Introduction
As a type of common multi-onset disease, stroke is
often characterized by high mortality and morbidity rates. Stroke
is generally considered as a major factor causing epilepsy in
adults (1). Epilepsy, a type of
recurrent cerebral dysfunction disease caused by abnormal neuron
discharges in the brain, is divided into subtypes according to
discharge site (e.g., frontal lobe epilepsy, temporal lobe
epilepsy, centrotemporal benign epilepsy and occipital lobe
epilepsy) (2). A few epilepsy after
stroke (EAS) patients can have their conditions controlled with
only short-term administration of antiepileptic drugs. However, the
majority of EAS patients require long-term medication to control
epileptic seizures. Generally, monotherapy can be effective, but
combination medication is required in many circumstances (3). Gastrodin, an active ingredient from the
Gastrodia elata Blume, is used extensively in Chinese
medicine, and is characterized by pacification, analgesia, and
sedative, effects. Study has shown that gastrodin has antagonistic
effects against epilepsy and can extend the epilepsy latency time
(4). Folate (FOL) and vitamin-B12
(V-B12) are key coenzymes involved in human HCY metabolism, and are
beneficial to neural function recovery in EAS patients (5). High-mobility group protein B1 (HMGB-1),
interleukin-2 (IL-2), and IL-6 play key roles in the occurrence and
development of EAS by participating in EAS pathology. Here, we
investigated the efficacy of gastrodin, FOL and V-B12 combination
therapy versus conventional antiepileptic drugs for EAS treatment,
and observed relatively satisfactory results.
Materials and methods
Patient information
We randomly selected 92 EAS patients who received
treatment between April, 2014 and March, 2016. Inclusion criteria
were as follows: i) patients diagnosed as epileptic according to
the classification methods stipulated by the International League
Against Epilepsy (ILAE) and electroencephalogram (EEG)
examinations; ii) patients who had never experienced epilepsy prior
to stroke, but suffered from epilepsy several times after the onset
of stroke; iii) patients who signed written informed consent.
Exclusion criteria were as follows: i) patients diagnosed with
secondary epilepsy caused by tumor, brain trauma, history of
craniocerebral operation or central nervous system infection; ii)
patients who were unable to receive the medication on time; iii)
patients who were allergic to the drugs used in this study. The
patients were randomly divided into control and observation groups
by computer software (SPSS Inc., Chicago, IL, USA) (n=46 each). No
statistically significant differences were identified between the
two groups in terms of general data (p>0.05) (Table I). The study was approved by the
Ethics Committee of the First People's Hospital of Xuzhou.
 | Table I.General patient data. |
Table I.
General patient data.
|
| Observation group
(n=46) | Control group
(n=46) | t/χ2 | P-value |
|---|
| Gender
(male/female) | 25/21 | 22/24 | 0.174 | 0.676 |
| Age (years) | 40–80 | 40–85 |
|
|
| Average age
(years) | 58.34±8.49 | 59.15±7.52 | 0.484 | 0.629 |
| Disease course
(year) | 7.35±2.49 | 6.96±2.37 | 0.769 | 0.443 |
| Degree of education
(n, %) |
| Junior
middle school or below | 9 (19.57) | 12 (26.09) | 0.118 | 0.731 |
| Senior
middle school or technical secondary school | 21 (45.65) | 19 (41.30) | 0.061 | 0.804 |
| Junior
college or above | 16 (34.78) | 15 (32.61) | 0.646 | 0.421 |
| Epilepsy subtype (n,
%) |
| Temporal
lobe epilepsy | 5 (10.86) | 6 (13.04) | 0.001 | 0.999 |
| Occipital
lobe epilepsy | 13 (28.26) | 10 (21.74) | 0.232 | 0.630 |
| Frontal
lobe epilepsy | 17 (36.96) | 19 (41.30) | 0.045 | 0.831 |
|
Centrotemporal benign
epilepsy | 13 (28.26) | 11 (23.91) | 0.056 | 0.812 |
Methods
Antiepileptic drug regimens
Patients in both groups received medication in
accordance with their epilepsy type. Patients with partial seizures
took carbamazepine (Jiangsu Yellow River Pharmaceutical Limited by
Share Ltd. (Jiangsu, China); approval no.: Chinese Drug Approval
no. H32020638) at an initial dosage of 100 mg twice daily, which
was augmented to 300 mg thrice a day within 1–4 weeks. Patients
with generalized seizures took sodium valproate orally [Hunan
Xiangzhong Pharmaceutical Co., Ltd. (Hunan, China); approval no.:
Chinese Drug Approval no. H43020874] at a dose of 300 mg thrice
daily. Patients with absence seizures took lamotrigine [Sanjin
Group Hunan Sanjin Pharmaceutical Co., Ltd. (Hunan, China);
approval no.: Chinese Drug Approval no. H20050596] at an initial
dosage of 25 mg once daily, which was augmented to 50 mg twice a
day within 1–4 weeks. Patients with myoclonic seizures took
clonazepam [Jiangsu Nhwa Saide Pharmaceutical Co., Ltd. (Jiangsu,
China); approval no.: Chinese Drug Approval no. H32020591] at an
initial dosage of 0.5 mg thrice per day, which was augmented to 2
mg thrice a day within 1–4 weeks. Patients with generalized tonic
clonic seizures took phenytoin sodium [CSPC Ouyi Pharmaceutical
Co., Ltd. (Shijiazhuang, China); approval no.: Chinese Drug
Approval no. H13020977] at an initial dosage of 100 mg twice daily,
which was augmented to 150 mg thrice per day within 1–4 weeks.
Combination medication treatment
In the observation group, patients took gastrodin
tablets orally [Tonghua Renmin Pharmaceutical Stock Co., Ltd.
(Jilin, China); Approval no.: Chinese Drug Approval no. H22025759]
in addition to antiepileptic drugs at a dosage of 50 mg thrice a
day. FOL tablets were orally administered daily [Jiangsu Yabang
Epson Pharmaceutical Co., Ltd. (Jiangsu, China); Approval no.:
Chinese Drug Approval no. H32023288] at a dose of 5 mg/day. V-B12
tablets were administered [Yunpeng Shanxi Pharmaceutical Co., Ltd.
(Shanxi, China); Approval no.: Chinese Drug Approval no. H14023321]
at a dosage of 25 µg thrice per day.
Detection of serum HCY, FOL and V-B12 levels
After 3 months post-treatment, we collected 3–5 ml
of venous blood from patients who did not eat or drink anything for
8 h prior to collection. Samples, after being placed at room
temperature for 1 h, were centrifuged at 4°C for 15 min at 2,100 ×
g, with the supernatant collected and refrigerated at −20°C.
Detection of serum HCY levels was performed using an HCY kit
(Beijing Wantai DRD Co., Ltd., Beijing, China) in strict accordance
with manufacturer's instructions via a biochemical analyzer
(Beckman Coulter Co., Ltd., Brea, CA, USA). Additionally, serum FOL
and V-B12 levels were detected using FOL and V-B12 detection kits
respectively (Beckman Coulter Co., Ltd.) via chemiluminescence in
accordance with manufacturer's instructions.
Detection of serum HMGB-1, IL-2 and IL-6
levels
At one day prior to treatment and on the 7th, 21st,
30th and 90th days after treatment, we collected 3–5 ml of venous
blood of patients who did not eat or drink anything for 8 h prior
to collection. Samples, after being placed at room temperature for
1 h, were centrifuged at 4°C for 20 min at 1,600 × g, with the
supernatant at −80°C. Enzyme-linked immunosorbent assay (ELISA) was
used to detect serum HMGB-1, IL-2 and IL-6 levels, and all kits
used in this procedure were manufactured by Sangon Biotech Shanghai
Co., Ltd. (Shanghai, China). All operations were in strict
accordance with manufacturer's instructions. OD values at a
wavelength of 450 nm were detected using a microplate reader
(Jiangsu Potebio Co., Ltd., Jiangsu, China) and concentrations of
HMGB-1, IL-2 and IL-6 were calculated accordingly.
Observation and follow-up
After medication, patient adverse reactions, such as
gastrointestinal reactions, dizziness, changes in weight,
dermatitis, somnolence and mental symptoms, were observed at all
times. Mild symptoms were alleviated via dosage adjustments, while
patients with severe symptoms were delivered to the hospital for
treatment. A 1-year follow-up was carried out for all patients
discharged from the hospital. Long-term medication was required for
patients, and dosage reduction and/or drug withdrawal was carried
out in accordance with the principal of gradual reduction only
after epilepsy was fully controlled.
Evaluation indexes
Evaluation criteria for efficacy of
treatment
i) Excellent effectiveness: Seizure frequency was
reduced by 75–99% within 6 months. ii) Effective: Seizure frequency
was reduced by 50–74% within 6 months. iii) Improvement: Seizure
frequency was reduced by 25–49% within 6 months. iv) Ineffective:
Seizure frequency was reduced by <25% or increased within 6
months. Total effectiveness rate = (‘excellent effectiveness’ rate
+ ‘effective’ rate + ‘improvement’ rate) ×100%.
Comparisons were performed regarding patient
epileptic seizure frequencies and Montreal cognitive assessment
(MoCA) scores
Twenty-eight days was set as the unit of seizure
frequency. The MoCA scale was used to evaluate patient condition in
8 domains (spatial and executive abilities, memory, attention and
concentration, naming, language, recall, abstract thinking and
orientation) before and after treatment, with a maximum score of 30
points.
Statistical analysis
Data were processed using SPSS 19.0 software (SPSS
Inc., Chicago, IL, USA). Measurement data were presented as mean ±
standard deviation (SD), and t-tests were performed. Ranked sum
tests were adopted in comparisons of treatment efficacy, and
Pearson's correlation coefficient was adopted in correlation
analysis. p<0.05 is considered to indicate a statistically
significant difference.
Results
After 6 months of treatment, we compared treatment
efficacy between the two groups, and found that total treatment
effectiveness rate was 95.65% in the observation group, which was
significantly higher than the control group (73.91%, p<0.05)
(Table II).
 | Table II.Comparison of efficacy (n, %). |
Table II.
Comparison of efficacy (n, %).
| Group | n | Excellent
effectiveness | Effective | Improvement | Ineffective | Total
effectiveness |
|---|
| Observation | 46 | 34 (73.91) | 7 (15.22) | 3 (6.52) | 2 (4.35) | 44 (95.65) |
| Control | 46 | 19 (41.30) | 9 (19.57) | 6 (13.04) | 12 (26.09) | 34 (73.91) |
Comparison of epileptic seizure frequency and MoCA
scores: At 6 months post-treatment, ameliorations were detected in
patient epileptic seizure frequency and MoCA scores in both groups.
The observation group showed significantly better improvement in
both parameters compared to the control group (p<0.05) (Table III).
 | Table III.Comparison of epileptic seizure
frequency and MoCA scores. |
Table III.
Comparison of epileptic seizure
frequency and MoCA scores.
|
| MoCA (points) | Epileptic seizure
frequency (time) |
|---|
|
|
|
|
|---|
| Group | Before treatment | After treatment | t-test | P-value | Before treatment | After treatment | t-test | P-value |
|---|
| Observation | 25.4±2.3 | 14.5±1.9 | 24.781 | <0.0001 | 26.2±3.2 | 11.2±3.3 | 22.132 | <0.0001 |
| Control | 25.3±2.4 | 18.7±1.8 | 14.921 | <0.0001 | 25.8±3.3 | 17.6±3.4 | 21.218 | <0.0001 |
| t-test | 0.204 | 10.884 |
|
| 0.590 | 9.161 |
|
|
| P-value | 0.838 | <0.0001 |
|
| 0.556 | <0.0001 |
|
|
At 3 months after treatment, analysis of serum HCY,
FOL and V-B12 levels showed that serum HCY levels in observation
group patients were significantly lower than control group
patients. Serum FOL and V-B12 levels in the observation group were
significantly higher than in the control group (p<0.05)
(Table IV).
 | Table IV.Comparison of patient HCY, FOL and
V-B12 levels. |
Table IV.
Comparison of patient HCY, FOL and
V-B12 levels.
| Group | n | HCY (µmol/l) | FOL (µmol/l) | V-B12 (pmol/l) |
|---|
| Observation | 46 | 12.38±7.27 | 16.48±6.37 | 213.12±26.43 |
| Control | 46 | 26.46±7.16 | 8.62±6.35 | 126.45±17.52 |
| t-test |
| 9.359 | 5.297 | 18.538 |
| P-value |
| <0.0001 | <0.0001 | <0.0001 |
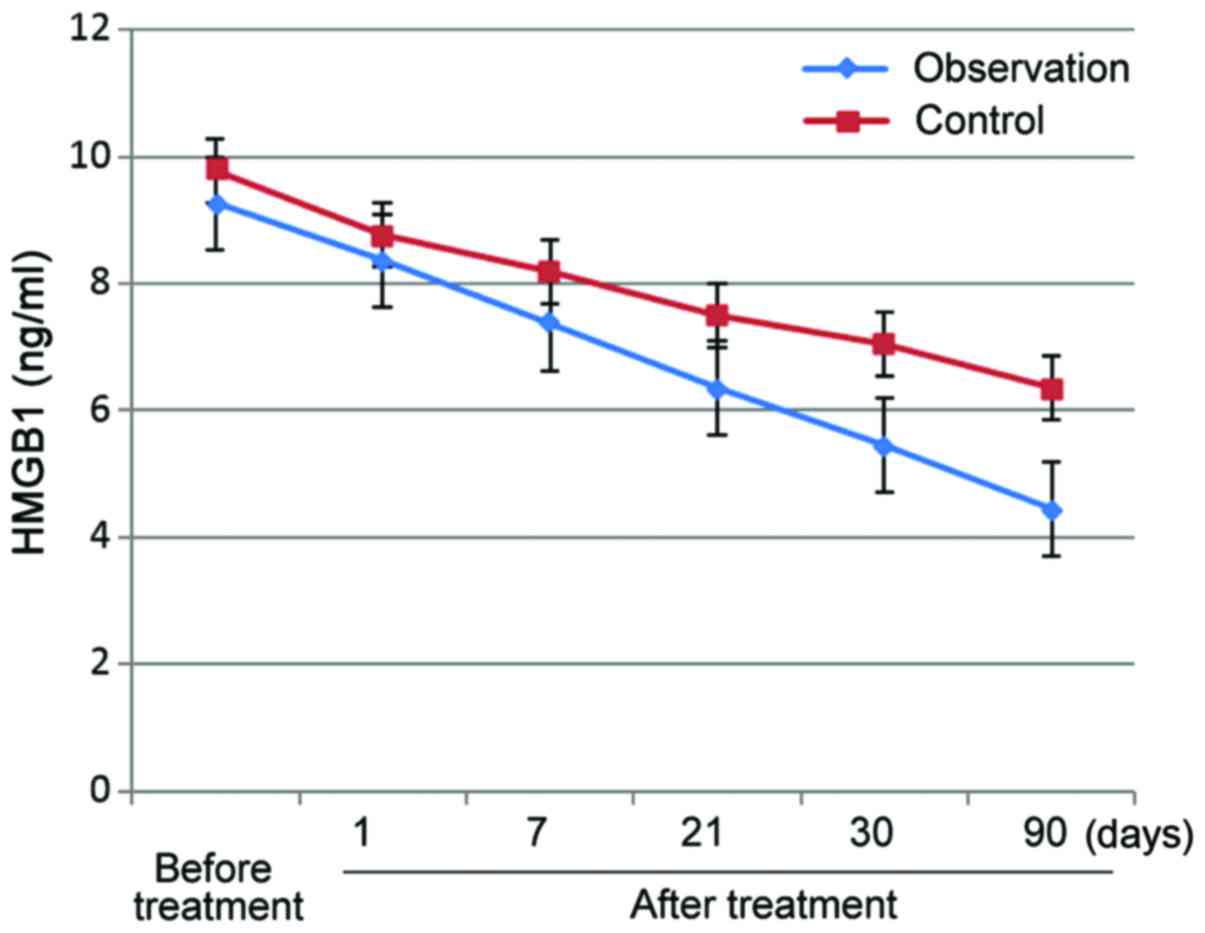
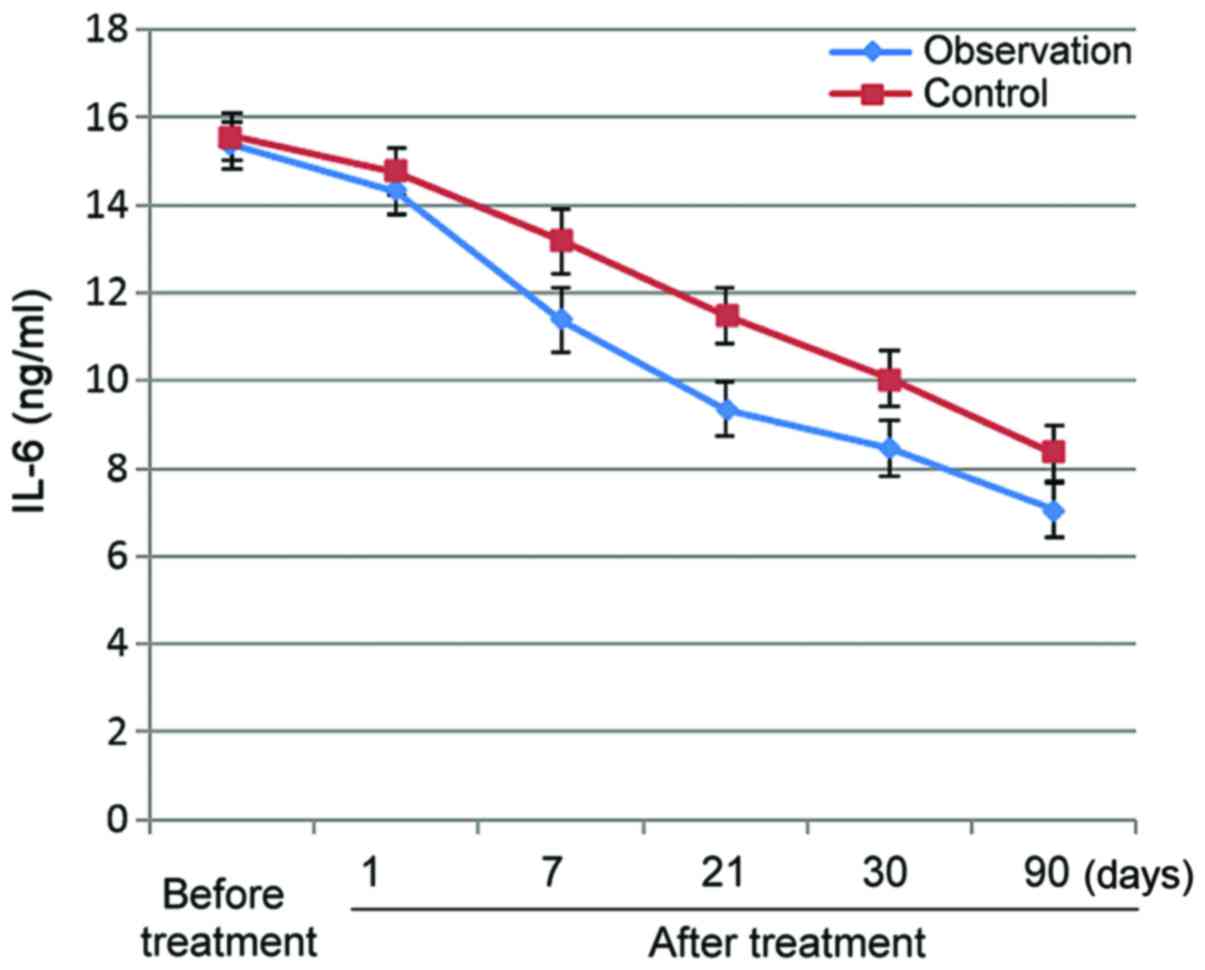
After treatment, serum HMGB-1, IL-2 and IL-6 levels
decreased in both groups, and this decreasing trend persisted over
time. Observation group levels were significantly lower for all
three parameters than control group levels (p<0.05) (Figs. 1–3).
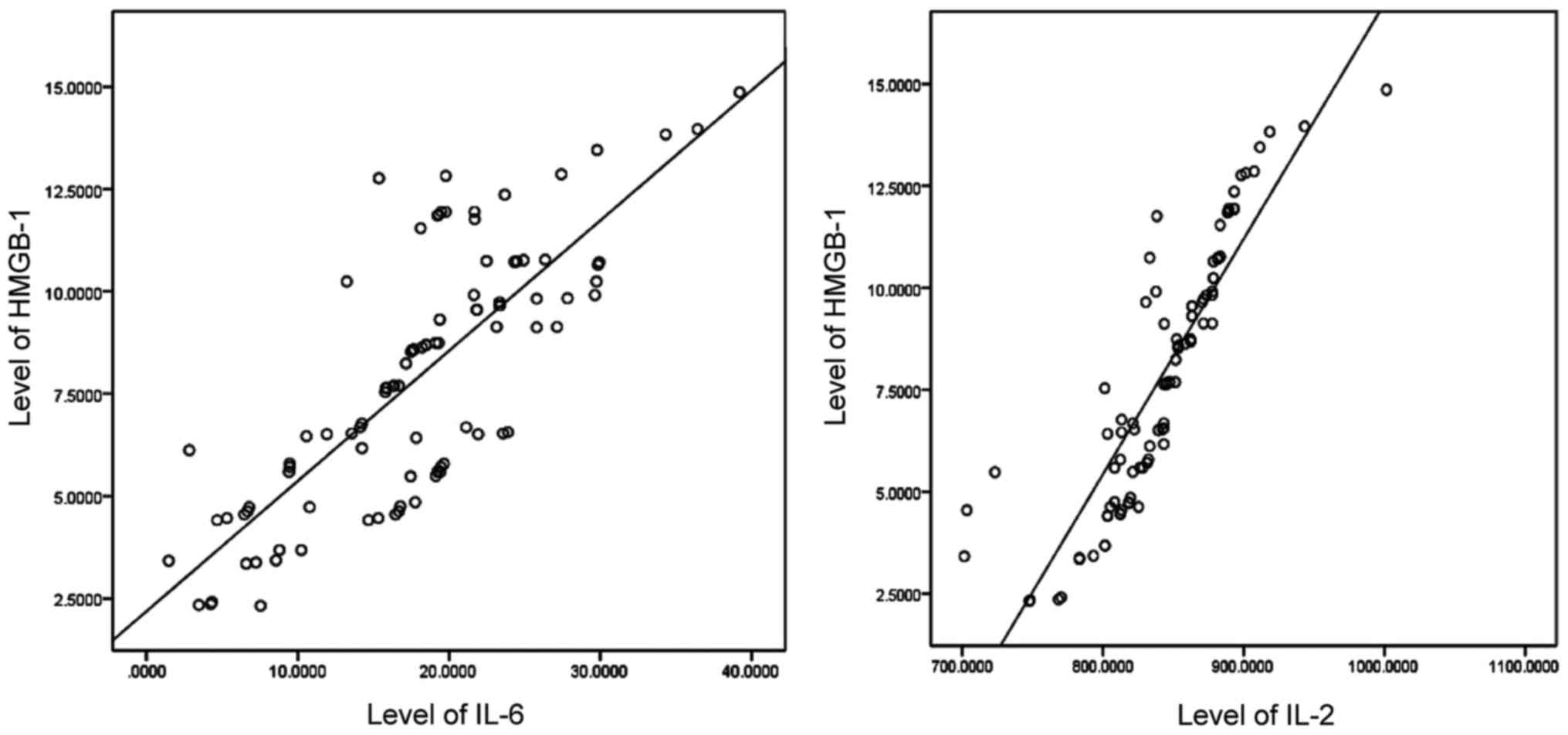
Correlation analysis for HMGB-1 levels and IL-2 and
IL-6 levels. Pearson's correlation coefficient analysis revealed
that HMGB-1 levels were positively correlated with IL-2 and IL-6
levels (p<0.05) (Table V and
Fig. 4).
 | Table V.Correlation analysis for HMGB-1 levels
and IL-2 and IL-6 levels. |
Table V.
Correlation analysis for HMGB-1 levels
and IL-2 and IL-6 levels.
| Item | Relevant
coefficient | P-value |
|---|
| IL-2 | 0.774 | 0.005 |
| IL-6 | 0.627 | 0.013 |
Discussion
Features and influencing factors of EAS. Stroke is
one of the major factors leading to adult epilepsy. Epilepsy can be
divided into two categories based on onset time: early-onset
epilepsy after stroke and late-onset epilepsy after stroke, with
the cut-off point at usually around 2 weeks (6). EAS can greatly affect patient
neurological function, which deleteriously impacts regular recovery
and can even lead to deterioration or death. Post-stroke injury to
the brain structure and function may induce neuronal changes,
necrosis, and/or deficiency, causing neurotransmitter balance
alterations, increased excitability, abnormal ion distribution, and
excessive cerebral neuron discharge, thus resulting in
deterioration of epilepsy or even persistent epilepsy, which
threatens the lives of patients and forms a vicious circle
(7). Additionally, EAS is
characterized by transient, abrupt, and recurrent features. The
onset of EAS may be associated with following factors: i) glial
tissue hyperplasia: Which can induce apoplectic cyst formation,
which replaces normal brain tissue to decrease neural cell
regulatory functions, weakens excitability regulation, and causes
excessive neuron discharge, thus resulting in epilepsy (8). ii) Factors affecting neural cell
membrane stability: after stroke, neural cell membrane instability
can give rise to abnormalities in the function of surrounding cells
and selective neuronal mutations, leading to an increase in neural
cell excitability and synchronous discharge, thus resulting in
epilepsy (9). iii) Inflammatory
factors: after stroke, long-term inflammatory responses can
generate changes in morphology, increasing the amount of neuroglia,
thus causing or aggravating epilepsy (10). iv) Immunological factors: Disorders
in immunological functions have been found in EAS patients,
including abnormal humoral and cellular immunological functions,
which can induce the onset of epilepsy (11).
EAS treatment. So far, the pathogenesis of EAS
remains unclear, and treatment has also presented problems.
Generally, EAS treatment is focused on the primary disease and
customized medication can be additionally used based on the
features of the epileptic seizures present. Long-term medication is
also required, mainly carbamazepine and sodium valproate (12). EAS patients are susceptible to
shortages of FOL and V-B12, which can cause disorders in HCY
metabolism, leading to increased HCY levels. Thus, medication
should be used to combat this condition in specific treatments
(13). This study showed that
supplementation of FOL and V-B12 significantly reduced serum HCY
levels, suggesting that this medication schedule, from the
perspective of improvement in primary disease, is beneficial to the
treatment and control of epilepsy.
Gastrodin mechanism of action in EAS. Gastrodin is a
major active ingredient in Gastrodia elata Blume. Study has
indicated that gastrodin, with anticonvulsive and antiepileptic
functions, can prolong the latency of epileptic seizures, shorten
onset course, alleviate degree of attack, accelerate recovery and
decrease mortality rate. Thus, gastrodin can be used as an
auxiliary drug to antagonize absence seizures and severe grand mal
seizures for the amelioration of patient clinical symptoms
(14). The potential mechanism of
action of gastrodin may lie in an inhibition of the action and
expression of inhibitory and amino acid neurotransmitters (glutamic
acid) in the temporal lobe and hippocampus. This suppresses
abnormal gap junction formation and decreases cerebral cortex
excitability, thus generating the antiepileptic effect. Gastrodin
can also alleviate damage to endothelial cells, astrocytes, and
neurons in hippocampal vasculature, which can protect brain tissue
(15). After combination therapy
using gastrodin and antiepileptic drugs, the total treatment
effectiveness rate significantly increased (95.74% in the
observation group vs. 74.47% in the control group, p<0.05).
Significance of serum HMGB-1, IL-2, and IL-6 after
EAS. HMGB-1, as a type of nonhistone chromosomal binding protein,
is involved in the pathogenesis of various diseases, including
tissue damage, respiratory system diseases, sepsis, ischemic brain
damage and carcinoma (16). Study
has indicated that HMGB-1 expression is elevated inside and outside
astrocytes and hippocampal neurons in epilepsy patients (17). IL-6 is an early-stage inflammatory
factor, and despite its protective effect on brain tissue,
long-term overexposure will not only result in the augmentation of
neuroglia, but also cause variations in tissue morphology, thus
aggravating epilepsy (18). IL-2 can
regulate the balance of lymphocytes, shorten epilepsy latency and
decrease the threshold value for extending the onset of epileptic
discharge (19). This study showed
that serum HMGB-1 levels were positively correlated with the levels
of IL-2 and IL-6. HMGB-1 functions as a central link in the network
of pro-inflammatory factors, the release of HMGB-1 can induce the
secretion of IL-2 and IL-6 for the amplification of inflammatory
responses, while IL-2 and IL-6 can in turn promote the release of
HMGB-1, thus forming positive feedback loops (20). After treatment, patient serum levels
of HMGB-1, IL-2 and IL-6 in the observation group were
significantly lower than in the control group, suggesting that this
medication schedule was beneficial to the control of epilepsy.
In conclusion, treatment with a combination of
gastrodin, FOL, and V-B12 in addition to standard antiepileptic
treatment can effectively control epileptic seizures and increase
treatment efficacy for EAS patients.
Acknowledgements
This study was supported by the Clinical Science and
Technology Development Fund of Jiangsu University (no. JLY20160140)
and the Xuzhou Science and Technology Planning Project (no.
KC16SH025).
References
|
1
|
Keller L, Hobohm C, Zeynalova S, Classen J
and Baum P: Does treatment with t-PA increase the risk of
developing epilepsy after stroke? J Neurol. 262:2364–2372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma R and Pachori RB: Classification of
epileptic seizures in EEG signals based on phase space
representation of intrinsic mode functions. Expert Syst Appl.
42:1106–1117. 2014. View Article : Google Scholar
|
|
3
|
Schmidt D and Schachter SC: Drug treatment
of epilepsy in adults. BMJ. 348:g254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang R, Peng Z, Wang H, Xue F, Chen Y,
Wang Y, Wang H and Tan Q: Gastrodin ameliorates depressive-like
behaviors and up-regulates the expression of BDNF in the
hippocampus and hippocampal-derived astrocyte of rats. Neurochem
Res. 39:172–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eldayem SMA, Saleh ON, Emara NA, Kandil ME
and Shatla RH: Evaluation of homocysteine, folate and vitamin B12
levels among Egyptian children with idiopathic epilepsy. Maced J
Med Sci. 7:109–113. 2014.
|
|
6
|
Hsu CJ, Weng WC, Peng SS and Lee WT:
Early-onset seizures are correlated with late-onset seizures in
children with arterial ischemic stroke. Stroke. 45:1161–1163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beghi E, D'Alessandro R, Beretta S,
Consoli D, Crespi V, Delaj L, Gandolfo C, Greco G, La Neve A,
Manfredi M, et al: Epistroke Group: Incidence and predictors of
acute symptomatic seizures after stroke. Neurology. 77:1785–1793.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattiangady B and Shetty AK: Neural stem
cell grafting in an animal model of chronic temporal lobe epilepsy.
Curr Protoc Stem Cell Biol: Chapter. 2:Unit2D.72011.
|
|
9
|
Bouthour W, Leroy F, Emmanuelli C, Carnaud
M, Dahan M, Poncer JC and Lévi S: A human mutation in Gabrg2
associated with generalized epilepsy alters the membrane dynamics
of GABAA receptors. Cereb Cortex. 22:1542–1553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu N, Liu H and Di Q: Modulation of
immunity and the inflammatory response: A new target for treating
drug-resistant epilepsy. Curr Neuropharmacol. 11:114–127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kan AA, van Erp S, Derijck AAHA, de Wit M,
Hessel EV, O'Duibhir E, de Jager W, Van Rijen PC, Gosselaar PH, de
Graan PN, et al: Genome-wide microRNA profiling of human temporal
lobe epilepsy identifies modulators of the immune response. Cell
Mol Life Sci. 69:3127–3145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwan J, Wood E, Kinton L and Cordonnier C:
Antiepileptic drugs for the primary and secondary prevention of
seizures after stroke. Cochrane Database Syst Rev. 1:CD005398.
2010.
|
|
13
|
Wang YP, Lin HP, Chen HM, Kuo YS, Lang MJ
and Sun A: Hemoglobin, iron, and vitamin B12 deficiencies and high
blood homocysteine levels in patients with anti-thyroid
autoantibodies. J Formos Med Assoc. 113:155–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang C, Wang L, Liu X, Cheng M, Qu Y and
Xiao H: Comparative pharmacokinetics of gastrodin in rats after
intragastric administration of free gastrodin, parishin and
Gastrodia elata extract. J Ethnopharmacol. 176:49–54. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsieh CL, Chen CL, Tang NY, Chuang CM,
Hsieh CT, Chiang SY, Lin JG and Hsu SF: Gastrodia elata BL
mediates the suppression of nNOS and microglia activation to
protect against neuronal damage in kainic acid-treated rats. Am J
Chin Med. 33:599–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Tian J, Fu X, Chen Y, Zhang W, Yao H
and Hao Q: Serum high mobility group box protein 1 as a clinical
marker for ovarian cancer. Neoplasma. 61:579–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang JS, Wu Y, Huang Q, Li SJ, Ye JM, Wei
X, Liu QD, Liu Y and Ma MG: Expression level and distribution of
HMGB-1 in Sombati's cell model and kainic acid-induced epilepsy
model. Eur Rev Med Pharmacol Sci. 19:2928–2933. 2015.PubMed/NCBI
|
|
18
|
Uludag IF, Duksal T, Tiftikcioglu BI,
Zorlu Y, Ozkaya F and Kirkali G: IL-1β, IL-6 and IL1Ra levels in
temporal lobe epilepsy. Seizure. 26:22–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steinborn B, Zarowski M, Winczewska-Wiktor
A, Wójcicka M, Młodzikowska-Albrecht J and Losy J: Concentration of
IL-1β, IL-2, IL-6, TNFα in the blood serum in children with
generalized epilepsy treated by valproate. Pharmacol Rep.
66:972–975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwak MS, Lim M, Lee YJ, Lee HS, Kim YH,
Youn JH, Choi JE and Shin JS: HMGB-1 binds to lipoteichoic acid and
enhances TNF-α and IL-6 production through HMGB-1-mediated Transfer
of lipoteichoic acid to CD14 and TLR2. J Innate Immun. 7:405–416.
2015. View Article : Google Scholar : PubMed/NCBI
|