Introduction
Commonly observed chronic cutaneous venous ulcers,
diabetic foot ulcers and pressure ulcers heal with difficulty and
cause significant morbidity for patients due to a delayed healing
process and secondary complications. These skin injuries pose a
huge financial burden upon national health systems and constitute
one of the important issues that require immediate attention
(1). At present, the clinical
understanding is insufficient and the development of novel
treatments to accelerate wound healing is required (2).
In response to full-thickness skin lesions, a
complex, multi-phase process is activated, involving inflammation,
proliferation and maturation. This eventually results in the
formation of scar tissue and leads to the proper restoration of
skin integrity and barrier function (3). In the initial stage of inflammation,
inflammatory cells participate in hemostasis and the clearing of
wound debris. This inflammatory process is primarily meditated by
factors, including platelet-derived growth factor or tumor necrosis
factor α, released from mast cells and platelets, which initiate
angiogenesis and preliminary repair within 48 h of injury (4–6). During
the subsequent proliferation stage, granulation tissue (GT)
provides the growth of a new provisional wound matrix and promotes
angiogenesis. Proliferation of epithelial progenitor cells helps
achieve re-epithelialization of the wound bed and re-establishes
the outer protective barrier (4).
Together, keratinocytes and fibroblasts stimulate each other to
release growth factors, which in turn stimulate cellular growth at
the wound site, leading to proper wound closure (3,7).
Finally, the maturation stage involves wound contraction to
decrease the size of an open wound and synthesis of collagen by
fibroblasts to re-build dermal tissue. However, this process
requires the proper alignment of the collagen bundles and
fibroblasts, which is also meditated by GT (8). Full completion of wound healing may
take months or years, requiring dermal tissue remodeling and
building of tensile strength, and in most cases, full recovery is
not achieved and permanent scars remain at wound sites.
A previous investigation indicated the participation
of hair follicle stem cells (HFSCs) in wound healing. Under normal
conditions, they are not involved in the regular turnover of
cutaneous epithelium (9). Upon
wounding, HFSCs residing in the hair follicles migrate to the
epidermis to aid in re-epithelialization (10). In this regard, the role of HFSCs in
skin re-epithelialization suggests their potential application in
an approach of exogenous stimulation of wound healing.
Prompt re-epithelialization and the closure of open
wounds are crucial to prevent excessive inflammation, scar
formation and opportunistic infection. A therapeutic approach is to
facilitate the proliferation of epithelial progenitor cells by
growth factors. A good example of such a growth factor is
platelet-derived growth factor (PDGF). Previously, a topical gel
formulation containing PDGF has been shown to be capable of
stimulating epithelial cell proliferation and accelerating wound
healing (11). Such growth factor
therapy has been used in the clinic to treat patients with
diabetes-associated skin ulcers with delayed healing (12).
Pigment epithelium-derived factor (PEDF) is a
protein containing peptide domains responsible for neurotrophic and
anti-angiogenesis functions. PEDF is produced by keratinocytes in
the wound area and changes in PEDF levels have been correlated with
skin ageing and pathological injuries (13). Therefore, it has been postulated that
PEDF may have an important role in early wound repair and is
associated with tissue homeostasis. A previous study by our group
identified that a 44-amino-acid fragment of PEDF, which is known to
have a neurotrophic function, stimulates progenitor cell
proliferation and corneal epithelial wound healing (14). The study indicated that this PEDF
fragment stimulated progenitor cell proliferation within the limbal
stem cell niche and in the basal layer of healing epithelium. A
recent study by our group found that the 44-amino-acid fragment of
PEDF also promoted muscle regeneration by stimulating the
proliferation of muscle stem cells and satellite cells (15). These finding suggested that PEDF
fragments may also be able to facilitate skin wound healing. In the
present study, the previously used 44-amino-acid peptide was
shortened to 18-, 20- and 29-amino-acid peptides and their effect
in promoting the healing of full-thickness skin wounds in mice and
the proliferation of human follicle stem cells was assessed. It was
revealed that the peptide fragments not only promoted
re-epithelization of skin wounds but also increased the local
accumulation of dermal collagen.
Materials and methods
Materials
HEPES-buffered Dulbecco's modified Eagle's medium
(DMEM), Ham's/F12 medium, trypsin-EDTA, fetal bovine serum (FBS),
bovine pituitary extract, antibiotic-antimycotic solutions and
trypsin were from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Masson's trichrome stain, dimethyl sulfoxide
(DMSO), mitomycin C (MMC), 5-bromo-2′-deoxyuridine (BrdU),
insulin-transferrin-sodium selenite (ITSE) media supplement and
Hoechst 33258 dye were all from Sigma-Aldrich (Merck-Millipore,
Darmstadt, Germany). Collagenase A and dispase II were obtained
from Roche (Indianapolis, IN, USA). Antibodies against leucine-rich
repeat-containing G protein-coupled receptor 6 (Lgr6; cat. no.
sc-99123; Santa Cruz Biotechnology, Dallas, TX, USA) and BrdU (cat.
no. GTX42641; GeneTex, San Antonio, TX, USA) were used as primary
antibodies. Rhodamine-conjugated donkey anti-mouse immunoglobulin
(Ig)G (cat. no. AP192R; EMD Millipore, Billerica, MA, USA) and
fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG
were used as secondary antibodies (cat. no. 406403; BioLegend, San
Diego, CA, USA). Human plasma was obtained from two of the authors
of the current study (YPT and TCH). PEDF was purified from this
human plasma via collagen I-sepharose resin, as described
previously (16) and preserved in a
buffer composed of 20 mM sodium phosphate, pH 6.4, 0.2 M sodium
chloride and 1 mM dithiothreitol. Short synthetic PEDF peptides,
including the 29-mer (residues Ser93-Thr121), 20-mer (Ser93-Leu112)
and 18-mer (Glu97-Ser114) were synthesized, modified by acetylation
at the NH2 termini and amidation at the COOH termini for
stability, and characterized by mass spectrometry (>90% purity),
to order by GenScript (Piscataway, NJ, USA).
Analysis of skin wound healing
Experimental procedures were approved by the Mackay
Memorial Hospital Review Board (Taipei, Taiwan) and performed
according to national animal welfare regulations (Council of
Agriculture, R.O.C). To perform full-thickness skin excision, 64
8–10 week-old female C57BL/6 mice (20±2 g) were purchased from
BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan) and housed in a 25°C
temperature-controlled room with a 12-h light/dark cycle. Mice had
ad libitum access to water and pelleted standard laboratory
chow (BioLASCO Taiwan Co., Ltd.). Mice were anesthetized by an
intraperitoneal injection of a mixture of zoletil (6 mg/kg; Virbac
Laboratories, Carros Cedex, France) and Rompun® (3
mg/kg; Rompun Bayer Korea Ltd., Seoul, Korea). Two full-thickness
skin excisional wounds 4 mm in diameter were made on each side of
the dorsal midline using a Sklar Tru-Punch disposable biopsy punch
(Sklar, West Chester, PA, USA). PEDF-derived short peptide was
reconstituted in DMSO as stock (5 mM) and mixed with TOBREX eye
ointment containing 0.3% tobramycin and 0.5% chlorobutanol (Alcon,
Fort Worth, TX, USA) to achieve a PEDF fragment concentration of 50
µM. In each treatment group, the skin wound was treated with 25 µl
of this ointment topically (containing 0.25 µl DMSO as vehicle)
once daily after scraping of the skin. In the vehicle control
group, the ointment only contained an equal volume of DMSO. Mice
were housed individually during the healing period.
The dimensions of the wound areas were quantified
from images captured using a computer-assisted image analyzer
(Adobe Photoshop CS3 10.0; Adobe Systems, San Jose, CA, USA) and
the percentage of residual epithelial defect at each time-point vs.
the initial wound area was calculated.
Prior to histological analysis, mice (n=8 per time
point and 2 wounds per mouse) were euthanized by CO2
(flow rate: 2 l/min; final CO2 concentration >70%;
euthanasia confirmed by cardiac and respiratory arrest). The
complete wound covering 2 mm of the epithelial margins was
isolated, bisected, fixed overnight in 4% paraformaldehyde in PBS
and embedded in paraffin. Sections (5 µm) from the middle of the
wound were stained using the Masson's trichrome procedure as
described by the manufacturer, in which the epithelium was stained
red and connective tissue was stained blue. Images were captured
using a Nikon Eclipse 80i microscope (Nikon Corporation, Tokyo,
Japan) equipped with a Leica DC 500 camera (Leica Microsystems,
Wetzlar, Germany).
To measure cell proliferation, BrdU (Sigma-Aldrich;
Merck Millipore) was used after reconstitution in DMSO at (80 mM).
Of this BrdU stock, 10 µl was mixed with 90 µl PBS and injected
intraperitoneally into the mice at 6 h prior to euthanasia with
CO2. . DNA synthesis in proliferating cells was assessed
by measurement of BrdU incorporation.
Cultivation of human HFSCs
Human hair follicles were isolated from the hairy
scalp skin of healthy donors. Written informed consent was obtained
from all donors Human tissue was handled according to the tenets of
the Declaration of Helsinki. The protocol was approved by the
Institutional Review Board of the Mackay Memorial Hospital (Taipei,
Taiwan). The follicles were dissected with tweezers under
microscopy and the majority of adipose and connective tissue was
removed. The bulb region of the hair follicle under the sebaceous
gland was cut and bulb fragments were transferred into a 35-mm dish
containing 1 ml collagenase A (1 mg/ml), followed by incubation for
1.5 h at 37°C to digest the collagen capsule. Subsequently, the
bulb fragments were transferred into a fresh cell culture dish
containing dispase II (2.4 U/ml)/0.05% trypsin and incubated for
1.5 h at 37°C to obtain a single-cell suspension.
After mechanical dissection and enzymatic digestion,
the suspended cells isolated from five bulb fragments were seeded
into one well of a 12-well culture plate and incubated with a basal
medium supplement containing four parts calcium-free DMEM and one
part Ham's F12 medium (final calcium concentration, 0.25 mM). The
basal medium was further supplemented with 10 ng/ml human epidermal
growth factor (cat. no. AF-100-15; PeproTech EC Ltd., Rocky Hill,
NJ), 500 mg/l L-glutamine (Gibco; Thermo Fisher Scientific, Inc.),
0.2% bovine pituitary extract, 0.18 g/ml hydrocortisone (Gibco;
Thermo Fisher Scientific, Inc.), 1% ITSE as well as
antibiotic-antimycotic solution and 10% FBS to support cell growth.
The cultivation method was based on that of a previous study
(17). The medium was changed every
2–3 days. The bulb cells were co-cultured with MMC (4 µg/ml; 2
h)-treated NIH/3T3 fibroblast feeder cells (CRL-1658™;
ATCC, Manassas, VA, USA). At the third passage, near-confluent
cells were harvested with 0.25% trypsin for 5 min at 37°C for use
in the BrdU labeling assay.
BrdU labeling
Approximately 1×105 human bulb cells were
transferred to a fresh culture dish and allowed to grow for 2 days.
Subsequently, cells were cultured in a basal medium (untreated; UT)
or basal medium containing either 4.5 nM of the 44-amino-acid
fragment of PEDF or 50 nM of the 22-amino-acid fragment of PEDF for
24 h. BrdU (final concentration, 10 µM) was added to the culture
for 2 h. Following fixation with 4% paraformaldehyde at room
temperature for 2 h, cells were exposed to cold methanol at 4°C for
2 min and then treated with 1 N HCl at RT for 1 h prior to
immunofluorescence microscopy.
Immunofluorescence
Deparaffinized tissue sections or 4%
paraformaldehyde-fixed human bulb cells were blocked with 10% goat
serum (Gibco; Thermo Fisher Scientific, Inc.) and 5% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) in PBS containing
0.1% Tween-20 for 1 h. Staining was performed using primary
antibodies against Lgr6 (1:100 dilution) or BrdU (1:100 dilution)
at 37°C for 2 h, followed by incubation with the appropriate
rhodamine- or FITC-conjugated donkey IgG (1:500 dilution) for 1 h
at room temperature. Images were captured using a epifluorescence
microscope (Zeiss Axioplan 2 imaging; Zeiss, Oberkochen, Germany)
equipped with a charge-coupled device camera (Zeiss AxioCam HRm,
Zeiss) and quantification was performed using Axiovert software
(Zeiss AxioVision Release 4.8.2, Zeiss).
Statistical analysis
Data were analyzed using Microsoft Excel 2000
version 9 (Microsoft Corporation, Redmond, WA, USA) and expressed
as the mean ± standard error of the mean. One-way analysis of
variance was used for statistical comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
PEDF-derived short peptides accelerate
skin wound healing
To investigate the effects of the PEDF-derived short
peptides on skin wound healing, 4-mm punch wounds were made on the
dorsal side of C57BL/6 mice and subsequently treated with ointment
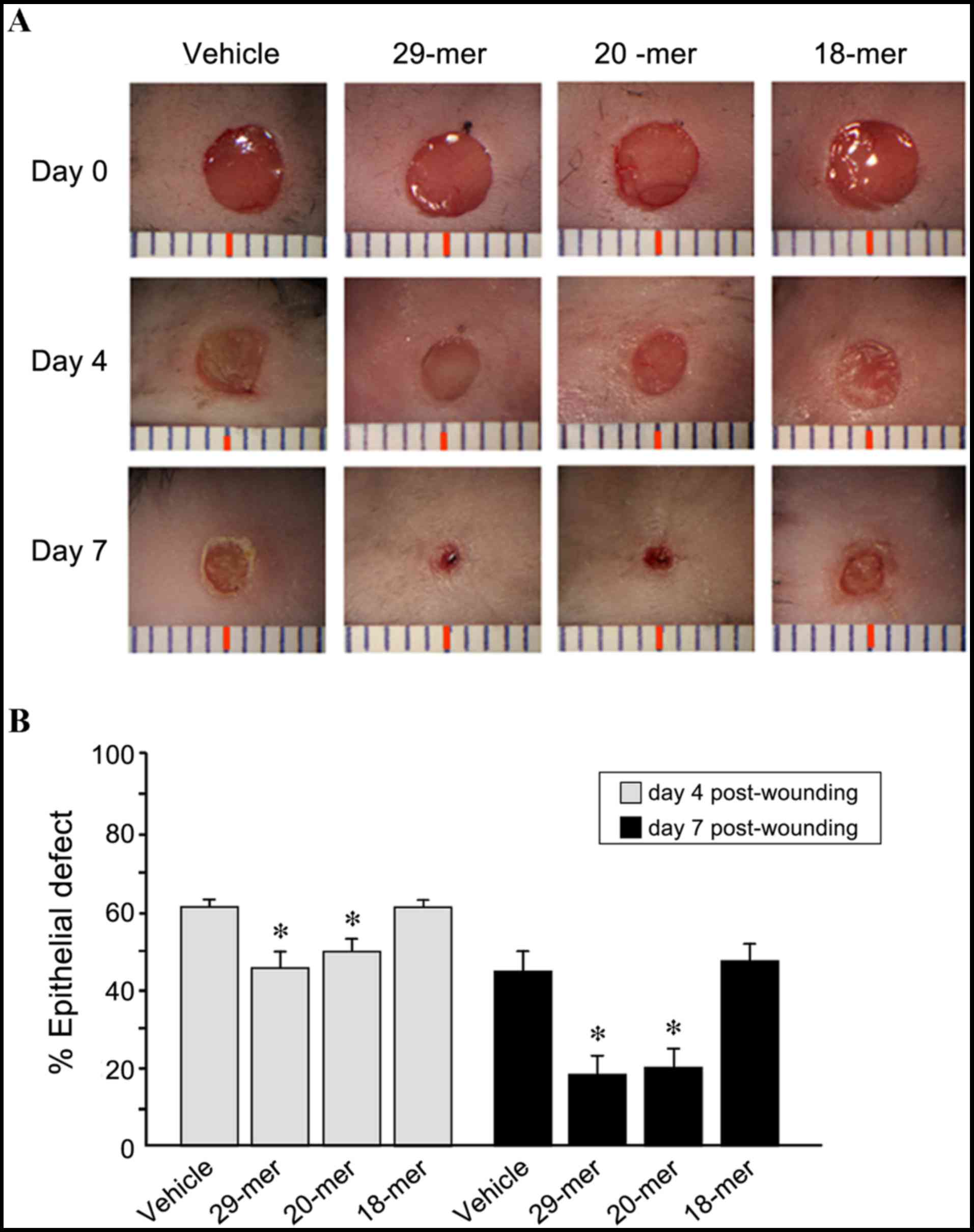
containing 29-mer, 20-mer, 18-mer or DMSO vehicle. As shown in
Fig. 1, 29- and 20-mer-treated
groups showed significantly enhanced wound healing at day 4
post-injury compared to the vehicle group (46.3±3.7 and 50.1±3.8
vs. 61.5±3.2% epidermal defect remaining). At day 7 post-injury,
skin wounds receiving 29- and 20-mer peptides showed almost
complete healing and substantially less scar tissue developed,
compared to vehicle-treated wounds (18.2±4.3 and 20.0±4.9 vs.
44.5±5.3% epidermal defect remaining). The results of the current
study indicated that control peptide (18-mer) treatment was not
able to promote wound healing. The results indicated that the 29-
and 20-mer benefit skin re-epithelialization.
Histology of partially healed wounds
treated with PEDF-derived short peptides
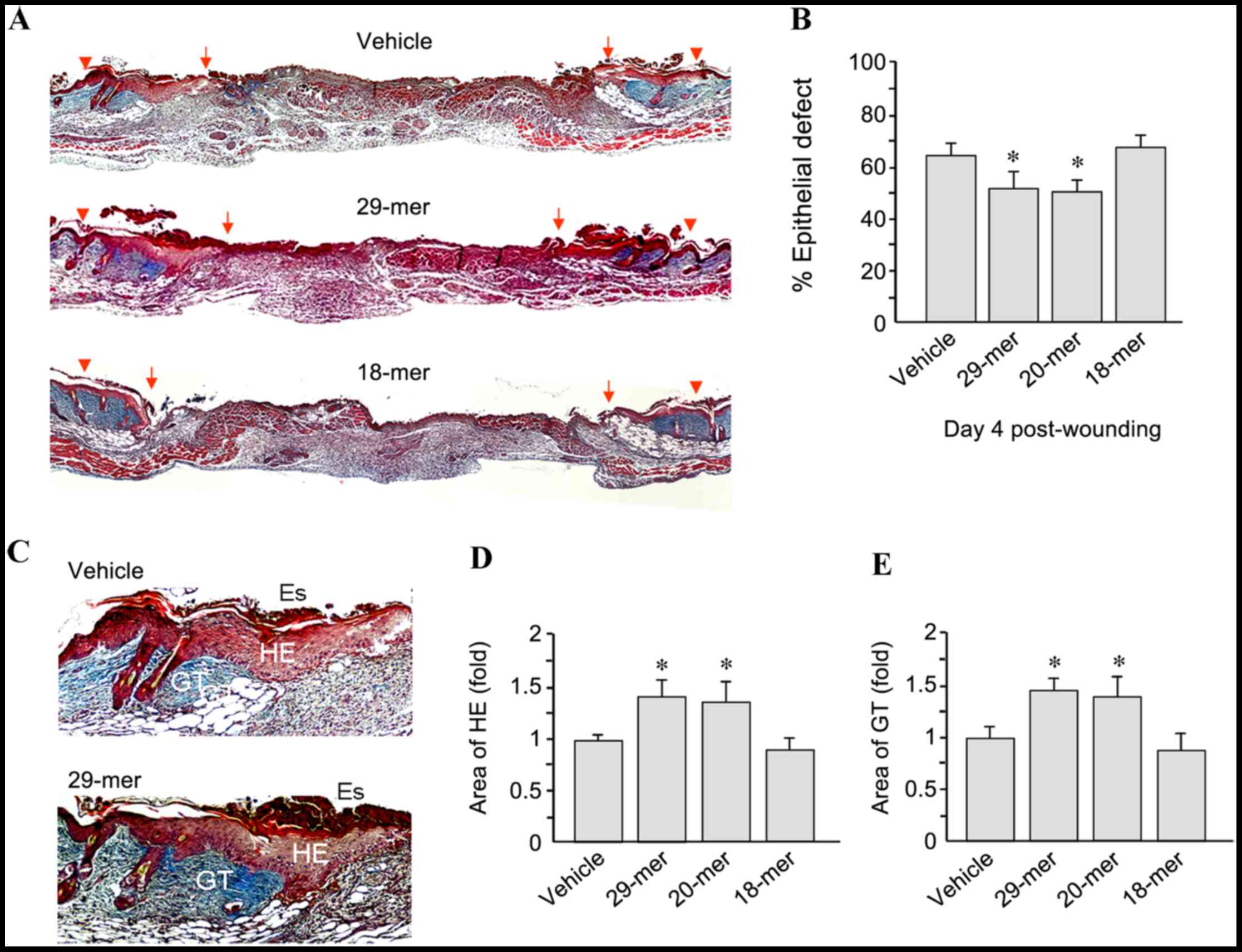
To confirm the efficacy of PEDF-derived short
peptides in skin wound repair, sections of wound tissue from day 4
after wounding were stained using Masson's trichrome. As shown in
Fig. 2A, 29-mer-treated skin wounds
showed improved wound healing than those in the vehicle-treated
group. The residual epithelial defects in the 29- and 20-mer
treated groups were 52.7±6.2 and 49.7±5.6 compared to 63.8±5.8% for
the vehicle group (Fig. 2B).
In addition, PEDF 29-mer treatment increased the
thickness of the hyperproliferative epithelium (HE) and GT at the
wound edge (Fig. 2C). Quantification
of the HE tissue area revealed that total area growth in the 29-
and 20-mer-treated groups was 1.41±0.25- and 1.32±0.21-fold higher,
respectively, than that in the vehicle-treated group (Fig. 2D). Furthermore, quantification of the
area of GT indicated that the 29- and 20-mer-treated groups had a
1.45±0.23 and 1.37±0.17-fold higher area recovery than the vehicle
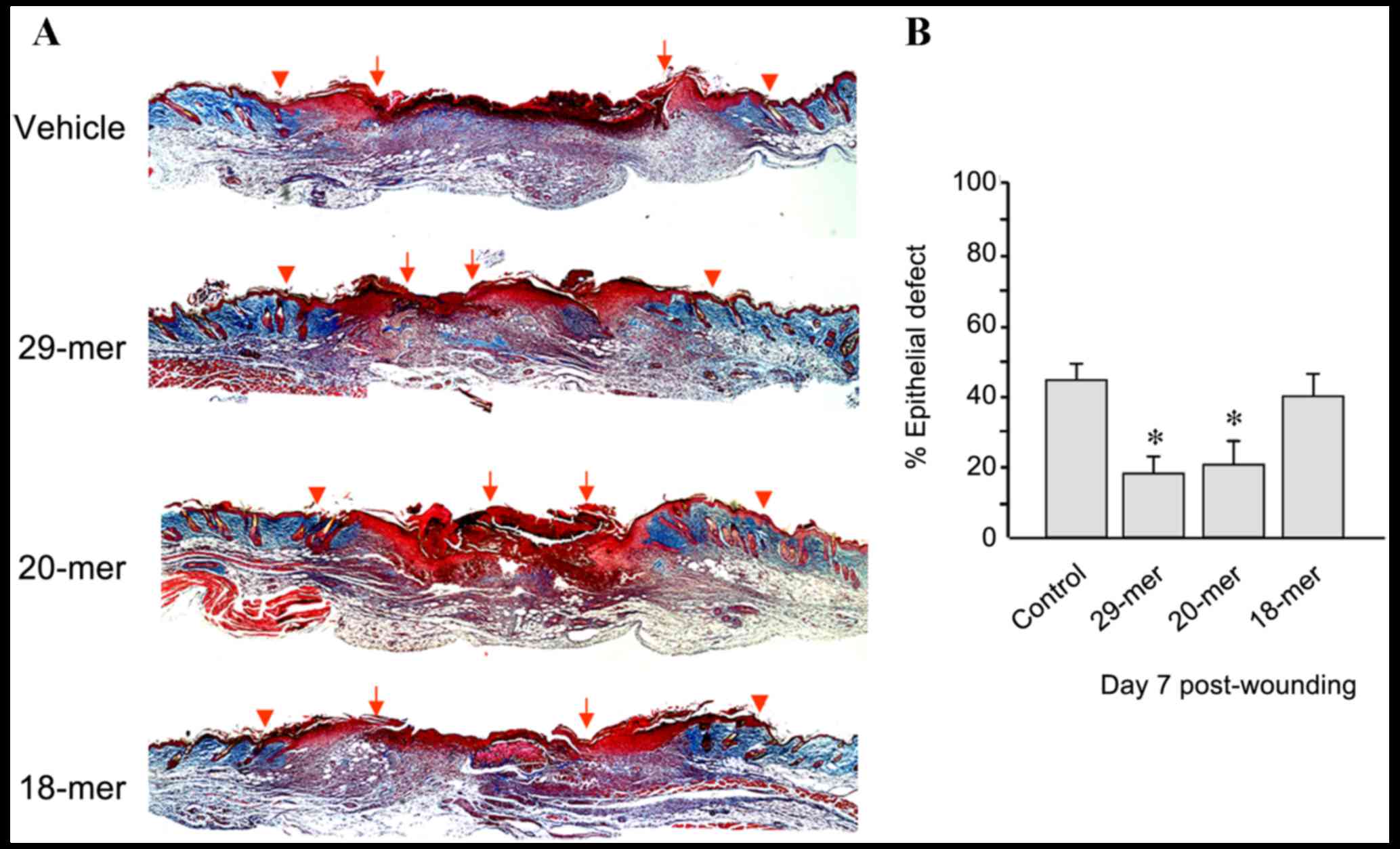
group (Fig. 2E). Skin sections from
day 7 after trauma were stained using Masson's trichrome to measure
wound closure. The 29- and 20-mer-treated wounds showed better
re-epithelialization evidenced by the smaller residual epithelial
defects compared with those of vehicle ointment-treated wounds
(17.9±3.3 and 17.2±7.3 vs. 43.5±6.5%; Fig. 3). Collectively, the 29-mer and the
20-mer increased the growth of HE and GT, resulting in the
acceleration of wound closure.
PEDF-derived short peptides accelerate
skin wound healing by promoting proliferation of the basal cells of
the HE
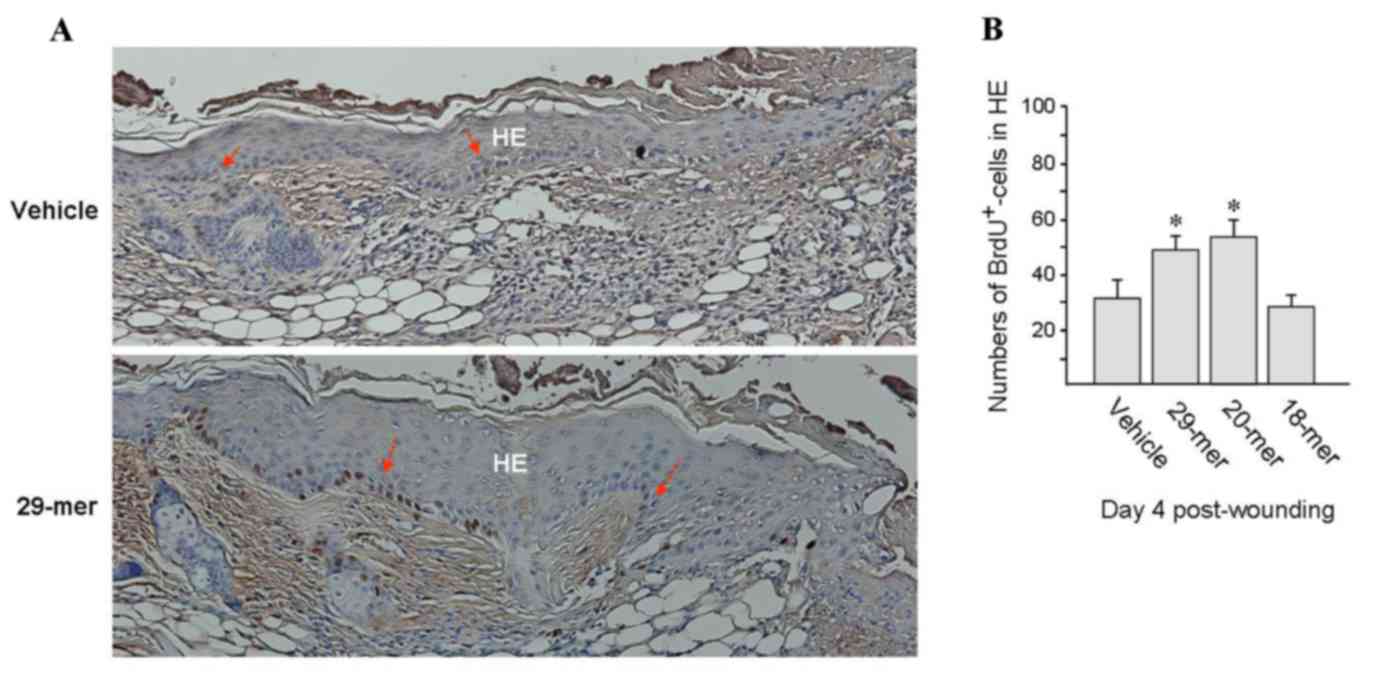
To investigate whether skin resurfacing accelerated
by treatment with PEDF-derived short peptides involves epithelial
basal cell replication, wounds were treated with PEDF-derived
peptide ointments for 4 days and the mice were then
intraperitoneally injected with BrdU and sacrification at 6 h
thereafter. Immunohistochemical analysis of skin specimens using
the anti-BrdU antibody showed that the BrdU-positive cells were are
mostly distributed in the basal layer of HE tissue and the bulb
region of hair follicles (Fig. 4A).
The numbers of BrdU-positive cells were significantly increased in
29- and 20-mer-treated wounds compared to those in vehicle-treated
wounds (47.9±5.0 and 53.1±6.5 vs. 30.8±8.1%; Fig. 4B). Noticeably, HE tissues in wounds
treated with PEDF 29-mer peptides were thicker than vehicle-treated
group (Fig. 4A), which would be
expected due to the increase of actively proliferating basal cells.
These evidences indicated that PEDF peptides stimulate epithelial
basal cell proliferation in healing wounds.
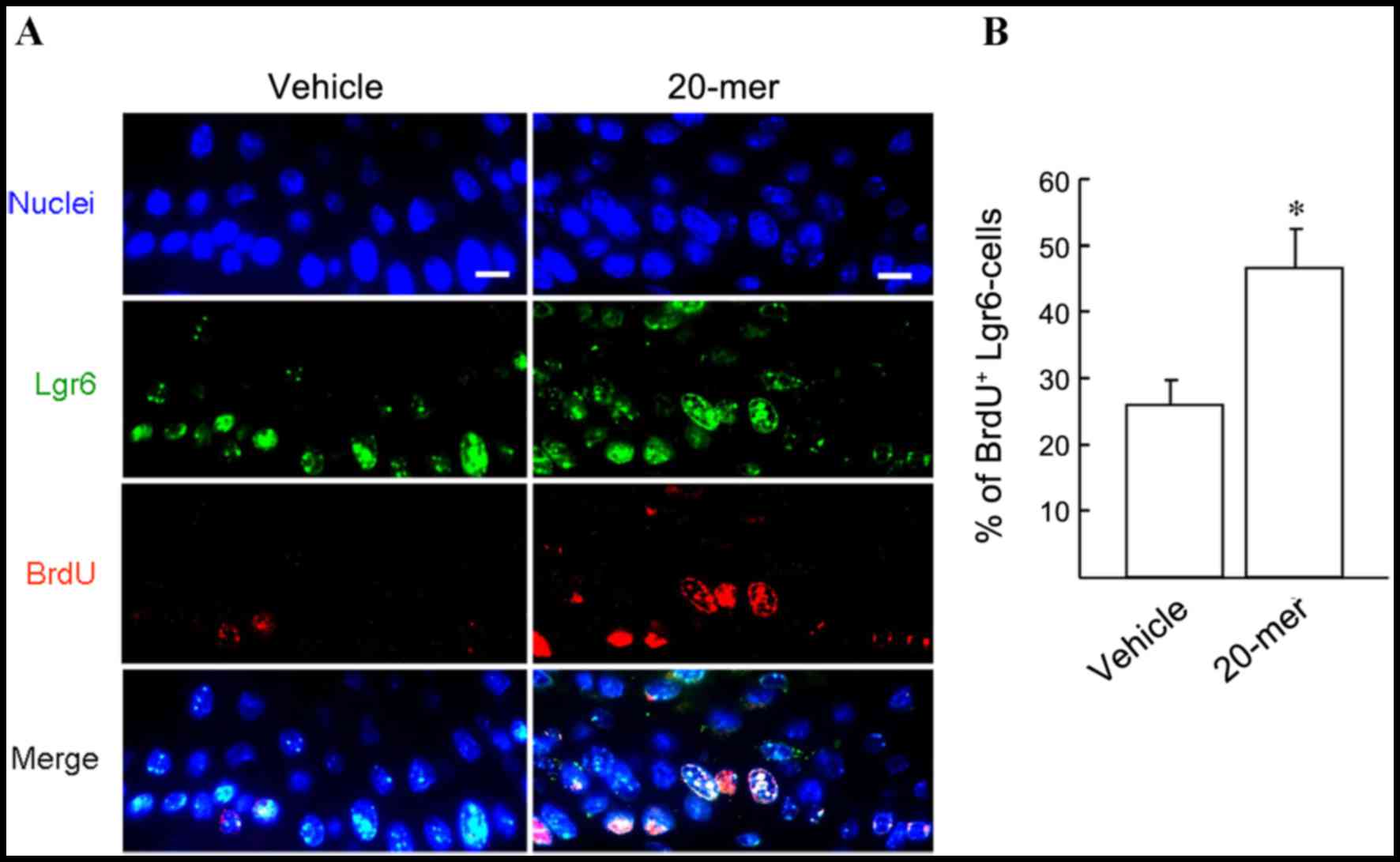
PEDF-derived peptide promotes
Lgr6-positive cell proliferation during skin wound healing
Lgr6-positive HFSCs are important precursor cells
for the repair of epithelium during skin wound healing (18). To investigate the participation of
HFSCs in wound healing, a Lgr6 and BrdU double immunostaining
experiment was performed and the involvement of proliferating
Lgr6-positive HFSCs was determined. As shown in Fig. 5A, immunostaining confirmed that
Lgr6-positive cells (green) were distributed in HE tissue after
skin injury. Moreover, dual immunostaining analysis of the skin
specimens from wounds treated with 20-mer ointment for 4 days
showed that the BrdU/Lgr6-double positive cells were markedly
increased in HE tissue, compared to those in vehicle-treated wounds
(46.7±5.8 vs. 26.0±3.6%; Fig. 5B).
This indicates the participation of HFSCs in wound healing
following stimulation by the 20-mer fragment of PEDF.
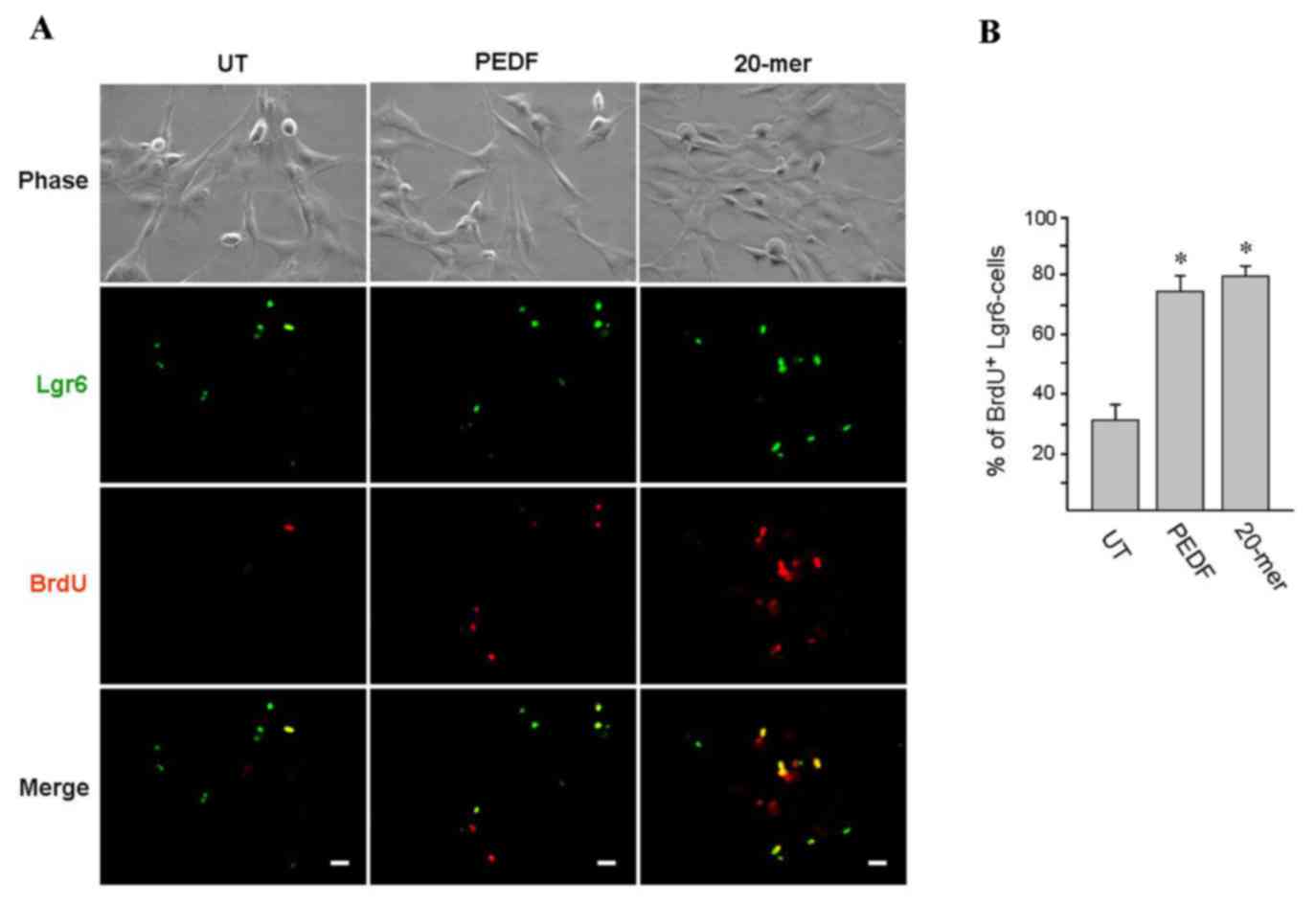
PEDF and 29-mer promote HFSC
proliferation in vitro
To determine the influence of the 44-mer fragment of
PEDF and its short derivatives on HFSC proliferation, human hair
follicles cultured and treated with PEDF were stained for HFSC
markers. As shown in Fig. 6A, BrdU
pulse-labeling (2 h; red color) was performed and the HFSC
phenotype was confirmed by immunostaining of Lgr6 marker (green
color). Human HFSCs cultured in medium containing PEDF or 20-mer
exhibited a significantly higher proliferative rate than the
untreated group cultivated in control medium (73.8±6.0 and
79.5±2.6, respectively, vs. 31.9±5.6%; Fig. 6B). Collectively, these results
indicated that PEDF or its derived 20-mer enhanced HFSC
proliferation in vitro.
Discussion
In the present study, by exposing cutaneous punch
wounds to PEDF peptide, improved wound healing and HFSC expansion
was demonstrated in vivo. This was observed at 4 and 7 days
after wounding. Furthermore, histological analysis indicated faster
wound coverage by keratinocytes and accumulation of GT under PEDF
peptide fragment treatment. To the best of our knowledge, the
present study was the first to describe the healing of
full-thickness skin wounds following treatment with the PEDF
peptide derivatives.
In the present study active proliferation of
epithelium basal cells under PEDF peptide treatment was observed,
particularly around the wound margin. One of the possible
explanations for this is that PEDF peptide stimulates basal cell
proliferation. Another possibility is that these basal cells were
newly generated from a stem cell niche, such as the bulb of the
hair follicle, and maintained their progenitor characteristic (Lgr6
marker) and superior proliferative potential. In PEDF
peptide-assisted cornea wound healing, basal cells in the newly
regenerated corneal epithelium have been previously reported to
proliferate actively and maintain a high level of cornea progenitor
cell marker ∆Np63α (14). The
similar findings for skin epithelial basal cells and corneal basal
cells suggest that skin epithelial basal cells may retain certain
properties of progenitor cells under the influence of the PEDF
peptide.
In addition to the stem cell proliferative effect,
PEDF also has an anti-angiogenesis domain that lies within the
34-mer region (amino acid positions Asp44-Asn77) (19). However, angiogenesis is a function
that participates in wound healing, via mast cell recruitment and
subsequent initiation of GT and keratinocyte proliferation. By
contrast, the anti-angiogenesis function is responsible for
arresting neovascularization through reducing local vascular
endothelial growth factor (VEGF) synthesis and suppression of VEGF
receptor expression, which promotes endothelial cell apoptosis
(20,21). Therefore, the full-length PEDF
protein containing the responsible 34-mer anti-angiogenesis domain
may not be a good candidate for wound healing therapy. PEDF levels
were found to be reduced locally after wound injuries, and
exogenous PEDF was found to delay wound healing in diabetic animals
(22). However, the synthetic 29- or
20-mer peptides did not include the 34-mer anti-angiogenesis domain
of PEDF, and were successfully demonstrated to be accountable for
the wound healing function of PEDF.
Previously, the documented association regarding
PEDF and stem cell proliferation was limited to neural stem cells
(NSCs). It has been reported that PEDF promotes the formation of
neurospheres in vitro from NSCs derived from the hippocampus
and neuroretina (23). Moreover,
PEDF promotes NSC self-renewal by symmetrical division in the
subventricular zone of the dentate gyrus in the hippocampus
(24). A previous study by our group
identified the expansion of limbal corneal epithelial stem cells by
PEDF peptide (14). This indicated
that the stem cell-promoting function of PEDF is not limited to the
neuronal system. The observations made in the present study further
expanded the scope of the function of PEDF to the proliferation of
cutaneous epithelial stem cells and wound healing. PEDF appears to
be functional in stem cell proliferation and expansion in multiple
systems. Based on this knowledge, PEDF peptide may be useful for
tissue regeneration therapy in various disease conditions.
Growth factors are important promoters of wound
healing by controlling the growth, differentiation and metabolism
of cells. Interleukin (IL)-1 released by keratinocytes induces
fibroblasts to secrete important cytokines and growth factors, such
as keratinocyte growth factor, fibroblast growth factor 7, IL-6
granulocyte-macrophage colony-stimulating factor and hepatocyte
growth factor during wound repair (25–27).
These factors in turn promote keratinocyte proliferation, in a
paracrine-fashioned feedback loop, which is essential for proper
healing of the wound site (3). PEDF
was also found to impair skin wound healing in diabetic animals
(22). The results of the present
study may suggest that PEDF is a physiological reparative factor.
However, it remains elusive whether PEDF is essential for wound
healing, since the wound healing function of PEDF may be
replaceable by other growth factors. However, effect of the topical
application of short PEDF fragments shown in the present study
indicated that they, similar to other reparative cytokines, promote
wound healing.
HFSC is a cellular source for re-epithelialization.
The present study provided evidence for HFSC proliferation through
PEDF peptide stimulation at the wound site, suggesting that this is
one of the mechanisms through which PEDF participates in wound
healing. This idea opens up another frontier in promoting the wound
healing process and may provide a therapeutic option for external
wounds.
In addition to epithelium enhancement at the wound
site, increased GT formation was observed under treatment with PEDF
fragments. The mechanism of the accumulation of fibroblasts and
extracellular matrix in GT remains to be determined. One of the
postulated mechanisms may be associated with the fibroblast
chemotactic function of PEDF (28);
however, further evidence is required to determine the precise
mechanism of action.
Acknowledgements
The authors would like to thank Dr Tim J Harrison
for kindly proofreading this manuscript. Furthermore, the authors
thank Chu-Ping Ho and Chin-Min Wang (Departments of Medical
Research, Mackay Memorial Hospital, Taipei 10449) for assistance
with animal experiments. The present study was supported by grants
from the National Science Council, Taiwan (grant no.
NSC-101-2314-B-195-006-MY3) and Mackay Memorial Hospital
(MMH-E-101-006).
References
|
1
|
Wysocki AB: Wound fluids and the
pathogenesis of chronic wounds. J Wound Ostomy Continence Nurs.
23:283–290. 1996. View Article : Google Scholar
|
|
2
|
Stojadinovic O, Ito M and Tomic-Canic M:
Hair cycling and wound healing: To pluck or not to pluck? J Invest
Dermatol. 131:292–294. 2011. View Article : Google Scholar
|
|
3
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar
|
|
4
|
Lau K, Paus R, Tiede S, Day P and Bayat A:
Exploring the role of stem cells in cutaneous wound healing. Exp
Dermatol. 18:921–933. 2009. View Article : Google Scholar
|
|
5
|
Szpaderska AM, Egozi EI, Gamelli RL and
DiPietro LA: The effect of thrombocytopenia on dermal wound
healing. J Invest Dermatol. 120:1130–1137. 2003. View Article : Google Scholar
|
|
6
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003.
|
|
7
|
Smola H, Thiekötter G and Fusenig NE:
Mutual induction of growth factor gene expression by
epidermal-dermal cell interaction. J Cell Biol. 122:417–429. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stewart KJ: A quantitative ultrastructural
study of collagen fibrils in human skin normal scars and
hypertrophic scars. Clin Anat. 8:334–338. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ito M and Cotsarelis G: Is the hair
follicle necessary for normal wound healing? J Invest Dermatol.
128:1059–1061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito M, Liu Y, Yang Z, Nguyen J, Liang F,
Morris RJ and Cotsarelis G: Stem cells in the hair follicle bulge
contribute to wound repair but not to homeostasis of the epidermis.
Nat Med. 11:1351–1354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Persson U, Willis M, Odegaard K and
Apelqvist J: The cost-effectiveness of treating diabetic lower
extremity ulcers with becaplermin (Regranex): A core model with an
application using Swedish cost data. Value Health. 3 Suppl
1:S39–S46. 2000. View Article : Google Scholar
|
|
12
|
Steed DL: Clinical evaluation of
recombinant human platelet-derived growth factor for the treatment
of lower extremity ulcers. Plast Reconstr Surg. 117 7
Suppl:143S–151S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L and DiPietro LA: Production and
function of pigment epithelium-derived factor in isolated skin
keratinocytes. Exp Dermatol. 23:436–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho TC, Chen SL, Wu JY, Ho MY, Chen LJ,
Hsieh JW, Cheng HC and Tsao YP: PEDF promotes self-renewal of
limbal stem cell and accelerates corneal epithelial wound healing.
Stem Cells. 31:1775–1784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho TC, Chiang YP, Chuang CK, Chen SL,
Hsieh JW, Lan YW and Tsao YP: PEDF-derived peptide promotes
skeletal muscle regeneration through its mitogenic effect on muscle
progenitor cells. Am J Physiol Cell Physiol. 309:C159–C168. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petersen SV, Valnickova Z and Enghild JJ:
Pigment-epithelium-derived factor (PEDF) occurs at a
physiologically relevant concentration in human blood: Purification
and characterization. Biochem J. 374:199–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blazejewska EA, Schlötzer-Schrehardt U,
Zenkel M, Bachmann B, Chankiewitz E, Jacobi C and Kruse FE: Corneal
limbal microenvironment can induce trans differentiation of hair
follicle stem cells into corneal epithelial-like cells. Stem Cells.
27:642–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Snippert HJ, Haegebarth A, Kasper M, Jaks
V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel
H, Vries RG, et al: Lgr6 marks stem cells in the hair follicle that
generate all cell lineages of the skin. Science. 327:1385–1389.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Filleur S, Volz K, Nelius T, Mirochnik Y,
Huang H, Zaichuk TA, Aymerich MS, Becerra SP, Yap R, Veliceasa D,
et al: Two functional epitopes of pigment epithelial-derived factor
block angiogenesis and induce differentiation in prostate cancer.
Cancer Res. 65:5144–5152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsui T, Nishino Y, Maeda S and Yamagishi
S: PEDF-derived peptide inhibits corneal angiogenesis by
suppressing VEGF expression. Microvasc Res. 84:105–108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai J, Jiang WG, Grant MB and Boulton M:
Pigment epithelium-derived factor inhibits angiogenesis via
regulated intracellular proteolysis of vascular endothelial growth
factor receptor 1. J Biol Chem. 281:3604–3613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi W, Yang C, Dai Z, Che D, Feng J, Mao Y,
Cheng R, Wang Z, He X, Zhou T, et al: High levels of pigment
epithelium-derived factor in diabetes impair wound healing through
suppression of Wnt signaling. Diabetes. 64:1407–1419. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Marzo A, Aruta C and Marigo V: PEDF
promotes retinal neurosphere formation and expansion in vitro. Adv
Exp Med Biol. 664:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramírez-Castillejo C, Sánchez-Sánchez F,
Andreu-Agulló C, Ferrón SR, Aroca-Aguilar JD, Sánchez P, Mira H,
Escribano J and Fariñas I: Pigment epithelium-derived factor is a
niche signal for neural stem cell renewal. Nat Neurosci. 9:331–339.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boxman ILA, Ruwhof C, Boerman OC, Löwik
CWGM and Ponec M: Role of fibroblasts in the regulation of
proinflammatory interleukin IL-1, IL-6 and IL-8 levels induced by
keratinocyte-derived IL-1. Arch Dermatol Res. 288:391–398. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Waelti ER, Inaebnit SP, Rast HP, Hunziker
T, Limat A, Braathen LR and Wiesmann U: Co-culture of human
keratinocytes on post-mitotic human dermal fibroblast feeder cells:
Production of large amounts of interleukin 6. J Invest Dermatol.
98:805–808. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Florin L, Maas-Szabowski N, Werner S,
Szabowski A and Angel P: Increased keratinocyte proliferation by
JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell
Sci. 118:1981–1989. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sarojini H, Estrada R, Lu H, Dekova S, Lee
MJ, Gray RD and Wang E: PEDF from mouse mesenchymal stem cell
secretome attracts fibroblasts. J Cell Biochem. 104:1793–1802.
2008. View Article : Google Scholar : PubMed/NCBI
|