Introduction
Gardenia jasminoides Ellis belongs to the
Rubiaceae family and Gardenia genus, and its dried ripe fruit can
be used as a medicine, with bitter and cold taste, and nourishing
the heart, lungs and San Juao meridian according to the principles
of Chinese Medicine (1). Gardenia
fruit mainly protects the liver and nourishes the gallbladder, and
its active constituent is geniposide, which belongs to the iridoid
glycosides (2). It is also rich in
other constituents such as organic acids, pigments and volatile
oils (3). A pharmacokinetic study
has found that geniposide, the active constituent of Gardenia
jasminoides Ellis, is hydrolyzed by β-glucosidase produced by
intestinal microorganisms to generate genipin (4). Studies have proved that genipin also
significantly reduces inflammation, lipid peroxidation and
angiogenesis, with low cytotoxicity, sound biocompatibility and
high anti-degradation ability (5,6).
Gardenia jasminoides Ellis has a low genipin content, which
only accounts for 0.005–0.01%, and mainly exists in the form of its
precursor-geniposide, accounting for 3–5%. At present, geniposide
is extracted with organic solvents, and 100 g gardenia fruit
provide ~4 g geniposide. Therefore, it is of great significance to
ferment Gardenia by microorganisms to produce genipin
(7). In addition, the use of
microorganisms and geniposide may achieve a better control of the
concentration of active constituents, which may enhance the
pharmacological effect. The transformation of geniposide contained
in Gardenia jasminoides by fermentation with microorganisms
is carried out by β-glucosidase produced by bacteria, which breaks
chemical bonds in geniposide to produce genipin (8). In the present study, high-yield
lactobacillus produced by β-glucosidase was used to react
with geniposide and the joint effect on cancer cells was
observed.
Organisms balance the number of cells in their
tissues by proliferation and apoptosis. When the balance is
disturbed, certain diseases, such as cancer, may occur (9). Elucidation of the association between
apoptosis and cancer may provide a new reference for the treatment
of cancer. In the last 30–50 years, cytotoxic radiotherapy and
chemotherapy have been used as the main treatment measures of
cancer, which have certain therapeutic effects for numerous
hematological malignancies and certain types of solid tumor,
particularly germ cell tumors and certain pediatric malignant tumor
types (10). However, malignant
tumors have certain resistances to these measures; while high-dose
chemotherapy may overcome the resistance, it may not be curative
and also damage normal tissues and cells. For a long time, it was
thought that tumors can be treated by selectively killing target
cells which divide rapidly, but it is not considered to be
satisfactory in clinical practice, as certain treatable cancer
cells grow slowly and those with resistance divide rapidly
(11). Furthermore, the treatments
may induce apoptosis of tumor cells, while various cells have
different apoptosis thresholds, and their responses to treatment
differ accordingly. As the induction and regulation of apoptosis
are complex processes, the mechanisms of apoptosis induction by
various anti-tumor drugs may not be the same (12). The present study observed the effect
of Lactobacillus rhamnosus GG strain (LGG) combined with
geniposide on the apoptosis of cancer cells and analyzed the
underlying mechanisms, providing evidence for the clinical
application of LGG combined with geniposide.
Materials and methods
Preparation of experimental
samples
The LGG strain was provided by Culturelle Probiotics
(i-Health, Cromwell, CT, USA). Geniposide standard was purchased
from Shanghai Jinsui Biotechnology Co., Ltd. (Shanghai, China).
Cell lines
Human oral keratinocytes (HOK) were supplied by
Bioleaf Co. (Shanghai, China) and the HSC-3 human oral squamous
cell carcinoma cell line was supplied by EK-Bioscience Co.
(Shanghai, China). These cells were cultured in RPMI-1640 medium
(HyClone; GE Healthcare, Little Chalfont, UK) with 10% fetal bovine
serum (HyClone; GE Healthcare) at 37°C in an incubator with 5%
CO2, and the medium was changed every day.
MTT assay
Culture solution was added to a suspension of the
cancer cells in the logarithmic growth phase to adjust the
concentration to 2×104 cells/well, which were then added
to the 96-well culture plate at 50 µl per well, and incubated at
37°C for 24 h. Drugs (1.0×103 CFU/ml LGG + 25 or 50
µg/ml geniposide) were added at 50 µl per well, to adjust the
concentration of cancer cells to 10 µg/ml eventually, while cells
in the control group were treated with 50 µl culture solution only,
followed by culture for 48 h. All culture solution in wells was
discarded, then MTT solution was added in the wells and plates were
incubated for another 4 h. After the supernatant was removed, 100
µl DMSO was added to the blank control group and following
agitation for 30 min, the absorbance of the wells was detected at
570 nm using a 680 Microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) (13).
Flow cytometric assay
A single-cell suspension was centrifuged to remove
the supernatant, cells were washed with 3 ml PBS twice and then
centrifuged (1,490 × g, 25°C) for 5 min. Propidium iodide (PI, 10
µg/ml) staining solution (1 ml) was added and samples were
incubated at 4°C for 30 min in the dark. Subsequent to filtration
through a 500-hole copper mesh (pore width, 8.2 mm), flow
cytometric detection using an argon ion laser with 15 mA excitation
light source and 488 nm wavelength, and a 630-nm band-pass filter
was performed. A total of 10,000 cells were collected in the
forward vs. side scatter dot plot diagram, with gating technology
used to exclude adhesive cells and cell debris, to analyze the
percentage of apoptotic cells in a PI fluorescence histogram
(13).
Nuclear staining with DAPI
HSC-3 cells were cultured as an MTT assay, then all
groups of HSC-3 cells were collected and washed with PBS, followed
by fixing in 3.7% paraformaldehyde (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in PBS for 10 min at 25°C. The fixed cells were
stained with the DAPI solution (1 mg/ml; Sigma-Aldrich; Merck KGaA)
for 10 min at 25°C. Finally, all the cells were washed three times
with PBS analyzed using a fluorescence microscope (IX73; Olympus
Corp., Tokyo, Japan) (13).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
RNAzol reagent (Sigma-Aldrich; Merck KGaA) was used
to extract the total RNA from cancer cells, and RNase-free DNase
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
adopted to digest total RNA at 37°C for 15 min. The RNeasy kit
(74104; Qiagen GmbH, Hilden, Germany) was used to purify RNA to
adjust its concentration to 1 µg/µl. RNA (2 µg) was used as the
template to synthetize complementary (c)DNA by reacting with
reverse transcriptase at 37°C for 120 min, at 99°C for 4 min and at
4°C for 3 min, respectively. PCR (50°C for 2 min, then 40 cycles of
95°C for 30 sec, 95°C for 5 sec and 60°C for 34 sec) was then
performed to amplify the cDNA using primers listed in (Table I). The housekeeping gene GAPDH was
used as an internal control. Finally, agarose electrophoresis with
1% ethidium bromide was performed to quantify the PCR-amplified
products (13).
 | Table I.Sequences of primers used for
polymerase chain reaction. |
Table I.
Sequences of primers used for
polymerase chain reaction.
| Gene | Sequence |
|---|
| Caspase-3 | Forward,
5′-CAAACTTTTTCAGAGGGGATCG-3′ |
|
| Reverse,
5′-GCATACTGTTTCAGCATGGCA-3′ |
| Caspase-8 | Forward,
5′-CCCCACCCTCACTTTGCT-3′ |
|
| Reverse,
5′-GGAGGACCAGGCTCACTTA-3′ |
| Caspase-9 | Forward,
5′-GGCCCTTCCTCGCTTCATCTC-3′ |
|
| Reverse,
5′-GGTCCTTGGGCCTTCCTGGTAT-3′ |
| Bax | Forward,
5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ |
|
| Reverse,
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-2 | Forward,
5′-CTCGTCGCTACCGTCGTGACTTGG-3′ |
|
| Reverse,
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-xL | Forward,
5′-CCCAGAAAGGATACAGCTGG-3′ |
|
| Reverse,
5′-GCGATCCGACTCACCAATAC-3′ |
| Fas | Forward,
5′-GAAATGAAATCCAAAGCT-3′ |
|
| Reverse,
5′-TAATTTAGAGGCAAAGTGGC-3′ |
| FasL | Forward,
5′-GGATTGGGCCTGGGGATGTTTCA-3′ |
|
| Reverse,
5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′ |
| p53 | Forward,
5′-GCTCTGACTGTACCACCATCC-3′ |
|
| Reverse,
5′-CTCTCGGAACATCTCGAAGCG-3′ |
| p21 | Forward,
5′-CTCAGAGGAGGCGCCATG-3′ |
|
| Reverse,
5′-GGGCGGATTAGGGCTTCC-3′ |
| hIAP-1 | Reverse,
5′-GCCTGATGCTGGATAACTGG-3′ |
|
| Forward,
5′-GGCGACAGAAAAGTCAATGG-3′ |
| hIAP-2 | Reverse,
5′-GCCTGATGCTGGATAACTGG-3′ |
|
| Forward,
5′-GCTCTTGCCAATTCTGATGG-3′ |
| NF-κB | Forward,
5′-CACTTATGGACAACTATGAGGTCTCTGG-3′ |
|
| Reverse,
5′-CTGTCTTGTGGACAACGCAGTGGAATTTTAGG-3′ |
| IκB-α | Forward,
5′-GCTGAAGAAGGAGCGGCTACT-3′ |
|
| Reverse,
5′-TCGTACTCCTCGTCTTTCATGGA-3′ |
| COX-2 | Reverse,
5′-TTAAAATGAGATTGTCCGAA-3′ |
|
| Forward,
5′-AGATCACCTCTGCCTGAGTA-3′ |
| iNOS | Reverse,
5′-AGAGAGATCGGGTTCACA-3′ |
|
| Forward,
5′-CACAGAACTGAGGGTACA-3′ |
| GAPDH | Reverse,
5′-CGGAGTCAACGGATTTGGTC-3′ |
|
| Forward,
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Western blot analysis
Subsequent to treatment, cells of each group were
lysed using RIPA lysis and extraction buffer (Gibco; Thermo Fisher
Scientific, Inc.) to obtain total protein extracts. The Bradford
method was adopted to determine the concentration of proteins. A
10% separation gel and a 5% stacking gel were prepared for
SDS-PAGE, then 30 µg protein was subjected to SDS-PAGE and
transferred to nitrocellulose filter membranes. Subsequent to
blocking in 5% nonfat milk for 2 h, membranes were incubated in
primary antibody (1:1,500 dilution; caspase-3, ab13847; caspase-8,
ab25901; caspase-9, ab52298; Bax, ab32503; Bcl-2, ab59348; Bcl-xL,
ab32370; Fas, ab82419; FasL, ab15285; p53, ab1431; p21, ab188224;
HIAP-1, ab2399; HIAP-2, ab234323; NF-κB; ab220803; IκB-α, ab32518;
COX-2, ab52237; iNOS, ab15323; all Abcam, Cambridge, MA, USA) at
4°C overnight. After washing with Tris-buffered saline containing
Tween-20 (TBST) for 3 times, membranes were incubated with
secondary antibody (1:2,000 dilution, ab131368; Abcam) at room
temperature for 2 h, and then rinsed with TBST for 3 times.
Enhanced chemiluminescence was used to develop the blots, and a GIS
gel imager system (E-Gel Imager; Thermo Fisher Scientific, Inc.)
was used to analyze and process the images (13).
Statistical analysis
The results were expressed as the mean ± standard
deviation. Differences between groups were analyzed using Duncan's
multiple-range test using SAS version 9.2 (SAS Institute Inc.,
Cary, NC, USA). P<0.05 was considered to indicate a significant
difference between groups.
Results
Growth inhibitory effects of LGG and
geniposide in HOK and HSC-3 cells
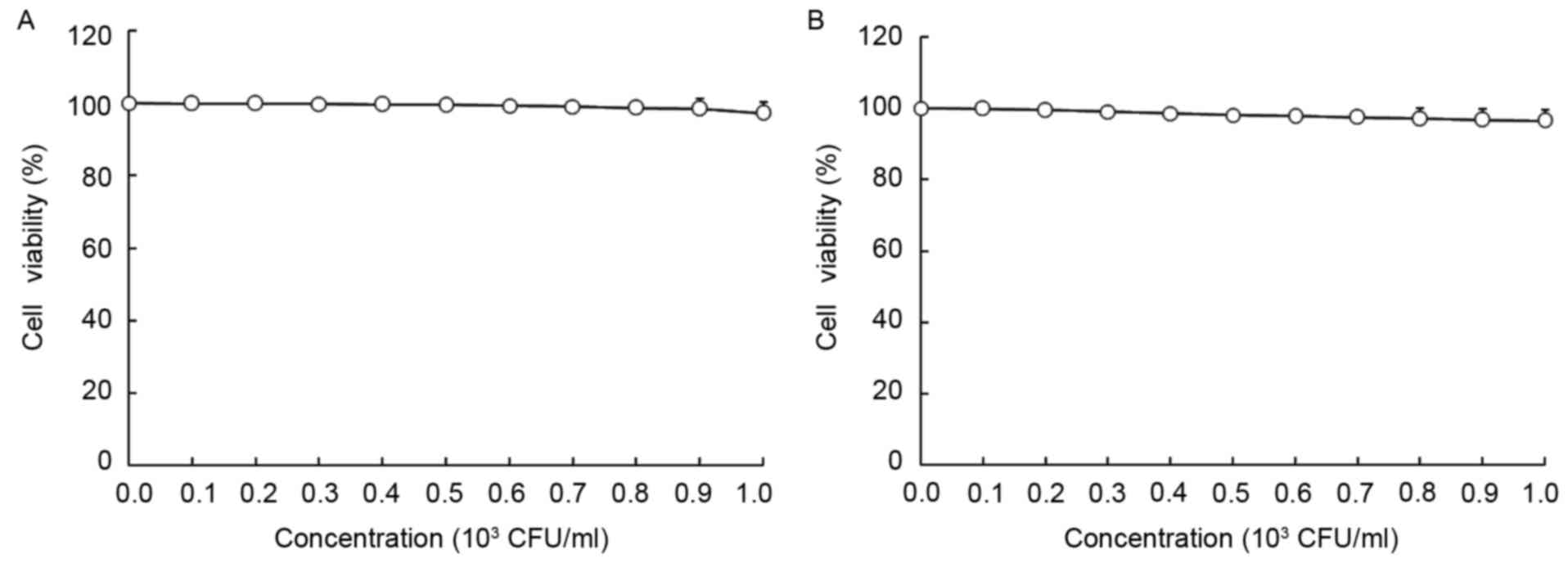
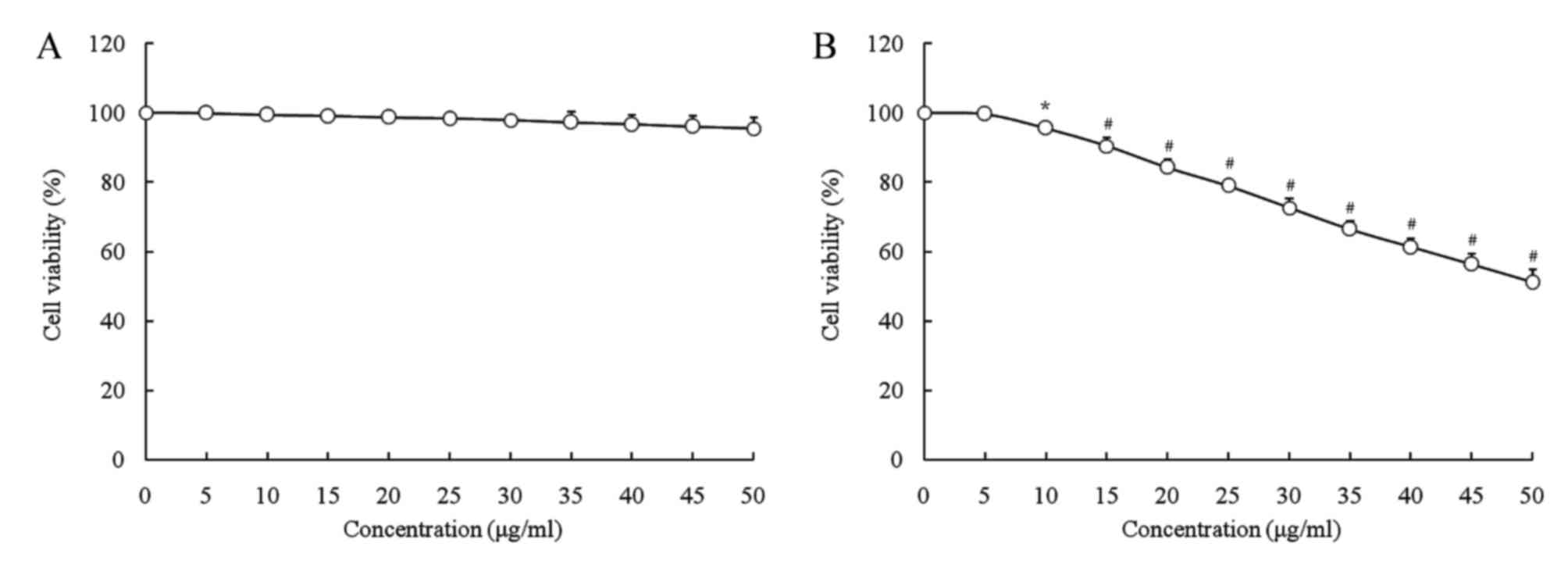
As presented in Figs.
1 and 2, LGG at the
concentration of 0–103 CFU/ml and geniposide at 0–50
µg/ml did not inhibit the growth of normal oral cells (HOK cells),
and LGG at 0–103 CFU/ml did not inhibit the growth of
HSC-3 cells, while geniposide at 0–50 µg/ml exerted
concentration-dependent growth inhibitory effects. Based on these
results, 1.0×103 CFU/ml of LGG and 25 or 50 µg/ml of
geniposide were selected for use in the subsequent experiments. The
MTT assay revealed that only geniposide treatment at 25 and 50
µg/ml had a significant inhibitory effect [optical density at 570
nm (OD570), 0.361±0.010 and 0.231±0.011, respectively,
vs. 0.466±0.004 for the control group]. In addition LGG treatment
at 1.0×103 CFU/ml further decreased the OD570
value resulting from geniposide treatment at 25 and 50 µg/ml to
0.332±0.009 and 0.162±0.008, respectively. The inhibitory rates of
the low concentration of geniposide (geniposide-L; 25 µg/ml),
LGG-geniposide-L (LGG at 1.0×103 CFU/ml and geniposide
at 25 µg/ml), high concentration of geniposide (geniposide-H; 50
µg/ml) and LGG-geniposide-H (LGG at 1.0×103 CFU/ml and
geniposide at 50 µg/ml) were 22.5±1.8, 28.8±2.3, 50.4±2.7 and
65.2±3.3%, respectively (Table
II).
 | Table II.Growth inhibitory effects of
geniposide and LGG in human oral squamous carcinoma HSC-3
cells. |
Table II.
Growth inhibitory effects of
geniposide and LGG in human oral squamous carcinoma HSC-3
cells.
| Treatment | OD540
value | Inhibitory rate
(%) |
|---|
| Control | 0.466±0.004 | – |
| Geniposide-L |
0.361±0.010a |
22.5±1.8b |
|
LGG-geniposide-L |
0.332±0.009a |
28.8±2.3b |
| Geniposide-H |
0.231±0.011a |
50.4±2.7b |
|
LGG-geniposide-H |
0.162±0.008a | 65.2±3.3 |
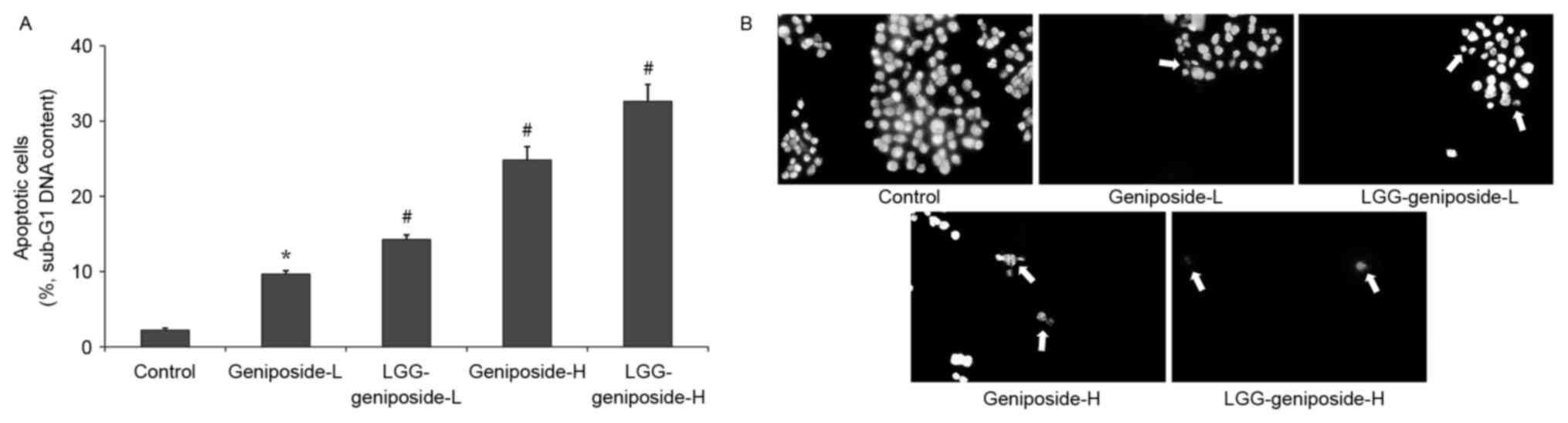
Sub-G1 content of HSC-3 cells
The flow cytometry experiment revealed that the
apoptotic cells (sub-G1 DNA content) in the control, geniposide-L,
LGG-geniposide-L, geniposide-H and LGG-geniposide-H-treated HSC-3
cells were 2.2±0.3, 9.7±0.4, 14.3±0.6, 24.8±1.8 and 32.6±2.3%,
respectively (Fig. 3A).
Apoptotic HSC-3 cell observation
Images of the control HSC-3 cells revealed intact
cells and homogeneous chromatin nuclei, but after treatment with
geniposide, the cells revealed chromatin condensation and nuclear
fragmentation. Additional LGG treatment led to the rupture of
certain cells, with the high concentration of geniposide causing
the largest percentage of apoptotic cells (Fig. 3B).
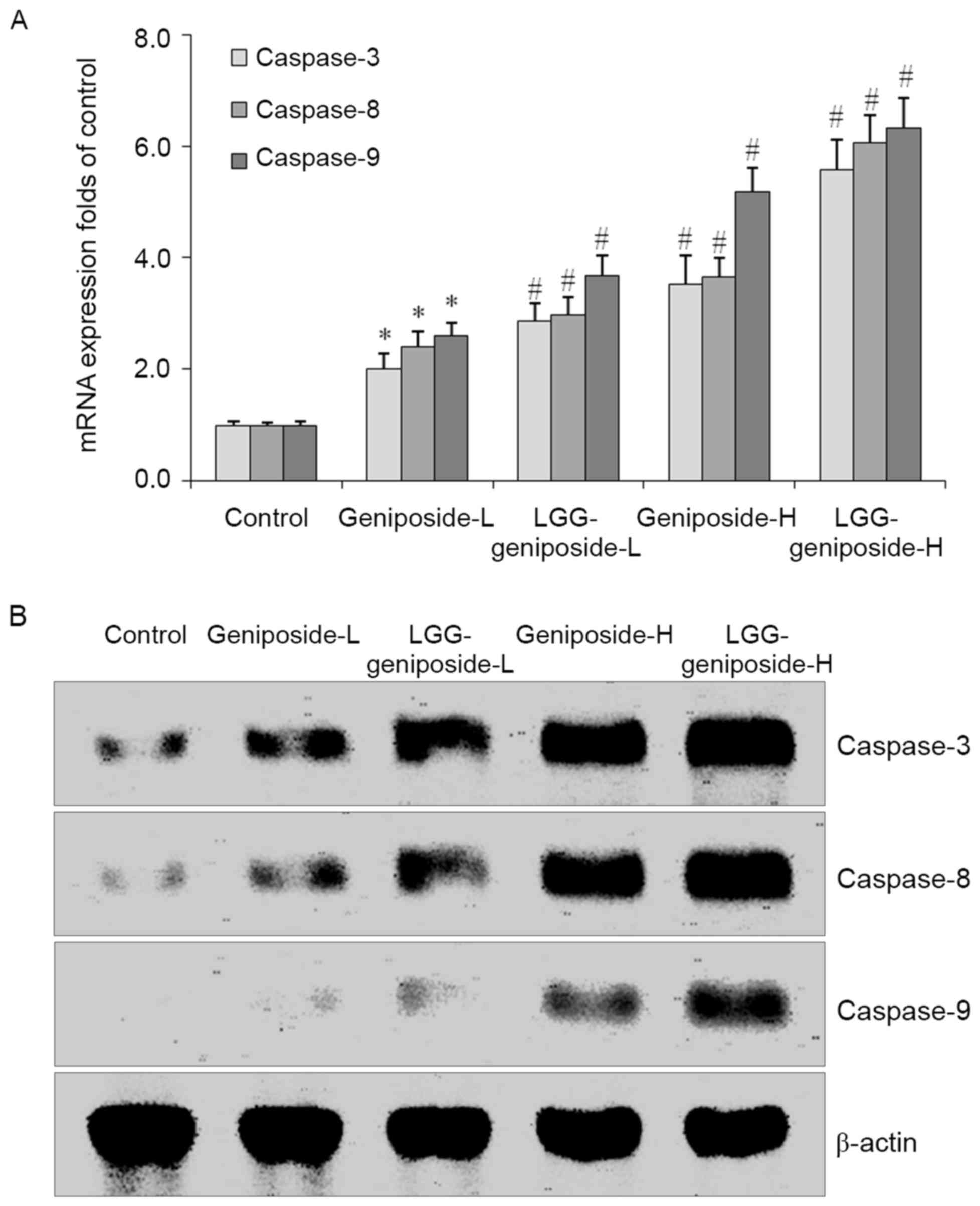
mRNA and protein expression of
caspase-3, −8 and −9 in HSC-3 cells
Geniposide treatment raised the mRNA and protein
expression of caspase-3, −8 and −9 compared to that in the control
cells in a dose-dependent manner. LGG enhanced the increasing
effects geniposide treatment on caspase-3, −8 and −9 expressions
(Fig. 4).
mRNA and protein expression of B-cell
lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and Bcl-extra
large protein (Bcl-xL) in HSC-3 cells
After treatment with geniposide-L, LGG-geniposide-L,
geniposide-H and LGG-geniposide-H, Bax mRNA and protein expression
was increased, while Bcl-2 and Bcl-xL expression was decreased in
order (Fig. 5).
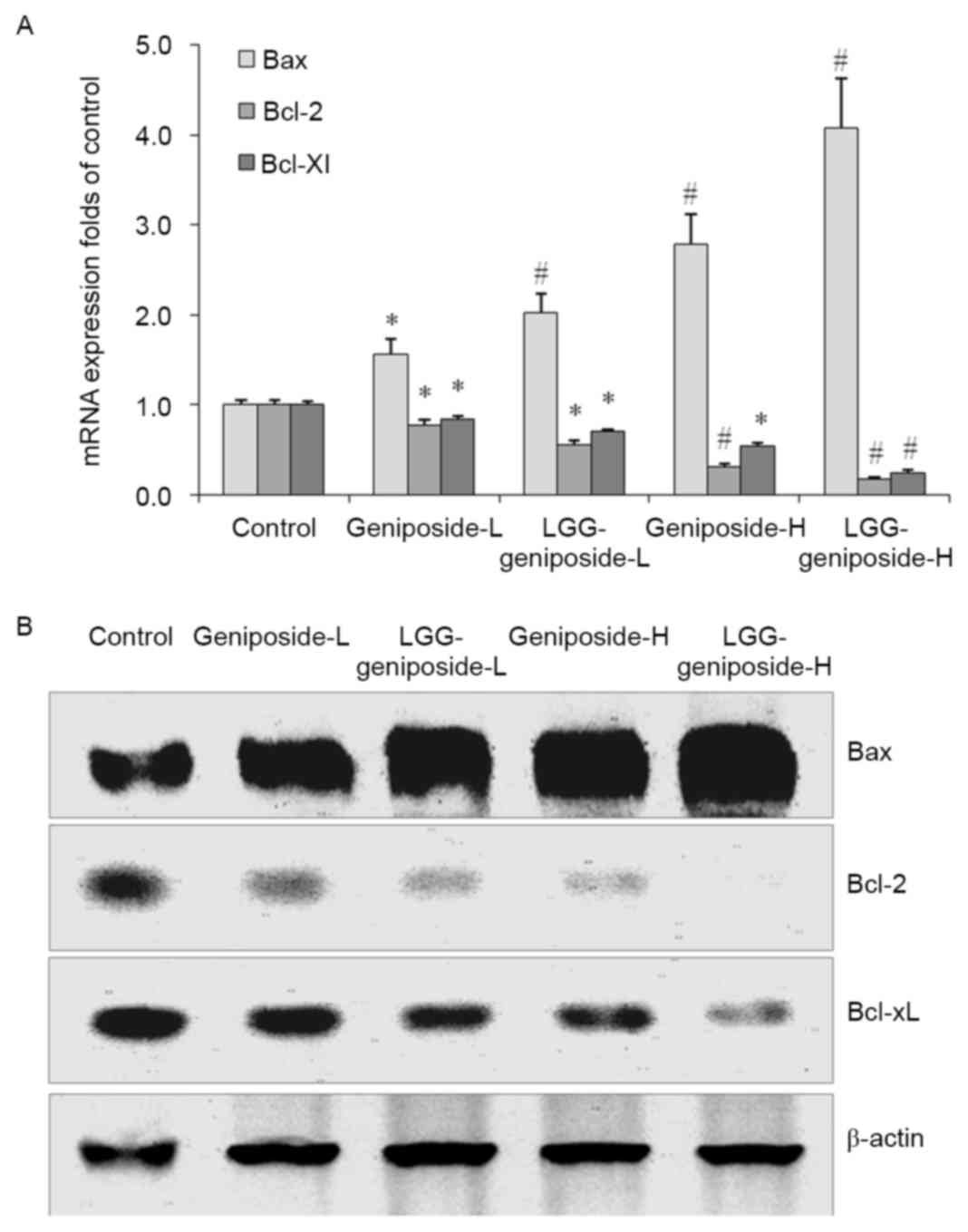 | Figure 5.(A) mRNA and (B) protein expression of
Bax, Bcl-2 and Bcl-xL in human oral squamous carcinoma HSC-3 cells.
*P<0.05 and #P<0.01 vs. control group. Groups:
Geniposide-L, 25 µg/ml geniposide; LGG-geniposide-L,
1.0×103 CFU/ml LGG + 25 µg/ml geniposide; Geniposide-H,
50 µg/ml geniposide; LGG-geniposide-H, 1.0×103 CFU/ml
LGG + 50 µg/ml geniposide. LGG, Lactobacillus rhamnosus GG
strain; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
Bcl-xL, Bcl-extra large protein; CFU, colony-forming units. |
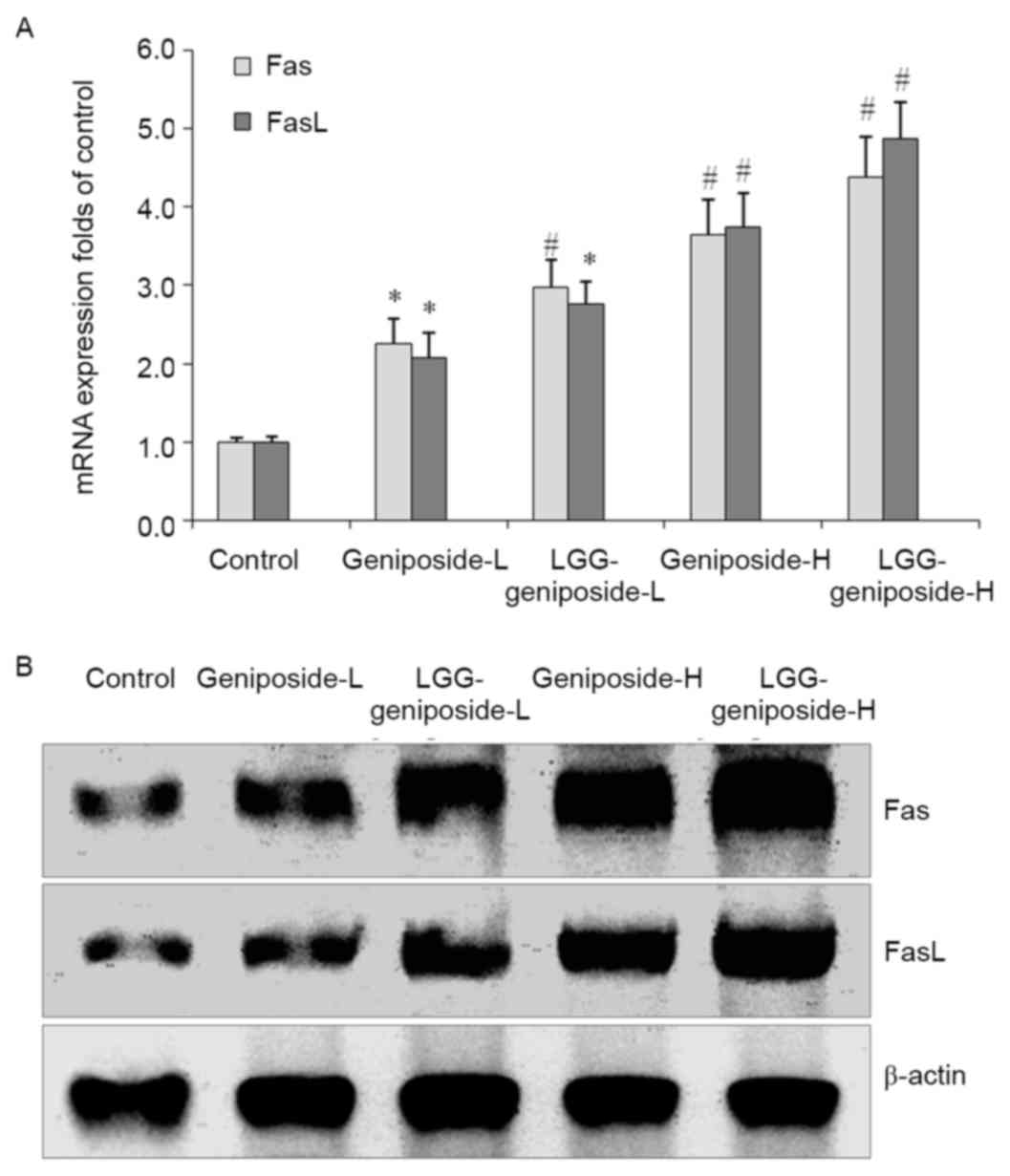
mRNA and protein expression of Fas and
Fas ligand (FasL) in HSC-3 cells
The Fas and FasL mRNA and protein expression was
increased by geniposide treatment, and upon additional LGG
teratment, Fas and FasL expression was further increased. The high
concentration of geniposide and LGG resulted in the higheset Fas
and FasL expression (Fig. 6).
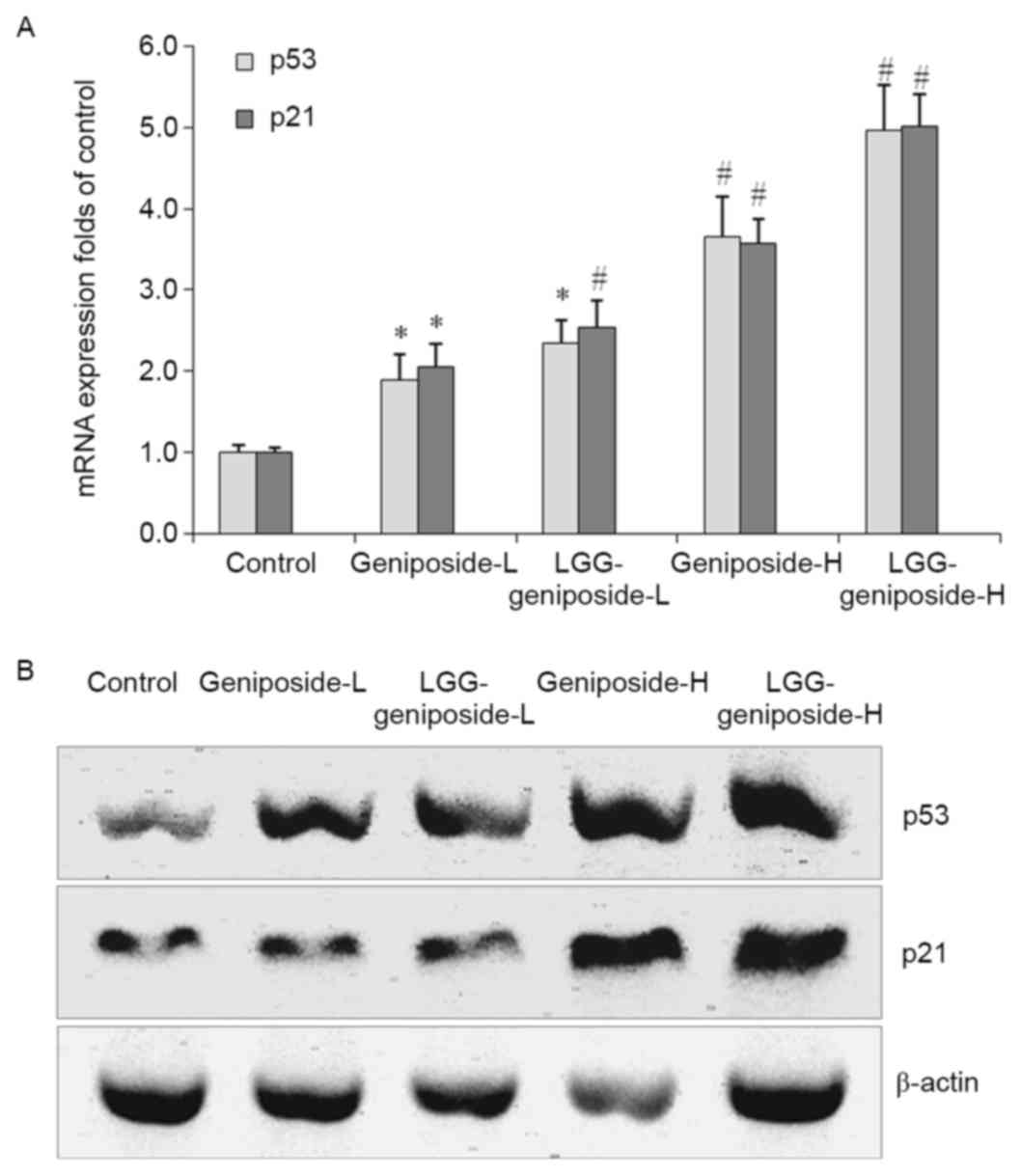
mRNA and protein expression of p53 and
p21 in HSC-3 cells
The p53 and p21 mRNA and protein expression in the
LGG-geniposide-H group was higher than that in the other groups,
and LGG/geniposide combination treatment resulted in a higher p53
and p21 expression than treatment with geniposide only (Fig. 7).
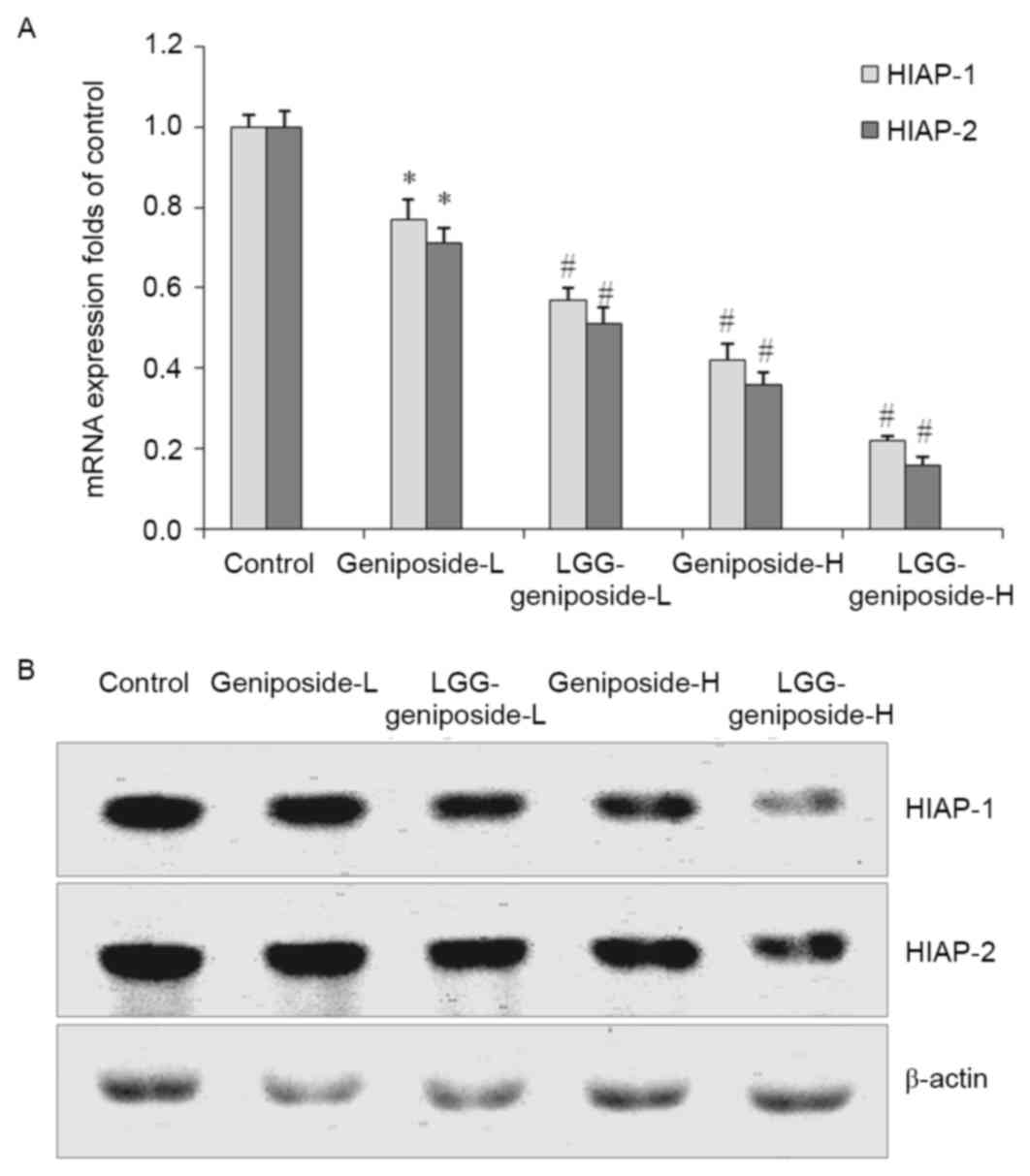
mRNA and protein expression of human
inhibitor of apoptosis (HIAP)-1 and HIAP-2 in HSC-3 cells
Geniposide reduced the mRNA and protein expression
of HIAP-1 and HIAP-2 in HSC-3 cells compared with that in the
control cells (Fig. 8). Treatment
with 50 µg/ml geniposide resulted in a the lower HIAP-1 and HIAP-2
expression than 25 g/ml geniposide, and LGG-geniposide-H-treated
cells had the lowest expression.
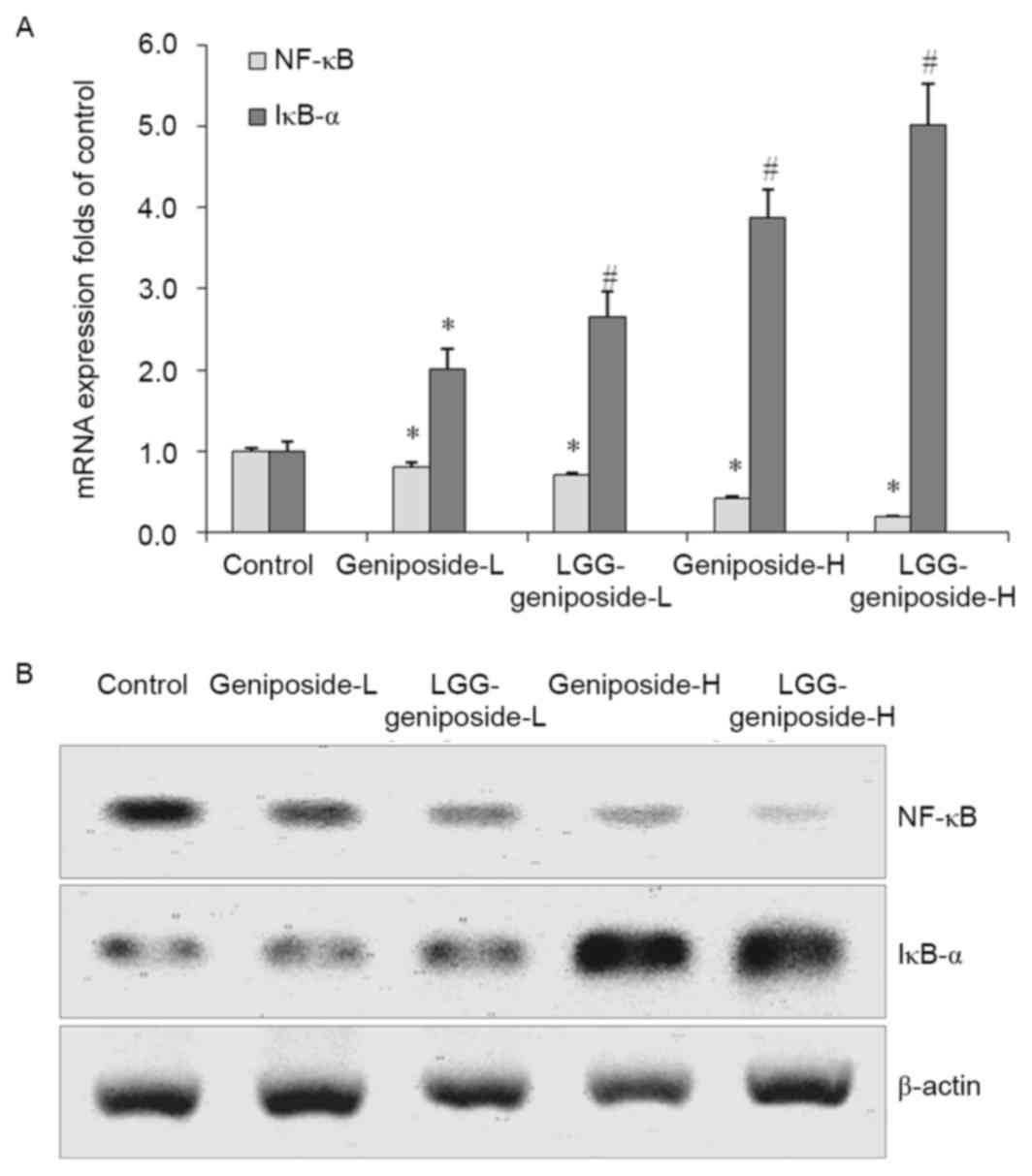
mRNA and protein expression of nuclear
factor-κB (NF-κB) and inhibitor of NF-κB α (IκB-α) in HSC-3
cells
Geniposide reduced NF-κB mRNA and protein expression
and raised IκB-α expression compared with that in the untreated
control cells, which was further enhanced in the presence of LGG.
Therefore, LGG-geniposide-H-treated cells had the lowest NF-κB
expression and highest IκB-α expression (Fig. 9).
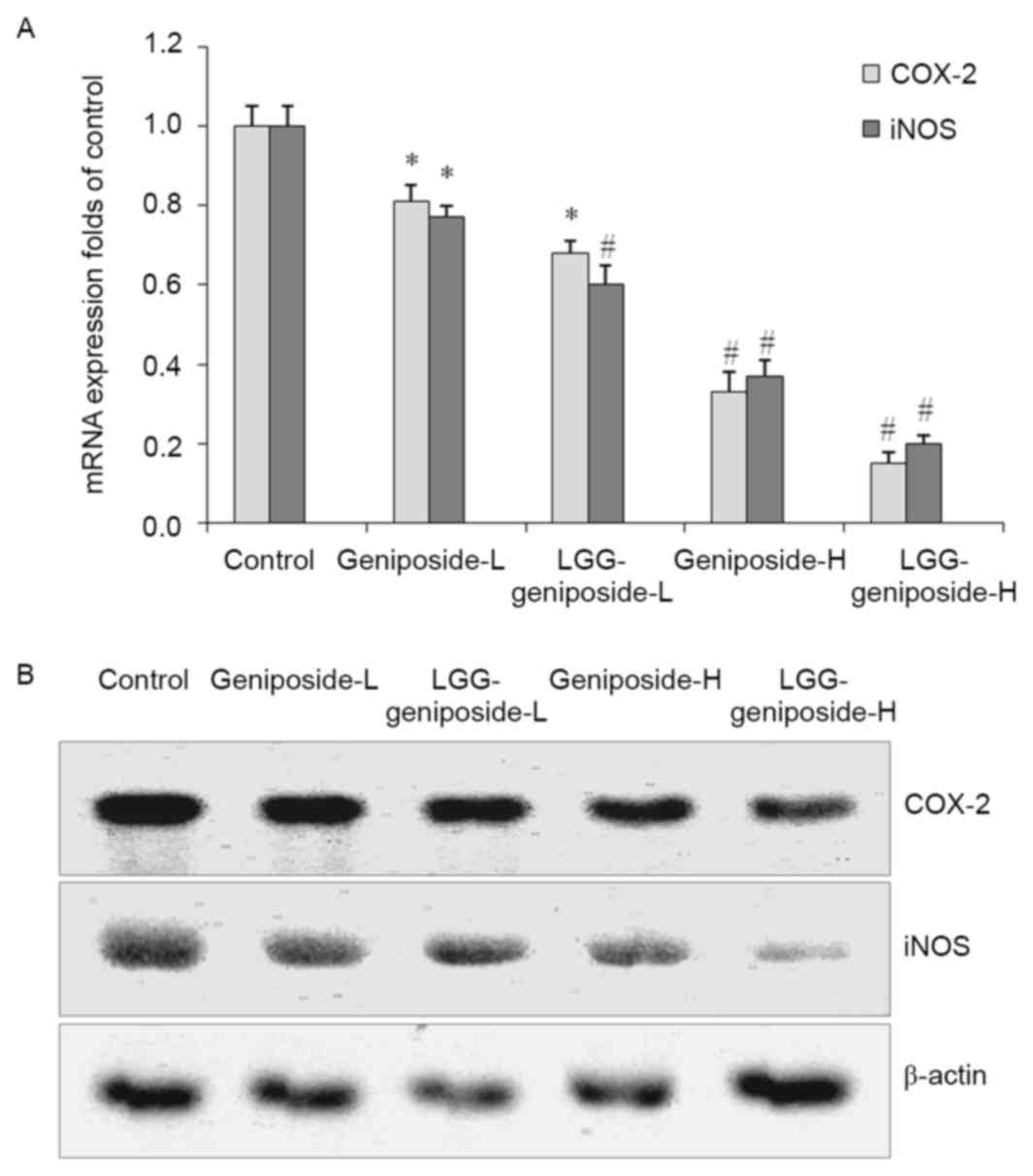
mRNA and protein expression of
cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS)
in HSC-3 cells
LGG-geniposide-H resulted in the lowest COX-2 and
iNOS mRNA and protein expression compared with that in the other
groups, and in the LGG-geniposide-L group, the expression was lower
than that in the geniposide-L group (Fig. 10).
Discussion
The ability of cancer cells to induce apoptosis is
an important index for the determination of anticancer cancer
effects (13). In the present study,
geniposide and LGG had no effects on normal oral cells (HOK cells),
but geniposide on its own or in combination with LGG had marked
apoptotic effects on HSC-3 oral cancer cells.
As an upstream protein involved in exogenous
apoptosis, caspase-8 shears and activates downstream
apoptosis-inducing proteins such as caspase-3, −6 and −7, causing
cell apoptosis (14). Apoptotic
protease activating factor 1 binds to the original structural
domain of the precursor of caspase-9 through the complementary
domain of caspase, leading to the self-activation of caspase-9,
which further activates the downstream caspase-3, −6 and −7,
inducing endogenous apoptosis of cells (15). Caspase-3 is involved in exogenous as
well as endogenous apoptosis, and numerous apoptotic factors
interact with downstream effector caspase-3 to ultimately induce
cell apoptosis (16).
The inhibition of apoptosis is vital for the
incidence and development of cancer, and Bcl-2 family proteins have
important roles in regulating the apoptosis of cancer cells. The
Bcl-2 family is made up of the apoptosis inhibitory factors Bcl-2
and Bcl-xL, and the apoptosis-promoting factor Bax, and their ratio
determines whether the cell is able to accept the apoptotic signal
(17). To a certain extent,
apoptosis or apoptosis inhibition are regulated by the above two
genes. Disturbed apoptosis regulation is crucial to the development
of tumors, and the Bcl-2 family has a major role in this process
(18). As the main members of the
Bcl-2 family, Bcl-2, Bax and Bcl-xL mainly regulate the apoptosis
of cells by affecting the mitochondrial pathway. When cells receive
death signals, Bax, which is bound to Bcl-2 or Bcl-xL, is
displaced, increasing the permeability of the mitochondrial
membrane and leading to the release of a series of substances, thus
eventually causing the death of cells (19).
Fas, FasL and caspase-3 are the important proteins
mediating the apoptosis of cells. FasL may be induced by certain
stress responses, such as ultraviolet light and DNA damage, and the
interactions between FasL and Fas may induce programmed death of
cells, which is an important mechanism of the body to clear mutated
cells (20). FasL is expressed on
the surface of tumor cells, and tumor-specific antigen may induce
tumor-infiltrating T lymphocytes to express Fas in large
quantities, enhancing the sensitivity of T cells to apoptosis. T
cells induce apoptosis of T lymphocytes, which causes high
expression of Fas by FasL, resulting in immunosuppression.
Fas-mediated apoptosis is also associated with numerous other
factors, such as p53 gene mutations or the lack of co-stimulatory
factor (21).
p53, the major protein regulating the Bcl-2 family,
controls different proteins of Bcl-2 family in various ways,
affecting the biological behavior of pancreatic cancer. p53 may
upregulate Bax and downregulate Bcl-2 or Bcl-xL, affecting the
apoptosis of cancer cells, and changing the permeability of
mitochondria, thus affecting the function of downstream
pro-apoptotic genes (22). As the
clumping factor of cyclin D kinase (CDK), low concentrations of
tumor suppressor gene p21 positively regulate the function of CDK,
facilitating the development of cells and promoting the transition
from G1 to S stage, but highly expressed p21 protein and cyclin
bind to CDK competitively to inhibit the activity of CDK, causing
the cell development to stagnate in G1 stage, thus inhibiting cell
proliferation or inducing cell apoptosis (23). p73 and p53 protein are homologous in
target gene binding, but their functions have great differences. As
p73 causes cell cycle arrest and induces apoptosis, it may inhibit
tumors to a certain extent (24).
The apoptosis-inhibiting genes HIAP-1 and HIAP-2
inhibit caspase to weaken its function to induce apoptosis.
Therefore, regulating and weakening the functions of HIAP-1 and
HIAP-2 genes is conducive to the activation of caspase, inducing
the apoptosis of cancer cells (25).
The NF-κB system is composed of the NF-κB family and
its inhibitor IκB-α. NF-κB is an extremely important
transcriptional activator, and IκB-α is the inhibitory protein of
NF-κB (26). NF-κB is vital to the
inflammation process, and is also the key regulatory protein to the
development of cancer. It has an important role in information
transmission in association with tumor growth, and is closely
associated with the incidence and development of tumors (27). Studies have found that NF-κB is
highly expressed in numerous types of tumor, and activated NF-κB
promotes the expression of a variety of genes involved in the
development of cancer (28,29). Helicobacter pylori infection
was found to activate NF-κB and the expression of COX-2, which have
important roles in the incidence and development of cancer
(30).
COX-2 and iNOS are not only the target molecules of
inflammation, but are also closely associated with the development
of tumors, particularly colon cancer. The increased expression of
COX-2 and iNOS affects signal transduction pathways, leading to the
occurrence, invasion and metastasis of tumors (31). At the same time, iNOS induces the
expression of COX-2 and catalyzes the production of NO to enhance
the activity of COX-2. Therefore, COX-2 and iNOS complement each
other to cause cancer. Inhibiting the expression of inflammatory
factors COX-2 and iNOS and the synthesis of induced products may
block the proliferation of tumor cells and may provide approaches
for treating cancer (32).
Genipin a major anticancer agent, which is produced
by transformation of geniposide by β-glycosidase produced by lactic
acid bacteria (33). LGG is a good
probiotic producing β-glycosidase, and in the present study, LGG
was likely to transform geniposide into genipin, leading to an
increased inhibitory effect on oral cancer cells.
In present study, the anticancer enhancement effects
of LGG on geniposide in HSC-3 cancer cells were determined by an
MTT assay, flow cytometry, RT-qPCR and western blot experiments.
Geniposide demonstrated a strong anticancer effect on HSC-3 cancer
cells, which was enhanced by a relatively low concentration of LGG.
These results indicated that LGG enhances the anticancer effects
for geniposide, and that this combination may be used in cancer
treatment.
References
|
1
|
Wu S, Jin Y, Liu QA, Wu JX, Bi YA, Wang ZZ
and Xiao W: Acousto-optic tunable filter near-infrared spectroscopy
for in-line monitoring liquid-liquid extraction of Gardenia
jasminoides Ellis based on statistical analysis. Pharmazie.
70:640–645. 2015.PubMed/NCBI
|
|
2
|
Kim SJ, Kim JK, Lee DU, Kwak JH and Lee
SM: Genipin protects lipopolysaccharide-induced apoptotic liver
damage in D-galactosamine-sensitized mice. Eur J Pharmacol.
635:188–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu H, Yin R, Han F, Guan J, Zhang X, Mao
X, Zhao L, Li Q, Hou X and Bi K: Characterization of chemical
constituents in Zhi-Zi-Da-Huang decoction by ultra high performance
liquid chromatography coupled with quadrupole time-of-flight mass
spectrometry. J Sep Sci. 37:3489–3496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong G, Zheng Z, Liu H, Wang L, Diao J,
Wang P and Zhao G: Purification and characterization of a
β-glucosidase from aspergillus niger and its application in the
hydrolysis of geniposide to genipin. J Microbiol Biotechnol.
24:788–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM,
Kim SJ, Kim BC, Jin C, Lim CJ and Park EH: Antiinflammatory effects
of genipin, an active principle of gardenia. Eur J Pharmacol.
495:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho HI, Kim SJ, Choi JW and Lee SM:
Genipin alleviates sepsis-induced liver injury by restoring
autophagy. Br J Pharmacol. 173:980–991. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Xiao Y, Li L, Li W and Yin X:
Comparative studies on contents of iridoid in different parts of
fruit from Gardenia jasminoides. Zhongguo Zhong Yao Za Zhi.
34:1949–1951. 2009.(In Chinese). PubMed/NCBI
|
|
8
|
Yang YS, Zhang T, Yu SC, Ding Y, Zhang LY,
Qiu C and Jin D: Transformation of geniposide into genipin by
immobilized β-glucosidase in a two-phase aqueous-organic system.
Molecules. 16:4295–4304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katakwar P, Metgud R, Naik S and Mittal R:
Oxidative stress marker in oral cancer: A review. J Cancer Res
Ther. 12:438–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mirzaei HR, Sahebkar A, Salehi R, Nahand
JS, Karimi E, Jaafari MR and Mirzaei H: Boron neutron capture
therapy: Moving toward targeted cancer therapy. J Cancer Res Ther.
12:520–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song S, Hao Y, Yang X, Patra P and Chen J:
Using gold nanoparticles as delivery vehicles for targeted delivery
of chemotherapy drug fludarabine phosphate to treat hematological
cancers. J Nanosci Nanotechnol. 16:2582–2586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu A and Liu S: Noncoding RNAs in growth
and death of cancer cells. Adv Exp Med Biol. 927:137–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Ju JH, Kim HM and Park KY:
Antimutagenic activity and in vitro anticancer effects of bamboo
salt on HepG2 human hepatoma cells. J Environ Pathol Toxicol Oncol.
32:9–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen G, Cheng X, Zhao M, Lin S, Lu J, Kang
J and Yu X: RIP1-dependent Bid cleavage mediates TNFα-induced but
Caspase-3-independent cell death in L929 fibroblastoma cells.
Apoptosis. 20:92–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guerrero AD, Chen M and Wang J:
Delineation of the caspase-9 signaling cascade. Apoptosis.
13:177–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agostini-Dreyer A, Jetzt AE, Stires H and
Cohick WS: Endogenous IGFBP-3 mediates intrinsic apoptosis through
modulation of Nur77 phosphorylation and nuclear export.
Endocrinology. 156:4141–4151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakazawa M, Matsubara H, Matsushita Y,
Watanabe M, Vo N, Yoshida H, Yamaguchi M and Kataoka T: The human
Bcl-2 family Member Bcl-rambo localizes to mitochondria and induces
apoptosis and morphological aberrations in drosophila. PLoS One.
11:e01578232016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tiwari P and Khan MJ: Molecular and
computational studies on apoptotic pathway regulator, Bcl-2 gene
from breast cancer cell line MCF-7. Indian J Pharm Sci. 78:87–93.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Neill KL, Huang K, Zhang J, Chen Y and
Luo X: Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak
through the outer mitochondrial membrane. Genes Dev. 30:973–988.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen SQ, Lin JP, Zheng QK, Chen SJ, Li M,
Lin XZ and Wang SZ: Protective effects of paeoniflorin against
FasL-induced apoptosis of intervertebral disc annulus fibrosus
cells via Fas-FasL signalling pathway. Exp Ther Med. 10:2351–2355.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin EM, Kim S, Merfort I and Kim YS:
Glycyrol induces apoptosis in human Jurkat T cell lymphocytes via
the Fas-FasL/caspase-8 pathway. Planta Med. 77:242–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Huang K, O'Neill KL, Pang X and
Luo X: Bax/Bak activation in the absence of Bid, Bim, Puma, and
p53. Cell Death Dis. 7:e22662016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gongpan P, Lu Y, Wang F, Xu Y and Xiong W:
AS160 controls eukaryotic cell cycle and proliferation by
regulating the CDK inhibitor p21. Cell Cycle. 15:1733–1741. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wang X, Flores ER, Yu J and Chang
S: Dysfunctional telomeres induce p53-dependent and independent
apoptosis to compromise cellular proliferation and inhibit tumor
formation. Aging Cell. 15:646–660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2, and IAP
families and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang C, Wang J, Lu X, Hu W, Wu F, Jiang
B, Ling Y, Yang R and Zhang W: Z-guggulsterone negatively controls
microglia-mediated neuroinflammation via blocking IκB-α-NF-κB
signals. Neurosci Lett. 619:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He G, Li LI, Guan E, Chen J, Qin YI and
Xie Y: Fentanyl inhibits the progression of human gastric carcinoma
MGC-803 cells by modulating NF-κB-dependent gene expression in
vivo. Oncol Lett. 12:563–571. 2016.PubMed/NCBI
|
|
28
|
Lu YX, Ju HQ, Wang F, Chen LZ, Wu QN,
Sheng H, Mo HY, Pan ZZ, Xie D, Kang TB, et al: Inhibition of the
NF-κB pathway by nafamostat mesilate suppresses colorectal cancer
growth and metastasis. Cancer Lett. 380:87–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McLoed AG, Sherrill TP, Cheng DS, Han W,
Saxon JA, Gleaves LA, Wu P, Polosukhin VV, Karin M, Yull FE, et al:
Neutrophil-derived IL-1β impairs the efficacy of NF-κB inhibitors
against lung cancer. Cell Rep. 16:120–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS,
Lin JT, Inoue H and Chen GH: Helicobacter pylori promote gastric
cancer cells invasion through a NF-kappaB and COX-2-mediated
pathway. World J Gastroenterol. 11:3197–3203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hasan SK, Siddiqi A, Nafees S, Ali N,
Rashid S, Ali R, Shahid A and Sultana S: Chemopreventive effect of
18β-glycyrrhetinic acid via modulation of inflammatory markers and
induction of apoptosis in human hepatoma cell line (HepG2). Mol
Cell Biochem. 416:169–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiarugi V, Magnelli L and Gallo O: Cox-2,
iNOS and p53 as play-makers of tumor angiogenesis (review). Int J
Mol Med. 2:715–719. 1998.PubMed/NCBI
|
|
33
|
Wan LH, Yao Z, Ni F, Wei M, Zhou Z, Wang
HQ, Sun Y and Zhong ZX: Biosynthesis of genipin from gardenoside
catalyzed by β-glucosidase in two-phase medium. CIESC J.
65:3583–3591. 2014.
|