Introduction
Atrial fibrillation (AF) is the most prevalent form
of sustained arrhythmia (1).
Treatment strategies for AF (120–180 times/min) aim to regulate the
heart rate to a near normal range (60–100 times/min), typically
with drug therapy (2). Abnormal
heart rhythm may lead to heart palpitations, fainting, shortness of
breath, chest pain and fatality. Risk factors for AF include heart
failure, dementia and stroke, and the incidence of AF increases
with age (2). AF is an important
focus of research into heart disease. In China, the prevalence of
AF is 0.77, and 7.5% of all patients with AF are >80 years
(3). The pathogenesis of AF is not
yet fully understood, and the efficacy of current treatment
strategies is poor.
Cardiac electrophysiological processes are essential
for heart function, and are regulated by changes in the expression
and activity of ion channel membrane proteins and connexin (Cx)
proteins (4,5). Electrical and structural remodeling are
among the primary characteristics of cellular electrophysiology in
AF (6). The majority of heart
disease is caused by abnormal expression of ion channel proteins
and Cx proteins (7–9). Previous studies have investigated the
underlying molecular mechanisms that affect the development of AF
(10–13), revealing that the occurrence and
maintenance of chronic AF is regulated by multiple genes and
proteins, including L-type calcium channel and sarcoplasmic
reticulum Ca2+-ATPase (10). However, the association between these
multiple genes and proteins remains unclear.
Recent studies have indicated that potassium
voltage-gated channel subfamily A member 5 (KCNA5) and Cx proteins
serve a role in the pathogenesis of AF (11,14,15). In
turn, a number of drugs that target KCNA5 and Cx40 have been
identified (16–20). For instance, Vernakalant (RSD1235;
Cardiome Pharma Corp., Vancouver, BC, Canada) is a drug that
exhibits high affinity for KCNA5 and has arrhythmia-specific
effects (21). Furthermore,
intravenous administration of Vernakalant in phase II and III
clinical trials caused cardioversion effects (22). However, the electrophysiological
properties of atrial ion channels, and the regulation of KCNA5 and
Cx40, during AF are not well understood.
KCNA5 and Cx40 may exert combined effects on
electrophysiological function during AF. Therefore, the present
study used RNA interference to investigate the effects of KCNA5 and
Cx40 on cardiomyocyte function during AF. The results of the
present study may improve understanding of the pathogenesis of AF,
and aid in the prevention and treatment of AF.
Materials and methods
Patients
A total of 60 patients were included in the present
study. Of these patients, 30 presented with persistent AF, while 30
presented with rheumatic heart disease and were used as a control
group. The patients were enrolled between September 2014 and
September 2015 in the Department of Cardiothoracic Surgery, Nanshan
People's Hospital of Shenzhen (Shenzhen, Guangdong). The clinical
characteristics of all patients are presented in Table I. Patients with persistent AF and
patients with rheumatic heart disease were matched based on typical
clinical symptoms. In patients with AF, the ventricular rates were
fast (up to 120–180 times/min) and irregular. The rhythm was
irregular with unequal heart sounds and short pulses (the pulse
rate was lower than the heart rate). In patients with rheumatic
heart disease, valve lesions observed included mitral stenosis or
mitral regurgitation. AF was diagnosed by evaluating patient
medical records and the results of a 12-lead electrocardiogram. In
atrial fibrillation, the P-wave disappeared and was replaced by the
atrial fibrillation wave. Furthermore, the R-R interval was
irregular and the ventricular rate was irregular (120–180
times/min). Additionally, the QRS complex was deformed and
rheumatic heart disease was diagnosed using Doppler
echocardiography. In rheumatic heart disease, mitral valve leaflets
were thickened and were observed on ultrasound as a hyperechoic
area. Furthermore, the activity range was decreased and the
diastolic anterior lobe was bulging in a balloon-shaped. The tip of
the front and rear leaf distance was notably shortened.
Additionally, the distance between the front and back of the valve
tip was shortened and the opening area was reduced. Atrial muscle
tissues from the left atrial appendage section were collected
during heart valve replacement surgery. Written informed consent
was obtained from all patients, and the experimental procedures
were approved by the Ethics Committee of the Nanshan People's
Hospital of Shenzhen (Shenzhen, China) and performed according to
their guidelines.
 | Table I.Clinical characteristics of the
patients included in the present study. |
Table I.
Clinical characteristics of the
patients included in the present study.
|
| Type of disease (mean
± standard deviation) |
|---|
|
|
|
|---|
| Characteristics | RHD (n=30) | AF (n=30) |
|---|
| Sex (n) |
| Male | 13 | 15 |
|
Female | 17 | 15 |
| Age (years) | 47.33±6.78 | 52.68±10.65 |
| LA (mm) | 37.52±4.71 | 34.98±5.78 |
| RA (mm) | 33.91±3.99 | 35.16±4.88 |
| EF (%) | 60.62±7.54 |
43.06±10.62a |
Cell culture
Atrial myocytes from the left side of the heart were
obtained from 3 enrolled patients. Briefly, a 1 mm3
atrial muscle segment was washed in PBS (Sangon Biotech Co., Ltd.,
Shanghai, China). The 1 mm3 atrial muscle segments were
placed in cell culture flasks (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and incubated at 37°C in an atmosphere containing
5% CO2. Once the cells reached up to 80% confluence, the
cells adhered to the flask were washed with PBS and 0.25% Trypsin
(Sangon Biotech Co., Ltd., Shanghai, China) for 5 min. The washed
cells were centrifuged for 3 min at room temperature 500 × g/min to
obtain the passage cells. Cells were adhered to cell culture
flasks. Cells were incubated at 37°C with 5% CO2 for 90
min, prior to the addition of Dulbecco's modified Eagle's medium
(DMEM) supplemented with 15% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The medium was replaced
every 3 days. Upon reaching ~80% confluence, cells were passaged
into new flasks. The following experiments were conducted on the
third generation of passaged cells.
RNA interference
Cells were plated into 96-well plates at a
concentration of 1.0×105 cells/ml in DMEM and cultured
for 24 h at 37°C with 5% CO2. Cells were subsequently
transfected with Cx40-small interfering RNA (siRNA) (cat. no.
HSS104129) and KCNA5-siRNA (cat. no. HSS105670) (both from
Invitrogen; Thermo Fisher Scientific, Inc.). Briefly, cells were
treated with 10 pmol Cx40-siRNA or KCNA5-siRNA and 0.5 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h at 37°C. Untransfected cells were used
as the control. Following treatment, the transfection solution was
replenished with fresh DMEM medium. Total RNA was extracted from
cells and levels of target gene inhibition were determined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions, and the quantity of extracted RNA was
measured using an ultraviolet spectrophotometer (NanoDrop; Thermo
Fisher Scientific, Inc.). The A260/280 was used to analyze the RNA
purity (when A260/280=1.8–2.1, the sample was used). The RNA
quantification was based on Beer-Lambert law: A=εcl (A=absorbance,
ε=extinction coefficient, c=concentration and l=path length)
according to the instruction of manufacturers. Reverse
transcription was performed using a PrimeScript RT reagent kit with
gDNA Eraser (Takara Bio, Inc., Otsu, Japan) with 1 µg total RNA
according to the manufacturer's instructions. qPCR was performed
using a Takara SYBR Green PCR kit (DRR820A; Takara Bio, Inc.) with
an ABI 7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR thermocycling conditions were as
follows: 95°C for 15 min; 40 cycles at 95°C for 10 sec, 60°C for 20
sec and 72°C for 15 sec. Primers were designed using Primer Express
software (version 2.0.0; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers used were as follows: Cx40 forward,
5′-CCGGCCCACAGAGAAGAATGT-3′ and reverse,
5′-TCTGACCTTGCCTTGCTGCTG-3′; KCNA5 forward,
5′-CAGAGTCTCCAAGCAGAAGG-3′ and reverse, 5′-CCAGGTGTGGCTTATCTTCG-3′;
and GAPDH forward, 5′-ACTCTGGCAAAGTGGATATTGTCG-3′ and reverse,
5′-CAGCATCACCCCATTTGATG-3′. Levels of gene expression relative to
that of GAPDH were calculated using the 2−ΔΔCq method
(23). Four replicates were
performed for each qPCR reaction.
Western blot analysis
Following transfection, proteins were extracted from
cells using RIPA lysis buffer (Beyotime Institute of Biotechnology,
Shanghai, China) and separated using 10% SDS-PAGE. Proteins were
then transferred onto polyvinylidene difluoride membranes (Merck
KGaA, Darmstadt, Germany). The blocking of proteins was performed
at room temperature in 5% non-fat dry milk for 1 h. The following
polyclonal rabbit primary antibodies were used: Cx40 (1:500, cat.
no. ab38580), KCNA5 (1:500, cat. no. ab181798) and GAPDH (1:1,000,
cat. no. ab9485). Following incubation with primary antibodies for
18 h at 4°C, the membrane was rinsed 3 times with wash buffer 1X
phosphate-buffered saline with Tween-20 (PBST) wash buffer
(Beyotime Institute of Biotechnology, Shanghai, China). The
secondary antibody was goat polyclonal anti-rabbit immunoglobulin G
(1:2,000, cat. no. ab150077), which was incubated with membrane at
room temperature for 2 h following washing with 1X PBST wash buffer
3 times. All antibodies were purchased from Abcam (Cambridge, UK).
Protein expression was analyzed using ECL Western Blotting
substrate (Pierce; Thermo Fisher Scientific, Inc.). Western blot
images were captured using a ChemiDoc XRS system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein levels were
determined relative to GAPDH using Image-Pro Plus software (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. Data are expressed
as the mean ± standard deviation. An independent samples t-test was
used to compare levels of gene and protein expression between
groups. Pearson's correlation coefficient was used to measure the
association between Cx40 and KCNA5 expression in atrial myocytes.
All analyses were conducted as two-tailed tests. P<0.05 was
considered to indicate a statistically significant difference.
Linear regression analysis was plotted using GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA) to indicate the
correlation between Cx40 and KCNA5 expression.
Results
Expression of Cx40 and KCNA5 is
decreased in patients with AF
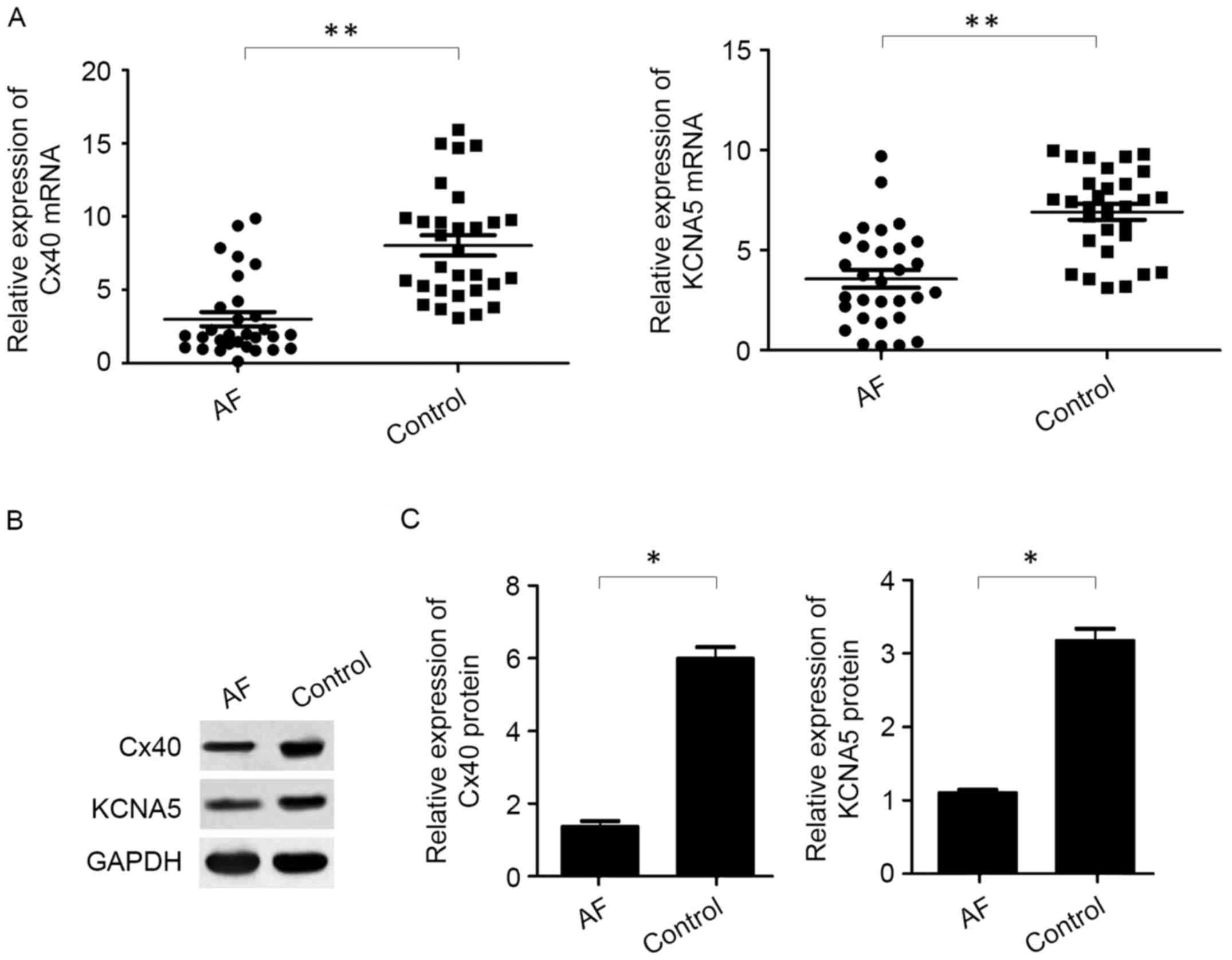
To evaluate the expression of Cx40 and KCNA5 in
patients with AF, RT-qPCR and western blotting were used to measure
the levels of Cx40 and KCNA5 mRNA and protein, respectively, in the
atrial myocytes of patients with AF and rheumatic heart disease. It
was observed that levels of Cx40 and KCNA5 mRNA (P<0.01;
Fig. 1A) and protein (P<0.05;
Fig. 1B) were significantly reduced
in the atrial myocytes of patients with AF patients compared with
the control patients with rheumatic heart disease.
Cx40 and KCNA5 mRNA expression is
positively correlated in patients with AF
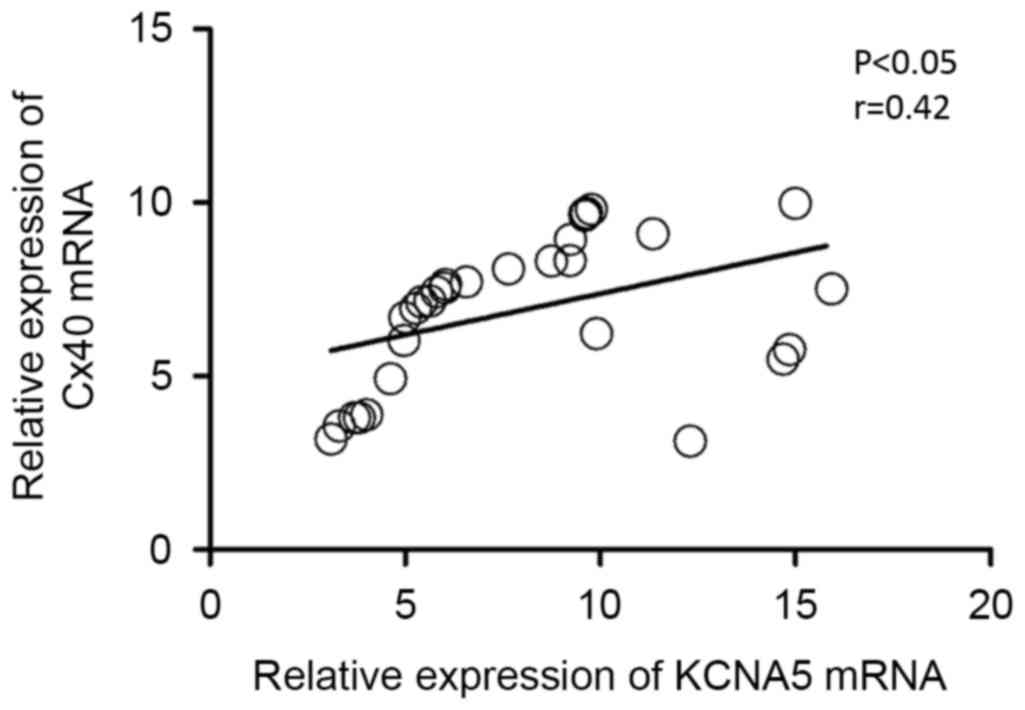
To determine whether mRNA levels of Cx40 and KCNA5
were associated in patients with AF, the Pearson's correlation
coefficient test was performed on the expression data from 30
patients with AF. It was observed that levels of Cx40 and KCNA5
mRNA were positively correlated (P<0.05, r=0.42; Fig. 2), indicating a potential association
between the expression of Cx40 and KCNA5 in patients with AF. In
addition, using linear regression analysis, the line of best fit
was determined to be y=0.237×+5.003, indicating a positive
correlation between Cx40 and KCNA5 expression.
Inhibition of Cx40 expression reduces
KCNA5 expression in atrial myocytes
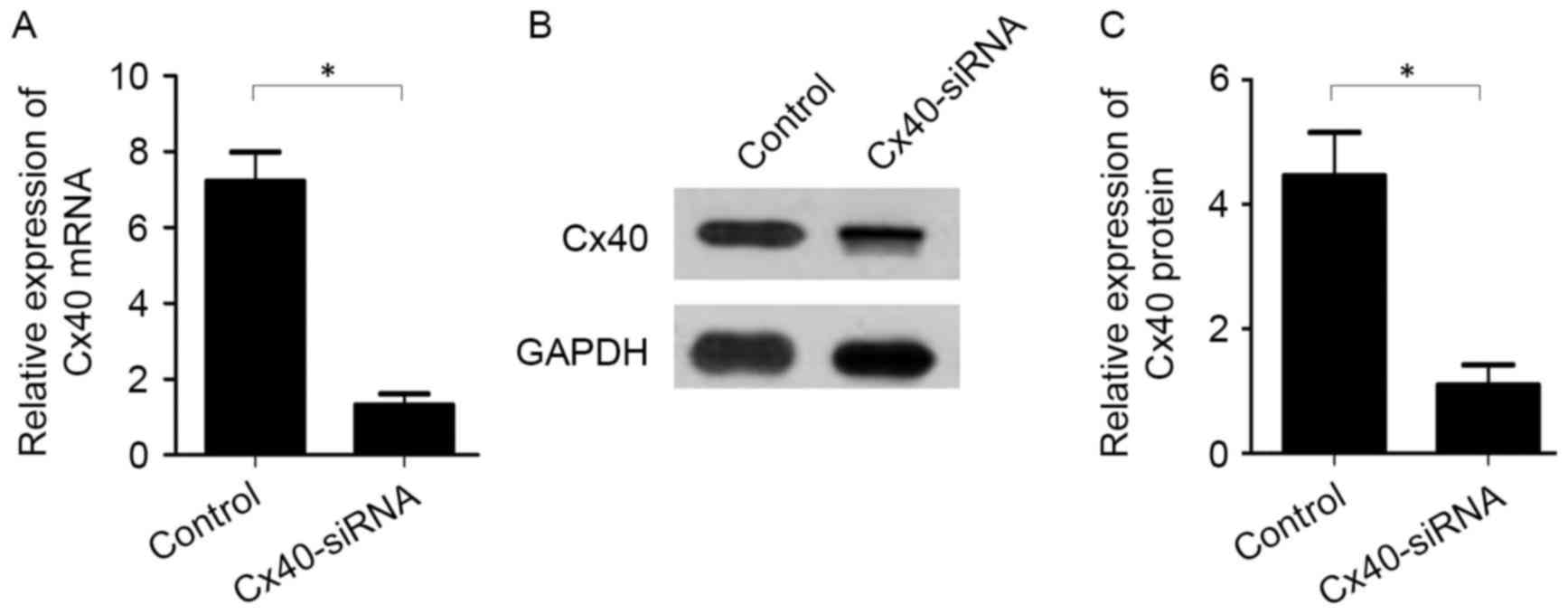
To evaluate the association between Cx40 and KCNA5
expression in patients with AF, Cx40-siRNA was transfected into
atrial myocytes collected from the patients. Knockdown of Cx40 was
subsequently validated by RT-qPCR and western blot analysis. The
results indicated that Cx40 mRNA (1.31±0.53 vs. 7.21±1.33,
P<0.05; Fig. 3A) and protein
(1.10±0.56 vs. 4.46±1.21, P<0.05; Fig. 3B and C) expression was significantly
decreased by Cx40-siRNA transfection compared with the
untransfected control cells.
In the atrial myocytes transfected with Cx40-siRNA,
mRNA (1.54±0.52 vs. 10.44±2.34, P<0.05; Fig. 4A) and protein (1.10±0.08 vs.
3.21±0.43, P<0.05; Fig. 4B and C)
levels of KCNA5 were significantly decreased compared with the
control cells.
Inhibition of KCNA5 expression reduces
Cx40 expression in atrial myocytes
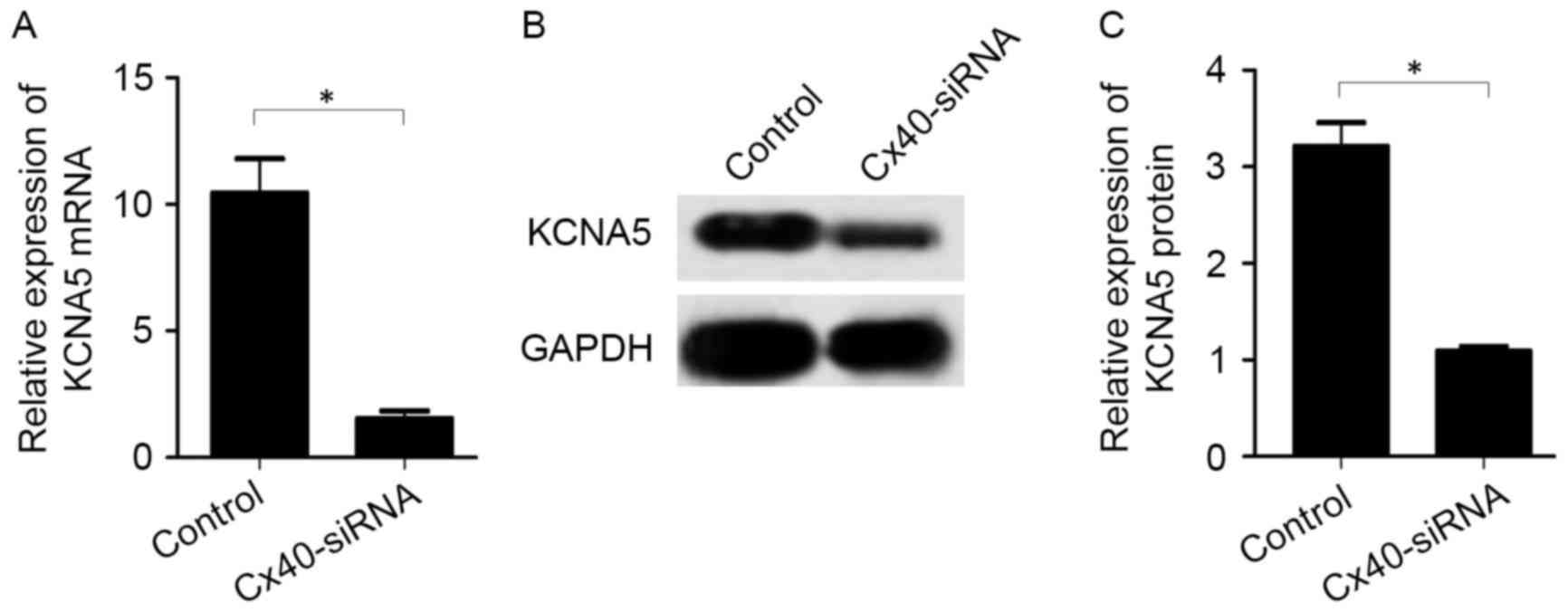
The effects of KCNA5 inhibition on the expression of
Cx40 were assessed by transfecting KCNA5-siRNA into atrial
myocytes. Following KCNA5-siRNA transfection, knockdown of KCNA5
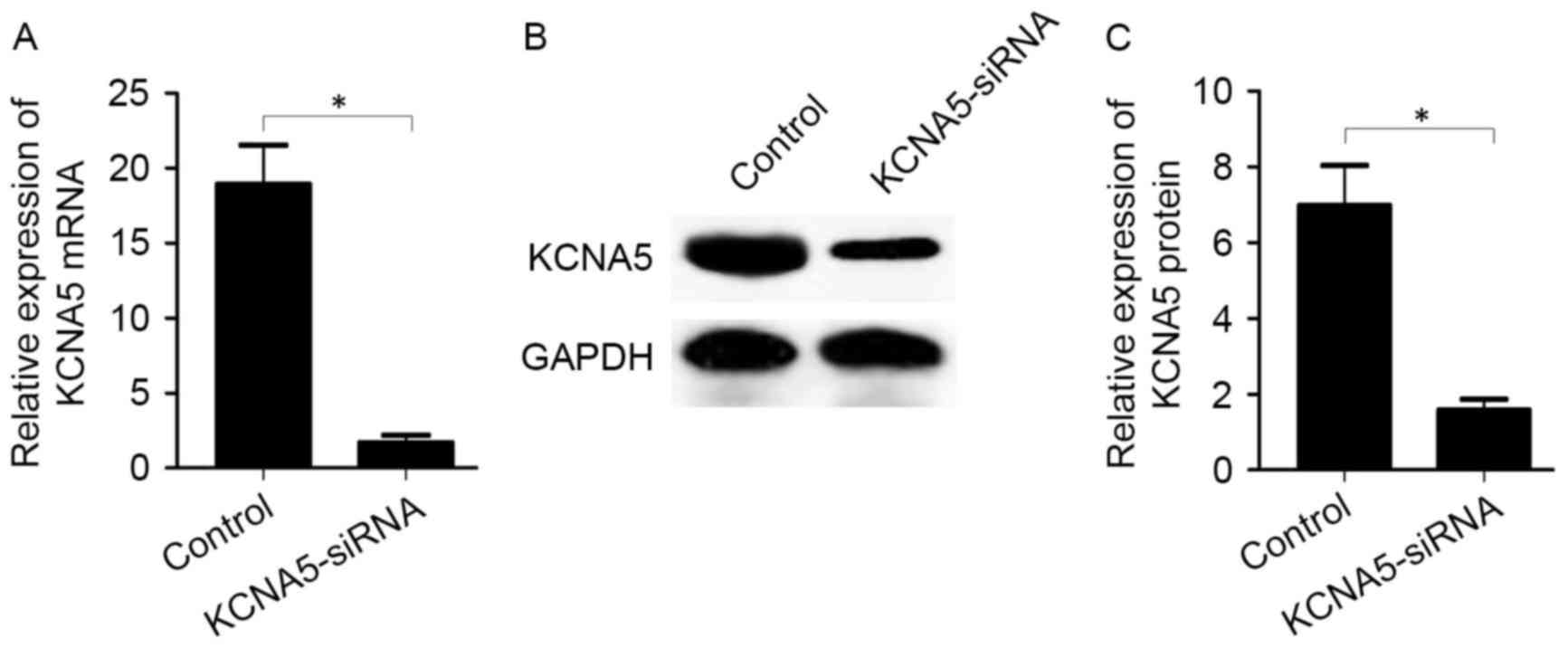
was validated by RT-qPCR and western blot analysis. It was observed
that KCNA5-siRNA transfection significantly reduced the expression
of KCNA5 mRNA (1.69±0.87 vs. 18.90±4.56, P<0.05; Fig. 5A) and protein (1.58±0.50 vs.
6.98±1.82, P<0.05; Fig. 5B and C)
compared with the control group.
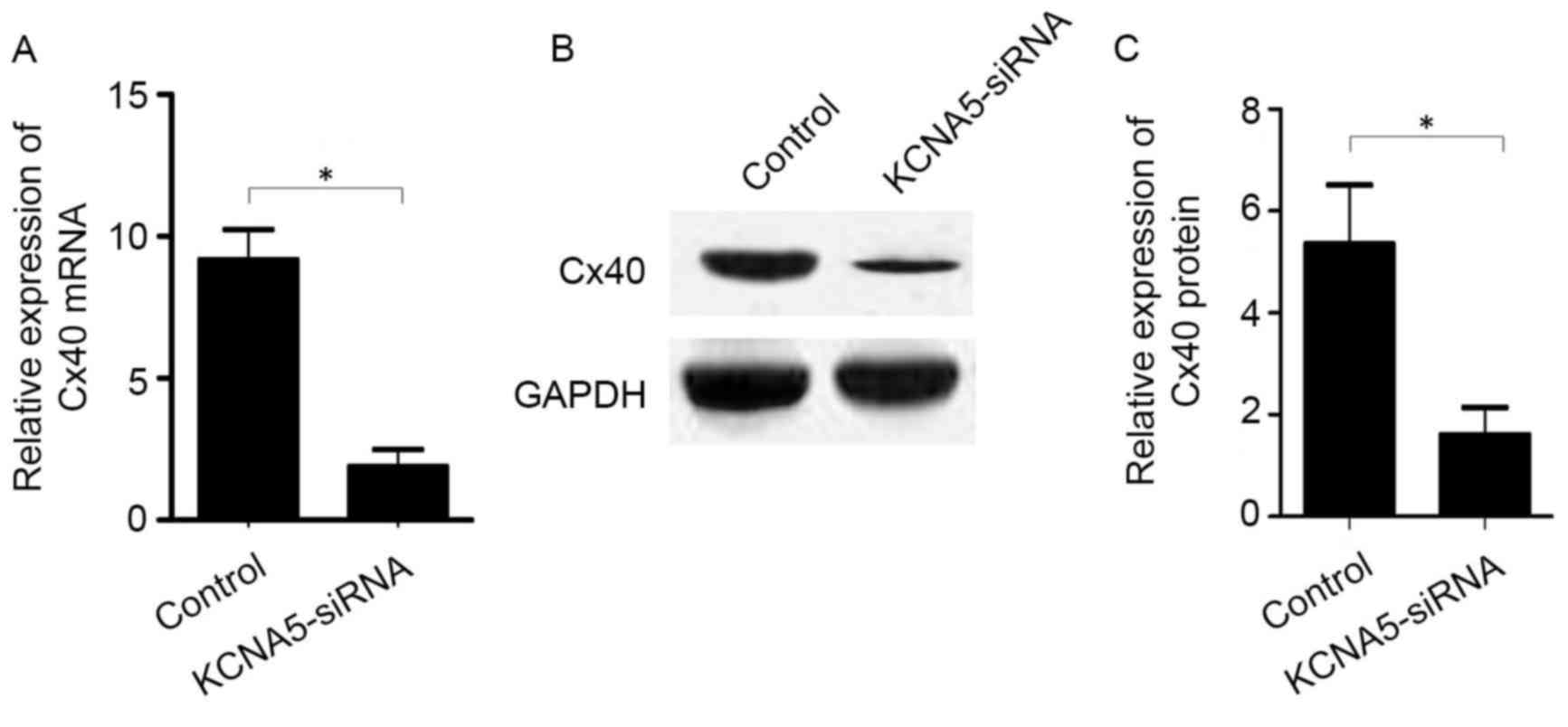
In the atrial myocytes transfected with KCNA5-siRNA,
levels of Cx40 mRNA (P<0.05; Fig.
6A) and protein (P<0.05; Fig. 6B
and C) were significantly decreased compared with the control
cells.
Discussion
AF is the most prevalent type of arrhythmia,
although it's the molecular mechanisms underlying its pathogenesis
are not well understood. Compared with rheumatic heart disease, AF
presents with more severe clinical symptoms, including a markedly
lower left ventricular ejection fraction (24). In the present study, levels of Cx40
and KCNA5 expression in atrial myocytes from patients with AF were
evaluated. It was observed that Cx40 and KCNA5 were significantly
downregulated in the atrial myocytes of patients with AF when
compared with those in patients with rheumatic heart disease.
Similarly, previous results have indicated that Cx40 expression is
reduced during AF, and that the distribution of Cx40 may be altered
in AF (25). These data suggest that
Cx40 expression is altered in AF. Furthermore, reduced expression
of KCNA5 has been observed in AF (26). Cx40 and KCNA4 are considered to
regulate structural and electrical remodeling during AF (11,12);
however, the association between the expression of Cx40 and KCNA4
is remains unclear.
AF may lead to long-term structural remodeling
within atrial myocytes and the myocardial interstitium (27). Structural remodeling typically occurs
in the left atrium (28), and is
considered to worsen the pathological changes, including
disregulated cellular energy balance and an increased inflammatory
response (28). Within the
myocardium, gap junctions provide cytoplasmic continuity between
myocytes (29). A number of
transmembrane proteins within gap junctions belong to the Cx
protein family, including Cx40, 43 and 45 (30). In AF, structural remodeling
associated with Cx proteins may alter the composition of gap
junctions and distribution of connective fibers (31). Notably, aberrant distribution of Cx40
protein has been documented in AF, and mutant Cx40 protein has been
associated with a higher risk of AF (12).
Electrical remodeling of the atrial myocardium leads
to decrease in contractility and increases the risk of stroke
(32). Rapid atrial contraction may
also result in heart failure. Therefore, reversal of the electrical
remodeling that occurs may be a novel treatment strategy for AF.
Previous studies have documented that AF may be induced by a
shortening of action potential duration (1,2,6). Ion channels serve a key role in changes
to membrane potential. KCNA5 is a member of the potassium
voltage-gated channel family, which is considered to be the most
complex class of voltage-gated ion channel (33). KCNA5 encodes a potassium channel that
serves a role in vascular function by regulating smooth muscle
contraction, neurotransmitter release and epithelial electrolyte
transport (11).
In the present study, Cx40 and KCNA5 were
downregulated in the atrial myocytes of patients with AF. These two
genes have been associated with the worsening of symptoms and an
increased risk of morbidity in patients with AF (11,12). The
present study assessed the potential association between Cx40 and
KCNA5 expression at the transcriptional and translational levels.
In patients with AF, it was observed that mRNA and protein levels
of Cx40 and KCNA5 were significantly positively correlated,
indicating that there is an association between Cx40 and KCNA5
expression. Previous studies have indicated that structural and
electrical remodeling results in a poor prognosis for patients with
AF (34,35). However, the link between these
changes is not well understood.
The present study was the first, to the best of our
knowledge, to assess the association between structural and
electrical remodeling in AF, and observed that structural and
electrical changes to the atrial myocardium were correlated, which
was indicated by the correlation between Cx40 and KCNA5 in the
present study. During the initial stage AF, electrophysiological
changes in the atrial myocytes occur, typically within the first
few h of sustained atrial tachycardia (36,37).
Structural remodeling then occurs at a slower rate following the
initial electrophysiological changes (6). Subsequently, structural remodeling
alters the architecture of the atrial myocardium. In the present
study, downregulation of KCNA5 inhibited the expression of Cx40,
suggesting that key molecules associated with electrophysiological
changes may regulate Cx40 expression. Therefore,
electrophysiological changes may induce structural remodeling by
altering the expression of Cx40. Furthermore, it was observed that
downregulation of Cx40 reduced the expression of KCNA5, indicating
that structural remodeling may also affect electrical remodeling.
These data indicate that synergistic regulation may occur between
Cx40 and KCNA5 expression to form a network of electrical and
structural remodeling during AF.
In conclusion, the present study identified that
there was reduced expression of Cx40 and KCNA5 in the atrial
myocytes of patients with AF. A positive correlation was also
observed between the expression of Cx40 and KCNA5 at the mRNA level
and protein levels, and using siRNA transfection a potential
regulatory relationship between Cx40 and KCNA5 was identified.
These data suggest that there is an association between Cx40 and
KCNA5 expression in the atrial myocytes of patients with AF. The
present results may be useful in improving the understanding and
treatment of AF. Future studies are required to fully elucidate the
use of Cx40 and KCNA5 connection in treating AF.
Acknowledgements
The present study was supported by the Shenzhen
Scientific Research Program of the People's Republic of China
(grant nos. JCYJ20130322154529556 and 2012003).
References
|
1
|
Frustaci A, Chimenti C, Bellocci F,
Morgante E, Russo MA and Maseri A: Histological substrate of atrial
biopsies in patients with lone atrial fibrillation. Circulation.
96:1180–1184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le Heuzey JY, Breithardt G, Camm J, Crijns
H, Dorian P, Kowey PR, Merioua I, Prystowsky EN, Schwartz PJ,
Torp-Pedersen C and Weintraub W: The RecordAF study: Design,
baseline data, and profile of patients according to chosen
treatment strategy for atrial fibrillation. Am J Cardiol.
105:687–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Z and Hu D: An epidemiological study
on the prevalence of atrial fibrillation in the Chinese population
of mainland China. J Epidemiol. 18:209–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verheijck EE, van Kempen MJ, Veereschild
M, Lurvink J, Jongsma HJ and Bouman LN: Electrophysiological
features of the mouse sinoatrial node in relation to connexin
distribution. Cardiovasc Res. 52:40–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jalife J, Morley GE and Vaidya D:
Connexins and impulse propagation in the mouse heart. J Cardiovasc
Electrophysiol. 10:1649–1663. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allessie M, Ausma J and Schotten U:
Electrical, contractile and structural remodeling during atrial
fibrillation. Cardiovasc Res. 54:230–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar NM and Gilula NB: The gap junction
communication channel. Cell. 84:381–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goodenough DA and Paul DL: Beyond the gap:
Functions of unpaired connexon channels. Nat Rev Mol Cell Biol.
4:285–294. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schram G, Pourrier M, Melnyk P and Nattel
S: Differential distribution of cardiac ion channel expression as a
basis for regional specialization in electrical function. Circ Res.
90:939–950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brundel BJ, Van Gelder IC, Henning RH,
Tuinenburg AE, Deelman LE, Tieleman RG, Grandjean JG, van Gilst WH
and Crijns HJ: Gene expression of proteins influencing the calcium
homeostasis in patients with persistent and paroxysmal atrial
fibrillation. Cardiovasc Res. 42:443–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olson TM, Alekseev AE, Liu XK, Park S,
Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A and
Terzic A: Kv1.5 channelopathy due to KCNA5 loss-of-function
mutation causes human atrial fibrillation. Hum Mol Genet.
15:2185–2191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gollob MH, Jones DL, Krahn AD, Danis L,
Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AF, et al:
Somatic mutations in the connexin 40 gene (GJA5) in atrial
fibrillation. N Engl J Med. 354:2677–2688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia M, Jin Q, Bendahhou S, He Y, Larroque
MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, et al: A Kir2.1
gain-of-function mutation underlies familial atrial fibrillation.
Biochem Biophys Res Commun. 332:1012–1019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Li J, Lin X, Yang Y, Hong K, Wang
L, Liu J, Li L, Yan D, Liang D, et al: Novel KCNA5 loss-of-function
mutations responsible for atrial fibrillation. J Hum Genet.
54:277–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christophersen IE, Olesen MS, Liang B,
Andersen MN, Larsen AP, Nielsen JB, Haunsø S, Olesen SP, Tveit A,
Svendsen JH and Schmitt N: Genetic variation in KCNA5: Impact on
the atrial-specific potassium current IKur in patients with lone
atrial fibrillation. Eur Heart J. 34:1517–1525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wickenden AD: K(+) channels as therapeutic
drug targets. Pharmacol Ther. 94:157–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wulff H, Castle NA and Pardo LA:
Voltage-gated potassium channels as therapeutic targets. Nat Rev
Drug Discov. 8:982–1001. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
King TJ and Bertram JS: Connexins as
targets for cancer chemoprevention and chemotherapy. Biochim
Biophys Acta. 1719:146–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravens U, Poulet C, Wettwer E and Knaut M:
Atrial selectivity of antiarrhythmic drugs. J Physiol.
591:4087–4097. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Losa D, Chanson M and Crespin S: Connexins
as therapeutic targets in lung disease. Expert Opin Ther Targets.
15:989–1002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bronis K, Metaxa S, Koulouris S and
Manolis AS: Vernakalant: Review of a novel atrial selective
antiarrhythmic agent and its place in current treatment of atrial
fibrillation. Hosp Chron. 7:171–181. 2012.
|
|
22
|
Roy D, Pratt CM, Torp-Pedersen C, Wyse DG,
Toft E, Juul-Moller S, Nielsen T, Rasmussen SL, Stiell IG, Coutu B,
et al: Vernakalant hydrochloride for rapid conversion of atrial
fibrillation: A phase 3, randomized, placebo-controlled trial.
Circulation. 117:1518–1525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dries DL, Exner DV, Gersh BJ, Domanski MJ,
Waclawiw MA and Stevenson LW: Atrial fibrillation is associated
with an increased risk for mortality and heart failure progression
in patients with asymptomatic and symptomatic left ventricular
systolic dysfunction: a retrospective analysis of the SOLVD trials.
Studies of left ventricular dysfunction. J Am Coll Cardiol.
32:695–703. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Velden HM, Ausma J, Rook MB,
Hellemons AJ, van Veen TA, Allessie MA and Jongsma HJ: Gap
junctional remodeling in relation to stabilization of atrial
fibrillation in the goat. Cardiovasc Res. 46:476–486. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ou XH, Li ML, Liu R, Fan XR, Mao L, Fan
XH, Yang Y and Zeng XR: Remodeling of Kv1.5 channel in right atria
from Han Chinese patients with atrial fibrillation. Med Sci Monit.
21:1207–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burstein B and Nattel S: Atrial fibrosis:
Mechanisms and clinical relevance in atrial fibrillation. J Am Coll
Cardiol. 51:802–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Casaclang-Verzosa G, Gersh BJ and Tsang
TS: Structural and functional remodeling of the left atrium:
Clinical and therapeutic implications for atrial fibrillation. J Am
Coll Cardiol. 51:1–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang P, Su J and Mende U: Cross talk
between cardiac myocytes and fibroblasts: From multiscale
investigative approaches to mechanisms and functional consequences.
Am J Physiol Heart Circ Physiol. 303:H1385–H1396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Söhl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Camelliti P, Borg TK and Kohl P:
Structural and functional characterisation of cardiac fibroblasts.
Cardiovasc Res. 65:40–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carnes CA, Chung MK, Nakayama T, Nakayama
H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM,
et al: Ascorbate attenuates atrial pacing-induced peroxynitrite
formation and electrical remodeling and decreases the incidence of
postoperative atrial fibrillation. Circ Res. 89:e32–e38. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pongs O: Voltage-gated potassium channels:
From hyperexcitability to excitement. FEBS Lett. 452:31–35. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Everett TH, Li H, Mangrum JM, McRury ID,
Mitchell MA, Redick JA and Haines DE: Electrical, morphological,
and ultrastructural remodeling and reverse remodeling in a canine
model of chronic atrial fibrillation. Circulation. 102:1454–1460.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benjamin EJ, Wolf PA, D'Agostino RB,
Silbershatz H, Kannel WB and Levy D: Impact of atrial fibrillation
on the risk of death the Framingham heart study. Circulation.
98:946–952. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Allessie MA, Boyden PA, Camm AJ, Kléber
AG, Lab MJ, Legato MJ, Rosen MR, Schwartz PJ, Spooner PM, Van
Wagoner DR and Waldo AL: Pathophysiology and prevention of atrial
fibrillation. Circulation. 103:769–777. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morillo CA, Klein GJ, Jones DL and
Guiraudon CM: Chronic rapid atrial pacing. Structural, functional,
and electrophysiological characteristics of a new model of
sustained atrial fibrillation. Circulation. 91:1588–1595. 1995.
View Article : Google Scholar : PubMed/NCBI
|