Introduction
The cervix is often referred to as the uterus gate,
due to the fact that it functions as a defense and guard. Due to
the special status of the uterine cervix, it is vulnerable to
attack from external bacteria and viruses. Unique morphological
changes occurring in the cervical epithelial tissue that comprises
the cervical epithelium may result in malignant transformation or
inflammation (1). Cervical cancer is
the fourth most common type of cancer and the fourth most common
cause of cancer-associated mortality in women worldwide (2,3). There
are different types. of cervical cancer: About 90% of all cervical
cancer cases are squamous cell carcinomas, ~10% are adenocarcinomas
and a small proportion of cases are other types, including small
cell carcinoma and clear cell carcinoma (4). Recently, the number of cervical cancer
cases detected in younger women (women aged 45 years or younger is
defined as here) has increased from 18.02/100,000 in 2012 to
19.71/100,000 in 2013 (5). Tumors
occur as the result of a number of factors, including a weakening
of the body's immune system, exposure to chemical carcinogenic
factors, physical carcinogenic factors or biological carcinogenic
factors and a variety of chronic stimulation (6).
Current treatment for cervical cancer consists of a
combination of surgery, radiotherapy and chemotherapy (7) and the five-year survival rate for
patients with cervical cancer in the United States is 68%, which is
a high 5-year survival rate compared with pancreatic cancer or lung
cancer (8). Outcomes, however,
depend heavily on how early the cancer is detected. It has been
reported that the cervical cancer microenvironment often has low
levels of T cell immunity, which is important as T cell immunity
serves a vital role in recognizing and inhibiting the growth and
proliferation of tumor cells (9).
B7 homolog 1 (B7-H1) is a recently identified family
of B7 co-stimulatory molecules that serve an important role in
inhibiting T cell activation and promoting T cell apoptosis
(10). It has been reported that
B7-H1 is exploited by tumors to evade immune responses (11). In many different types of human
cancer, B7-H1 is expressed on the cell surface and a correlation
between its expression and poor clinical prognosis has been
identified in gastric, renal, breast, ovarian and esophageal
carcinoma (12–16). A number of studies have investigated
the association between B7-H4 and cervical cancer (17–19).
However, few studies have focused on the role of the B7-H1 molecule
in cervical cancer. The present study attempted to identify the
association between B7-H1 and cervical cancer, and the role B7-H1
serves in the immune regulation of cervical cancer.
A total of 12 specimens of cervical cancer tissue
were investigated in the present study and using
immunohistochemistry, it was determined that they all expressed
B7-H1. However, immunohistochemistry indicated that normal cervical
epithelium taken from healthy controls was negative for B7-H1. It
has been demonstrated that cervical cancer cells can inhibit the
activation of T lymphocytes (17).
However, following the downregulation of B7-H1 expression using
specific blocking antibodies, suppression of T lymphocytes was
inhibited. When B7-H1 was downregulated using a lentivirus carrying
a small interfering RNA (siRNA) specific for B7-H1, the ratio of
human cervical cancer cells (the green fluorescent protein ratio in
mice injected with green fluorescent protein cancer cells) was
decreased, whereas the immune response was increased. Therefore,
the results of the present study suggest that B7-H1 may be
developed as a novel target for the gene therapy of cervical
cancer.
Materials and methods
Ethics statement
All methods were performed in accordance with the
approved guidelines. In the present study, samples were collected
with the written consent of subjects and the written approval of
the Ethical Review Board of the Suzhou Hospital, Affiliated with
Nanjing Medical University (Suzhou, China). Mice used in the
present study were handled in strict accordance with the
appropriate animal practices. All experimental procedures using
mice in the present study were reviewed and approved by the Ethical
Review Board of Nanjing Medical University.
Isolation and culture of normal
cervical epithelium and cervical cancer cells
A total of 27 patients were studied in the present
study. From these 27 patients, 15 normal cervical epithelial
samples (from 15 female patinents with hysteromyoma aged 45 years
or younger who had undergone routine womb excision) and 12 cervical
cancer samples were obtained (from 12 female patients with cervical
cancer aged 45 or younger who had undergone colposcopy and biopsy
of the suspicious cancerous area and pathological diagnosis to
confirm cervical cancer) at Suzhou Hospital, which is Affiliated
with Nanjing Medical University (Suzhou, China), between September
2015 to September 2016. Normal cervical epithelial and cervical
cancer cell samples were isolated by a two-step combined
dissociation method using dispase and trypsin prior to being plated
into a 10-cm culture dish (Corning Inc., Corning, NY, USA) and
expanded (20). The cells were
cultured in keratinocyte serum-free medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
incubator in 5% CO2 at 37°C and were passaged by
trypsinization (0.25% trypsin, 0.1% EDTA) and expanded serially
with a split ratio of 1:3 at 80% confluence after 10 days. Cells
were maintained in a humidified incubator in 5% CO2 at
37°C for ~1 week. In the present study, all procedures were
performed following the guidelines established by Suzhou
Hospital-Affiliated Nanjing Medical University Ethics Boards and
written approval was granted from the Ethical Review Board of
Suzhou Hospital. Written consent was obtained from all women, after
they were informed that the samples would be used for study
purposes.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using the RNeasy Mini
extraction kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's instructions. A total of 1 µg RNA was used for
reverse transcription and cDNA was synthesized from total RNA
following the manufacturer's instructions (Qiagen, Inc.). For qPCR,
primer mixes were loaded in duplicate wells in 96-well plates and
PCR was performed following the addition of SYBR Green PCR Master
mix (Thermo Fisher Scientific, Inc.) and 1 µg (final) cDNA. The
following thermocycling conditions were applied: Pre-denaturation
at 95°C for 5 min; followed by 40 cycles of denaturation for 15 sec
at 95°C; and annealing and elongation at 65°C for 35 sec. (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). As an internal control,
GAPDH levels were quantified in parallel with target genes.
Relative quantification of the target was determined by the
2−ΔΔCq method (21). The
primers used were as follows: B7-H1 forward,
5′-TGGCATTTGCTGAACGCATTT-3′ and reverse,
5′-TGCAGCCAGGTCTAATTGTTTT-3′; B7-H2 forward,
5′-TGGCATTTGCTGAACGCATTT-3′ and reverse,
5′-AAAGTTGCATTCCAGGGTCAC-3′; B7-H3 forward,
5′-CCCACAGGTTGCTTTGCTTAA-3′ and reverse, 5′-GCAGACCCCTGGAGAACCA-3′;
B7-H4 forward, 5′-TCTGGGCATCCCAAGTTGAC-3′ and reverse,
5′-TCCGCCTTTTGATCTCCGATT-3′; interleukin (IL)-1β forward,
5′-TTCAGGCAGGCAGTATCACTC-3′ and reverse,
5′-GAAGGTCCACGGGAAAGACAC-3′; IL-6 forward,
5′-CTGCAAGAGACTTCCATCCAG-3′ and reverse,
5′-AGTGGTATAGACAGGTCTGTTGG-3′; tumor necrosis factor (TNF)-α
forward, 5′-CAGGCGGTGCCTATGTCTC-3′ and reverse,
5′-CGATCACCCCGAAGTTCAGTAG-3′; GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′.
Flow cytometry analysis
The expression of cell surface antigens on cervical
cancer cells was analyzed by flow cytometry. A total of
2×105 cells were detached by trypsin and re-suspended in
phosphate-buffered saline (PBS) containing 1% bovine serum albumin.
They were then counted using a hemocytometer and 2×105
cells were incubated at 37°C for 30 min with diluted pre-immune
immunoglobulin (Ig) G to block nonspecific binding of the
antibodies (22). Following
incubation at 4°C for 1 h with a primary antibody specific for
B7-H1 (cat. no. ab210931, dilution 1:100; Abcam, Cambridge, UK),
the cells were washed three times in PBS. Cells were then incubated
with phycoerythrin (PE)-labeled goat anti-mouse IgG secondary
antibody (cat. no. ab5881, dilution 1:100; Abcam) at 37°C for 1 h.
Negative controls were conducted by performing incubation without
primary antibodies. The results were analyzed using a
FACSCalibur™ flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). All data were analyzed using FlowJo 7.2.2 software
(Tree Star Inc, Ashland, OR, USA), following recently updated
guidelines (23).
Histological and immunochemical
analysis
Cervical cancer tissue from human patients and mice,
respectively were immediately fixed at room temperature in 4%
paraformaldehyde for 24 h and were subsequently embedded in
paraffin. Consequently, 5-µm thick paraffin sections were evaluated
using, immunohistochemistry and the light microscope
(magnification, ×20) was used to evaluate staining. Mouse
anti-human B7-H1 monoclonal antibody (cat. no. ab210931, dilution
1:100; Abcam) was used for immunohistochemistry.
Immunohistochemistry was performed according to the indirect
streptavidin-biotin-peroxidase method (24). For immunostaining, the samples were
incubated with a PE-conjugated secondary antibody (cat. no. ab5881,
dilution 1:100; Abcam) and then counterstained with DAPI (Southern
Biotech, Birmingham, AL, USA) and observed under a fluorescence
microscope (magnification, ×10; Leica DM 2500; Leica Microsystems,
Inc., Buffalo Grove, IL, USA).
Preparation of peripheral blood
mononuclear cells (PBMCs) to obtain T lymphocytes
Whole blood collected from healthy adult donors was
provided by the Suzhou Red Cross Blood Center (Suzhou, China).
PBMCs were isolated from blood using Ficoll-Biocoll Separation
Solution (Jingyang, Tianjin, China), as previously described
(25). PBMCs were added to RPMI-1640
medium (Hyclone, Logan, UT, USA) and centrifuged at ~600 × g for 5
min at room temperature. The supernatant was discarded, cells were
resuspended in RPMI-1640 medium and counted using a light
microscope. PBMCs were seeded in triplicate at 2×105
cells/well in 96-well plates and incubated at 37°C for 3 days in a
humidified 5% CO2 environment.
T lymphocyte proliferation assay
To assess the various effects of cervical cancer
cells and the normal cervical epithelium on T cell proliferation,
both types of cells were treated with 10 µg/ml mitomycin C
(Sigma-Aldrich; Merck kGaA, Darmstadt, Germany) for 2 h at 37°C.
For the proliferation assay, 2×104 cervical cancer
cells/well and normal cervical epithelium were plated in triplicate
into 96-well plates, respectively. T lymphocytes were stimulated
using 0.4 µg/ml anti-human cluster of differentiation CD3 (cat. no.
10977-H001, dilution 1:100; BD Pharmingen; BD Biosciences) and CD28
antibodies (cat. no. 560684, dilution 1:100; BD Pharmingen; BD
Biosciences). The ratios of T cells to cervical cancer cell or
normal cervical epithelium were both 5:1. Cell proliferation was
measured at 48 and 72 h independently following incubation using
the Cell Counting Kit-8 (CCK-8) (Takara Biotechnology Co., Ltd.,
Dalian, China) assay.
Infection
Green fluorescent protein (GFP)-positive cervical
cancer cells and B7H1-downregulated GFP positive cervical cancer
cells were constructed. A lentivirus containing the GFP gene
(LV-EGFP) was obtained from Stemcell Technologies, Inc. (Shanghai,
China). Lentivirus containing the GFP gene and interfering RNA for
B7H1 downregulation were constructed using the same vector. A total
of 2×105 cells were seeded and then added
1.3×106 IU/ml of lentivirus with 10 mg/ml polybrene.
After 24 h at 37°C, the medium was changed, the cells were cultured
in a humidified incubator in 5% CO2 at 37°C. The culture
medium was consisted of 90% Dulbecco's modified Eagle's medium
(Hyclone) and 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.). When the cervical cancer cells and B7H1
downregulated cervical cancer cells were ~80% confluent,
GFP-positive cells were detected using a flow cytometer (BD
Biosciences).
MTT assay
B7H1 downregulated cervical cancer cells and
cervical cancer cells were seeded in 96-well culture plates at an
optimal density of 5×103 cells/well in triplicate wells
(15). Following incubation at 37°C
for 0, 24, 48, 72, 96 and 120 h, the cells were stained with 20 µl
MTT (5 mg/ml) at 37°C for 4 h and subsequently solubilized with 150
ml dimethyl sulfoxide. Absorbance was measured at 490 nm using a
microtitration plate spectrophotometer and calculated using
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA). Cell growth curves were calculated as the mean values of each
group.
Immunofluorescence
Slides (Nalgene Nunc International, Naperville, IL,
USA) that were 5-µm thick were obtained via frozen section at −20°C
were washed twice in PBS and fixed in acetone for 5 min. Cells were
blocked for 30 min in 3% BSA (Sigma-Aldrich; Merck kGaA) in PBS.
Nuclei were counterstained with 4′,6-diamindino-2-phenylindole-2
(Sigma-Aldrich; Merck KGaA) for 3 min at room temperature.
Preparations were observed under a fluorescence microscope
(magnification, ×10; Leica DM 2500; Leica Microsystems, Inc.).
ELISA assay
The ELISA reagents for interferon (IFN)-γ (cat. no.
RAB0223), IL-10 (cat. no. RAB0244) and transforming growth factor
(TGF)-β1 (cat. no. RAB0460) were all purchased from Sigma-Aldrich
(Merck kGaA). Experiments were performed according to the
manufacturer's instructions. Supernatants were collected from the T
cells of the cervical cancer cells and B7-H1 downregulated cells.
The cells were cultured for 12, 24 and 48 h. The experiments were
processed as triplicate samples and the analysis was completed
using a Bio-Tek ELX800 microplate reader at 450 nm (Bio-Tek
Instruments, Inc., Winnoski, VT, USA).
Animal model
A total of 20 BALB/c female mice (weigh, 25–30 g;
age, 4 weeks old) were obtained from the Chinese Academy of
Sciences (Beijing, China). The temperature of the housing room was
22°C, with 60% humidity. Mice had free access to food and water
every day and were observed for 5 weeks. Mice were housed
individually and observed every day. Mice were randomly divided
into 2 groups (n=10 per group). Briefly, 40 mg/kg nembutal
(Sigma-Aldrich; Merck kGaA) was used to anesthetize mice. A total
of 2×1010/l GFP-positive cervical cancer cells or
2×1010/l B7-H1 downregulated cells were hypodermically
injected into 10 mice. The end-point of the experiment was 35 days,
which meant that days the mice were sacrificed and the samples were
obtained at days 30 and 35. Subsequently, mice were sacrificed via
overdose using 80 mg/kg nembutal intraperitoneal injection. Both
B7H1 downregulated cervical cancer cell and cervical cancer cell
tumors in mice experienced the following treatment: Fixing, frozen
section and the images were captured of GFP cells using a
fluorescent microscope. Both tumor samples were digested into
single cells and the GFP ratio was measured by flow cytometry.
Furthermore, the expression levels of IL-1β, IL-6 and TNF-α in both
tumor samples, were analyzed using mouse specific primers. The
detail methods involved in animal experiment were described in the
previous sections.
Statistical analysis
Data were presented as the mean ± standard deviation
of at least three independent experiments and analyzed using Prism
5 software (Graph Pad Software, Inc., La Jolla, CA, USA).
Differences between each group were analyzed using one-way analysis
of variance and the Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of B7-H1 in cervical cancer
cells
To assess the expression of the B7 family of
negative co-stimulatory molecules in cervical cancer, cervical
cancer cells were studied. As presented in Fig. 1A, cervical cancer cells expressed the
negative co-stimulatory molecules B7-H1, B7-H2, B7-H3 and B7-H4, of
which, expression of B7-H1 was the highest. Results from flow
cytometry indicated that in cervical cancer cells, B7-H1 protein
was expressed at higher levels compared with B7H2, B7H3 or B7H4
(Fig. 1B). Thus, the present study
focused on B7-H1. The expressions of B7-H1 in the 12 cervical
cancer samples were measured using immunohistochemistry. The
results demonstrated that B7-H1 was highly expressed in patients
with cervical cancer (inside the red cycle; Fig. 1C), whereas B7-H1 was not expressed in
paracancerous tissue and normal cervical tissue. In addition to the
cancer tissue, immunostaining demonstrated that the cervical cancer
cells expressed B7-H1. Conversely, the normal cervical epithelium
was negative for B7-H1 expression (Fig.
1D).
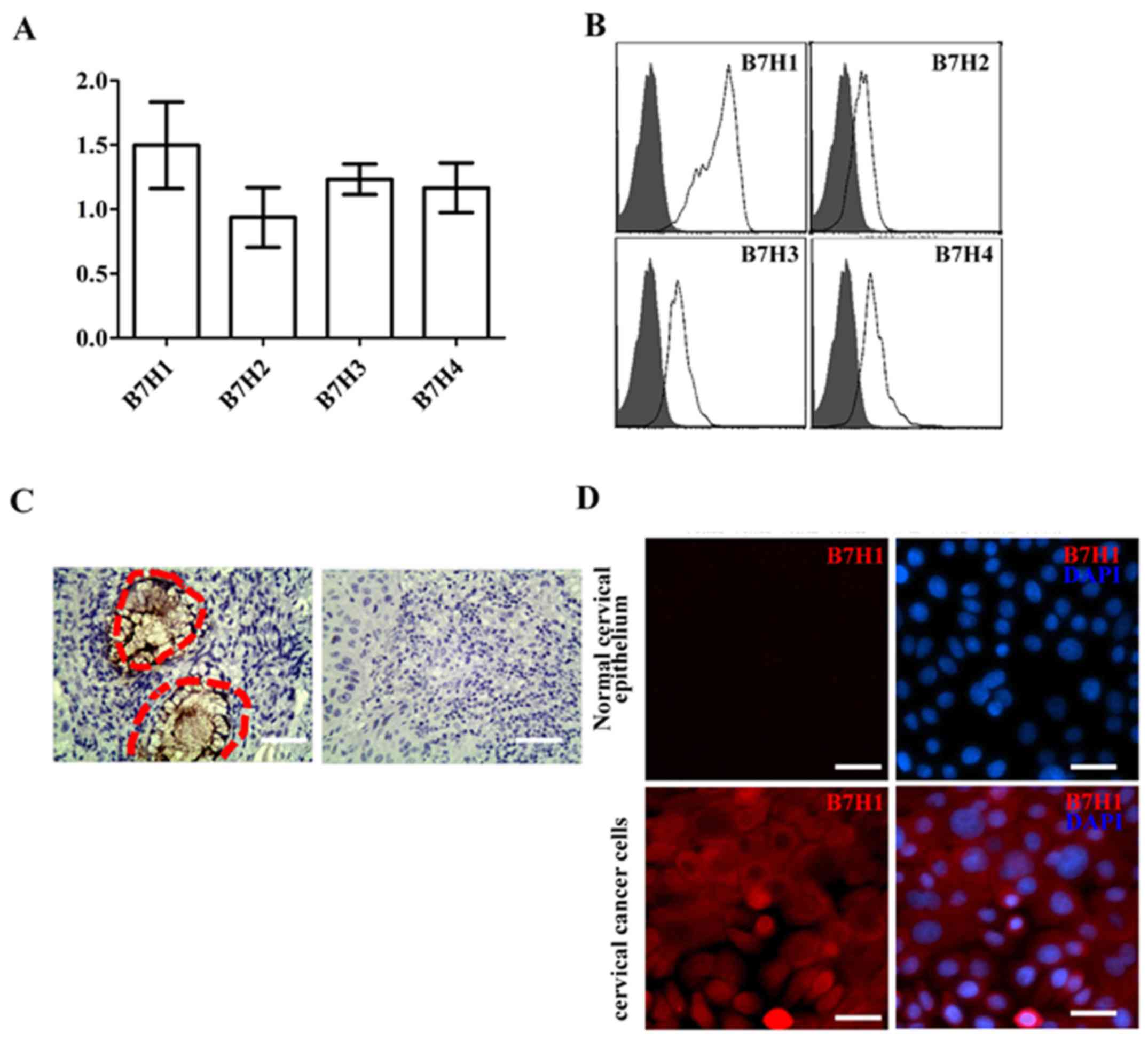 | Figure 1.High expression of B7-H1 in cervical
cancer cells and tissue. (A) mRNA expression levels of B7-H1,
B7-H2, B7-H3 and B7-H4 in cervical cancer cells. (B) Levels of
B7-H1, B7-H2, B7-H3 and B7-H4 protein in cervical cancer cells. (C)
Immunohistochemistry results indicated that B7-H1 was highly
expressed in cervical cancer cells (right-hand side; indicated by
the red dotted-lined circles). However, no B7-H1 expression was
observed in paracancerous tissue or normal cervical tissue
(left-hand side; magnification, ×10). (D) Immunostaining of B7-H1
(red) in normal cervical epithelium and cervical cancer cells.
Nuclei (blue) were stained with 4′,6-diamindino-2-phenylindole-2
(magnification, ×20). Scale bar, 40 µm. B7-H, B7 homolog. |
The role of B7-H1 in the inhibitory
effect of cervical cancer cells on human lymphocytes
To investigate the role of B7-H1 in the inhibitory
effect of the immune response to T cells, cervical cancer cells
were co-cultured with human T lymphocytes. CD3 was also used to
stimulate the activation of T lymphocytes. Mitomycin-treated
cervical cancer cells inhibited the activity of T lymphocytes,
whereas normal cervical epithelial tissue did not have any marked
inhibitory effects (Fig. 2).
Following the use of the specific blocking antibodies of B7-H1 and
siRNA to downregulate the expression of B7-H1, and specific
blocking antibodies of B7-H3 to downregulate the expression of
B7-H3, it was demonstrated that the B7-H1 blocking antibody and
siRNA are able to inhibit the suppression of T lymphocytes by
cervical cancer cells to a greater extent than the B7-H3 blocking
antibody. Furthermore, B7-H1 and B7-H3 combined did not exert a
significantly greater effect than B7-H1 or B7-H3 alone (Fig. 2; cells represented by each bar are
shown in figure legend). It was therefore concluded that B7-H1 is
an important molecule in inhibiting the immune response in cervical
cancer cells.
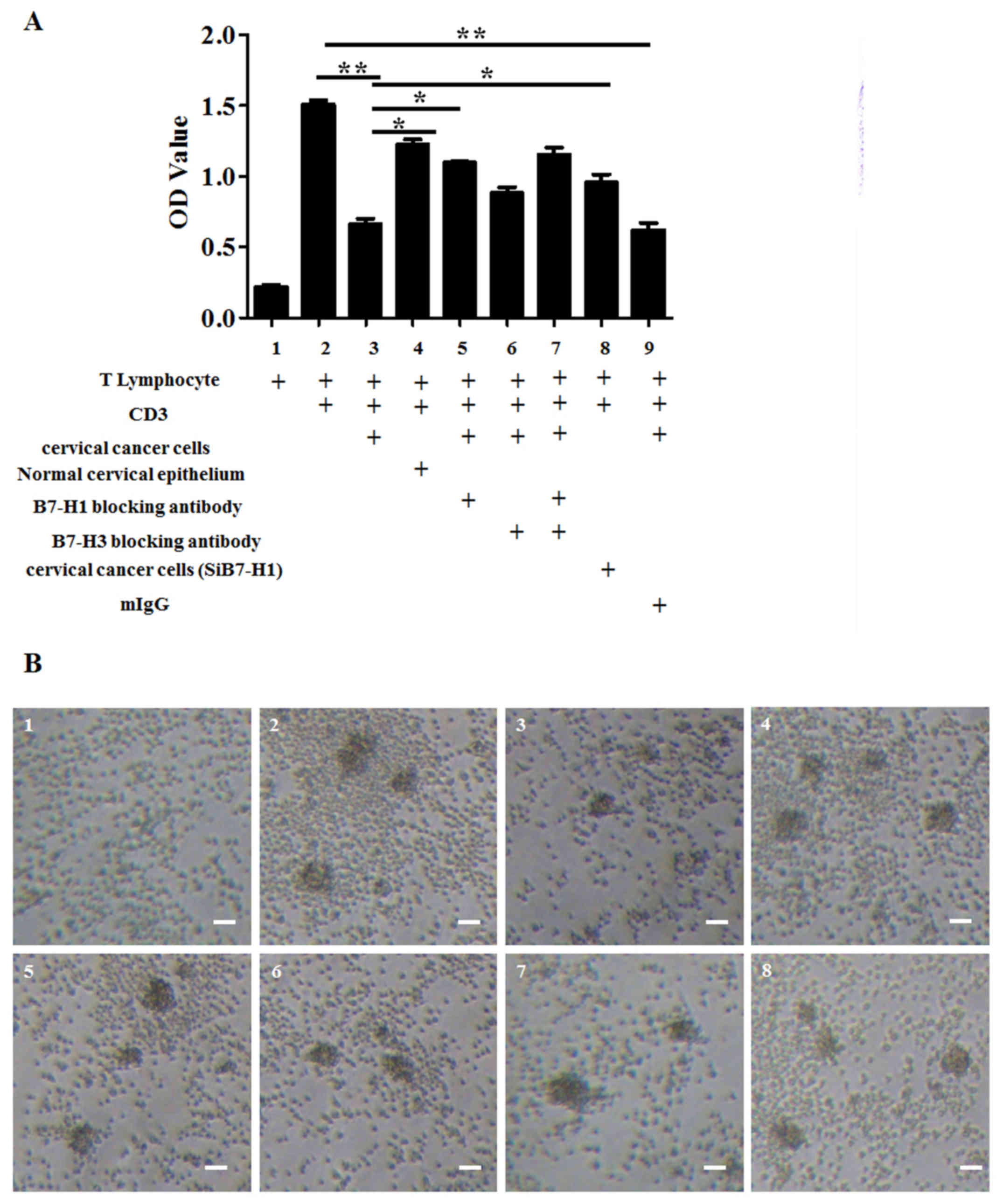 | Figure 2.Downregulation of B7-H1 in cervical
cancer suppresses the inhibitory ability of cervical cancer cells
on human T lymphocytes. (A) Analysis of the inhibitory effect of
cervical cancer cells on human lymphocytes and the effect of the B7
family blocking antibody on the inhibitory ability of cervical
cancer cells. Cell Counting Kit-8 data are presented as the mean ±
standard deviation of 3 independent experiments. Analysis was
performed with GraphPad Prism; *P<0.05; **P<0.01. (B) The
corresponding cell images: 1, T lymphocyte; 2, T lymphocyte + CD3;
3, T lymphocyte + CD3 + cervical cancer cells; 4, T lymphocyte +
CD3 + normal cervical epithelium; 5, T lymphocyte + CD3 + cervical
cancer cells + B7-H1 blocking antibody; 6, T lymphocyte + CD3 +
cervical cancer cells + B7-H3 blocking antibody; 7, T lymphocyte +
CD3 + cervical cancer cells + B7-H1 blocking antibody + B7-H3
blocking antibody. 8, T lymphocyte + CD3 + B7-H1 downregulated
(SiB7-H1) cervical cancer cells (magnification, ×10). Scale bar, 40
µm. CD, cluster of differentiation; B7-H, B7 homolog; OD, optical
density; Ig, immunoglobulin. |
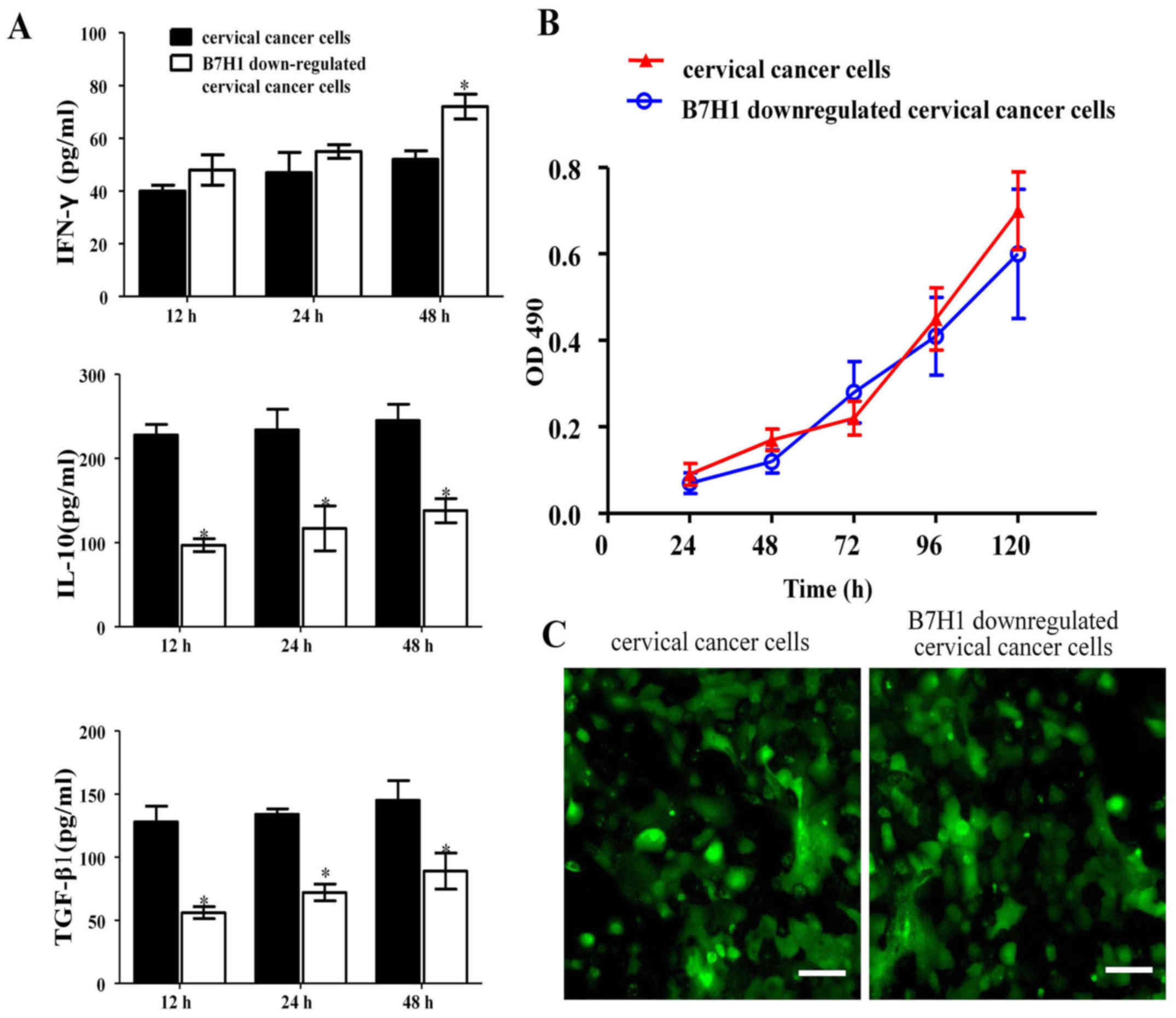
The role of B7-H1 in the proliferation
and cytokine secretion by T cell of cervical cancer cells
The results from ELISA demonstrated that following
co-culture with T cells, the concentration of IFN-γ in the B7-H1
downregulated group increased slightly following 12 h co-culture.
At 24 and 48 h, the concentration gradually increased and at 48 h
was significantly higher in the B7-H1 group compared with the
control (P<0.05). However, IL-10 and TGF-β1 secretion was
significantly lower than in the cervical cancer cell group at all
time points measured (P<0.05; Fig.
3A). B7-H1 is associated with a number of tumor-infiltrating
CD8+ T lymphocytes and therefore increases IFN-γ
production (26).
Results from the MTT assay demonstrated that when
B7-H1 was downregulated in cervical cancer cells, cell viability
was the same as in cervical cancer cells, which implies that the
proliferation ability of both types of cells did not differ
significantly (Fig. 3B). To further
investigate the underlying mechanism, a GFP reporter gene was
introduced into cervical cancer cells and B7-H1 downregulated
cervical cancer cells using a lentiviral vector. These cells were
then injected into BALB/c nude mice. Immunofluorescence showed that
almost 100% of the cells were GFP positive, confirming the
credibility of the following experiment (Fig. 3C). The aforementioned results
suggested that when these two types of cells (B7H1 downregulated
cervical cancer cells and cervical cancer cells) were transplanted
into the subcutis of BALB/c nude mice, the difference in the
ability to resist immune rejection was only influenced by the B7H1
downregulated cervical cancer cells and cervical cancer cells.
In the present study, the delitescence period of
tumor formation was ~4 weeks. To observe the proportion of
GFP-positive cells engrafted into the mice, the tumor and
surrounding normal tissue were excised. Results from
immunofluorescence staining indicated that the cervical cancer cell
group had a larger number of GFP-positive cells than the B7-H1
downregulated cervical cancer cell group on days 30 and 35
(Fig. 4A). Flow cytometry analysis
indicated that the proportion of GFP-positive cells in the cervical
cancer cell group was ~25% on day 30, with this proportion
increasing to ~36% on day 35. Conversely, the proportion of
GFP-positive cells in the B7-H1 downregulated cervical cancer cell
group was ~12% on day 30 and declined to ~3% by day 35 (Fig. 4B). Additionally, tissue from cervical
cancer cells and B7-H1 downregulated cells with the surrounding
precancerous lesions were excised and dispersed into a single-cell
suspension, respectively. The expression of IL-1β, IL-6 and TNF-α
was then analyzed using mouse specific primers. As presented in
Fig. 4C, the expression of IL-1β in
cervical cancer cells was significantly lower than in the B7-H1
downregulated cervical cancer cells on the first day (P<0.05).
On day 14, the expression of IL-1β in B7-H1 downregulated cervical
cancer cells reached a peak. A similar pattern was observed
regarding the significant difference in expression of IL-6 on day 1
and TNF-α on day 7 (P<0.05). This indicates that B7-H1 aids the
evasion of tumors from the immune system.
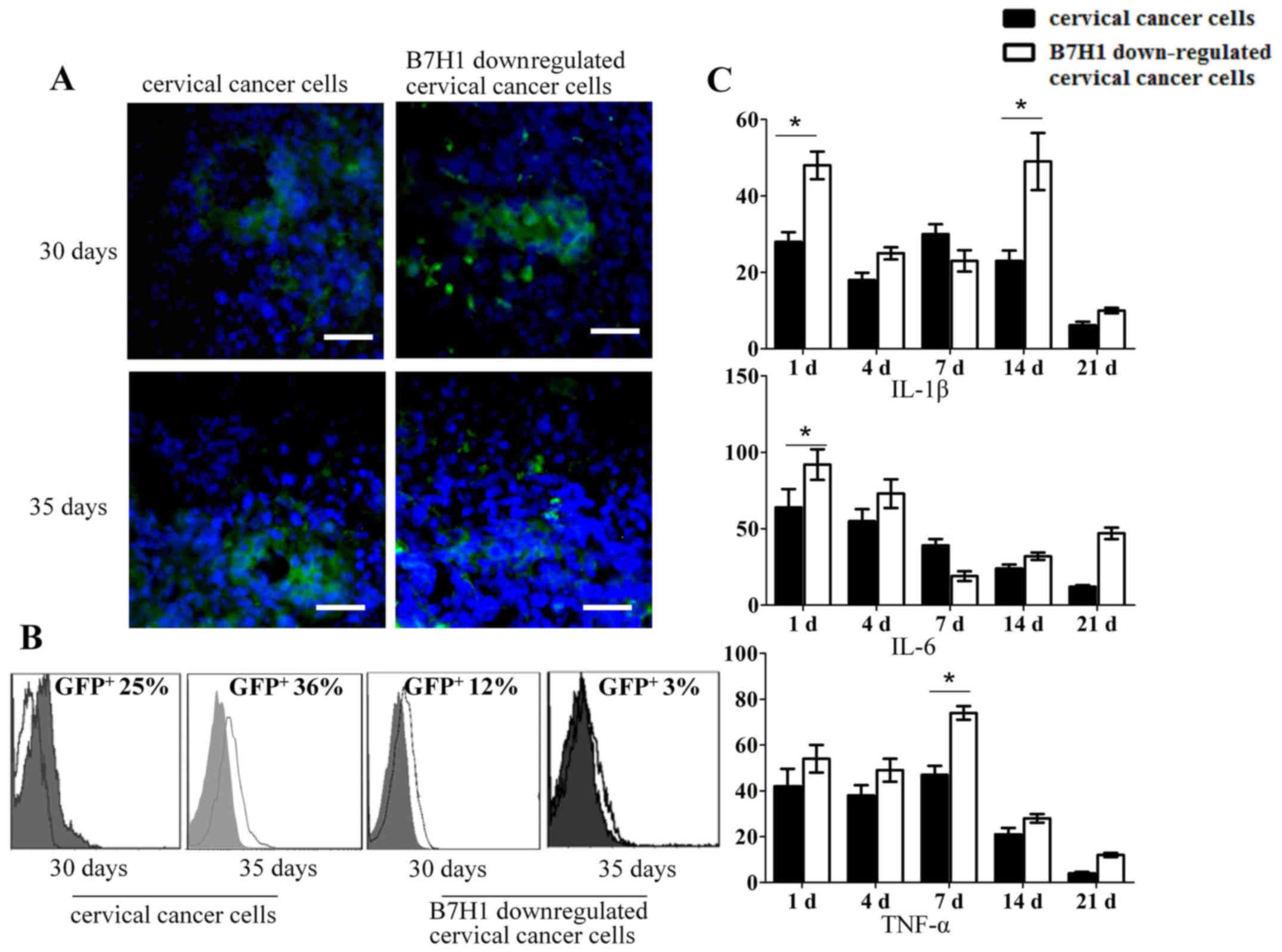 | Figure 4.B7-H1 may aid the evasion of tumors
from the immune system. (A) Immunofluorescence analysis of the
presence of GFP-positive cells in cervical cancer cells and B7-H1
downregulated cervical cancer cells after 30 and 35 days' injection
in mice. Scale bar, 40 µm. (B) Flow cytometry of GFP-positive cells
from cervical cancer cell and B7-H1 downregulated cervical cancer
cell-formed cervical cancer tissues after cell injection into
BALB/c nude mice for 30 and 35 days, respectively (magnification,
×10). (C) Reverse transcription-quantitative polymerase chain
reaction analysis of IL-1β, IL-6 and TNF-α mRNA levels on days 1,
4, 7, 14 and 21 in the cervical cancer cells and B7-H1
downregulated cervical cancer cells group. The data are presented
as the mean ± standard deviation of three independent experiments.
Analysis was performed with GraphPad Prism; *P<0.05. B7-H, B7
homolog; IFN, interferon; IL, interleukin; TGF, transforming growth
factor; GFP, green fluorescent protein; TNF, tumor necrosis
factor. |
Discussion
Cervical cancer is the most common malignant tumor
detected in the female genital tract and its incidence rate has
recently increased (1,2). T cell immunity serves a vital role in
the recognition and killing of tumor cells, as well as the
inhibition of tumor growth and proliferation (27). The occurrence and development of
cervical cancer is associated with the low immune function of the
local microenvironment of the host, particularly the immune
function of T cells (28). B7-H1,
also known as programmed death ligand, is a member of the B7
family. The binding of B7-H1 (programmed death-ligand 1) to its
receptor, programmed cell death protein 1, can inhibit the
proliferation of T cells and the secretion of some cytokines in
vitro (29–31). B7-H1 acts as a negative
co-stimulatory molecule in the process of T cell activation and may
serve a major role in suppressing the immune system during certain
events including pregnancy, tissue allografts (32), autoimmune disease (33) and other disease states such as
hepatitis (34). A number of studies
have detected elevated B7-H1 expression in numerous tumors and
cancer cell lines, including non-small cell lung cancer (35), melanoma (36), colon cancer (37), renal cell carcinoma (38), ovarian cancer (39), pancreatic cancer (40), gastric cancer (41) and breast cancer (42).
The present study found that B7-H1 was highly
expressed in cervical cancer cells and also in cervical cancer
tissue, whereas normal cervical epithelium and the paracancerous
tissue did not express B7-H1. Furthermore, it was demonstrated that
when B7-H1 was downregulated via its specific blocking antibody and
siRNA, it significantly inhibited the suppression of T lymphocytes.
When co-cultured with B7-H1 downregulated cervical cancer cells,
the levels of the cytokines IFN-γ, IL-10 and TGF-β1 secreted by T
cells differed significantly compared with cervical cancer cells.
Cervical cancer cells can form tumors more easily than B7-H1
downregulated cervical cancer cells in vivo and elicit a
more moderate immune response. Therefore, B7-H1 is able to mediate
the immune escape of cervical cancer cells.
It has been demonstrated that when B7-H1 is blocked
effectively in vivo, the lethality of immune cells in cancer
tissue can be strengthened (43) and
this could be potentially applied to treat B7-H1-positive tumors.
Thus, the immunoregulatory function of B7-H1 holds important
clinical value for treatment and for the molecular targeted therapy
of gynecological malignant tumors.
In conclusion, findings of the present study
indicated that B7-H1 mediated the inhibition of T lymphocyte
activation of cervical cancer cells. When B7-H1 was downregulated,
the cell viability of B7-H1 downregulated cervical cancer cells did
not change compared with cervical cancer cells, whereas the soluble
factors that are secreted by T cells changed between cervical
cancer cells and B7-H1 downregulated cervical cancer cells. In an
in vivo animal model, injected B7-H1 downregulated cervical
cancer cells exhibited a potent immune response, whereas cervical
cancer cells provoked the weak immune response. The findings
suggest that B7-H1 mediated the low immunogenicity of cervical
cancer and evaded attack from the immune system.
Acknowledgements
Funding for the present study was provided by
Special Funds for Industrial Technology Innovation of Suzhou (grant
no. SYS201567), Technical Specification for Diagnosis and Treatment
of Clinical Diseases of Suzhou (grant no. LCZX201410), Clinical
Medical Centers of Suzhou (grant no. SZZX201505) and Maternal and
Child Health Program of Jiangsu Province (grant nos. F201410 and
F201680).
References
|
1
|
Hammes LS, Tekmal RR, Naud P, Edelweiss
MI, Kirma N, Valente PT, Syrjänen KJ and Cunha-Filho JS:
Macrophages, inflammation and risk of cervical intraepithelial
neoplasia (CIN) progression-clinicopathological correlation.
Gynecol Oncol. 105:157–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu SY, Zheng RS, Zhao FH, Zhang SW, Chen
WQ and Qiao YL: Trend analysis of cervical cancer incidence and
mortality rates in Chinese women during 1989–2008. Zhongguo Yi Xue
Ke Xue Yuan Xue Bao. 36:119–125. 2014.(In Chinese). PubMed/NCBI
|
|
3
|
Wu YY, Liang MR, Li LY and Zeng SY:
Analysis of 4223 hospitalized patients with cervical cancer during
1990–2007. Zhonghua Fu Chan Ke Za Zhi. 43:433–436. 2008.(In
Chinese). PubMed/NCBI
|
|
4
|
Human papillomavirus testing for triage of
women with cytologic evidence of low-grade squamous intraepithelial
lesions: Baseline data from a randomized trial. The Atypical
Squamous Cells of Undetermined Significance/Low-Grade Squamous
Intraepithelial Lesions Triage Study (ALTS) Group. J Natl Cancer
Inst. 92:397–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo XM, Song L, Wu JL, Liu Y, Di JL, Song
B, Zheng RM and Ma L: Analysis of the reported data of national
rural cervical cancer screening project from 2012 to 2013, China.
Zhonghua Yu Fang Yi Xue Za Zhi. 50:346–350. 2016.(In Chinese).
PubMed/NCBI
|
|
6
|
Anisimov VN: Syndrome of accelerated aging
induced by carcinogenic environmental factors. Ross Fiziol Zh Im I
M Sechenova. 96:817–833. 2010.(In Russian). PubMed/NCBI
|
|
7
|
Stroh EL, Besa PC, Cox JD, Fuller LM and
Cabanillas FF: Treatment of patients with lymphomas of the uterus
or cervix with combination chemotherapy and radiation therapy.
Cancer. 75:2392–2399. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park HJ, Chang AR, Seo Y, Cho CK, Jang WI,
Kim MS and Choi C: Stereotactic body radiotherapy for recurrent or
oligometastatic uterine cervix cancer: A cooperative study of the
Korean radiation oncology group (KROG 14–11). Anticancer Res.
35:5103–5110. 2015.PubMed/NCBI
|
|
9
|
Kim KH, Dishongh R, Santin AD, Cannon MJ,
Bellone S and Nakagawa M: Recognition of a cervical cancer derived
tumor cell line by a human papillomavirus type 16 E6 52-61-specific
CD8 T cell clone. Cancer Immun. 6:92006.PubMed/NCBI
|
|
10
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chew V, Toh HC and Abastado JP: Immune
microenvironment in tumor progression: Characteristics and
challenges for therapy. J Oncol. 2012:6084062012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghebeh H, Mohammed S, Al-Omair A, Qattan
A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah
A, et al: The B7-1 (PD-L1) T lymphocyte-inhibitory molecule is
expressed in breast cancer patients with infiltrating ductal
carcinoma: Correlation with important high-risk prognostic factors.
Neoplasia. 8:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating
CD8+ T lymphocytes are prognostic factors of human
ovarian cancer. Proc Natl Acad Sci USA. 104:3360–3365. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson RH, Kuntz SM, Leibovich BC, Dong
H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H,
et al: Tumor B7-1 is associated with poor prognosis in renal cell
carcinoma patients with long-term follow-up. Cancer Res.
66:3381–3385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG and
Xu N: Immunohistochemical localization of programmed death-1
ligand-1 (PD-L1) in gastric carcinoma and its clinical
significance. Acta Histochem. 108:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Wang T, Xu M, Xiao L, Luo Y, Huang
W, Zhang Y and Geng W: B7-H4 overexpression impairs the immune
response of T cells in human cervical carcinomas. Hum Immunol.
75:1203–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han S, Li Y, Zhang J, Liu L, Chen Q, Qian
Q, Li S and Zhang Y: Roles of immune inhibitory molecule B7-H4 in
cervical cancer. Oncol Rep. 37:2308–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Shibata K, Koya Y, Kajiyama H,
Senga T, Yamashita M and Kikkawa F: B7-H4 overexpression correlates
with a poor prognosis for cervical cancer patients. Mol Clin Oncol.
2:219–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, He W, Shi Y, Gu H, Li M, Liu Z,
Feng Y, Zheng N, Xie C and Zhang Y: High expression of KIF20A Is
associated with poor overall survival and tumor progression in
Early-stage cervical squamous cell carcinoma. PLoS One.
11:e01674492016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pilz GA, Braun J, Ulrich C, Felka T,
Warstat K, Ruh M, Schewe B, Abele H, Larbi A and Aicher WK: Human
mesenchymal stromal cells express CD14 cross-reactive epitopes.
Cytometry A. 79:635–645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herzenberg LA, Tung J, Moore WA,
Herzenberg LA and Parks DR: Interpreting flow cytometry data: A
guide for the perplexed. Nat Immunol. 7:681–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinto AE, Areia F, Pereira T, Cardoso P,
Aparício M, Silva GL, Ferreira MC and André S: Clinical relevance
of the reappraisal of negative hormone receptor expression in
breast cancer. Springerplus. 2:3752013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen
B, Greiner J, Götz M, Guillaume P, Döhner H, Bunjes D and Schmitt
M: Dasatinib exerts an immunosuppressive effect on CD8+
T cells specific for viral and leukemia antigens. Exp Hematol.
36:1297–1308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou Q, Jin J, Xiao Y, Zhou X, Hu H, Cheng
X, Kazimi N, Ullrich SE and Sun SC: T Cell Intrinsic USP15
deficiency promotes excessive IFN-γ production and an
immunosuppressive tumor microenvironment in MCA-induced
fibrosarcoma. Cell Rep. 13:2470–2479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan M, Himoudi N, Pule M, Sebire N, Poon
E, Blair A, Williams O and Anderson J: Development of cellular
immune responses against PAX5, a novel target for cancer
immunotherapy. Cancer Res. 68:8058–8065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen YM, He X, Deng HX, Xie YP, Wang CT,
Wei YQ and Zhao X: Overexpression of the hBiot2 gene is associated
with development of human cervical cancer. Oncol Rep. 25:75–80.
2011.PubMed/NCBI
|
|
29
|
Riella LV, Paterson AM, Sharpe AH and
Chandraker A: Role of the PD-1 pathway in the immune response. Am J
Transplant. 12:2575–2587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Z, Wang H, Meng F, Li J and Zhang S:
Combined trabectedin and anti-PD1 antibody produces a synergistic
antitumor effect in a murine model of ovarian cancer. J Transl Med.
13:2472015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weinstock M and McDermott DF: Emerging
role for novel immunotherapy agents in metastatic renal cell
carcinoma: From bench to bedside. Am Soc Clin Oncol Educ Book.
2015:e291–e297. 2015. View Article : Google Scholar
|
|
32
|
Wang YT, Zhao XY, Zhao XS, Xu LP, Zhang
XH, Wang Y, Liu KY, Chang YJ and Huang XJ: The impact of donor
characteristics on the immune cell composition of mixture
allografts of granulocyte-colony-stimulating factor-mobilized
marrow harvests and peripheral blood harvests. Transfusion.
55:2874–2881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilson CL, Murphy LB, Leslie J, Kendrick
S, French J, Fox CR, Sheerin NS, Fisher A, Robinson JH, Tiniakos
DG, et al: Ubiquitin C-terminal hydrolase 1 (UCHL1) A novel
functional marker for liver myofibroblasts and a therapeutic target
in chronic liver disease. J Hepatol. 63:1421–1428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie ZY, Chen YW, Fu XL, Yang CY and Wu YZ:
Expression of B7-H1 on peripheral blood mononuclear cells from
chronic hepatitis B patients. Nan Fang Yi Ke Da Xue Xue Bao.
27:1635–1637. 2007.(In Chinese). PubMed/NCBI
|
|
35
|
Jin X and Xie C: TH-AB-BRB-04: Dosimetric
and clinical benefits of conformal radiotherapy plus volumetric
modulated arc therapy in the treatment of non-small cell lung
cancer. Med Phys. 42:37042015. View Article : Google Scholar
|
|
36
|
Harshman LC, Choueiri TK, Drake C and Hodi
Stephen F Jr: Subverting the B7-H1/PD-1 pathway in advanced
melanoma and kidney cancer. Cancer J. 20:272–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M,
Meng YL, Yang AG and Wen WH: B7-H1 expression is associated with
poor prognosis in colorectal carcinoma and regulates the
proliferation and invasion of HCT116 colorectal cancer cells. PLoS
One. 8:e760122013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicator of tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci USA. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pfankuchen DB, Stölting DP, Schlesinger M,
Royer HD and Bendas G: Low molecular weight heparin tinzaparin
antagonizes cisplatin resistance of ovarian cancer cells. Biochem
Pharmacol. 97:147–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Srivastava SK, Bhardwaj A, Arora S, Tyagi
N, Singh S, Andrews J, McClellan S, Wang B and Singh AP:
MicroRNA-345 induces apoptosis in pancreatic cancer cells through
potentiation of caspase-dependent and -independent pathways. Br J
Cancer. 113:660–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karabulut M, Alis H, Afsar CU, Karabulut
S, Kocatas A, Oguz H and Aykan NF: Serum neural precursor
cell-expressed, developmentally down regulated 9 (NEDD9) level may
have a prognostic role in patients with gastric cancer. Biomed
Pharmacother. 73:140–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Q and Fang X, Jiang C, Yao N and Fang
X: Thrombospondin promoted anti-tumor of adenovirus-mediated
calreticulin in breast cancer: Relationship with anti-CD47. Biomed
Pharmacother. 73:109–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mirza N, Duque MA, Dominguez AL, Schrum
AG, Dong H and Lustgarten J: B7-H1 expression on old CD8+ T cells
negatively regulates the activation of immune responses in aged
animals. J Immunol. 184:5466–5474. 2010. View Article : Google Scholar : PubMed/NCBI
|