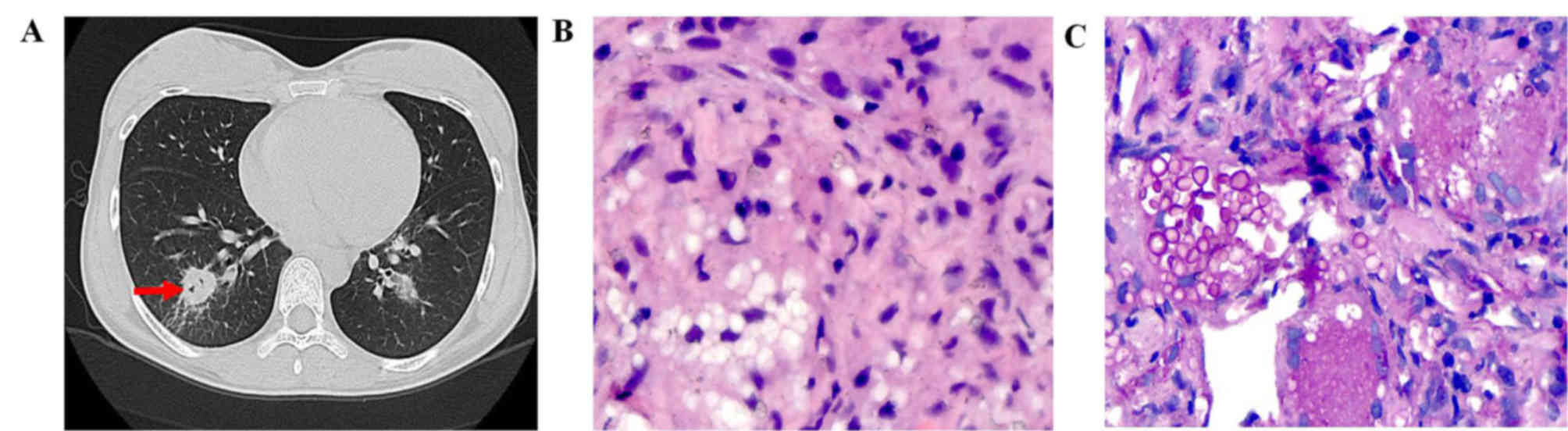
(Fig. 1), while multiple nodules
were observed in 63.64% (7 out of 11) of patients and were most
commonly bilateral in 71.43% (5 out of 7) of these patients. The
nodules were 6–20 mm in diameter in 6 patients and 21–30 mm in 5
patients. The majority of the nodules had poorly defined margins,
with peripheral predominance (outer third of the lung) close to the
pleura in 72.73% (8 out of 11) of patients. The second most common
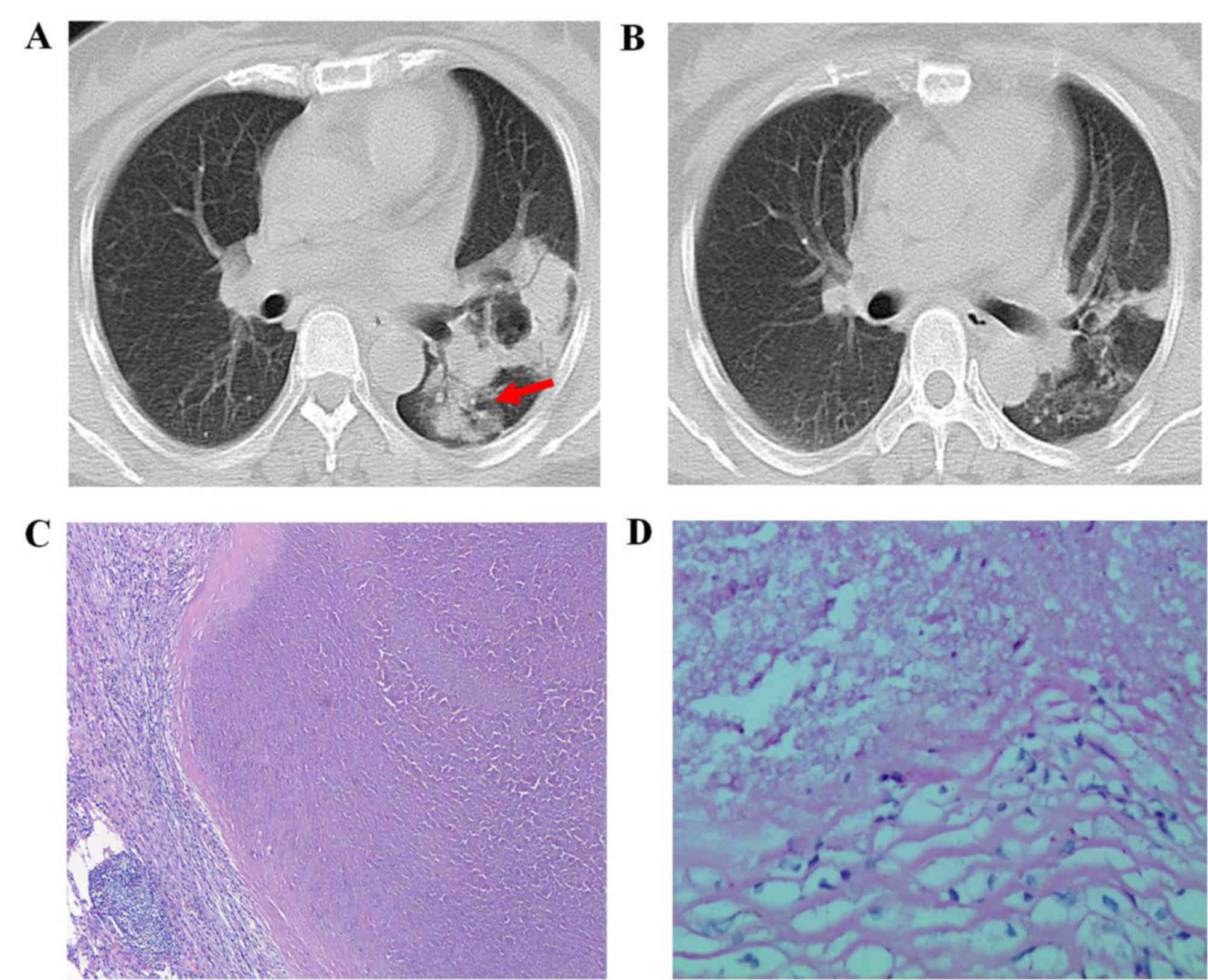
finding was consolidation (7 out of 27 patients; 25.93%; Fig. 2). Other associated findings included
ground-glass opacities (GGO; 6 out of 27; 22.22%) and presence of a
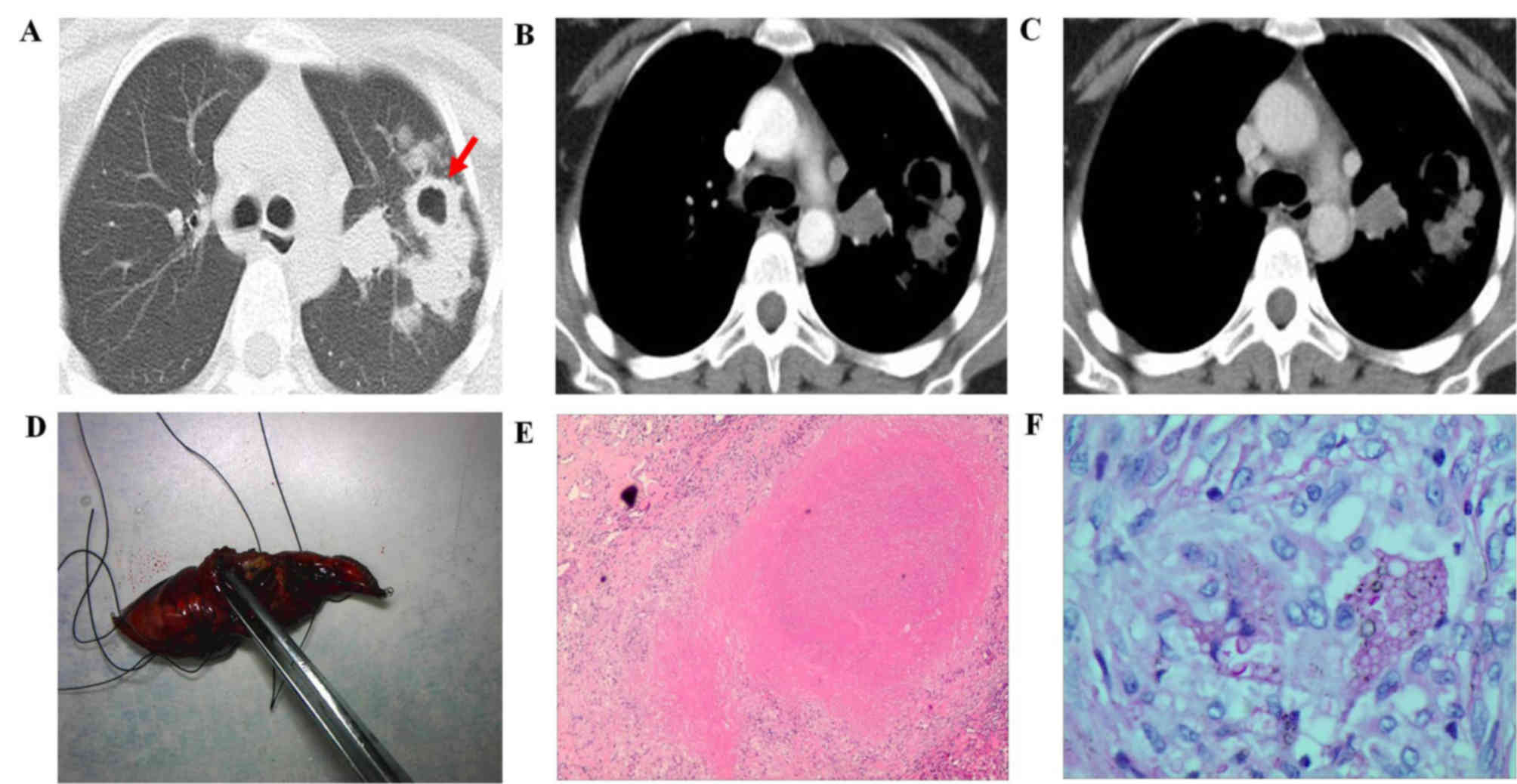
mass (3 out of 27; 11.11%; Fig.
3).
 | Table III.Radiological features of pulmonary
abnormalities of CT scans. |
Table III.
Radiological features of pulmonary
abnormalities of CT scans.
|
Characteristics | Nodule | Consolidation | GGO | Mass | Total no. (%) |
|---|
| Patient no.
(%) | 11 (40.74) | 7 (25.93) | 6 (22.22) | 3 (11.11) | 27 |
| Feature number |
|
|
|
|
|
|
Solitary | 4 | 2 | 2 | 3 | 11 (40.74) |
|
Multiple | 7 | 5 | 4 | 0 | 16 (59.26) |
| Lesion lung |
|
|
|
|
|
| Right
lung | 2 | 3 | 4 | 1 | 10 (37.04) |
| Left
lung | 4 | 2 | 2 | 2 | 10 (37.04) |
|
Bilateral lungs | 5 | 2 | 0 | 0 | 7 (25.92) |
| Lung area |
|
|
|
|
|
| Upper
lobes | 3 | 2 | 2 | 0 | 7 (25.93) |
| Middle
lobe | 2 | 1 | 1 | 1 | 5 (18.52) |
| Lower
lobes | 6 | 4 | 3 | 2 | 15 (55.55) |
| Predominant
distribution |
|
|
|
|
|
|
Central | 3 | 2 | 1 | 1 | 7 (25.93) |
|
Peripheral | 8 | 5 | 5 | 2 | 20 (74.07) |
| Margin |
|
|
|
|
|
| Poorly
defined | 7 | 7 | 4 | 2 | 20 (74.07) |
|
Smooth | 4 | 0 | 2 | 1 | 7 (25.93) |
| Halo
sign | 6 | 0 | 0 | 0 | 6 (22.22) |
|
Lobulated | 1 | 0 | 0 | 2 | 1 (3.70) |
|
Spiculated | 1 | 0 | 0 | 1 | 1 (3.70) |
| Internal
characteristics |
|
|
|
|
|
|
Air-bubble sign | 6 | 0 | 2 | 1 | 9 (33.33) |
| Air
bronchogram | 0 | 5 | 0 | 0 | 5 (18.52) |
|
Cavity | 0 | 2 | 0 | 2 | 4 (14.81) |
|
Calcification | 1 | 0 | 0 | 0 | 1 (3.71) |
|
Homogenous | 4 | 0 | 4 | 0 | 8 (29.63) |
| Concurrent
characteristics |
|
| Pleural
effusion | 0 | 2 | 0 | 0 | 2 (7.41) |
| Lymph
nodesa | 0 | 3 | 0 | 1 | 4 (14.81) |
|
None | 11 | 2 | 6 | 2 | 21 (77.78) |
| Maximal CT value
(Hu) |
|
|
Enhancement value | 20.92±5.67 | – | – | 35.61±8.32 | – |
| Lesion
enhancement | Ring | – | – | Inhomogeneous | – |
Lesions in the right lung were observed in 10
(37.04%) patients and in the left lung in 10 (37.04%) patients,
while bilateral lung lesions were observed in 7 (25.93%) patients.
The lower lobes were most frequently involved in 15 (55.55%)
patients, 7 (25.93%) patients had lesions involvement in the upper
lobes and 5 (18.52%) patients had middle lobe involvement.
Furthermore, lesions in the peripheral area were identified in 20
(74.07%) patients and in the central area in 7 (25.93%) patients.
These data suggested that the pulmonary lesions presented lower
lung lobe and peripheral predominance.
Air-bubble sign was a feature in the CT scans of 6
(54.55%) patients with a lung nodule (Fig. 1A), 2 (33.33%) patients with GGO and 1
patient with a mass. Nodules with calcification or halo sign were
observed in 1 (9.1%) or 6 (54.55%) patients, respectively. Air
bronchogram sign (Fig. 2A) was
identified in 5 (71.43%) patients with a consolidation lesion,
while a cavity was noted in 2 (28.57%) patients with consolidation
(Fig. 2A) and in 2 patients with a
mass lesion (Fig. 3A). In addition,
mediastinal lymph node enlargement was noted in 3 patients with a
consolidation lesion and in 1 patient with a mass lesion. Pleural
effusion was noted in 2 patients with a consolidation lesion,
however, pericardial effusion was not observed in any of the
patients. Thus, the air-bubble, halo, air bronchogram and cavity
signs were the most commonly reported features in CT scans (33.33,
22.22, 18.52 and 14.81%, respectively).
Out of the 27 participants, 11 patients with a
nodule lesion and 3 patients with mass lesions also underwent CT
contrast-enhancement evaluation. For the patients with nodule
lesions, the nodules presented ring enhancement with a mean maximal
enhancement value of 20.92±5.67 Hu (Fig.
3B). Among the 3 patients with mass lesions, the lesions
exhibited inhomogeneous enhancement with a mean maximal enhancement
value of 35.61±8.32 Hu (Fig.
3B).
Follow-up studies
The range of follow-up for the 27 immunocompetent
patients was 1–24 months (mean, 13.2 months). During the follow-up
period, no mortalities were reported as a result of pulmonary
cryptococcosis. The clinical or radiological characteristics were
significantly improved in 26 patients (96.30%). In 1 patient
(3.70%) who received no antifungal treatment due to rapid
progression of pancreatic cancer, the pulmonary lesions of multiple
nodules demonstrated disease progression over a 3-month follow-up
period. After a short time, the patient succumbed to pancreatic
cancer.
At the 3-month follow-up evaluation of the 23
patients who received antifungal therapy only, 16 patients
exhibited partial resolution of pulmonary abnormalities with a
decrease in the titer of serum cryptococcal antigen test or the
culture of respiratory specimens (Fig.
2), whereas the condition of 5 patients remained unchanged, and
2 patients presented radiographic deterioration and were re-treated
with antifungal therapy. At the 6- and 9-month follow-up
evaluation, 20 patients demonstrated radiographic improvement and 3
patients remained radiographically stable with a decrease in the
titer of serum cryptococcal antigen or the culture of respiratory
specimens. At the >12 months of follow-up, the radiographs in
all 23 patients presented significant resolution of pulmonary
abnormalities with a parallel decrease in the titer of serum
cryptococcal antigen or the culture of respiratory specimens.
At the 3- and 6-month follow-up evaluation of 3
patients without any therapy, CT revealed reduction in the size of
the parenchymal abnormalities. At the 9-month follow-up, the lung
lesions disappeared completely.
Discussion
Pulmonary cryptococcosis is an important
opportunistic fungal infection, with the majority of cases caused
by Cryptococcus neoformans or Cryptococcus gattii
infection (1,2). Epidemiological data indicate that
Cryptococcus neoformans occurs more frequently in
immunocompromised individuals, particularly those with AIDS. By
contrast, Cryptococcus gattii is more likely to cause
disease in healthy people (2), as
recently highlighted in the outbreaks in Canada (British Columbia)
and the United States Pacific Northwest (1). However, Cryptococcus gattii
infections are rare in China. Feng et al (16) reported that only 9 out of 110 (8.2%)
clinical Cryptococcus isolates from China were
Cryptococcus gattii, and the vast majority were
Cryptococcus neoformans, which was consistent with the data
of several studies of clinical molecular epidemiology in other
geographic areas (12,17).
Previously, pulmonary cryptococcosis was considered
to occur mainly in immunocompromised individuals, such as
individuals with AIDS, as well as in individuals that underwent
organ transplantation or had a hematologic malignancy, and to be
rare in immunocompetent patients (4). Recently, it has reported that the
incidence of pulmonary cryptococcosis is increasing (12). A epidemiological study indicated that
the annual incidence of pulmonary cryptococcosis has increased from
6 cases per million in 1999 to 38 cases per million in 2006 in
British Columbia, Canada (18). This
>6-fold increase cannot be explained by the stable incidence of
people with human immunodeficiency virus (HIV) infection. Thus, the
increased incidence is mainly due to infection of non-HIV
(immunocompetent) individuals (19,20).
Studies on the prevalence and epidemiology of
pulmonary cryptococcosis in China are relatively scarce. A recent
systemic review by Yuchong et al (21) summarized 1,032 reports of 8,769 cases
of pulmonary cryptococcosis in mainland China between 1985 and
2010. Notably, these rates were similar to those reported in
Taiwan, where the average annual incidence increased from 4.0 cases
per million individuals in 2000 to 5.5 per million individuals in
2007 (22). Considering the
aforementioned studies, it is evident that pulmonary cryptococcosis
cases in China have been increasing over the recent decades
(19,21,23). In
the present study of cases at a single-institution, the
retrospective review of 27 immunocompetent patients clinically
confirmed with pulmonary cryptococcosis revealed that pulmonary
cryptococcosis is not rare in immunocompetent people. In these
patients, the disease did not have specific clinical manifestations
and radiological findings, and the clinical prognosis was most
often favorable. The majority of patients in the current study
presented with respiratory symptoms, including coughing (33.33%),
expectoration (22.22%), fever (18.52%) and chest pain (14.81%),
while only 3 patients (11.11%) were asymptomatic. In contrast to
the present study, Kishi et al (10) reported that approximately one-third
of immunocompetent patients with pulmonary cryptococcosis were
asymptomatic. Depending on the host immune status, cryptococcosis
may disseminate from the lungs to other organs, including the CNS
and brain. One of the proposed mechanisms for cryptococcosis
crossing the blood-brain barrier is by hiding inside mononuclear
phagocytes via a ‘Trojan horse’ mechanism (24,25).
Thus, besides pulmonary cryptococcosis, Cryptococcus
infection can be frequently associated with cryptococcal
meningoencephalitis, resulting in high mortality and morbidity
rates. No patients in the current study presented with cryptococcal
meningoencephalitis. Compared with immunocompetent patients, the
symptoms of cryptococcosis infection in immunocompromised patients
are more evident (19,20,23).
Pulmonary cryptococcosis appears to predominantly
occur in adult males. In the current study, the 27 patients
included 16 males and 11 females. The male: female ratio (1.45:1)
in this group is similar to reported in previous studies (23,26).
Furthermore, in the present study, 33.33% (9 out of 27) of patients
had a history of direct exposure to pigeon droppings and 37.04% (10
out of 27) of patient had a history of keeping cats, dogs or
poultry. Only 29.63% (8 out of 27) patients had no exposure
history. These data suggest that more attention should be paid to
detailed questioning regarding the occupational and environmental
exposure history of patients in the diagnosis of pulmonary
cryptococcosis.
The radiological findings of pulmonary
cryptococcosis in plain chest CT scans are diverse. In the present
study, the most common manifestation of pulmonary cryptococcosis in
plain chest CT scans was pulmonary nodules, including single and
multiple nodules, which were observed in 40.74% (11 out of 27) of
patients, in comparison with 46.06% of patients in the study by Ye
et al (27) and 55.2% in the
study by Zhang et al (23).
Multiple nodules (63.64%) were more common compared with single
nodules (36.36%) and bilateral lung lesions were identified in
certain patients (45.45%), consistent with previous findings
(19). The margin of the nodules can
be ill-defined, smooth, lobulated or spiculated. In the present
study, the majority (63.64%) of the nodules in the patients had
poorly defined margins, with 54.55% (6 out of 11 patients) of the
nodules presenting a halo sign, while only 9.09% (1 out of 11
patients) of the nodules were lobulated or speculated, which were
misdiagnosed as lung cancer. Lee et al (26) reported that none of the nodules of 12
immunocompromised and immunocompetent patients demonstrated a
smooth margin. Furthermore, Lindell et al (28) reported that 77.0% (7 out of 10
immunocompetent patients) of the nodules were well-defined with
smooth margins, and Zinck et al (29) demonstrated that margination of
nodules in the reported cases was smooth (50.0%; n=5), poorly
defined (30.0%; n=3), lobulated (10.0%; n=1) or speculated (10.0%;
n=1) in 10 patients with immunocompromised or immunocompetent. The
halo sign is caused by various pathological conditions, including
hemorrhagic nodules of varying causes, tumor cell infiltration and
non-hemorrhagic inflammatory lesions (30). This sign in patients with pulmonary
cryptococcosis has been demonstrated to occur in association with
granulomatous inflammatory (20).
Other CT findings in the present study included
consolidation, GGO and a mass, with 7, 6 and 3 cases identified,
respectively. Internal characteristics in the nodules,
consolidation, GGO and mass lesions included air bubble (33.33%),
air bronchogram (18.52%), cavity (14.81%) and calcification (3.7%).
The air-bubble sign was observed in 6 patients with a lung nodule
lesion due to cryptococcosis erosion of a bronchiolus allowing air
to reach the space between granulomatous lesions. In addition, air
bronchogram was observed in 5 out of 7 patients with consolidation
due to inflammatory response composed of histiocytes,
multinucleated giant cells and lymphocytic infiltration without
destruction of tissue architectures (20). Cavitation was also evident on CT
scans in 4 (14.81%) of the 27 patients, including cavitating
consolidation in 2 patients and cavitating mass in two, which were
difficult to be distinguished from lung cancer and tuberculosis.
These aforementioned findings were similar to those of previous
studies (23,28,29).
Pulmonary cavitation has been more rarely reported in
immunocompetent patients compared with immunocompromised patients
(10). It has also been reported
that cavitation demonstrates a long-term localized pulmonary
abnormality, indicating that patients with cavitary pulmonary
lesions may experience a more severe cryptococcal infection that
requires a more aggressive antifungal therapy (31).
Regarding the distribution of the lesions of
pulmonary cryptococcosis, in agreement with prior radiographic
studies (15,23,28,30), the
pulmonary lesions in the present study were located predominantly
in the lower lung (55.55%) rather than in the middle (18.52%) or
upper lung (25.52%), and were identified mainly in the peripheral
areas (74.07%) rather than in the central areas (25.93%).
Enlargement of mediastinal lymph nodes and pleural effusion may
also occur, although this appears to be more likely in
immunocompromised patients rather than in immunocompetent ones
(26,27).
Based on the clinical practice guidelines for the
management of cryptococcal disease, initially published in 2000
(32) and updated in 2010 (33), it is essential to assess the hosts'
immune status and the anatomical sites of involvement in patients
with pulmonary cryptococcosis. For immunocompetent patients without
symptoms, observation alone is required since pulmonary
cryptococcosis may resolve spontaneously. When the lesions are
enlarged and symptoms develop during the follow-up period, timely
antifungal treatment is required. The preferred treatment regimens
include oral azole therapy (fluconazole or itraconazole) for 6–12
months. In the present study, 3 asymptomatic cases did not receive
any antifungal therapies but were observed. After 9 months, all
patients had complete resolution of radiographic findings. Among
the 23 patients who received antifungal therapy, significant
improvement or resolution of radiographic findings was observed. In
addition, 1 patient did not receive antifungal treatment due to
rapidly progressing pancreatic cancer and succumbed to pancreatic
cancer during the first 3-month follow-up.
In conclusion, pulmonary cryptococcosis in
immunocompetent patients may be associated with environmental
fungal exposure. The most common CT findings were multiple
pulmonary nodules with poorly defined margins. Other findings
included consolidation, GGO and mass. The majority of lesions
involved the periphery of the lower lung lobes with a halo sign,
air-bubble sign, air bronchogram and cavity. On contrast-enhanced
chest CT scans, the nodules presented ring enhancement, and masses
demonstrated inhomogeneous enhancement. Therefore, familiarity with
the CT findings and occupational environment exposure history will
assist in earlier and easier diagnosis of pulmonary cryptococcosis
in immunocompetent patients.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81571707,81071182), the
Program for Training Young Talents of Fujian Health (no.
2014-ZQN-ZD-35), the Natural Science Foundation of Fujian (no.
2015J01519), the Technology Foundation for Selected Overseas
Chinese Scholar, the Ministry of Human Resources and Social
Security of China (no. 2014-240) and the Scientific Research
Foundation for the Returned Overseas Chinese Scholars, Ministry of
Education of China (no. 2014-1685).
References
|
1
|
Smith IM, Stephan C, Hogardt M, Klawe C,
Tintelnot K and Rickerts V: Cryptococcosis due to Cryptococcus
gattii in Germany from 2004–2013. Int J Med Microbiol.
305:719–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Teodoro VL, Gullo FP, Sardi Jde C, Torres
EM, Fusco-Almeida AM and Mendes-Giannini MJ: Environmental
isolation, biochemical identification, and antifungal drug
susceptibility of Cryptococcus species. Rev Soc Bras Med
Trop. 46:759–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang W, Fa Z and Liao W: Epidemiology of
Cryptococcus and cryptococcosis in China. Fungal Genet Biol.
78:7–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamakawa H, Yoshida M, Yabe M, Baba E,
Okuda K, Fujimoto S, Katagi H, Ishikawa T, Takagi M and Kuwano K:
Correlation between clinical characteristics and chest computed
tomography findings of pulmonary cryptococcosis. Pulm Med.
2015:7034072015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alanio A, Desnos-Ollivier M and Dromer F:
Dynamics of Cryptococcus neoformans-macrophage interactions
reveal that fungal background influences outcome during
cryptococcal meningoencephalitis in humans. MBio. 2(4): e00158–11.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schelenz S, Barnes RA, Barton RC,
Cleverley JR, Lucas SB, Kibbler CC and Denning DW: British Society
for Medical Mycology: British Society for Medical Mycology best
practice recommendations for the diagnosis of serious fungal
diseases. Lancet Infect Dis. 15:461–474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perfect JR, Dismukes WE, Dromer F, Goldman
DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O,
Nguyen MH, et al: Clinical practice guidelines for the management
of cryptococcal disease: 2010 update by the infectious diseases
society of america. Clin Infect Dis. 50:291–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sider L and Westcott MA: Pulmonary
manifestations of cryptococcosis in patients with AIDS: CT
features. J Thorac Imaging. 9:78–84. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brizendine KD, Baddley JW and Pappas PG:
Predictors of mortality and differences in clinical features among
patients with cryptococcosis according to immune status. PLoS One.
8:e604312013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kishi K, Homma S, Kurosaki A, Kohno T,
Motoi N and Yoshimura K: Clinical features and high-resolution CT
findings of pulmonary cryptococcosis in non-AIDS patients. Respir
Med. 100:807–812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fox DL and Müller NL: Pulmonary
cryptococcosis in immunocompetent patients: CT findings in 12
patients. AJR Am J Roentgenol. 185:622–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Espinel-Ingroff A and Kidd SE: Current
trends in the prevalence of Cryptococcus gattii in the
United States and Canada. Infect Drug Resist. 8:89–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guarner J and Brandt ME: Histopathologic
diagnosis of fungal infections in the 21st century. Clin Microbiol
Rev. 24:247–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barton RC: Laboratory diagnosis of
invasive aspergillosis: From diagnosis to prediction of outcome.
Scientifica (Cairo). 2013:4594052013.PubMed/NCBI
|
|
15
|
Ye XD, Ye JD, Yuan Z, Li WT and Xiao XS:
Dynamic CT of solitary pulmonary nodules: Comparison of contrast
medium distribution characteristic of malignant and benign lesions.
Clin Transl Oncol. 16:49–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng X, Yao Z, Ling B, et al: Analysis of
the varieties, genotypes and mating types of 110 clinical
cryptococcal isolates from China. Chin J Microbiol Immunol.
28:193–197. 2008.
|
|
17
|
Wu SY, Lei Y, Kang M, Xiao YL and Chen ZX:
Molecular characterisation of clinical Cryptococcus neoformans
Cryptococcus gattii isolates from Sichuan province, China.
Mycoses. 58:280–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galanis E, Macdougall L, Kidd S and
Morshed M: British Columbia Cryptococcus gattii Working
Group: Epidemiology of Cryptococcus gattii, British
Columbia, Canada, 1999–2007. Emerg Infect Dis. 16:251–257. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie X, Xu B, Yu C, Chen M, Yao D, Xu X,
Cai X, Ding C, Wang L and Huang X: Clinical analysis of pulmonary
cryptococcosis in non-HIV patients in south China. Int J Clin Exp
Med. 8:3114–3119. 2015.PubMed/NCBI
|
|
20
|
Xie LX, Chen YS, Liu SY and Shi YX:
Pulmonary cryptococcosis: Comparison of CT findings in
immunocompetent and immunocompromised patients. Acta Radiol.
56:447–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuchong C, Fubin C, Jianghan C, Fenglian
W, Nan X, Minghui Y, Yalin S and Zhizhong Z: Cryptococcosis in
China (1985–2010): Review of cases from Chinese database.
Mycopathologia. 173:329–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YY and Lai CH: Nationwide
population-based epidemiologic study of cryptococcal meningitis in
Taiwan. Neuroepidemiology. 36:79–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Li N, Zhang Y, Li H, Chen X, Wang
S, Zhang X, Zhang R, Xu J, Shi J and Yung RC: Clinical analysis of
76 patients pathologically diagnosed with pulmonary cryptococcosis.
Eur Respir J. 40:1191–1200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Charlier C, Nielsen K, Daou S, Brigitte M,
Chretien F and Dromer F: Evidence of a role for monocytes in
dissemination and brain invasion by Cryptococcus neoformans.
Infect Immun. 77:120–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sorrell TC, Juillard PG, Djordjevic JT,
Kaufman-Francis K, Dietmann A, Milonig A, Combes V and Grau GE:
Cryptococcal transmigration across a model brain blood-barrier:
Evidence of the Trojan horse mechanism and differences between
Cryptococcus neoformans var. Grubii strain H99 and
Cryptococcus gattii strain R265. Microbes Infect. 18:57–67.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee WY, Wu JT, Jeng CM, HSU CY, Liu JS,
Wang YC, Wu CY, Chang HY, Huang JC, Yang CM and Kung CH: CT
Findings of pulmonary cryptococcosis: A series of 12 cases. Chin J
Radiol. 30:319–325. 2005.
|
|
27
|
Ye F, Xie JX, Zeng QS, Chen GQ, Zhong SQ
and Zhong NS: Retrospective analysis of 76 immunocompetent patients
with primary pulmonary cryptococcosis. Lung. 190:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lindell RM, Hartman TE, Nadrous HF and Ryu
JH: Pulmonary cryptococcosis: CT findings in immunocompetent
patients. Radiology. 236:326–331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zinck SE, Leung AN, Frost M, Berry GJ and
Müller NL: Pulmonary Cryptococcosis: CT and Pathologic Findings. J
Comput Assist Tomogr. 26:330–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK
and Heo JN: CT halo sign: The spectrum of pulmonary diseases. Br J
Radiol. 78:862–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang WC, Tzao C, Hsu HH, Lee SC, Huang
KL, Tung HJ and Chen CY: Pulmonary cryptococcosis: Comparison of
clinical and radiographic characteristics in immunocompetent
andimmunocompromised patients. Chest. 129:333–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saag MS, Graybill RJ, Larsen RA, Pappas
PG, Perfect JR, Powderly WG, Sobel JD and Dismukes WE: Practice
guidelines for the management of cryptococcal disease. Infectious
Diseases Society of America. Clin Infect Dis. 30:710–718. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perfect JR, Dismukes WE, Dromer F, Goldman
DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O,
Nguyen MH, et al: Clinical practice guidelines for the management
of cryptococcal disease: 2010 update by the infectious diseases
society of America. Clin Infect Dis. 50:291–322. 2010. View Article : Google Scholar : PubMed/NCBI
|