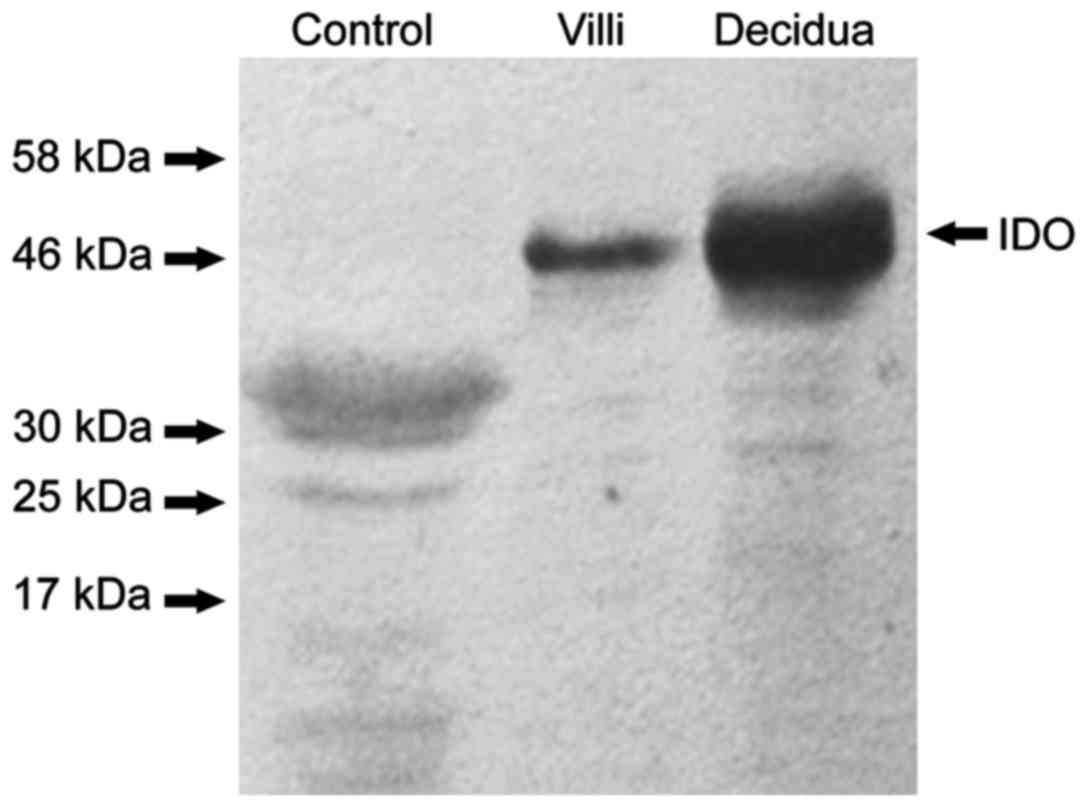
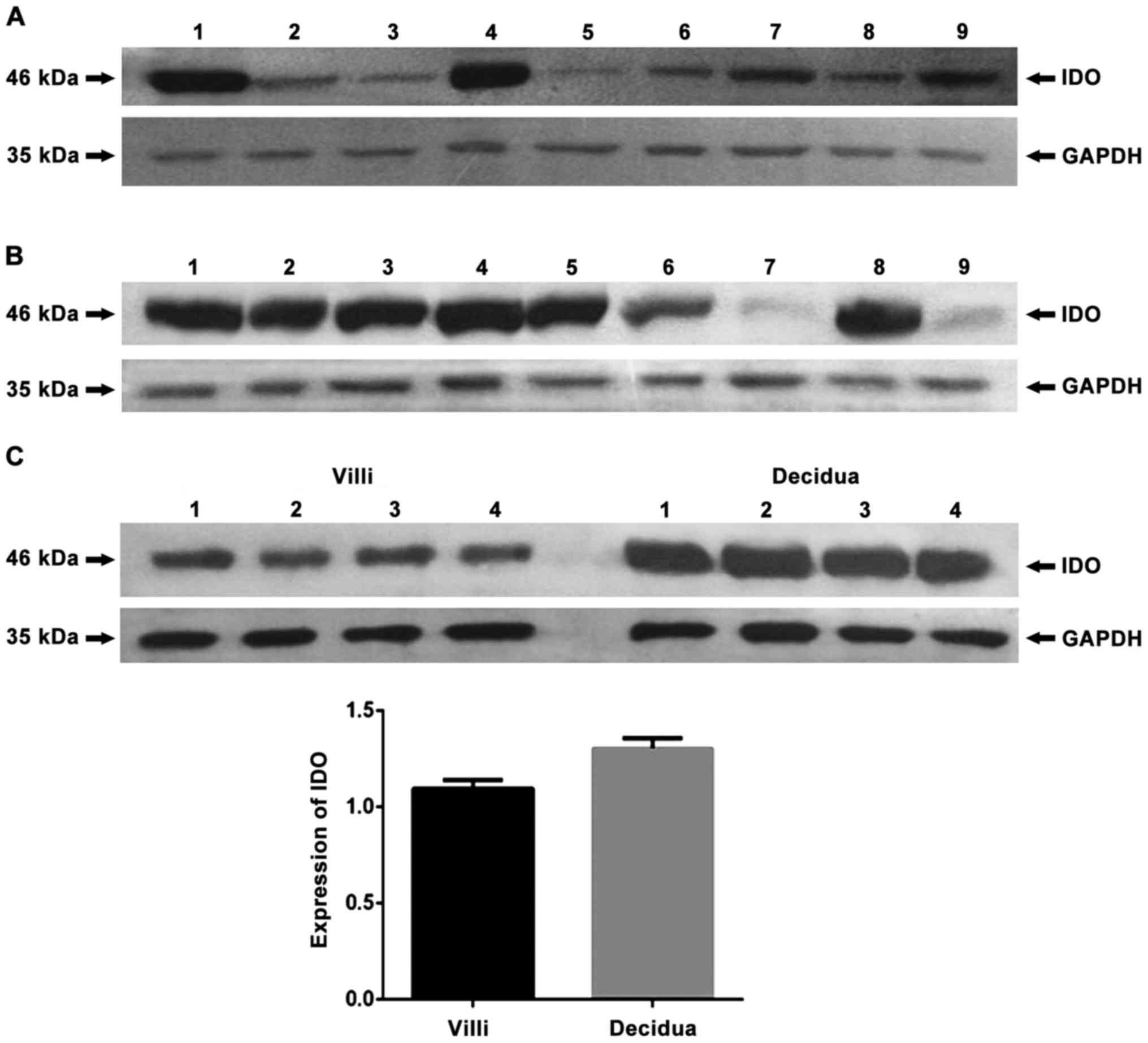
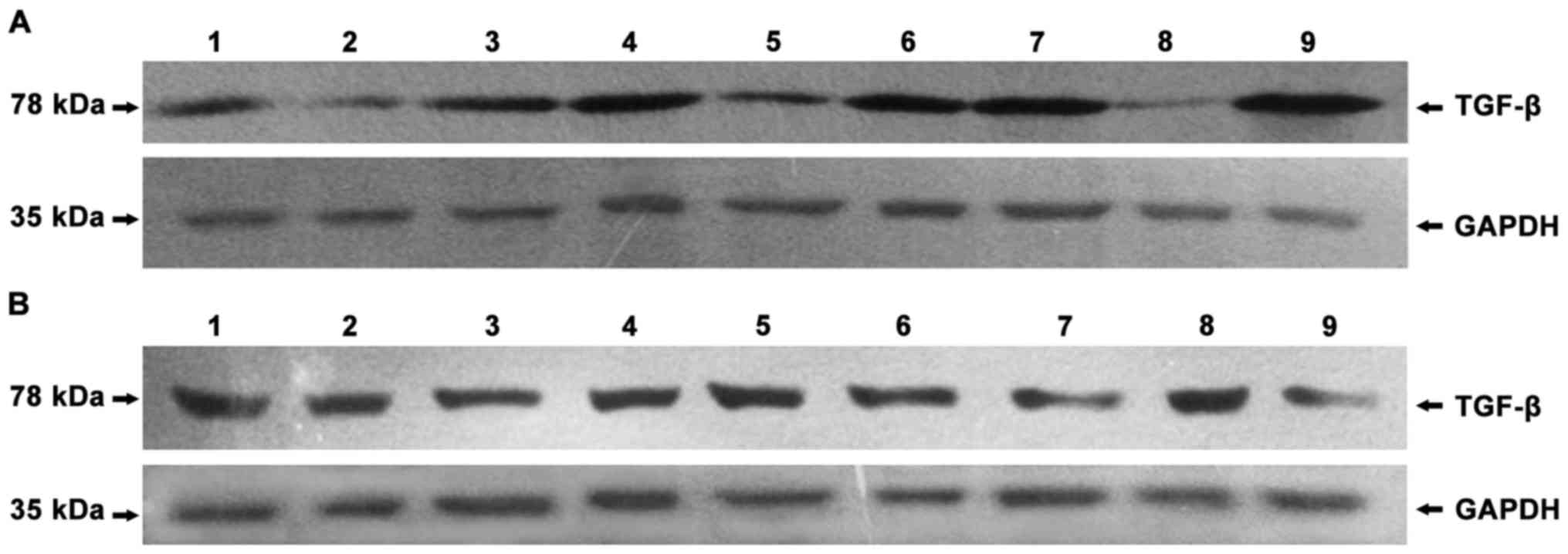
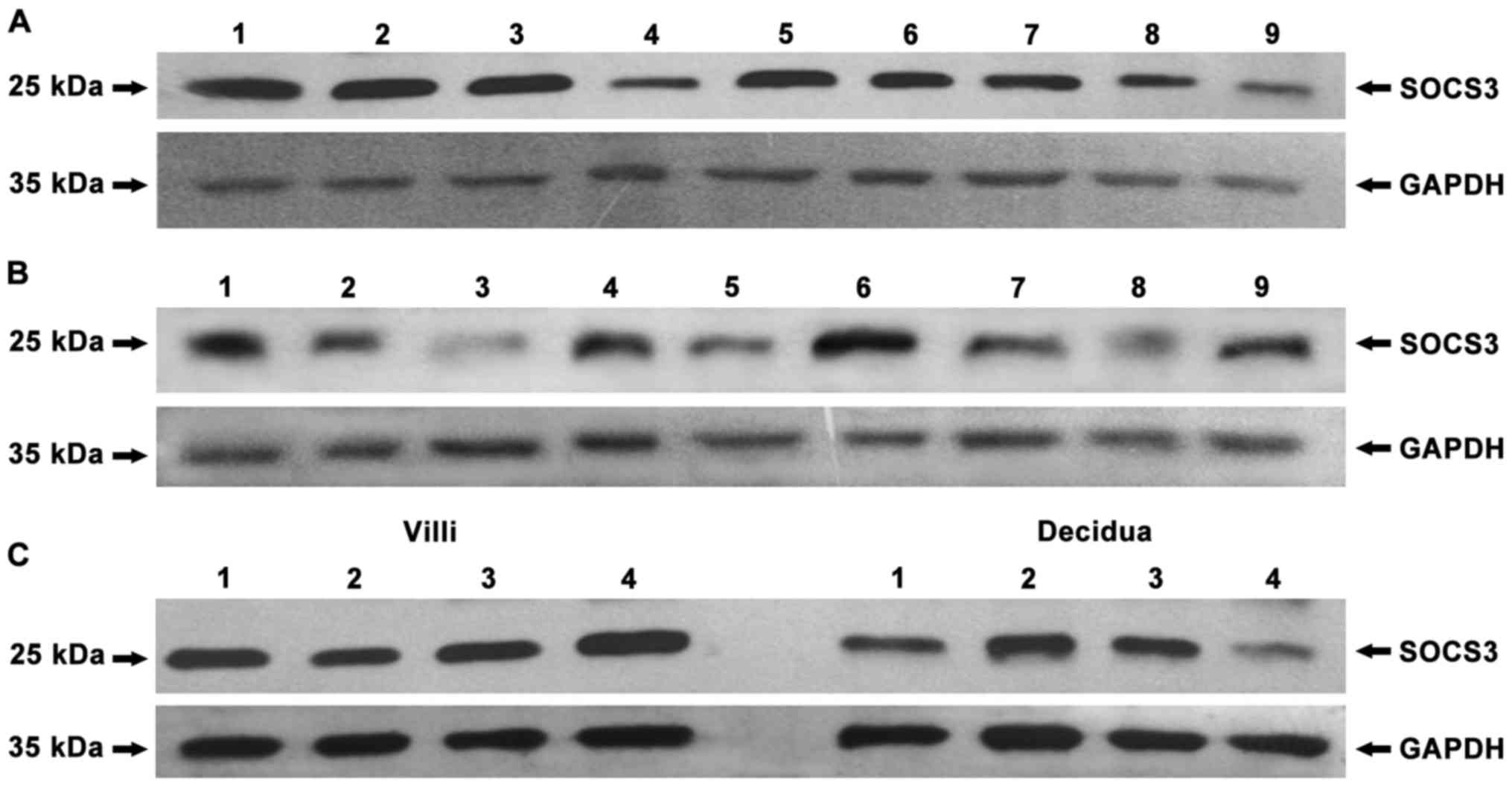
|
1
|
Kudo Y: The role of placental indoleamine
2,3-dioxygenase in human pregnancy. Obstet Gynecol Sci. 56:209–216.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi Y, Carpino N, Cross JC, Torres
M, Parganas E and Ihle JN: SOCS3: An essential regulator of LIF
receptor signaling in trophoblast giant cell differentiation. EMBO
J. 22:372–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carow B and Rottenberg ME: SOCS3, a major
regulator of infection and inflammation. Front Immunol. 5:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norris W, Nevers T, Sharma S and Kalkunte
S: Review: hCG, preeclampsia and regulatory T cells. Placenta. 32
Suppl 2:S182–S185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pazmany T and Tomasi TB: The major
histocompatibility complex class II transactivator is
differentially regulated by interferon-gamma and transforming
growth factor-β in microglial cells. J Neuroimmunol. 172:18–26.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pallotta MT, Orabona C, Volpi C, Vacca C,
Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M,
Bicciato S, et al: Indoleamine 2,3-dioxygenase is a signaling
protein in long-term tolerance by dendritic cells. Nat Immunol.
12:870–878. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu LL, Zhang YH and Zhao FX: Expression of
indoleamine 2,3-dioxygenase in pregnant mice correlates with
CD4+CD25+Foxp3+ T regulatory
cells. Eur Rev Med Pharmacol Sci. 21:1722–1728. 2017.PubMed/NCBI
|
|
8
|
Munn DH and Mellor AL: Indoleamine 2,3
dioxygenase and metabolic control of immune responses. Trends
Immunol. 34:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honig A, Rieger L, Kapp M, Sutterlin M,
Dietl J and Kammerer U: Indoleamine 2,3-dioxygenase (IDO)
expression in invasive extravillous trophoblast supports role of
the enzyme for materno-fetal tolerance. J Reprod Immunol. 61:79–86.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo Y, Boyd CA, Spyropoulou I, Redman CW,
Takikawa O, Katsuki T, Hara T, Ohama K and Sargent IL: Indoleamine
2,3-dioxygenase: distribution and function in the developing human
placenta. J Reprod Immunol. 61:87–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ban Y, Chang Y, Dong B, Kong B and Qu X:
Indoleamine 2,3-dioxygenase levels at the normal and recurrent
spontaneous abortion fetal-maternal interface. J Int Med Res.
41:1135–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boyle K and Robb L: The role of SOCS3 in
modulating leukaemia inhibitory factor signalling during murine
placental development. J Reprod Immunol. 77:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yen MC, Shih YC, Hsu YL, Lin ES, Lin YS,
Tsai EM, Ho YW, Hou MF and Kuo PL: Isolinderalactone enhances the
inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in
breast cancer. Oncol Rep. 35:1356–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mouratidis PX and George AJ: Regulation of
indoleamine 2,3-dioxygenase in primary human saphenous vein
endothelial cells. J Inflamm Res. 8:97–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orabona C, Pallotta MT and Grohmann U:
Different partners, opposite outcomes: A new perspective of the
immunobiology of indoleamine 2,3-dioxygenase. Mol Med. 18:834–842.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trabanelli S, Očadlíková D, Ciciarello M,
Salvestrini V, Lecciso M, Jandus C, Metz R, Evangelisti C,
Laury-Kleintop L, Romero P, et al: The SOCS3-independent expression
of IDO2 supports the homeostatic generation of T regulatory cells
by human dendritic cells. J Immunol. 192:1231–1240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pallotta MT, Orabona C, Volpi C, Grohmann
U, Puccetti P and Fallarino F: Proteasomal degradation of
indoleamine 2,3-dioxygenase in CD8 dendritic cells is mediated by
suppressor of cytokine signaling 3 (SOCS3). Int J Tryptophan Res.
3:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao MR, Qiu W, Li YX, Zhang ZB, Li D and
Wang YL: Dual effect of transforming growth factor β1 on cell
adhesion and invasion in human placenta trophoblast cells.
Reproduction. 132:333–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xuan YH, Choi YL, Shin YK, Ahn GH, Kim KH,
Kim WJ, Lee HC and Kim SH: Expression of TGF-β signaling proteins
in normal placenta and gestational trophoblastic disease. Histol
Histopathol. 22:227–234. 2007.PubMed/NCBI
|
|
20
|
Holzer U, Rieck M and Buckner JH: Lineage
and signal strength determine the inhibitory effect of transforming
growth factor β1 (TGF-β1) on human antigen-specific Th1 and Th2
memory cells. J Autoimmun. 26:241–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones RL, Stoikos C, Findlay JK and
Salamonsen LA: TGF-β superfamily expression and actions in the
endometrium and placenta. Reproduction. 132:217–232. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fallarino F, Orabona C, Vacca C, Bianchi
R, Gizzi S, Asselin-Paturel C, Fioretti MC, Trinchieri G, Grohmann
U and Puccetti P: Ligand and cytokine dependence of the
immunosuppressive pathway of tryptophan catabolism in plasmacytoid
dendritic cells. Int Immunol. 17:1429–1438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanks BA, Holtzhausen A, Evans KS,
Jamieson R, Gimpel P, Campbell OM, Hector-Greene M, Sun L, Tewari
A, George A, et al: Type III TGF-β receptor downregulation
generates an immunotolerant tumor microenvironment. J Clin Invest.
123:3925–3940. 2013. View
Article : Google Scholar : PubMed/NCBI
|