Introduction
Thyroid carcinoma is the cancer with the third
fastest increase in diagnosis in the US, and papillary thyroid
carcinoma (PTC) comprises 80% of thyroid carcinoma (1,2). PTC
occurs more often in women, and although it can occur at any age,
even in childhood, the peak incidence is in patients between 30 and
50 years of age (3). PTC typically
has an indolent clinical course and may be cured by thyroidectomy
and radioactive iodine therapy, even if metastatic (4). The majority of patients with PTC have a
good prognosis; however, a subset of patients may undergo
dedifferentiation and develop a more progressive disease that
consequently has a dismal prognosis (5).
The human cluster of differentiation (CD)44 gene
encodes type 1 transmembrane glycoproteins involved in cell-cell
and cell-matrix interactions (6).
The structural heterogeneity of the gene products is caused
primarily by alternative splicing of at least 10 out of 20 exons
(6). Certain CD44 variant isoforms,
in particular those containing CD44 variant domain 6 (CD44v6) have
been implicated in tumorigenesis, tumor cell invasion and
metastasis (6). A previous study
reported that CD44v6 is an important indicator in PTC cell
differentiation, and the expression of CD44v6 is significantly
higher in malignant lesions compared with non-malignant lesions
(7).
CD44+/CD24− cells are more aggressive than
CD44− cells, not only in PTC, but also breast and
ovarian cancer (8–10). However, the underlying mechanism that
promotes CD44+ cells to exhibit a more malignant
phenotype remains unknown.
In the present study, the expression of a p53 family
member transcription factor, p63, was demonstrated to be negatively
correlated with the expression of CD44. p63 is a well-known tumor
suppressor (11). In addition, the
present study indicated that CD44 inhibited the activity of p63 and
increased the proliferation and chemoresistance capacity of PTC
cells.
Materials and methods
Ethics statement
All animal experiments were approved by the Ethics
Committee of Fudan University Shanghai Medical College (Shanghai,
China) and followed the National Institutes of Health guidelines on
the care and use of animals.
Cell culture
TPC-1 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA) and maintained under
standard culture conditions (37°C, 5% CO2) in the
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
(v/v) fetal bovine serum (FBS).
Western blotting
TPC-1 cells were lysed in western
blot/immunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Haimen, Jiangsu, China) and all procedures were
conducted according to the manufacturer's protocol. Subsequently,
the cell lysates were boiled in 5X SDS-PAGE loading buffer for 10
min and then 20 µg of protein was resolved by 8% SDS-PAGE,
transferred to a nitrocellulose membrane and blocked with 5% (m/v)
bovine serum albumin (BSA; Shanghai Sangon Biological Engineering,
Shanghai, China) for 1 h. The membrane was subsequently incubated
with primary antibodies at 4°C for 12 h. The following antibodies
were used: CD44 (#3570, 1:1,000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA), CD24 (ab64064, 1:1,000
dilution; Abcam, Cambridge, UK), p63 (#13,109 1:1,000 dilution;
Cell Signaling Technology, Inc.) and GAPDH (1:5,000 dilution;
ProteinTech Group, Inc., Chicago, IL, USA). Specific secondary
antibodies mouse IgG (#7076, 1:2,000 dilution) and rabbit IgG
(#7074, 1:2,000) (both from Cell Signaling Technology, Inc.) were
used to probe the membrane at 37°C for 1 h. Bound antibodies were
subsequently visualized with an enhanced chemiluminescence kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions using a Bio-Rad ChemiDoc XRS+ system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Stable cell construction
The vector pcDNA3.1 (+) inserted with the ORF of p63
and empty vector were purchased from Fugene (Promega Corporation,
Madison, WI, USA). These plasmids were transfected into TPC-1 cells
with Fugene 9 (Promega Corporation) transfection system. After 48 h
transfection, these cells were treated with 1 mg/ml G418 (Shanghai
Sangon Biological Engineering) for 2 weeks. These selected cells
were considered stable cell lines.
Cell isolation
TPC-1 cells were washed once with PBS and then
harvested with 0.05% trypsin/0.025% EDTA (Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany). Detached cells were washed with PBS
containing 1% FBS (wash buffer) and then resuspended in the wash
buffer (106 cells/100 µl). Cells were incubated with 5
µl of Fc Blocker (#422301; BioLegend, Inc., San Diego, CA, USA) at
4°C for 20 min. Combinations of fluorochrome-conjugated monoclonal
antibodies were purchased from BD Biosciences (San Jose, CA, USA)
against human CD44 (fluorescein isothiocyanate conjugated; cat no.
555478) and CD24 (phycoerythrin conjugated; cat no. 555428) or
their respective isotype controls were added to the cell suspension
at 1:100 dilution and incubated at 4°C in the dark for 30 to 40
min. CD44+/CD24− cells were sorted by flow
cytometry on a FACSVantage cytometer (BD Biosciences) and analyzed
using FlowJo software (version 10.1; FlowJo LLC, Ashland, OR, USA).
The purity of sorted cells in this study was consistently more than
99%.
Cell Counting Kit-8 (CCK-8) cell
viability assays
TPC-1 cells were seeded into a 96-well plate at
3×103 cells/well with 100 µl DMEM containing 0, 2, 4, 6
and 8 µg/ml concentrations of 5-fluorouracil (5-FU; #F6627;
Sigma-Aldrich, Merck KGaA) and cultured at 37°C in an atmosphere
containing 5% CO2 for 24 h. The cell viability was
quantified by the addition of 10 µl CCK-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Following 1.5 h of incubation
at 37°C in an atmosphere containing 5% CO2 in the dark
for 30 to 40 min, the plates were monitored using a Power Wave XS
microplate reader (Omega Bio-Tek, Inc., Norcross, GA, USA) at an
absorbance 450 nm.
Colony formation assays
TPC-1 cells (500/well) were seeded in 6-well plates
and cultured in DMEM for 10 days at 37°C in an atmosphere
containing 5% CO2. After 10 days, colonies were fixed
and stained with 0.5% crystal violet (Beyotime Institute of
Biotechnology) and the number of colonies was counted. Only those
cell clusters containing more than 50 cells under a microscope were
considered as colonies. The assay was performed in triplicate.
In vivo tumor formation assay
In this assay, 10 BALB/c (nu/nu) 4-week-old male
mice were from Shanghai Laboratory Animal Center (Shanghai, China)
and housed in a specific pathogen-free facility. Mice were housed
with a 14/10 h light/dark cycle and provided with food and water
ad libitum. The mice were group-housed (3–5 per cage) and
maintained at a temperature of 24±1°C and a humidity of 50±10%. A
total of 1×106 p63-overexpressing (p63 group) or empty
vector (EV) CD44+/CD24− TPC-1 cells (control
group) were each subcutaneously injected into the right flank of
mice (n=5/group, weight 17.6±0.4 g vs. 18.2±0.6 g). Tumor sizes
were measured and calculated with the formula V = (length ×
width2)/2 once a week and mice were sacrificed for the analysis of
tumor burden after 4 weeks.
Immunohistochemical staining
The resected mice tumors were fixed in methanol and
embedded in paraffin and then tissues sectioned to 4 µm slides.
These slides were deparaffinized gradually using 50% xylene
(Meryer, Shanghai, China) and rehydrated. The sections were then
incubated with 0.3% hydrogen peroxide (Jianglaibio, Shanghai,
China) for 30 min at 37°C to inhibit endogenous peroxidase activity
and blocked with 10% BSA for 10 min 37°C. Sections were incubated
with an antibody targeting p63 (#13109, 1:200 dilution) or Ki-67
(#9449, 1:200 dilution) (both from Cell Signaling Technology, Inc.)
at 4°C overnight and subsequently reacted with horseradish
peroxidase-conjugated secondary antibody (#13109; 1:50; Beyotime
Institute of Biotechnology) for 20 min at 37°C. Sections were
stained with diaminobenzidine for 2 min at 37°C, counterstained
with hematoxylin for 30 sec at 37°C and mounted with neutral
balsam. A light microscope was used to capture representative
images (magnification, ×200) of the tumor tissues.
Statistics analysis
Data are expressed as the mean ± standard deviation
as indicated. Student's t-test was used for comparisons between
groups and P<0.05 was considered to indicate a statistically
significant difference.
Results
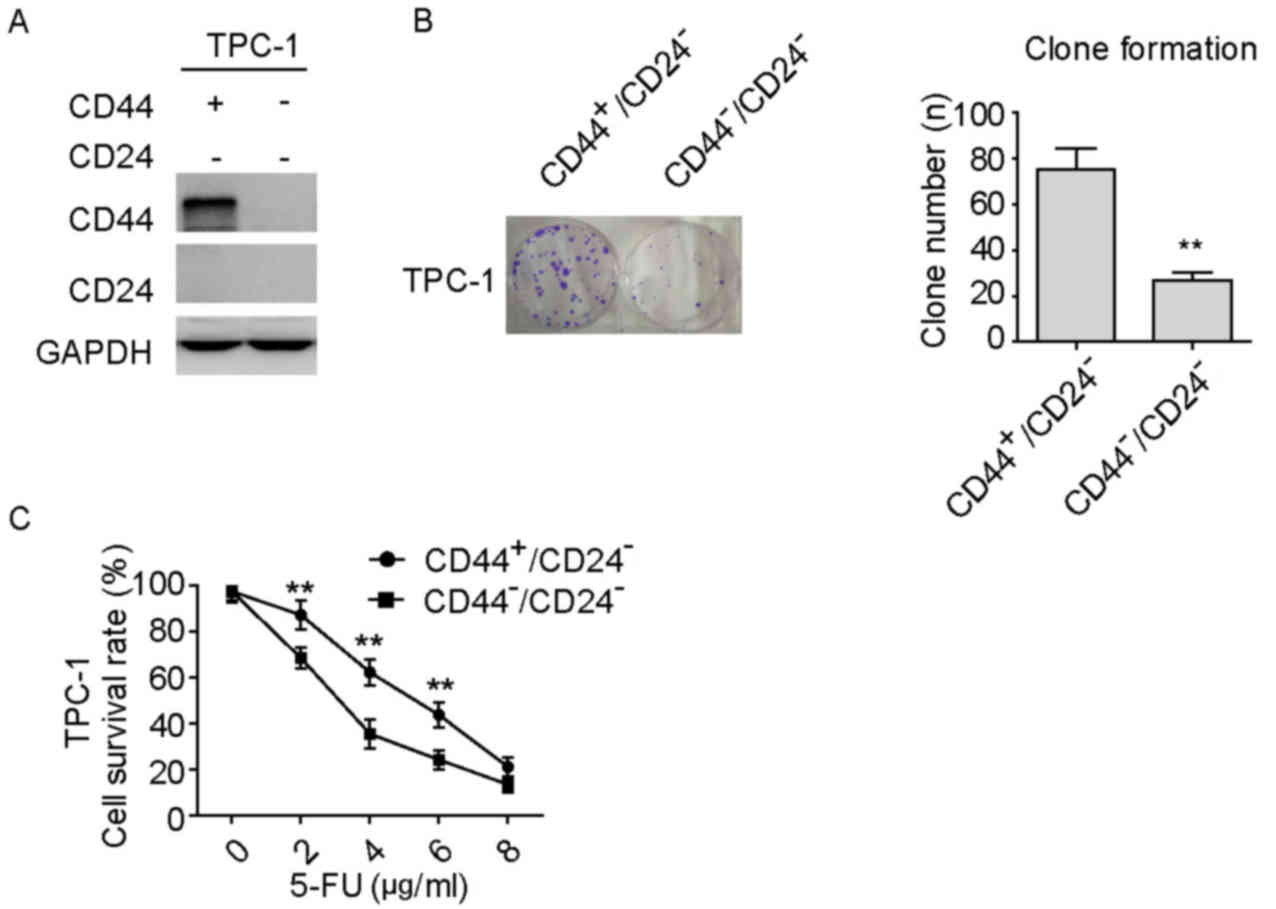
CD44+/CD24− PTC
cells exhibit increased proliferative and chemoresistant
capacity
It was previously reported that
CD44+/CD24− PTC cells obtain a more
progressive phenotype compared with
CD44−/CD24− cells in PTC progression
(7). In the present study,
CD44+/CD24− cells were isolated from the
TPC-1 PTC cell line, and the isolated cells were validated by
western blotting (Fig. 1A).
Subsequently, the cell proliferation and chemoresistance capacities
of the isolated CD44+/CD24− cells were
explored in comparison with those of
CD44−/CD24− cells. As shown in Fig. 1B-D, compared with
CD44−/CD24− cells,
CD44+/CD24− cells exhibited significantly
increased proliferative activity (Fig.
1B) and chemoresistance to 5-FU at specific doses (Fig. 1C and D). These data indicate that
CD44+/CD24− PTC cells have a greater
proliferative and chemoresistance capacity compared with
CD44−/CD24− cells.
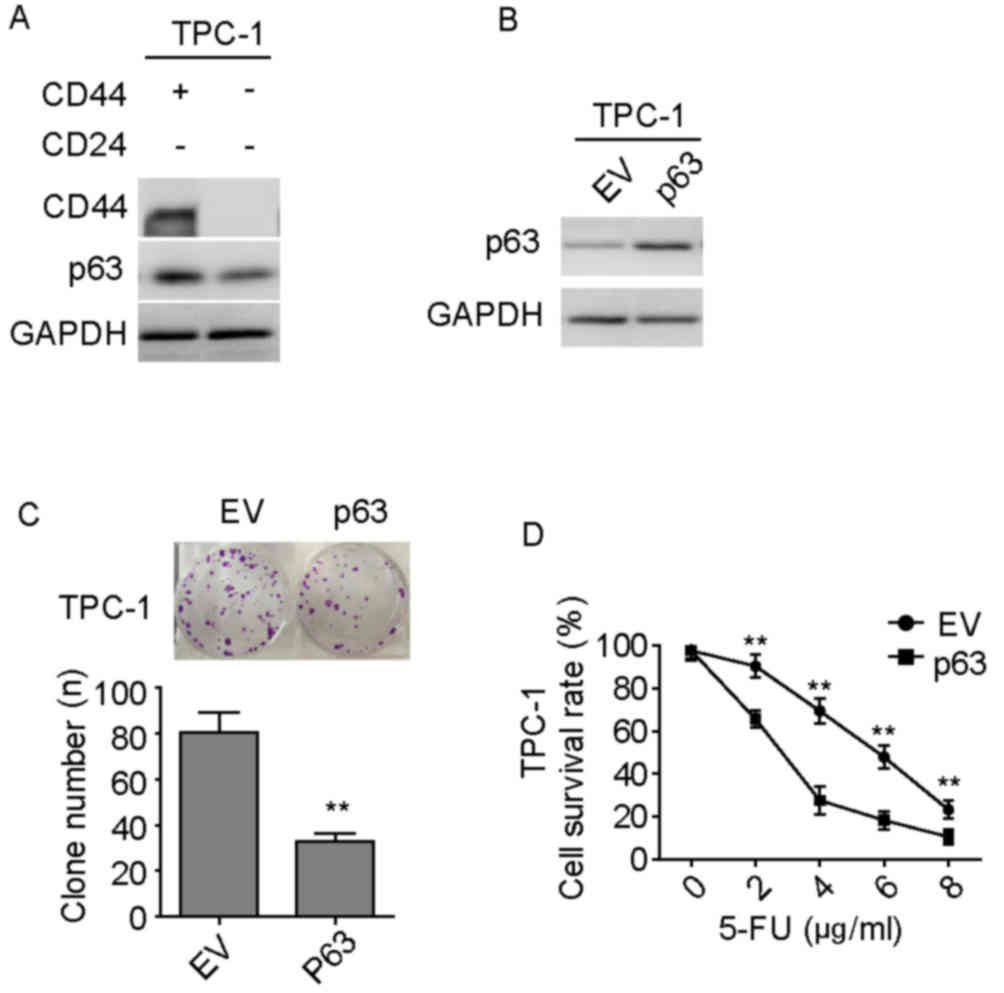
Expression of p63 is negatively
correlated with the expression of CD44
p63 is a member of the p53 family of transcription
factors and previous studies have revealed the suppressive roles of
p63 in the field of tumor biology (12,13). The
protein expression levels of p63 in TPC-1 isolated cell groups were
investigated, as shown in Fig. 2A.
CD44+/CD24− TPC-1 cells exhibited lower
expression levels of p63 compared with
CD44−/CD24− PTC cells. This finding suggested
that CD44 may inhibit the activity of p63. Subsequently, the
effects of overexpression of p63 in these isolated
CD44+/CD24− PTC cell lines were investigated;
the overexpression was demonstrated by western blotting (Fig. 2B). Cellular clone formation assays
and CCK-8 cell viability assays revealed that overexpression of p63
significantly attenuated the capacities of clone formation
(Fig. 2C) and chemoresistance to
5-FU at specific doses (Fig. 2D) in
CD44+/CD24− TPC-1 cells compared with that in
the EV group. These data suggest that
CD44+/CD24− cells inhibit the activity of p63
to be more progressive than CD44−/CD24−
cells.
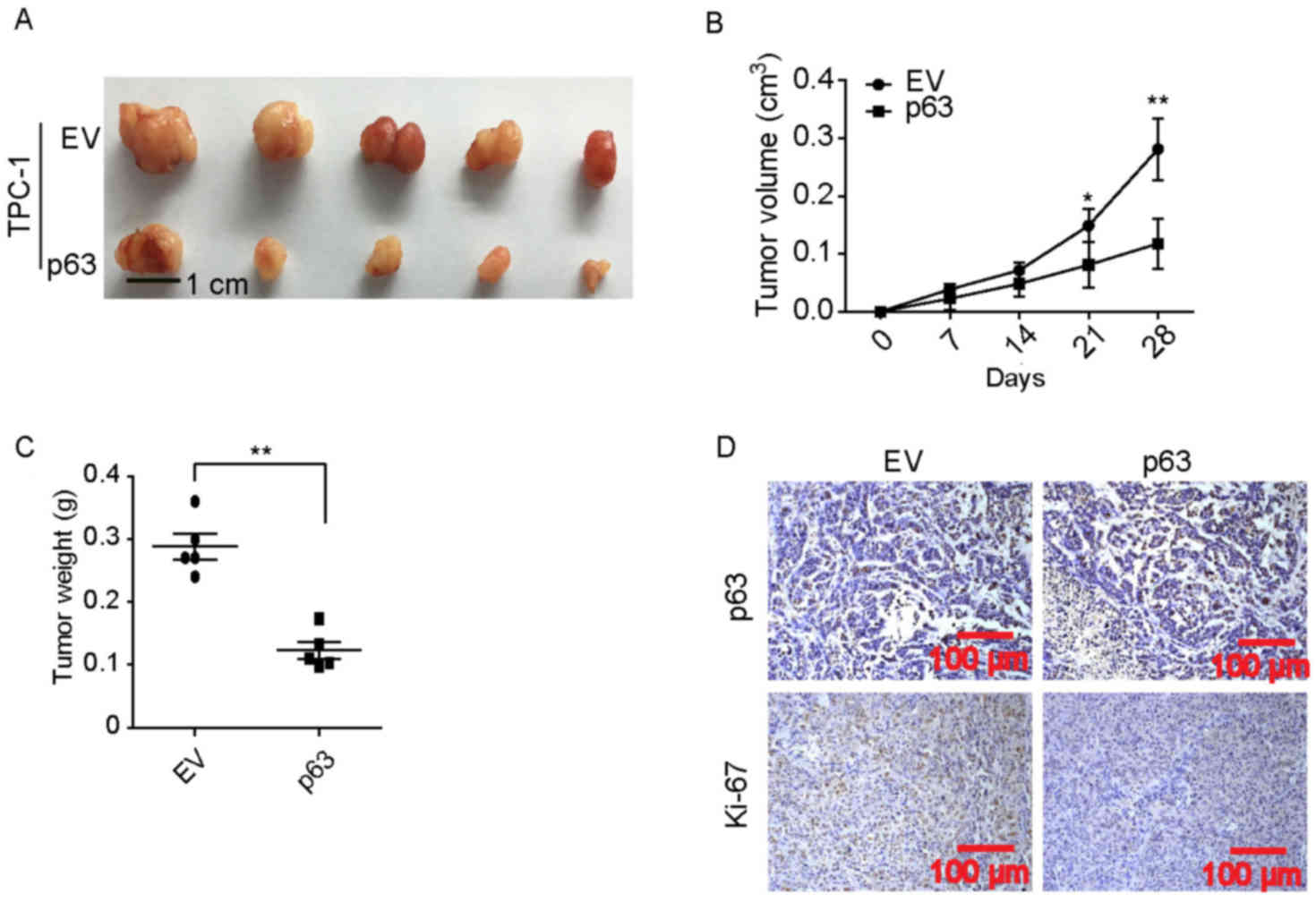
Ectopic expression of p63 in
CD44+/CD24− PTC cells inhibits xenograft tumor
formation
To further investigate the inhibitory effects of
p63, CD44+/CD24− p63-overexpressing TPC-1
cells and EV control TPC-1 cells were used to perform a xenograft
subcutaneous tumor formation assays. As shown in Fig. 3A-C, when tumors derived from
CD44+/CD24− TPC-1 cells were analyzed, those
overexpressing p63 exhibited reduced tumor growth in vivo
compared with those in the control group. There was a significant
difference in tumor volume between the groups on days 21 and 28
(Fig. 3B) and tumor weight on day 28
(Fig. 3C). Furthermore,
immunohistochemical staining indicated that the expression of
Ki-67, a cell proliferative marker (14), was markedly lower in the
p63-overexpressing group compared with the control group (Fig. 3D). These results suggest that
CD44+/CD24− cells may inhibit the activity of
p63, which results in increased cell proliferation.
Discussion
In the present study,
CD44+/CD24− PTC cells were indicated to have
a lower activity of p63 compared with
CD44−/CD24− PTC cells, which increased the
aggressive malignant phenotype of these cells, particularly
regarding proliferation and chemoresistance. CD44 s are a
polymorphic family of cell surface glycoproteins that are
implicated in cell-to-cell and cell-to-matrix adhesion interactions
(15). CD44 has been reported to be
involved in tumor metastasis and invasion in breast cancer, the
regulation of epithelial-mesenchymal transition and modulation of
phosphoinositide 3-kinase/Akt/mechanistic target of rapamycin and
activation of Wnt canonical signaling (15–17). In
the present study, CD44+/CD24− cells were
isolated from the PTC cell line TPC-1. The present study findings,
which indicated that CD44+/CD24− cells are
more proliferative and chemoresistant to 5-FU than are
CD44−/CD24− cells, are consistent with
previous studies (15,16). These findings suggest that CD44 may
be a pivotal oncoprotein in patients with malignant PTC.
To the best of our knowledge, the mechanism by which
CD44 exerts an aggressive role in malignant PTC progression has not
been reported to date. In the present study, the
CD44+/CD24- cells exhibited significantly reduced
protein expression levels of p63 compared with
CD44−/CD24− cells. This finding indicated
that CD44+/CD24− cells may have an attenuated
expression of p63, which increased the aggressiveness of the cells.
The ectopic expression of p63 in CD44+/CD24−
cells supported this hypothesis; overexpression of p63 reduced
CD44+/CD24− cell proliferation in
vitro and in vivo. The results considered together
indicate that malignant PTC cells highly expressed CD44, and
exhibited aggressive malignant phenotypes, which were likely
associated with the inhibition of p63.
In conclusion, the present study indicates that
CD44+/CD24− cells primarily contribute to PTC
proliferation and chemoresistance. Moreover, the results suggested
that p63 was suppressed in CD44+/CD24− cells
and that this suppression was associated with PTC cell
proliferation and chemoresistance.
References
|
1
|
Veiga LH, Neta G, Aschebrook-Kilfoy B, Ron
E and Devesa SS: Thyroid cancer incidence patterns in Sao Paulo,
Brazil, and the US SEER Program, 1997–2008. Thyroid. 23:748–757.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vigneri R, Malandrino P and Vigneri P: The
changing epidemiology of thyroid cancer: Why is incidence
increasing? Curr Opin Oncol. 27:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma C: An analysis of trends of
incidence and cytohistological correlation of papillary carcinoma
of the thyroid gland with evaluation of discordant cases. J Cytol.
33:192–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cobin RH, Gharib H, Bergman DA, Clark OH,
Cooper DS, Daniels GH, Dickey RA, Duick DS, Garber JR, Hay ID, et
al: AACE/AAES medical/surgical guidelines for clinical practice:
Management of thyroid carcinoma. American Association of Clinical
Endocrinologists. American College of Endocrinology. Endocrine
practice: Official journal of the American College of Endocrinology
and the American Association of Clinical Endocrinologists.
7:202–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McLeod DS, Cooper DS, Ladenson PW, Ain KB,
Brierley JD, Fein HG, Haugen BR, Jonklaas J, Magner J, Ross DS, et
al: Prognosis of differentiated thyroid cancer in relation to serum
thyrotropin and thyroglobulin antibody status at time of diagnosis.
Thyroid. 24:35–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heider KH, Kuthan H, Stehle G and Munzert
G: CD44v6: A target for antibody-based cancer therapy. Cancer
Immunol Immunother. 53:567–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahn SH, Henderson YC, Williams MD, Lai SY
and Clayman GL: Detection of thyroid cancer stem cells in papillary
thyroid carcinoma. J Clin Endocrinol Metab. 99:536–544. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iida J, Clancy R, Dorchak J, Somiari RI,
Somiari S, Cutler ML, Mural RJ and Shriver CD: DNA aptamers against
exon v10 of CD44 inhibit breast cancer cell migration. PLoS One.
9:e887122014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan W, Chen Y, Yao Y, Zhang H and Wang T:
Increased invasion and tumorigenicity capacity of CD44+/CD24-
breast cancer MCF7 cells in vitro and in nude mice. Cancer Cell
Int. 13:622013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng E, Long B, Sullivan P, McClellan S,
Finan MA, Reed E, Shevde L and Rocconi RP: CD44+/CD24- ovarian
cancer cells demonstrate cancer stem cell properties and correlate
to survival. Clin Exp Metastasis. 29:939–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Como CJ, Urist MJ, Babayan I, Drobnjak
M, Hedvat CV, Teruya-Feldstein J, Pohar K, Hoos A and Cordon-Cardo
C: p63 expression profiles in human normal and tumor tissues. Clin
Cancer Res. 8:494–501. 2002.PubMed/NCBI
|
|
12
|
Deyoung M and Ellisen L: p63 and p73 in
human cancer: Defining the network. Oncogene. 26:5169–5183. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Cho SJ and Chen X: RNPC1, an
RNA-binding protein and a target of the p53 family, regulates p63
expression through mRNA stability. Proc Natl Acad Sci USA.
107:9614–9619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyauchi A, Kudo T, Hirokawa M, Ito Y,
Kihara M, Higashiyama T, Yabuta T, Masuoka H, Shindo H, Kobayashi K
and Miya A: Ki-67 labeling index is a predictor of postoperative
persistent disease and cancer growth and a prognostic indicator in
papillary thyroid carcinoma. Eur Thyroid J. 2:57–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown RL, Reinke LM, Damerow MS, Perez D,
Chodosh LA, Yang J and Cheng C: CD44 splice isoform switching in
human and mouse epithelium is essential for epithelial-mesenchymal
transition and breast cancer progression. J Clin Invest.
121:1064–1074. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheridan C, Kishimoto H, Fuchs RK,
Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S and
Nakshatri H: CD44+/CD24- breast cancer cells exhibit enhanced
invasive properties: An early step necessary for metastasis. Breast
Cancer Res. 8:R592006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang G, Zhang H, Wang J, Zhang Y, Xu H,
Wang C, Zhang H, Ma L, Li Q and Pang T: CD44 targets
Wnt/beta-catenin pathway to mediate the proliferation of K562
cells. Cancer Cell Int. 13:1172013. View Article : Google Scholar : PubMed/NCBI
|