Introduction
It is still not fully understood how a fetus escapes
from being attacked by the maternal immune system. Previous studies
have suggested that the enzyme indoleamine 2,3-dioxygenase (IDO) is
a key protein in the maintenance of maternal-fetal tolerance.
During pregnancy, IDO is secreted by placental trophoblast,
decidual cells and maternal monocyte-macrophages, which inhibit the
T-lymphocyte reaction and mediate the immune tolerance to the fetus
via tryptophan depletion and defective tryptophan catabolism
(1). Recent studies have mainly
focused on the expression and function of IDO. Here, we studied the
relationship of suppressor of cytokine signaling (SOCS)-3 and
transforming growth factor (TGF)-β with IDO expression in chorionic
villi and decidua during pregnancy to investigate the possibility
that the two proteins may regulate the expression of IDO at the
maternal-fetal interface.
SOCS3 is part of a protein family that binds
cytokine receptors, thereby suppressing cytokine signaling. SOCS3
is an essential regulator of leukemia inhibitory factor receptor
signaling in trophoblast differentiation. The expression of SOCS3
is decreased in the placentas of women with intrahepatic
cholestasis, demonstrating its essential role in placental
development (2,3).
TGF-β is abundantly expressed in the endometrium,
epithelial glands and trophoblasts. It plays an important role in
endometrial inflammatory events associated with menstruation and
repair in preparation for implantation, particularly in promoting
the decidualization of endometrial stroma, the maternal support of
embryo development, immunomodulation at the maternal-fetal
interface and the maintenance of normal pregnancy. TGF-β deficiency
can lead to miscarriage or fetal death (4,5).
Studies have shown that, when cells were transfected
with IDO, SOCS3 can downregulate the expression of IDO, while the
expression of IDO can be upregulated in cultured dendritic cells or
plasmacytoid dendritic cells (pDCs) to which TGF-β had been added
(6). Since IDO, SOCS3 and TGF-β are
expressed at the maternal-fetal interface, the aim of this study
was to analyze the expression and possible correlation of the three
proteins in chorionic villi and decidua.
Materials and methods
Ethics statement
This study was conducted according to the principles
expressed in the Declaration of Helsinki. The study was approved by
the Institutional Review Board of the Affiliated Hospital of
Guizhou Medical University (Guizhou, China). All patients provided
written informed consent for the collection of samples and
subsequent analysis.
Patients
Twelve normal pregnant women investigated in the
study were selected from those who underwent legal termination at
the Affiliated Hospital of Guizhou Medical University, Guiyang,
between December2014 and May 2015. This study was approved by the
Ethics Committee of Affiliated Hospital of Guizhou Medical
University. Signed written informed consents were obtained from the
patients and/or guardians. Age, 27.33±3.37 years; gestational age
of the subjects, 62.42±6.30 days. Of all the women, normal
embryonic development was revealed by ultrasonic examination and
those with abnormal reproductive and history of chronic diseases
associated with chronic hypertension, kidney disease and diabetes
were excluded. Aseptic collection of chorionic villi and decidua
(around 100 mg each) was followed by washing with
phosphate-buffered saline (PBS) to remove red blood cells. Tissue
samples were either preserved at −80°C prior to western blot
analysis or immersed in 10% neutral formalin to fix them prior to
immunohistochemical assay.
Western blot analysis
Tissue stored at −80°C was placed in a mortar
containing liquid nitrogen and ground to powder, after which
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck &
Co., Inc., Whitehouse Station, NJ, USA) was added with protease
inhibitors (P8340; Sigma-Aldrich; USA Merck & Co., Inc.). The
lysate was centrifuged twice at 4°C for 30 min at 18,514 × g and
the supernatants were collected. A bicinchoninic acid assay,
protein assay kit (Beyotime Institute of Biotechnology, Haimen,
China) was used to determine protein content separately. Sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was
performed with 12.5% gels to separate the proteins, and gels were
subsequently transferred onto polyvinylidene fluoride (PVDF)
membranes (PerkinElmer, Inc., Waltham, MA, USA) by wet transfer
method at 120 V(200 mA) for 1 h and 50 min. PVDF membranes were
blocked in blocking buffer (Beyotime Institute of Biotechnology)
overnight at 4°C. Blots were incubated with primary antibody
(rabbit anti-human IDO, SOCS3 and TGF-β polyclonal antibody;
1:1,500; cat. nos. 12006, 2932 and 3711, respectively; Cell
Signaling Technology, Inc., Danvers, MA, USA) or human
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal
antibody (1:6,000; cat. no. GTX100118; GeneTex, Inc., Irvine, CA,
USA) for 3.5 h at room temperature, then rinsed with tris-buffered
saline with Tween-20 and incubated with secondary horseradish
peroxidase (HRP)-labeled goat anti-rabbit immunoglobulin G antibody
(PerkinElmer, Inc.). Protein bands were visualized by using the ECL
kit followed by autoradiography.
To determine whether SOCS3 and TGF-β expression were
related to that of IDO, equal amounts of cell lysate extracted from
chorionic villi and decidua were transferred to PVDF membrane
(PerkinElmer, Inc.) and then checked with IDO polyclonal antibody
(1:1,500), following which the membrane was washed with stripping
buffer (Beyotime Institute of Biotechnology) and incubated with
SOCS3, TGF-β or GAPDH primary antibody (1:500).
Immunohistochemistry
Tissue specimens were embedded in paraffin wax and
each sample continuously sliced into 5 µm sections. After dewaxing
and rehydration, slides were immersed in ethylenediaminetetraacetic
acid solution and boiled in an electric pressure cooker (3 min) for
antigen retrieval. Cooling was performed at room temperature and
endogenous peroxidase activity was quenched with 3% hydrogen
peroxide (H2O2). Sections were washed with
0.1 MPBS (pH 7.4) between each step of the immunostaining process.
The primary antibody (mouse anti-human IDO, SOCS3, and TGF-β
monoclonal antibody; cat. nos. ab55305, ab14939 and ab64715,
respectively; Abcam, Cambridge, UK) were diluted with antibody
dilution buffer (Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China) before use (dilution concentration of IDO,
SOCS3 and TGF-β antibody was 1:200, 1:120 and 1:200, respectively)
then incubated overnight at 4°C. Sections were then incubated with
rabbit anti-mouse HRP antibody (1:1,000; cat. no. K5007; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) for 30 min at
37°C. After being washed three times with PBS (pH 7.4),
3,3′-diaminobenzidine (DAB) solution from a DAB kit (Dako; Agilent
Technologies, Inc.) was used as the chromogen. Slides were
counterstained with hematoxylin and dehydrated. Thereafter,
sections were mounted with coverslips. Immunohistochemical images
were evaluated using an Olympus microscope (Olympus, Tokyo, Japan).
The scores of immunohistochemical images were defined as -, +, ++
or +++ if 0–9, 10–24, 25–50 or >50% of the cells were stained
positively, respectively. Immunostaining was scored blindly by two
independent observers with experience in immunohistochemical
pathology.
Statistical analysis
All statistical analyses were performed using the
SPSS statistical software package, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). Pearson's correlation analysis and a paired
samples t-test was used to analyze protein expression from western
blot analysis and the Chi-square test was used to analyze the
results of immunohistochemistry. P<0.05 was considered to
indicate a statistically significant analysis.
Results
Analysis of IDO, SOCS3 and TGF-β
expression in chorionic villi and decidua by western blot
analysis
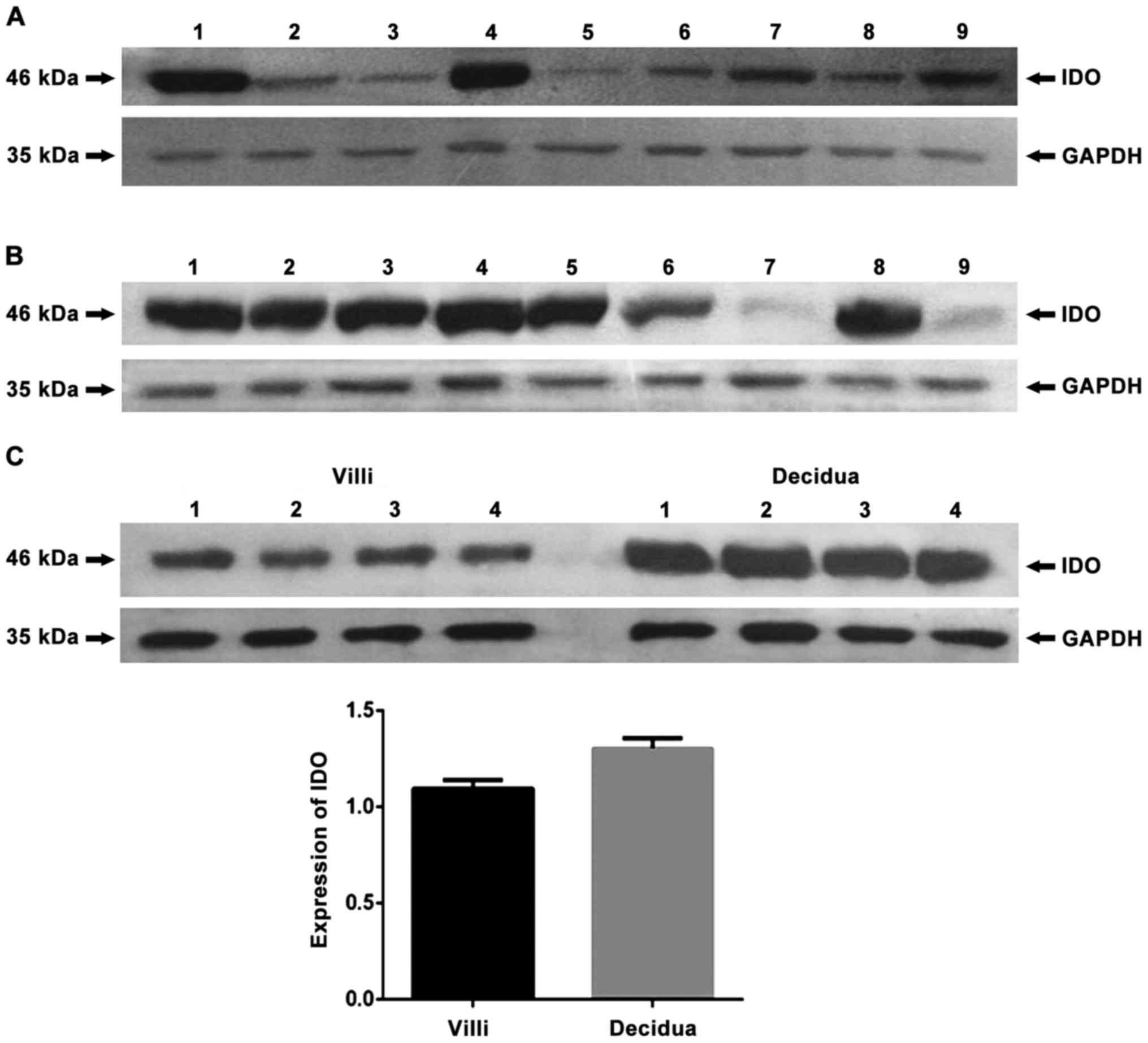
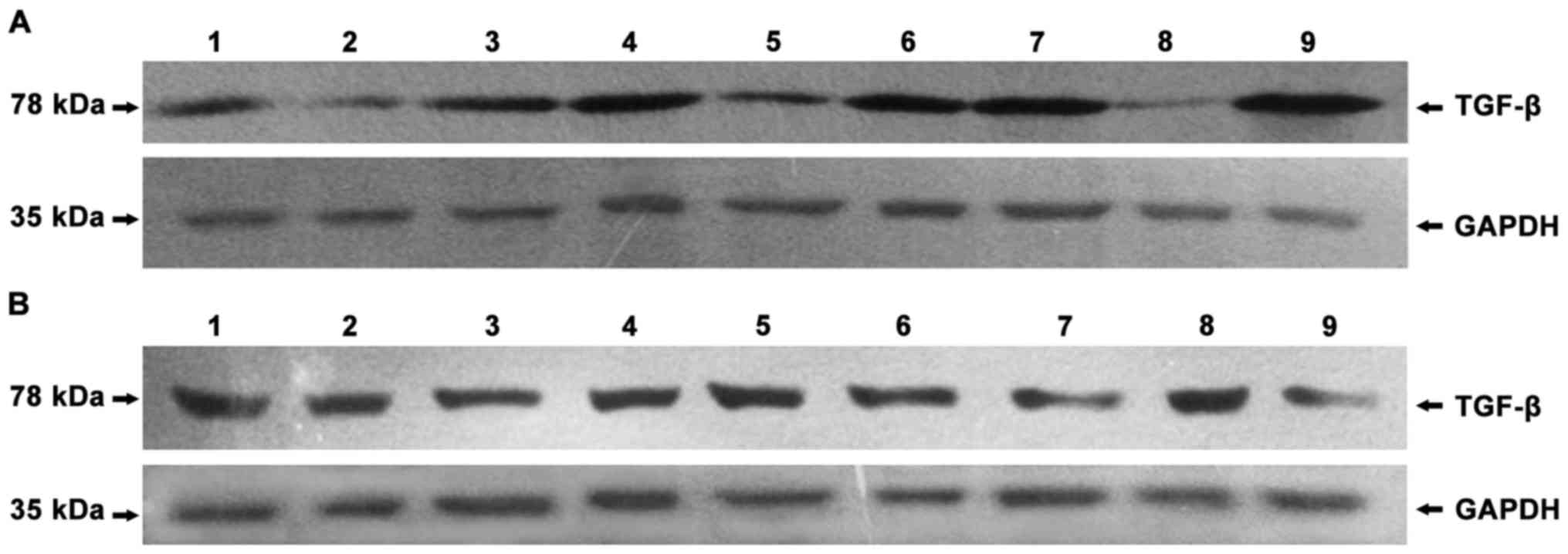
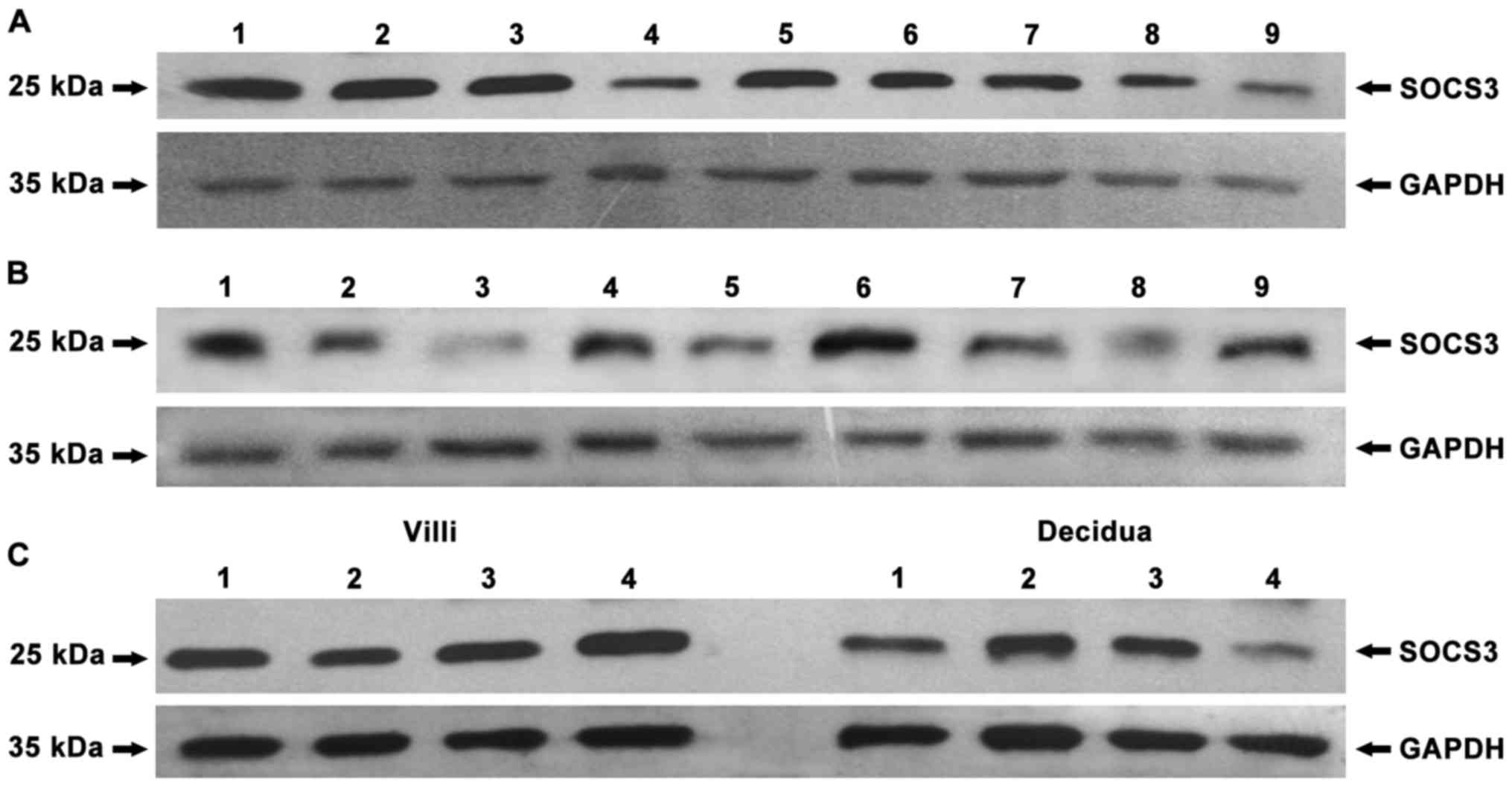
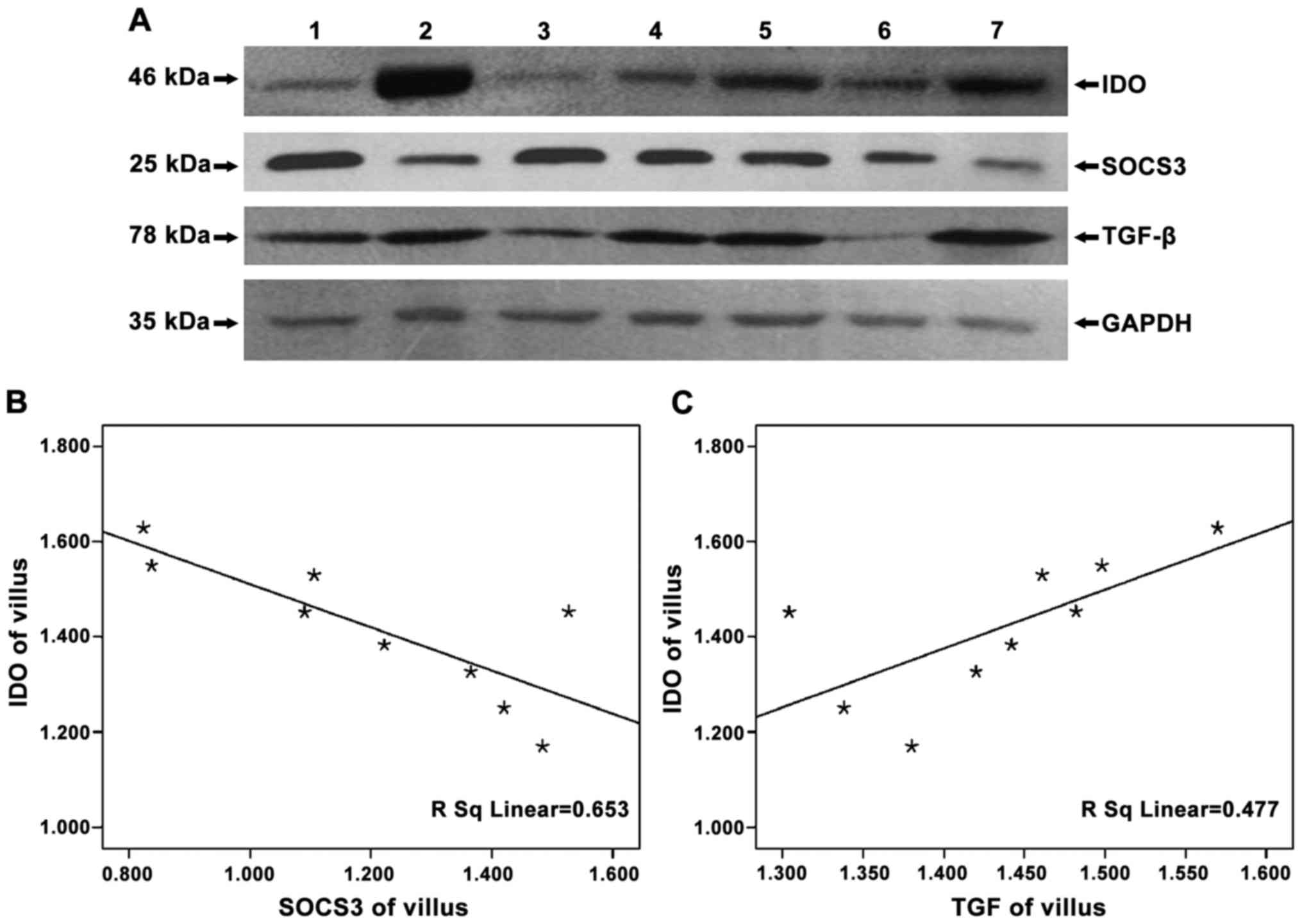
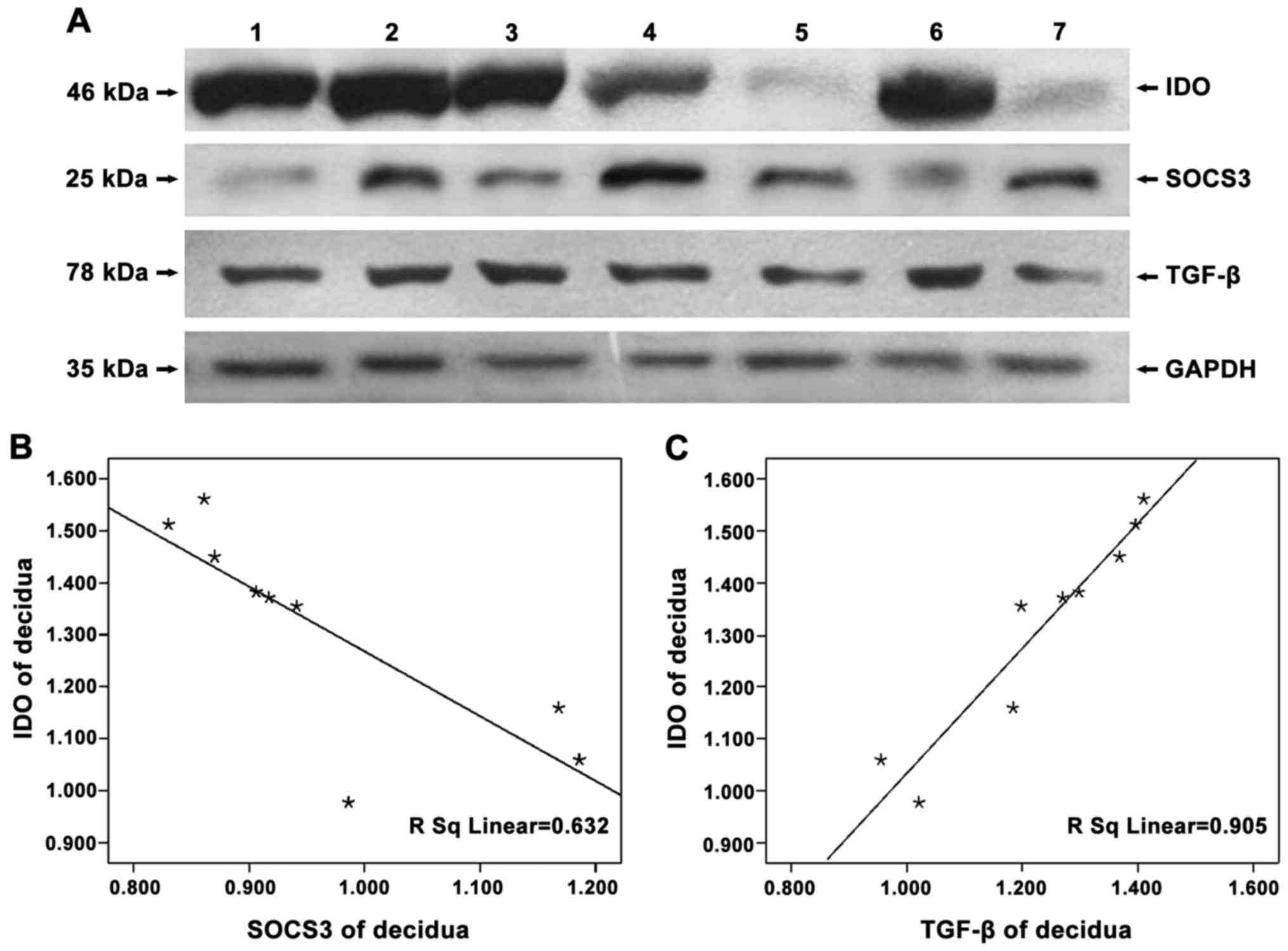
To detect the expression of IDO, SOCS3 and TGF-β in
chorionic villi and decidua of women in early pregnancy, equal
amounts of cell lysates were loaded onto an SDS-PAGE gel, followed
by western blot analysis with IDO, SOCS3 and TGF-β polyclonal
antibodies or rabbit anti-GAPDH polyclonal antibody. The results
showed that, in human peripheral blood mononuclear cells (PBMC),
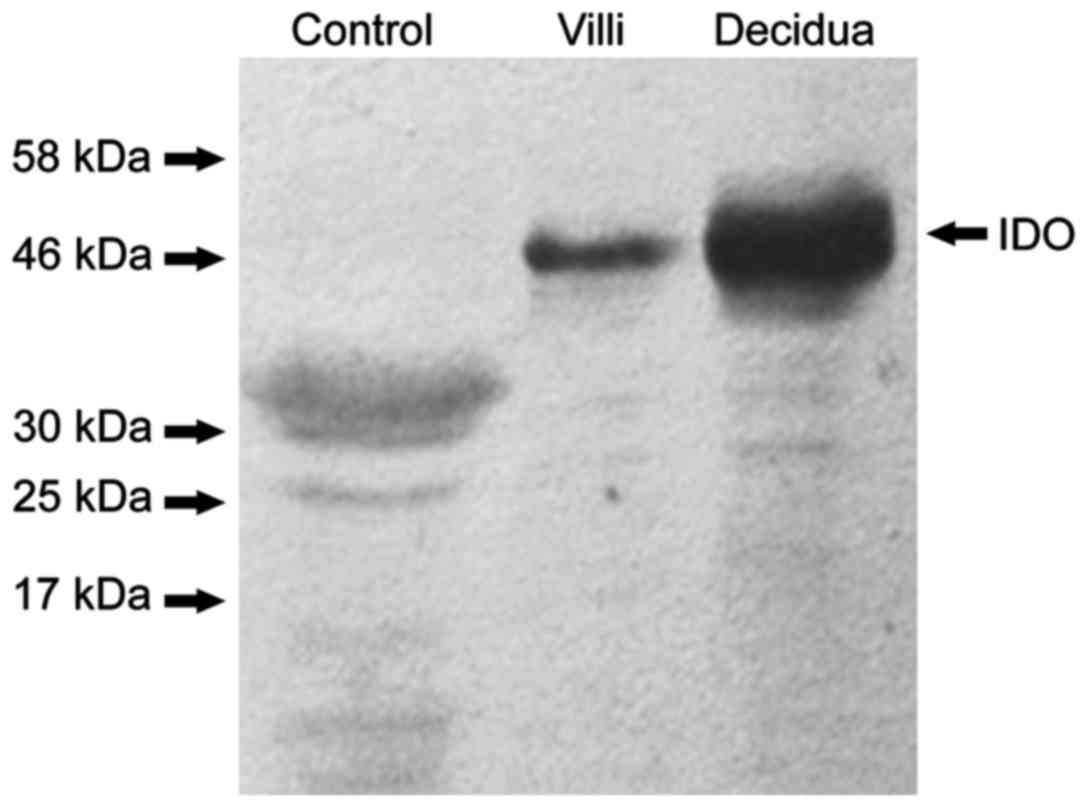
negative control, no protein bands of IDO were found (Fig. 1) and that IDO, SOCS3 and TGF-β were
expressed in chorionic villi (Figs.
2A–4A) and decidua (Figs. 2B–4B).
Expression of IDO was stronger in the decidua than in chorionic
villi (Fig. 2C, P=0.005). No
significant difference in SOCS3 expression was observed between
chorionic villi and decidua (Fig.
3C, P=0.993), while similar amounts of GAPDH were detected.
In examining whether SOCS3 and TGF-β expression were
related to that of IDO, we found that the expression of SOCS3 and
IDO were statistically negatively correlated (chorionic villi,
r=−0.808, P=0.004; decidua, r=−0.841, P=0.005), while that of TGF-β
and IDO were positively correlated (chorionic villi, r=0.690,
P=0.039; decidua, r=0.952, P<0.05) (Figs. 5 and 6).
Analysis of IDO, SOCS3 and TGF-β
expression in chorionic villi and decidua by
immunohistochemistry
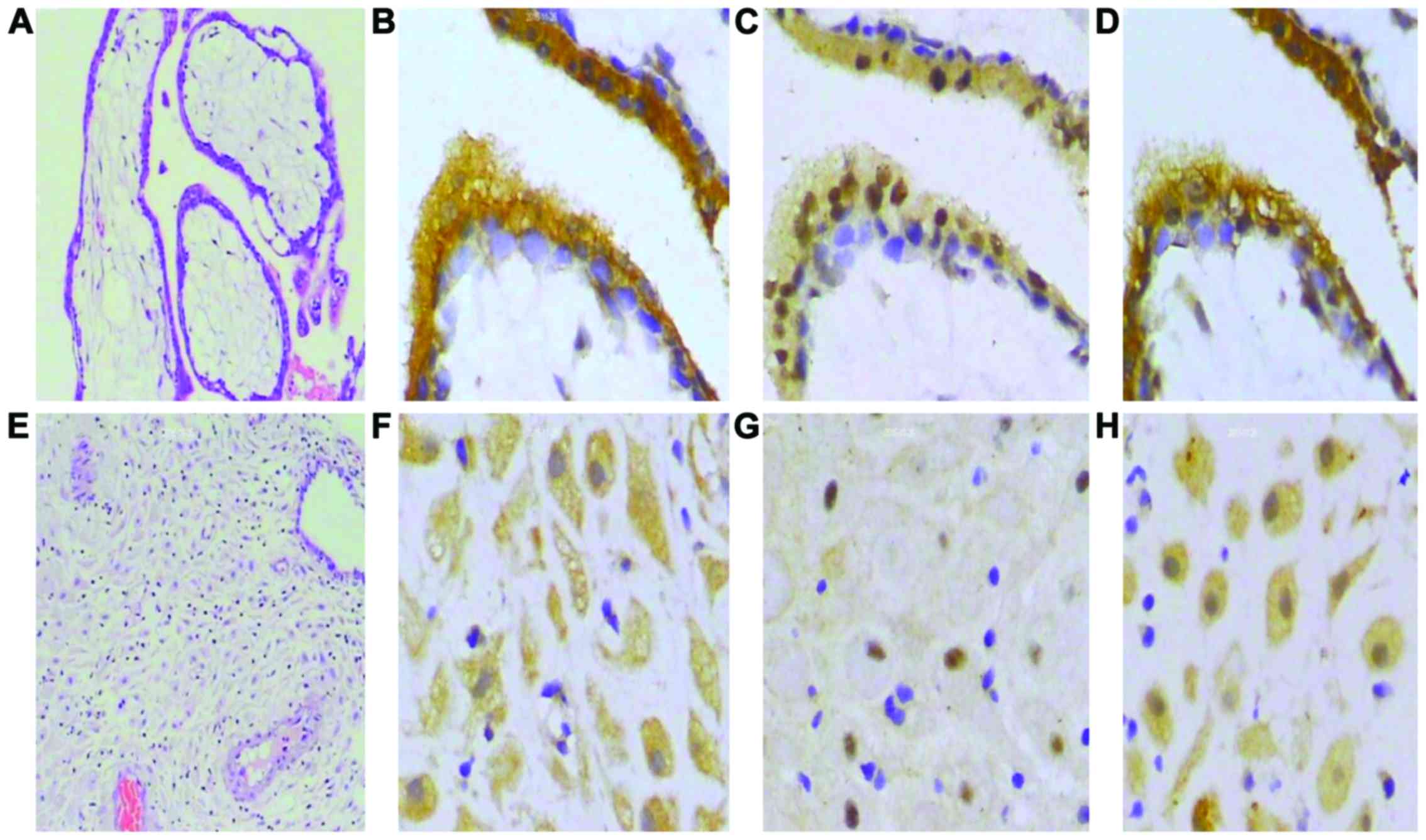
Immunocytochemical staining demonstrated that IDO,
SOCS3 and TGF-β are expressed in chorionic villi and decidua.
Results of immunohistochemistry were examined at ×400 and H&E
staining results were examined at ×100 magnification. Several
representative photomicrographs are shown in Fig. 7. Based on a semiquantitative scoring
system, the expression of IDO and SOCS3 were negatively correlated
(Table I), but there was a positive
correlation between IDO and TGF-β (Table II).
 | Table I.Immunohistochemical staining for IDO
and SOCS3 in chorionic villi and decidua from normal
pregnancies. |
Table I.
Immunohistochemical staining for IDO
and SOCS3 in chorionic villi and decidua from normal
pregnancies.
|
| Y (Intensity of IDO
immunostaining) |
|
|---|
|
|
|
|
|---|
| X (intensity of SOCS3
immunostaining) | + | ++ | +++ | ++++ | Total |
|---|
| + | 2 | 5 | 5 | 1 | 13 |
| ++ | 5 | 6 | 0 | 0 | 11 |
| Total | 7 | 11 | 5 | 1 | 24 |
| Linear-By-linear
association | 6.031 |
|
|
|
|
| Asymp. Sig.
(2-sided) | 0.014 |
|
|
|
|
| Spearman's
correlation | −0.511 |
|
|
|
|
| Approx. Sig. | 0.011 |
|
|
|
|
 | Table II.Immunohistochemical staining for IDO
and TGF-β in chorionic villi and decidua from normal
pregnancies. |
Table II.
Immunohistochemical staining for IDO
and TGF-β in chorionic villi and decidua from normal
pregnancies.
|
| Y(Intensity of IDO
immunostaining) |
|
|---|
|
|
|
|
|---|
| X (intensity of TGF-β
immunostaining) | + | ++ | +++ | Total |
|---|
| − | 2 | 0 | 0 | 2 |
| + | 1 | 5 | 0 | 6 |
| ++ | 2 | 10 | 2 | 14 |
| +++ | 0 | 1 | 1 | 2 |
| Total | 5 | 16 | 3 | 24 |
| Linear-By-linear
association | 6.264 |
|
|
|
| Asymp.Sig
(2-sided) | 0.012 |
|
|
|
| Spearman's
correlation | 0.474 |
|
|
|
| Approx. Sig. | 0.019 |
|
|
|
Discussion
Studies have found that IDO-dependent depletion of
L-tryptophan decreases the number of cytotoxic T-cells, natural
killer (NK) cells and tryptophan metabolites. In particular, the
tryptophan metabolite kynurenine is subsequently involved in the
inhibition of T and NK cells, inducing the generation of regulatory
T(Treg) cells, changing the immune microenvironment in vivo
and directly regulating the immune response (7,8). Our
results indicate that the increased expression of IDO in chorionic
villi and decidua may downregulate the immune response mediated by
local T-cells, which could prevent the rejection of the fetus by
the maternal immune response. Therefore, the factors that regulate
IDO protein levels in terms of mediating maternal-fetal tolerance
at this site are worthy of further investigation. Moreover, Hönig
et al (9)and Kudo et
al (10) have reported that IDO
is expressed in the chorionic villi and decidua of normal human
pregnancies, while IDO protein and mRNA expression in chorionic
villi and decidua have been detected by immunohistochemical and PCR
methods (11). Although different
technical methods were used, the results of our study are
consistent with these previous studies. Increasing evidence
suggests that IDO is expressed in endothelial cells and in
syncytiotrophoblasts of early or late placental tissues and that
IDO is highly expressed in invasive extravillous trophoblast cells
in close contact with the maternal immune system (9). Here, we have used western blot analysis
and immunohistochemical methods to confirm that the expression of
IDO in human decidua is stronger than that in human chorionic
villi, which is in keeping with the above previous studies.
Our results show that SOCS3 is present in chorionic
villi and decidua and that there is no significant difference in
the expression of this protein between the two tissues. Previous
studies have indicated that SOCS3 is involved in trophoblast
differentiation and the formation of the placenta, that
SOCS3-deficient placentas have decreased spongiotrophoblasts and
increased trophoblast secondary giant cells and that SOCS3
negatively regulates trophoblast giant cell differentiation. In
addition, SOCS3 regulates the development of the placenta by
negatively affecting receptor signaling of leukemia inhibitory
factor (2,12).
Studies have demonstrated that SOCS3 is able to
downregulate the expression of IDO in mouse unfractionated
dendritic cells transfected with IDO. The reason for the
downregulation may be that the IDO molecule contains two
immunoreceptor tyrosine-based inhibitory motifs (13,14)with
different molecular chaperone binding, either to promote the
degradation of IDO, or to activate its signaling activity and
maintain the original enzyme activity (15). If SOCS3 anchors in the phosphorylated
ITIM of IDO, IDO is bound to the E3 ubiquitin enzyme complex and
degraded (16,17). If the ITIM on IDO molecules combines
with tyrosine phosphatase, the expression of IDO protein is
enhanced. Our previous study demonstrates that the negative
correlation of expression between SOCS3 and IDO exists in the
chorionic villi and decidua of women in early pregnancy, and
reveals the possibility that SOCS3 may participate in immune
regulation via the degradation of IDO at the maternal-fetal
interface. It is tempting to speculate that the degradation of IDO
may be a new function of SOCS3 in human chorionic villi and
decidua. However, whether or not SOCS3 can degrade IDO at the
maternal-fetal interface requires further study. Although the
expression of SOCS3 and IDO is negatively correlated, the
expression of SOCS3 and IDO coexist. To some extent, IDO remains
stably expressed in human chorionic villi and decidua, suggesting
that other factors may be involved in regulating and maintaining
stable expression of IDO.
TGF-β transmits signals via the Smad and
mitogen-activated protein kinase signaling pathways, which regulate
the proliferation, differentiation and invasion of trophoblast
cells (18) and promote the process
of endometrial decidualization. In this study, western blot
analysis and immunohistochemistry were used to detect the
expression of TGF-β protein in human chorionic villi and decidua of
early gestation and the results were consistent with that of
previous studies (19). Studies have
shown that TGF-β induces immunotolerance by inhibiting B
lymphocytes (20), downregulating
major histocompatibility complex antigen expression in target cells
(5,21) and amplifying T-cells in vivo
and in vitro. Fallarino et al and Pallotta et
al (6,22) found that TGF-β could upregulate IDO
expression in vitro cultured cells. IDO gene silencing
technology can abolish increased expression of IDO by TGF-β. In
contrast, 1-methyl tryptophan, as an IDO inhibitor, cannot inhibit
the upregulation of induced TGF-β. In 2013, Hanks et al
(23) also demonstrated that the
expression of IDO was upregulated by TGF-β in the tumor
microenvironment in animals. Further studies have found that TGF-β
is able to phosphorylate the ITIM of IDO and induce its
upregulation. Our results show that the expression of IDO
concomitantly increases as the expression of TGF-β increases and
that the expression of TGF-β and IDO is positively correlated,
hinting that TGF-β may be involved in maintaining immune tolerance
by upregulating IDO expression at the maternal-fetal interface.
In conclusion, our study demonstrates that IDO,
SOCS3 and TGF-β are expressed in chorionic villi and decidua, where
IDO expression is negatively correlated with SOCS3 and positively
correlated with TGF-β. This suggests a possible role for SOCS3 and
TGF-β in maintaining immunotolerance by regulating IDO expression
at the maternal-fetal interface. Further investigation is needed to
elucidate the mechanisms behind the regulation of IDO expression by
SOCS3 and TGF-β.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360452). We thank
Dr Wenfeng Yu for his help in experimentation.
References
|
1
|
Kudo Y: The role of placental indoleamine
2,3-dioxygenase in human pregnancy. Obstet Gynecol Sci. 56:209–216.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi Y, Carpino N, Cross JC, Torres
M, Parganas E and Ihle JN: SOCS3: An essential regulator of LIF
receptor signaling in trophoblast giant cell differentiation. EMBO
J. 22:372–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carow B and Rottenberg ME: SOCS3, a major
regulator of infection and inflammation. Front Immunol. 5:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norris W, Nevers T, Sharma S and Kalkunte
S: Review: hCG, preeclampsia and regulatory T cells. Placenta. 32
Suppl 2:S182–S185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pazmany T and Tomasi TB: The major
histocompatibility complex class II transactivator is
differentially regulated by interferon-gamma and transforming
growth factor-β in microglial cells. J Neuroimmunol. 172:18–26.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pallotta MT, Orabona C, Volpi C, Vacca C,
Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M,
Bicciato S, et al: Indoleamine 2,3-dioxygenase is a signaling
protein in long-term tolerance by dendritic cells. Nat Immunol.
12:870–878. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu LL, Zhang YH and Zhao FX: Expression of
indoleamine 2,3-dioxygenase in pregnant mice correlates with
CD4+CD25+Foxp3+ T regulatory
cells. Eur Rev Med Pharmacol Sci. 21:1722–1728. 2017.PubMed/NCBI
|
|
8
|
Munn DH and Mellor AL: Indoleamine 2,3
dioxygenase and metabolic control of immune responses. Trends
Immunol. 34:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honig A, Rieger L, Kapp M, Sutterlin M,
Dietl J and Kammerer U: Indoleamine 2,3-dioxygenase (IDO)
expression in invasive extravillous trophoblast supports role of
the enzyme for materno-fetal tolerance. J Reprod Immunol. 61:79–86.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo Y, Boyd CA, Spyropoulou I, Redman CW,
Takikawa O, Katsuki T, Hara T, Ohama K and Sargent IL: Indoleamine
2,3-dioxygenase: distribution and function in the developing human
placenta. J Reprod Immunol. 61:87–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ban Y, Chang Y, Dong B, Kong B and Qu X:
Indoleamine 2,3-dioxygenase levels at the normal and recurrent
spontaneous abortion fetal-maternal interface. J Int Med Res.
41:1135–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boyle K and Robb L: The role of SOCS3 in
modulating leukaemia inhibitory factor signalling during murine
placental development. J Reprod Immunol. 77:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yen MC, Shih YC, Hsu YL, Lin ES, Lin YS,
Tsai EM, Ho YW, Hou MF and Kuo PL: Isolinderalactone enhances the
inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in
breast cancer. Oncol Rep. 35:1356–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mouratidis PX and George AJ: Regulation of
indoleamine 2,3-dioxygenase in primary human saphenous vein
endothelial cells. J Inflamm Res. 8:97–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orabona C, Pallotta MT and Grohmann U:
Different partners, opposite outcomes: A new perspective of the
immunobiology of indoleamine 2,3-dioxygenase. Mol Med. 18:834–842.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trabanelli S, Očadlíková D, Ciciarello M,
Salvestrini V, Lecciso M, Jandus C, Metz R, Evangelisti C,
Laury-Kleintop L, Romero P, et al: The SOCS3-independent expression
of IDO2 supports the homeostatic generation of T regulatory cells
by human dendritic cells. J Immunol. 192:1231–1240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pallotta MT, Orabona C, Volpi C, Grohmann
U, Puccetti P and Fallarino F: Proteasomal degradation of
indoleamine 2,3-dioxygenase in CD8 dendritic cells is mediated by
suppressor of cytokine signaling 3 (SOCS3). Int J Tryptophan Res.
3:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao MR, Qiu W, Li YX, Zhang ZB, Li D and
Wang YL: Dual effect of transforming growth factor β1 on cell
adhesion and invasion in human placenta trophoblast cells.
Reproduction. 132:333–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xuan YH, Choi YL, Shin YK, Ahn GH, Kim KH,
Kim WJ, Lee HC and Kim SH: Expression of TGF-β signaling proteins
in normal placenta and gestational trophoblastic disease. Histol
Histopathol. 22:227–234. 2007.PubMed/NCBI
|
|
20
|
Holzer U, Rieck M and Buckner JH: Lineage
and signal strength determine the inhibitory effect of transforming
growth factor β1 (TGF-β1) on human antigen-specific Th1 and Th2
memory cells. J Autoimmun. 26:241–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones RL, Stoikos C, Findlay JK and
Salamonsen LA: TGF-β superfamily expression and actions in the
endometrium and placenta. Reproduction. 132:217–232. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fallarino F, Orabona C, Vacca C, Bianchi
R, Gizzi S, Asselin-Paturel C, Fioretti MC, Trinchieri G, Grohmann
U and Puccetti P: Ligand and cytokine dependence of the
immunosuppressive pathway of tryptophan catabolism in plasmacytoid
dendritic cells. Int Immunol. 17:1429–1438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanks BA, Holtzhausen A, Evans KS,
Jamieson R, Gimpel P, Campbell OM, Hector-Greene M, Sun L, Tewari
A, George A, et al: Type III TGF-β receptor downregulation
generates an immunotolerant tumor microenvironment. J Clin Invest.
123:3925–3940. 2013. View
Article : Google Scholar : PubMed/NCBI
|