Introduction
The incidence of colorectal cancer (CRC) varies
worldwide, with the incidence of CRC being higher in North America,
Australia, northern and western Europe compared with other regions.
CRC is less prevalent in developing countries, particularly in
Africa and Asia (1). Over 90% of CRC
cases occur in people ≥50 years of age (2). Nevertheless, CRC incidence appears to
be increasing amongst the younger population and the incidence of
CRC in individuals of ≤40 years of age ranges from 1.6 to 23%
(3,4). The lowest incidences of CRC are in Asia
and countries in the Middle East, and the highest incidences of CRC
are in Europe and the United States. The incidence of CRC has not
been established in north Africa. Therefore, in order to improve
the survival rate in patients with CRC, there is an urgent
requirement to identify putative diagnostic markers, prognostic
factors and treatment strategies.
Through the activation of the Ras homolog gene
family member A (RhoA) by guanine nucleotide exchange factor (GEF)
and epithelial cell transforming 2 (Ect2), the central spindle
stimulates contractile ring formation (5). The central spindle complex is composed
of a heterotetramer of mitotic kinesin-like protein 1 and
MgcRacGAP/Cyk-4 (6,7) and helps to form the central spindle
during anaphase. Ect2 binds to Cyk-4 via N-terminal breast cancer 1
C-terminus domains, which recruits Ect2 to the central spindle
(8–10). The GEF activity of Ect2 is mediated
by the conserved Dbl homology (DH) and pleckstrin homology (PH)
domains in the C-terminus (11). The
DH domain catalyzes nucleotide exchange on RhoA and the PH domain
is involved in cortical localization of Ect2, although the
molecular function, including phospholipid or protein interactions,
is not known (11–14). In metaphase, cyclin dependent kinase
1 phosphorylation induces a conformational change in Ect2, which
prevents Cyk-4 binding and inhibits GEF activity (9,13).
Despite the knowledge of the involvement of Ect2 in the cell cycle,
the importance of the association between Ect2 expression levels
and CRC tumor diagnosis, prognosis and clinical and pathologic
features remains unclear.
The present study analyzed the expression levels of
Ect2 in CRC and non-cancerous tissues using western blot analysis
and immunohistochemistry. Furthermore, the relationship between
Ect2 overexpression and clinicopathological features and
post-resection survival was determined. The results of the present
study demonstrated that Ect2 may be used as an independent
prognostic factor in patients with CRC and may also be used as a
therapeutic target for CRC.
Materials and methods
Ethics statement
Written informed consent was provided by all
patients enrolled in the present study. The study was approved by
the Ethics Committee of the First Affiliated Hospital of China
Medical University (Shenyang, China).
Patients
CRC and paired non-cancerous tissues were collected
from 66 patients (38 males and 28 females) who had undergone
hepatectomies for CRC at The First Affiliated Hospital of China
Medical University and 202 Hospital of People's Liberation Army
(both Shenyang, China) between July 2007 and July 2012.
Histopathological analyses were performed independently by
pathologists from both hospitals. The median age of the patients
was 53 years (range, 37–81 years). The gender, age and
clinicopathological features of patients, including tumor size,
lymph node metastasis status, tumor differentiation, complication,
number of tumors, histopathological differentiation, recurrence,
invasion status and TNM staging are summarized in Table I. TNM staging was determined using
the United Network of Organ Sharing-modified TNM staging system for
CRC (15). Tumor differentiation was
based on the World Health Organization criteria (16).
 | Table I.Relationship between Ect2 expression
levels and clinicopathological characteristics in patients with
colorectal cancer. |
Table I.
Relationship between Ect2 expression
levels and clinicopathological characteristics in patients with
colorectal cancer.
|
|
| Ect2 expression
level |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Number | High | Low | χ2 | P-value |
|---|
| Total cases | 66 | 44 | 22 |
|
|
| Age (years) |
| ≥60 | 40 | 25 | 15 | 0.793 | 0.373 |
|
<60 | 26 | 19 | 7 |
|
|
| Gender |
| Male | 37 | 27 | 10 | 1.507 | 0.220 |
|
Female | 29 | 17 | 12 |
|
|
| Tumor size (cm) |
| ≥5 | 39 | 28 | 11 | 1.128 | 0.288 |
|
<5 | 27 | 16 | 11 |
|
|
| TNM stage |
| I+II | 30 | 18 | 12 | 1.100 | 0.294 |
|
III+IV | 36 | 26 | 10 |
|
|
| Lymph node metastasis
status |
|
Positive | 38 | 28 | 10 | 1.985 | 0.159 |
|
Negative | 28 | 16 | 12 |
|
|
| Tumor
differentiation |
| WD | 27 | 19 | 8 | 0.354 | 0.838 |
| MD | 24 | 15 | 9 |
|
|
| PD | 15 | 10 | 5 |
|
|
| Complications |
| Yes | 35 | 20 | 15 | 3.041 | 0.081 |
| No | 31 | 24 | 7 |
|
|
| Number of tumors |
|
Single | 36 | 23 | 13 | 0.275 | 0.600 |
| More | 30 | 21 | 9 |
|
|
| Recurrence |
| Yes | 37 | 29 | 8 | 5.198 | 0.023 |
| No | 29 | 15 | 14 |
|
|
| Invasion |
|
Yes | 39 | 31 | 8 | 7.051 | 0.008 |
| No | 27 | 13 | 14 |
|
|
Western blot (WB) analysis
Total protein was extracted from 66 pairs of fresh
tissue samples of CRC and paired non-cancerous tissues. Cells were
lysed in pre-chilled RIPA lysis buffer (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing protease inhibitor
cocktail (Roche Diagnostics, Basel, Switzerland) for 30 min.
Samples were centrifuged at 15,000 × g for 20 min to obtain the
supernatant. Following this, protein samples (30 µg/well) were
separated by 10% SDS-PAGE and transferred onto a 0.45-mm
nitrocellulose membrane. Subsequently, the membrane was blocked at
25°C overnight with a blocking buffer (pH 7.6) containing 5%
non-fat milk prior to incubation with primary rabbit anti-human
Ect2 polyclonal antibody (1:300, according to the instruction of
the reagent) for 2 h at room temperature. This antibody was
synthesized in the Central Laboratory of the First Affiliated
Hospital of China Medical University, with approval from the Ethics
Committee of the First Affiliated Hospital of China Medical
University and specifically recognizes an epitope in the N-terminus
of Ect2 (17). The membrane was
washed with TBST three times, for 5 min each. Mouse anti-human
β-actin antibody (1:200, 2 h at room temperature; sc-69879; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) was used as an internal
control. An incubation with secondary antibody (1:5,000, 1 h at
room temperature; ZB-2305, ZSGB-Bio Company, Beijing, China) was
performed immediately after the TBST washing. Again, the membrane
was washed with TBST three times, for 5 min each. Immunoreactive
proteins were stained using a chemiluminescence detection system
(Immunodetection System, T1046; Pierce; Thermo Fisher Scientific,
Inc.).
Semi-quantitative assessment and
scoring
Ect2-expressing samples were scored
semi-quantitatively based on the number of positively stained cells
and the staining intensity. Samples were considered positive for
Ect2 if the nucleus or cytoplasm of the sample cells had positive
staining. The percent positivity (positively stained cells out of
total cells in the microscopic visual field) was defined as 0 if
0%, 1 if 1–10%, 2 if 11–50%, 3 if 51–80 and 4 if >80% of cells
were stained. The staining intensity was scored as 3 (markedly
stained), 2 (moderately stained), 1 (weakly stained) or 0 (no
staining). Staining percentage and intensity were assessed by two
examiners in a double-blind manner. The Ect2 expression status
(scored from 0–12, by calculation of positivity percentage
multiplied by staining intensity score) was determined from the
combined results of the percentage positivity and staining
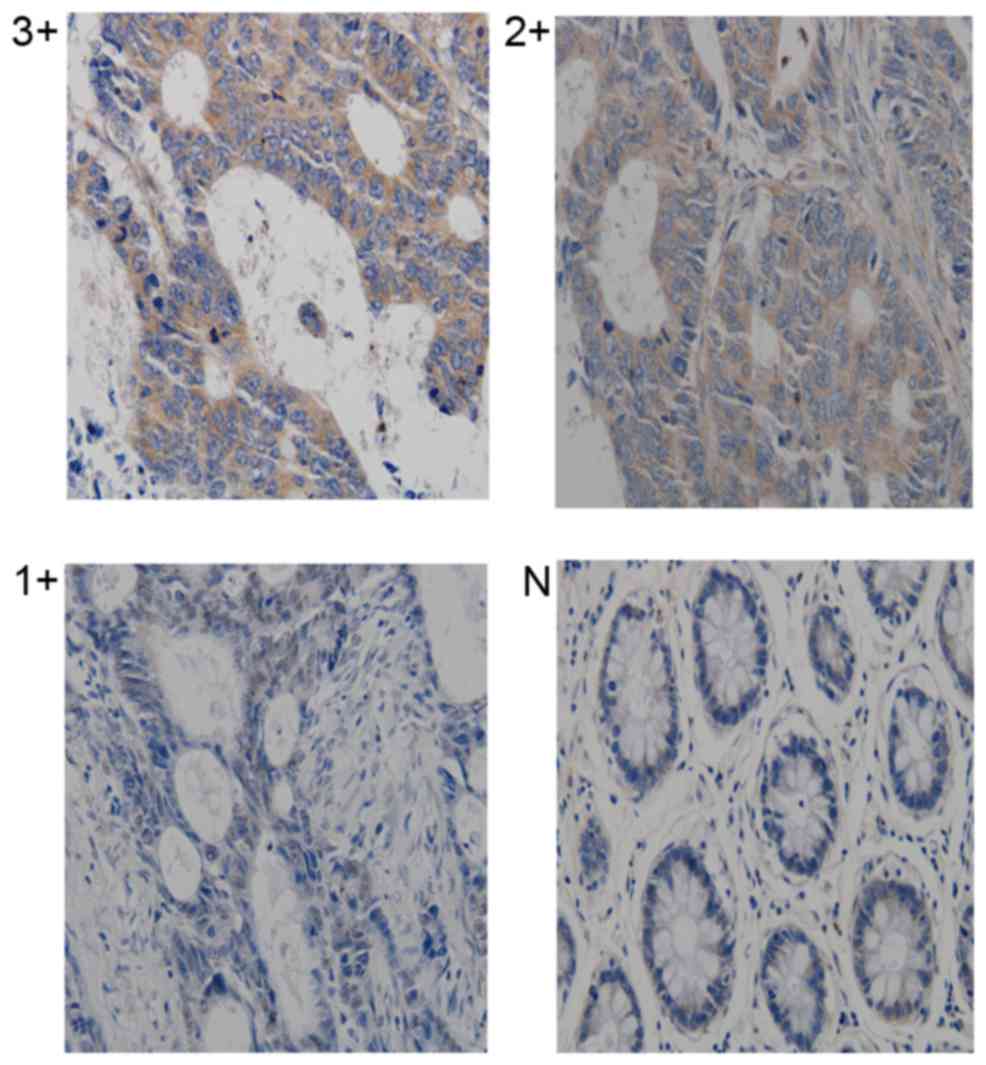
intensity scores, as follows: A score of 0 was considered a
negative sample; a score of 1–4 was considered to indicate low
expression (1+); a score of 5–8 was considered to indicate moderate
expression (2+); and a score of 9–12 was considered to indicate
strong expression (3+) (18). The
immunohistochemical results of Ect2 were divided into two groups
(low expression, 0–1; high expression, 2+).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen CRC and paired
non-cancerous tissue samples with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Reverse transcription (cat. no.
RR037A, PrimeScript RT reagent kit, Takara Biotechnology, Co.,
Ltd., Dalian, China) was performed using 500 µg total RNA from each
sample. qPCR was performed using a SYBR Green PCR master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and a Rotor
Gene 6000 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR cycling conditions were as follows: 30
sec at 95°C; followed by two-step PCR for 40 cycles of 95°C for 5
sec, 60°C for 60 sec and 85°C for 5 sec. Each reaction mixture
contained 2 µl cDNA sample, 1 µl of each of the forward and reverse
primers (Applied Biosystems; Thermo Fisher Scientific, Inc.), 8.5
µl RNase-free H2O and 12.5 µl SYBR Green, amounting to a
total reaction volume of 25 µl. β-actin was used as a reference for
gene normalization. The sequences of the primers used were as
follows: Ect2 forward, 5′-TCCTCCGGGTGGACCAGAG-3′, Ect2 reverse,
5′-CTGGCTTCATAATTGGAGTGC-3′; and β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′, β-actin reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′. The relative levels of gene
expression were determined using the 2−ΔΔCq method
(19). Experiments were repeated
three times.
Immunohistochemistry (IHC)
Ect2 expression was examined using
immunohistochemistry on paraffin-embedded samples from 66 patients
with CRC. Tumor samples were fixed in 10% formalin prior to
embedding the samples in paraffin. Following this, the embedded
samples were cut into 5-µm consecutive sections. After general
deparaffinization, antigen retrieval was implemented with an
autoclave using 0.01 mol/l sodium citrate buffer (pH 6.0) for 30
sec. H2O2, (0.3%) was added to samples to
block endogenous peroxidase activity for 30 min at 37°C.
Non-specific immunoglobulin binding sites were blocked by
incubating the samples with normal goat serum (ZSGB-Bio Company)
for 30 min at 37°C. Following this, sections were incubated at 4°C
overnight with a purified primary Ect2 rabbit polyclonal antibody
(1:150; NB100-74663; Novus Biologicals, LLC, Littleton, CO, USA).
After three 5-min washes with phosphate buffered saline, a
secondary biotinylated anti-rabbit immunoglobulin G (IgG) (NC-100,
1:300) or anti-mouse IgG antibody (NC-1390, 1:400) (both Fuzhou
MaiXin Biotech Co., Ltd., Fuzhou, China) was applied to the
sections for 30 min at room temperature. After washing with PBS
three times for 5 min each, streptavidin-biotin conjugated with
horseradish peroxidase (Reagent C of SP-9000, ZSGB-Bio Company) was
applied to the sections for 30 min at room temperature, and the
slides were colored with 3,3′-diaminobenzidine tetrahydrochloride.
The sections were then stained with Meyer's hematoxylin. Normal
rabbit/mouse IgG was used as the primary antibody at the same
dilution as a negative control.
Follow-up
A total of 66 patients were available for follow-up,
with follow-up time ranging from 3–53 months (median, 26 months)
after experiment initiation. On 31st May 2012 (the census date), 45
(68.18%) patients were alive and 21 (38.12%) patients had succumbed
to their disease.
Statistical analysis
SPSS v.17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses. The relationships between
tumor markers and other parameters were evaluated using t test and
a χ2 test. Patient survival was analyzed using the
Kaplan-Meier method and the log-rank test was used to analyze
survival differences. A Cox regression model was used for
univariate and multivariate analysis of prognostic parameters.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of Ect2 mRNA in
clinical tissue specimens
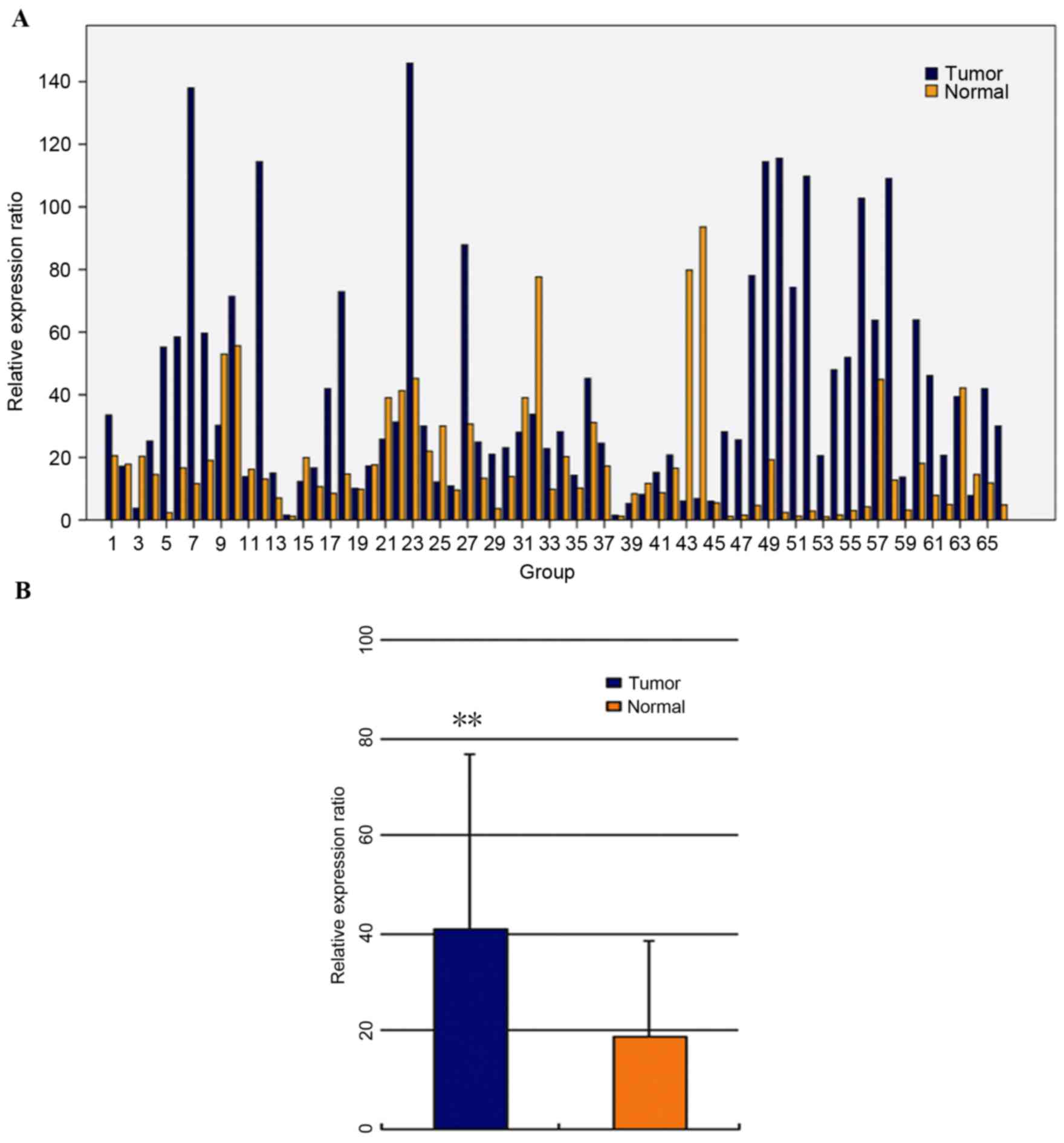
Ect2 mRNA expression levels in clinical samples were
determined using RT-qPCR. Of the 66 patients, 44 (66.67%)
demonstrated a higher level of Ect2 mRNA in CRC tissue than in the
paired non-cancerous tissue (Fig.
1). The mean level of Ect2 mRNA expression in CRC tissues (mean
± standard deviation, 41.75±35.10; standardized by β-actin gene
expression) was significantly higher compared with the level
(19.21±20.03) in the paired non-cancerous tissues (t=4.196,
P<0.01; Fig. 1).
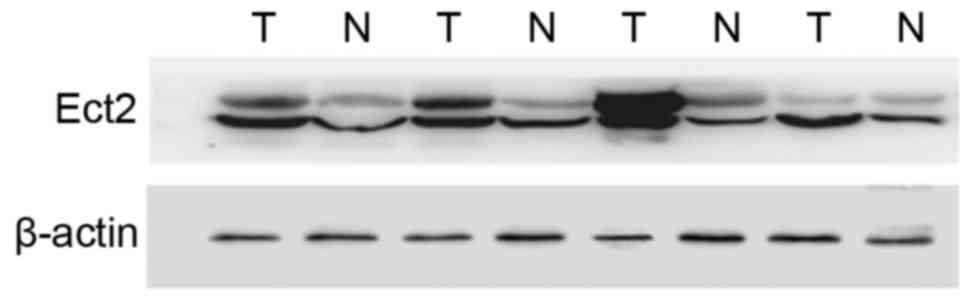
WB and IHC
WB was used to evaluate Ect2 protein expression
levels in CRC and paired non-cancerous tissues from 66 patients and
expression levels were normalized to β-actin (Fig. 2). Ect2 protein was detected by IHC in
45 CRC specimens. Overexpression of Ect2 was observed in ~two
thirds of tumor samples [29 of 45 (64.45%); 2–3+]. The remaining 16
cases (35.55%; Table I) demonstrated
low levels of Ect2 protein expression (0 to 1+). Ect2 protein
expression was observed in the nucleus and cytoplasm of tumor cells
at variable levels (Fig. 3). The
Ect2 staining scores were significantly influenced by recurrence
and vein invasion (Table I; P=0.022
and 0.008, respectively); however, other clinicopathological
factors did not significantly influence Ect2 staining scores
(Table I; P>0.05).
Influence of Ect2 expression levels on
overall survival (OS) and disease-free survival (DFS) in CRC
According to univariate analysis, gender, tumor
size, age, differentiation, histopathological type and family
history were not predictive for DFS or OS (Table II; P>0.05). Invasion and TNM
stage were significant predictors for DFS (P<0.001 and P=0.009,
respectively). Ect2, TNM stage, complication and invasion were
demonstrated to be significant independent prognostic factors for
OS (Table II; P=0.004, 0.007, 0.007
and P<0.001, respectively). Multivariate analysis demonstrated
that Ect2 expression levels [hazard ratio (HR)=1.745; 95%
confidence interval (CI), 1.121–2.574; P=0.015], complication
(HR=1.695; 95% CI, 1.079–2.525; P=0.019) and invasion (HR=2.654;
95% CI, 1.452–3.378; P<0.001) were significant independent
prognostic factors for OS in patients with CRC (Table III). Furthermore, Ect2 expression
levels (HR=1.671; 95% CI, 1.093–2.424; P=0.020), TNM stage
(HR=1.633; 95% CI, 1.216–2.284; P=0.038) and invasion (HR=2.710;
95% CI, 1.913–4.346; P<0.001) were significant independent
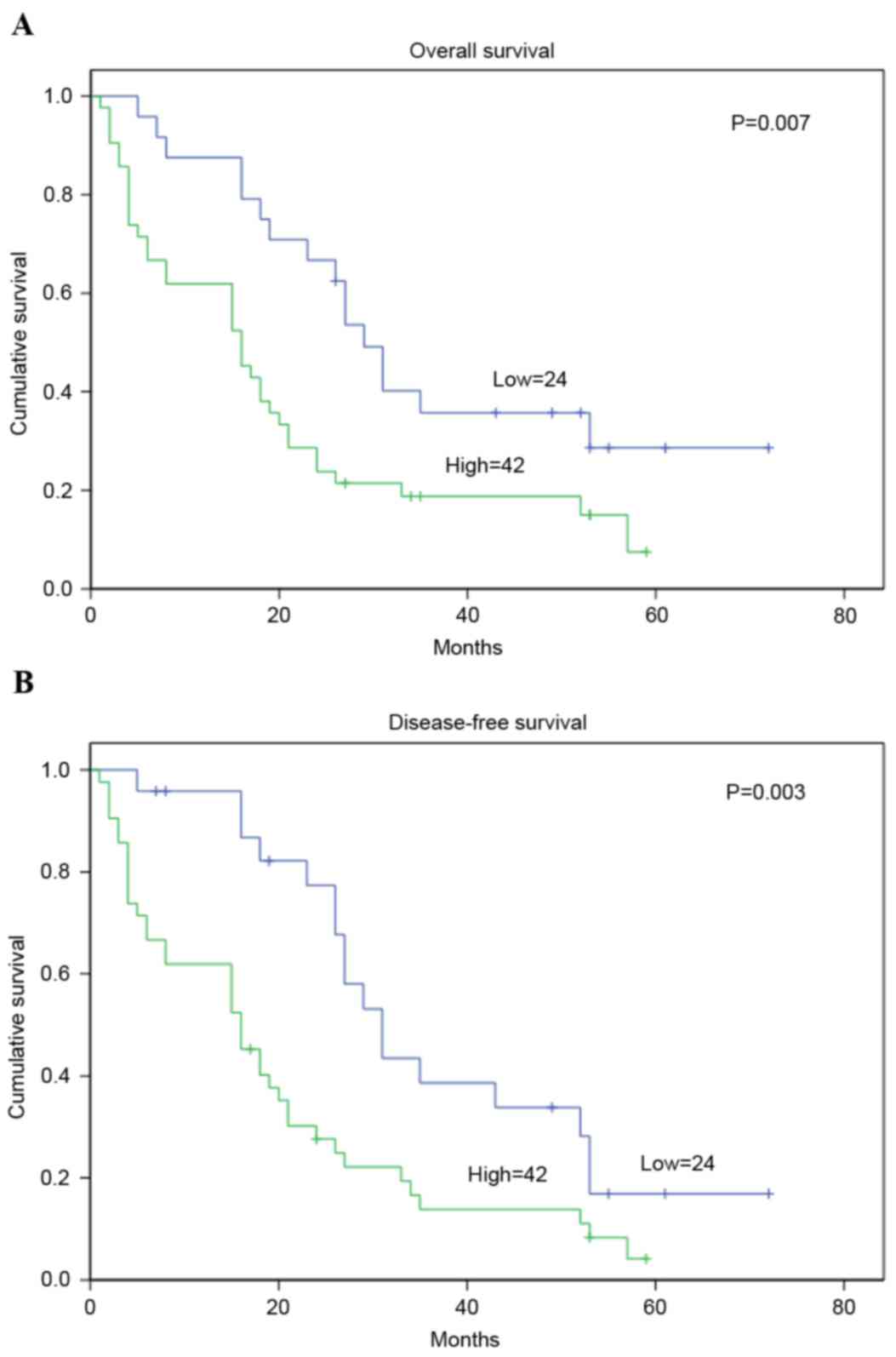
prognostic factors for DFS in patients with CRC (Table III). According to the Kaplan-Meier
method and log-rank test, CRC specimens with a higher level of Ect2
were demonstrated to have significantly shorter OS or DFS (log-rank
value=10.54 and 8.20; P=0.007 and 0.003, respectively; Fig. 4).
 | Table II.Univariate survival analyses of
individual parameters for correlations with DFS and OS. |
Table II.
Univariate survival analyses of
individual parameters for correlations with DFS and OS.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Variable | HR | CI (95%) | P-value | HR | CI (95%) | P-value |
|---|
| Ect2 | 1.823 | 1.192–2.633 | 0.004a | 1.654 | 1.085–2.374 | 0.008a |
| Age (years) | 1.142 | 0.754–1.571 | 0.875 | 1.021 | 0.700–1.424 | 0.912 |
| Gender | 1.241 | 0.734–1.762 | 0.524 | 1.101 | 0.699–1.754 | 0.722 |
| Tumor size | 1.331 | 0.899–1.995 | 0.201 | 1.241 | 0.810–1.891 | 0.207 |
| TNM stage | 1.754 | 1.275–2.531 | 0.007a | 1.568 | 1.012–2.372 | 0.009a |
| Histopathologic
differentiation | 0.935 | 0.704–1.206 | 0.701 | 0.896 | 0.712–1.304 | 1.002 |
| Family history | 1.254 | 0.703–1.507 | 0.824 | 1.145 | 0.785–1.593 | 0.513 |
| Complication | 1.721 | 1.135–2.643 | 0.007a | 1.401 | 0.937–2.081 | 0.097 |
| Invasion | 2.456 | 1.654–3.681 |
<0.001a | 3.245 | 2.112–4.897 |
<0.001a |
 | Table III.Multivariate survival analyses of
individual parameters for correlations with OS and DFS. |
Table III.
Multivariate survival analyses of
individual parameters for correlations with OS and DFS.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Variables | HR | CI (95%) | P-value | HR | CI (95%) | P-value |
|---|
| Ect2 | 1.745 | 1.121–2.574 | 0.015a | 1.671 | 1.093–2.424 | 0.020a |
| TNM stage |
|
|
| 1.633 | 1.216–2.284 | 0.038a |
| Complication | 1.695 | 1.079–2.525 | 0.019a |
|
|
|
| Invasion | 2.654 | 1.452–3.378 |
<0.001a | 2.710 | 1.913–4.346 |
<0.001a |
Discussion
Ect2, a Rho GEF, is a proto-oncogene that has
transforming ability in fibroblasts and is involved in cytokinesis
(13,20). Ect2 has been reported to be expressed
at elevated levels in various types of cancer, including esophageal
cancer, lung cancer, and glioma (21,22). A
study by Chalamalasetty et al (12) demonstrated that Ect2 is localized to
the central spindle and the cell cortex in mitotic cells. Depletion
of Ect2 impairs cleavage furrow formation and inhibits the
accumulation of RhoA and citron kinase at the cleavage furrow,
suggesting that Ect2 is essential for cytokinesis (23). A study by Hirata et al
(21) using tumor tissue microarray
analysis indicated that a high Ect2 expression levels are
associated with poor prognosis for patients with non-small cell
lung cancer. Furthermore, the study by Hirata et al
(21) also demonstrated that Ect2
knockdown by small interfering RNA was able to effectively suppress
lung cancer cell growth, suggesting a specific role for Ect2 in
lung cancer development. Interestingly, Ect2 protein expression
levels were significantly upregulated in the lungs of a murine
model of pulmonary squamous cancer, suggesting that increased Ect2
expression contributes to lung tumor development (24). Thus, Ect2 is an independent factor
that may affect patient prognosis; however, it has not been
determined if factors of this type are prognostic of CRC. Further
studies are required to determine if Ect2 may be applied to
estimate the prognosis of other types of cancer.
In the present study, IHC revealed that there was a
significant association between elevated Ect2 expression levels and
tumor invasion and recurrence in the 66 CRC specimens examined
according to χ2 tests; however, there was no significant
relationship with other clinicopathological parameters of CRC,
including age, gender, tumor size, TNM stage, lymph node metastasis
status, tumor differentiation, complication and number of tumors.
These findings therefore suggest that Ect2 expression level is
relative to CRC invasion and recurrence, and that Ect2 may have an
important role in tumor carcinogenesis and CRC progression.
Furthermore, patients who overexpressed Ect2 had a lower DFS and OS
following surgery than those who demonstrated reduced Ect2
expression levels, according to Kaplan-Meier analysis. Multivariate
Cox regression analysis indicated that, among the variables
analyzed, elevated Ect2 expression was an independent prognostic
factor for DFS and OS, without new recurrent tumors. According to
the data obtained, the results indicated that high Ect2 expression
levels may be associated with a poor prognosis, which also suggests
that Ect2 may be a novel independent prognostic indicator in
patients with CRC. Based on the staining results, it was
demonstrated that Ect2 may be important in predicting CRC
recurrence; however, the mechanism involved is unclear. A study by
Hayashi et al (25) reported
that recurrence of CRC in patients was associated with poor
prognosis. It is possible to hypothesize that when Ect2 expression
is stable, dephosphorylation of Ect2 is inhibited, which may
promote the recurrence of CRC; however, the signal pathway in which
the coding gene has been changed is unknown.
In conclusion, the present study demonstrated that
Ect2 is overexpressed in a great proportion of CRC cases, and that
high Ect2 expression levels are associated with the poor results in
CRC post resection. Therefore, Ect2 may be applied as an
independent biomarker to examine increased risk of recurrence.
Furthermore, Ect2 overexpression has been demonstrated to be an
independent prognostic indicator in CRC and Ect2 may therefore be a
novel molecular biomarker in the diagnosis and prognosis, or a
therapeutic target, of this deadly disease.
Acknowledgments
The present study was supported by the National
Natural Science Foundation Project (grant no. 30972939) and the
Doctoral Startup Fund of Science and Technology Agency, Liaoning
Province (grant no. 20061038).
References
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: GLOBOCAN 2008, Cancer incidence and mortality worldwide. IARC
cancer base No. 10International Agency for Research on Cancer.
Lyon: 2010 http://www.iarc.fr/en/media-centre/iarcnews/2010/globocan2008.phpAccessed.
January 5–2010, View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ries LAG, Melbert D, Krapcho M, Stinchcomb
DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ,
Altekruse SF, et al: SEER cancer statistics review, 1975–2005. 2008
National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2005/Accessed.
April 17–2008
|
|
3
|
Chung YF, Eu KW, Machin D, Ho JM, Nyam DC,
Leong AF, Ho YH and Seow-Choen F: Young age is not a poor
prognostic marker in colorectal cancer. Br J Surg. 85:1255–1259.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cusack JC, Giacco GG, Cleary K, Davidson
BS, Izzo F, Skibber J, Yen J and Curley SA: Survival factors in 186
patients younger than 40 years old with colorectal adenocarcinoma.
J Am Coll Surg. 183:105–112. 1996.PubMed/NCBI
|
|
5
|
Piekny A, Werner M and Glotzer M:
Cytokinesis: Welcome to the Rho zone. Trends Cell Biol. 15:651–658.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishima M, Kaitna S and Glotzer M: Central
spindle assembly and cytokinesis require a kinesin-like
protein/RhoGAP complex with microtubule bundling activity. Dev
Cell. 2:41–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mishima M, Pavicic V, Grüneberg U, Nigg EA
and Glotzer M: Cell cycle regulation of central spindle assembly.
Nature. 430:908–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Somers WG and Saint R: A RhoGEF and Rho
family GTPase-activating protein complex links the contractile ring
to cortical microtubules at the onset of cytokinesis. Dev Cell.
4:29–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuce O, Piekny A and Glotzer M: An
ECT2-centralspindlin complex regulates the localization and
function of RhoA. J Cell Biol. 170:571–582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao WM and Fang G: MgcRacGAP controls the
assembly of the contractile ring and the initiation of cytokinesis.
Proc Natl Acad Sci USA. 102:13158–13163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solski PA, Wilder RS, Rossman KL, Sondek
J, Cox AD, Campbell SL and Der CJ: Requirement for C-terminal
sequences in regulation of Ect2 guanine nucleotide exchange
specificity and transformation. J Biol Chem. 279:25226–25233. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chalamalasetty RB, Hümmer S, Nigg EA and
Silljé HH: Influence of human Ect2 depletion and overexpression on
cleavage furrow formation and abscission. J Cell Sci.
119:3008–3019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito S, Liu XF, Kamijo K, Raziuddin R,
Tatsumoto T, Okamoto I, Chen X, Lee CC, Lorenzi MV, Ohara N and
Miki T: Deregulation and mislocalization of the cytokinesis
regulator Ect2 activate Rho signaling pathways leading to malignant
transformation. J Biol Chem. 279:7169–7179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tatsumoto T, Xie X, Blumenthal R, Okamoto
I and Miki T: Human ECT2 is an exchange factor for Rho GTPases,
phosphorylated in G2/M phases, and involved in cytokinesis. J Cell
Biol. 147:921–928. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin J, Qiu M, Xu R and Dobs AS: Comparison
of survival and clinicopathologic features in colorectal cancer
among African American, Caucasian and Chinese patients treated in
the United States: Results from the surveillance epidemiology and
end results (SEER) database. Oncotarget;. 6:33935–33943. 2015.
View Article : Google Scholar
|
|
16
|
Kim JW, Shin MK and Kim BC:
Clinicopathologic impacts of poorly differentiated cluster-based
grading system in colorectal carcinoma. J Korean Med Sci. 30:16–23.
2015. View Article : Google Scholar
|
|
17
|
Wolfe BA, Takaki T, Petronczki M and
Glotzer M: Polo-like kinase 1 directs assembly of the HsCyk-4
RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation.
PLoS Bio. 7:e10001102009. View Article : Google Scholar
|
|
18
|
Engers R, Mueller M, Walter A, Collard JG,
Willers R and Gabbert HE: Prognostic relevance of Tiam1 protein
expression in prostate carcinomas. Br J Cancer. 95:1081–1086. 2006.
View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Miki T, Smith CL, Long JE, Eva A and
Fleming TP: Oncogene ect2 is related to regulators of small
GTP-binding proteins. Nature. 362:462–465. 1993. View Article : Google Scholar
|
|
21
|
Hirata D, Yamabuki T, Miki D, Ito T,
Tsuchiya E, Fujita M, Hosokawa M, Chayama K, Nakamura Y and Daigo
Y: Involvement of epithelial cell transforming sequence-2
oncoantigen in lung and esophageal cancer progression. Clin Cancer
Res. 15:256–266. 2009. View Article : Google Scholar
|
|
22
|
Sano M, Genkai N, Yajima N, Tsuchiya N,
Homma J, Tanaka R, Miki T and Yamanaka R: Expression level of ECT2
proto-oncogene correlates with prognosis in glioma patients. Oncol
Rep. 16:1093–1098. 2006.
|
|
23
|
Bui DA, Lee W, White AE, Harper JW,
Schackmann RC, Overholtzer M, Selfors LM and Brugge JS: Cytokinesis
involves a nontranscriptional function of the Hippo pathway
effector YAP. Sci Signal. 9:ra232016. View Article : Google Scholar
|
|
24
|
Woik N, Dietz CT, Schäker K and Kroll J:
Kelch-like ECT2-interacting protein KLEIP regulates late-stage
pulmonary maturation via Hif-2α in mice. Dis Model Mech. 7:683–692.
2014. View Article : Google Scholar
|
|
25
|
Hayashi M, Inoue Y, Komeda K, Shimizu T,
Asakuma M, Hirokawa F, Miyamoto Y, Okuda J, Takeshita A, Shibayama
Y and Tanigawa N: Clinicopathological analysis of recurrence
patterns and prognostic factors for survival after hepatectomy for
colorectal liver metastasis. BMC Surg. 10:272010. View Article : Google Scholar
|